Abstract
Background and purpose:
The histamine H4 receptor is widely expressed in cells of immune origin and has been shown to play a role in a variety of inflammatory processes mediated by histamine. In this report, we describe the in vitro and in vivo anti-inflammatory properties of a potent histamine H4 receptor antagonist, A-940894 (4-piperazin-1-yl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine).
Experimental approach:
We have analysed the pharmacological profile of A-940894 at mouse native, rat recombinant and human recombinant and native, histamine H4 receptors by radioligand binding, calcium mobilization, mast cell shape change, eosinophil chemotaxis assays and in the mouse model of zymosan-induced peritonitis.
Key results:
A-940894 potently binds to both human and rat histamine H4 receptors and exhibits considerably lower affinity for the human histamine H1, H2 or H3 receptors. It potently blocked histamine-evoked calcium mobilization in the fluorometric imaging plate reader assays and inhibited histamine-induced shape change of mouse bone marrow-derived mast cells and chemotaxis of human eosinophils in vitro. In a mouse mast cell-dependent model of zymosan-induced peritonitis, A-940894 significantly blocked neutrophil influx and reduced intraperitoneal prostaglandin D2 levels. Finally, A-940894 has good pharmacokinetic properties, including half-life and oral bioavailability in rats and mice.
Conclusions and Implications:
These data suggest that A-940894 is a potent and selective histamine H4 receptor antagonist with pharmacokinetic properties suitable for long-term in vivo testing and could serve as a useful tool for the further characterization of histamine H4 receptor pharmacology.
Keywords: histamine, H4, selective antagonist, A-940894, receptor binding, mast cells, eosinophils, shape change, chemotaxis, zymosan
Introduction
The histamine H4 receptor is the most recently identified member of the G-protein coupled histamine receptor family, displaying distinct molecular and pharmacological properties from H1, H2 and H3 histamine receptor subtypes (Oda et al., 2000; Morse et al., 2001; nomenclature follows Alexander, 2008). The mRNA for H4 receptors is expressed in a number of peripheral tissues, including bone marrow and spleen (Oda et al., 2000; Morse et al., 2001) and at the cellular level, histamine H4 receptor mRNA is also expressed by several immune cells, including eosinophils, monocytes, neutrophils, mast cells, dendritic cells, basophils and T-cells (Oda et al., 2000; Morse et al., 2001; Damaj et al., 2007) where the histamine H4 receptor may play a role in chemotactic and inflammatory processes (Thurmond et al., 2008), although the precise function of histamine H4 receptors in regulating inflammation is not well understood.
Histamine H4 receptors have been implicated in rodent models of acute inflammation, including zymosan-induced peritonitis and experimental colitis as well as in acute inflammatory pain (G.C. Hsieh et al., unpubl. experiments; Takeshita et al., 2004; Thurmond et al., 2004; Varga et al. 2005). In a rodent asthma model, blockade of H4 receptors reduced eosinophil infiltration while mast cell redistribution and eosinophilia have been associated with allergic rhinitis (Fokkens et al., 1992; Slater et al., 1996). Thus, it has been postulated that diseases associated with immune cell-mediated responses, such as asthma, allergic rhinitis, rheumatoid arthritis, inflammatory bowel disease and autoimmune diseases, may be potential targets for treatment with H4 receptor antagonists (Daugherty, 2004; Fung-Leung et al., 2004; Zhang et al., 2006; Thurmond et al., 2008).
Thioperamide, originally characterized as a histamine H3 receptor antagonist, was the first H4 antagonist identified when it was shown to block H4 receptor-mediated functional properties (Liu et al., 2001a; Morse et al., 2001), although at concentrations that also antagonize H3 receptors. The discovery of JNJ7777120 (1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine) as the first orally active, potent and selective non-imidazole H4 receptor antagonist (Jablonowski et al., 2003) allowed a significant advance in the understanding of the pharmacology and function of H4 receptors both in in vitro and in vivo (Thurmond et al., 2004). Other selective, non-imidazole H4 antagonists, such as the benzimidazole analogue VUF6002, have also been recently described (Coruzzi et al., 2007). More recently, structurally different non-imidazoles, such as A-943931 and A-987306 (Cowart et al., 2008; Liu et al., 2008; D. G. Witte et al., unpubl. experiments), have been shown to behave as potent and selective H4 receptor antagonists in vitro and in vivo. The aim of this report is to describe the pharmacological properties of A-940894, a potent and selective H4 receptor antagonist with favourable pharmacokinetic and in vivo anti-inflammatory properties in rodents.
Methods
Animals
Animals for experiments conducted in house were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities at Abbott Laboratories in a temperature-regulated environment with lights on between 6:00 AM and 6:00 PM. All experimental protocols were approved by Abbott's Institutional Animal Care and Use Committee. Male Sprague–Dawley rats were supplied by Charles River Laboratories (Portage, MI, USA). Female BALB/c mice were obtained from Taconic (Hudson, NY, USA), while WBB6F1/J-KitW/KitW-v (W/Wv mast cell deficient mice) and their normal controls, WBB6F1/J-KitW, were from Jackson Laboratories (Bar Harbor, ME, USA). The animals were acclimated to laboratory conditions for at least 1 week before testing.
Cloning and expression of histamine H4 receptors
All reagents used for cell culture were acquired from Invitrogen (Carlsbad, CA, USA) unless otherwise indicated. Human embryonic kidney (HEK293) cells were from ATCC (Manassas, VA, USA). Full-length human histamine H4 receptor cDNA was cloned using total RNA isolated from the HL-60 human promyelocytic leukaemia cell line (ATCC, Manassas, VA, USA) with RT-PCR methods using primers designed according to the published human H4 receptor gene sequences to give a sequence identical to accession # AY136745. Full-length rat and mouse H4 receptor cDNAs were cloned using either rat spleen poly-A+ (Clonetech, Mountain View, CA, USA) or mouse bone marrow total RNA (Zyagen, San Diego, CA, USA) to give sequences identical to accession # AF358860 and AF358859 respectively. All cDNAs were subcloned into the pCI-neo expression vector (Promega, Madison, WI, USA). Human, rat and mouse vectors were transfected into a clonal host HEK293 cell line stably expressing the chimeric Gαqi5 protein, to re-direct histamine H4 receptor signalling to intracellular calcium release (Yao et al., 2003), using LIPOFECTAMINE 2000 (Invitrogen, Carlsbad, CA, USA). Stable cell lines expressing these receptors were established using standard molecular techniques and were used in a fluorometric imaging plate reader (FLIPR) assay. In addition to that, rat histamine H4 receptor cDNA was subcloned into pFLAG-CMV-4 (Sigma, Saint Louis, MO, USA) and stable cell line expressing a FLAG-tagged rat H4 receptors (rat H4-FLAG) was also established using HEK293 cells. These cell lines were used in radioligand and [35S]-GTPγS binding assays.
Membrane preparations
Cells expressing histamine H4 receptors were harvested and homogenized in TE buffer (50 mmol·L−1 Tris-HCl, 5 mmol·L−1 EDTA, pH 7.4) using a Polytron at 20 000 r.p.m. for 2 × 10 s bursts in the presence of protease inhibitors [1 mmol·L−1 benzamidine, 2 µg·mL−1 aprotinin, 1 µg·mL−1 leupeptin and 1 µg·mL−1 pepstatin (Sigma-Aldrich)], followed by centrifugation at 40 000×g. The homogenization and centrifugation steps were repeated as described above to further purify the membrane pellets. Final membrane preparations were obtained by re-homogenizing the pellets in 6.25 volumes (weight/volume) of TE buffer and frozen at −70°C until used. For the rat H4-FLAG receptor cell lines, final membrane preparations included glycerol and bovine serum albumin (BSA) at a final concentration of 10% and 1% respectively.
Radioligand binding assays
For competition binding, membrane preparations from cells expressing human histamine H4 receptors or rat H4-FLAG receptors were incubated with [3H]-histamine (12–20 nmol·L−1) in the presence or absence of increasing concentrations of ligands for H4 receptors. Incubations were in a final volume of 0.5 mL TE buffer at 25°C and were terminated after 30 min by rapid filtration. Non-specific binding was determined in presence of 10 µmol·L−1 JNJ7777120. In saturation binding experiments Bmax of the human H4 receptor cell line was found to be 216 fmol·mg−1 and Kd of [3H]-histamine 26 nmol·L−1 (pKd= 6.83 ± 0.09). For the rat H4-FLAG cell line Bmax= 1624 fmol·mg−1 and Kd= 109 nmol·L−1 (pKd= 7.70 ± 0.04, data not shown). Radioligand binding assays for cloned human histamine H1, H2 and H3 receptors were performed as described (Strakhova and Esbenshade, 2006) using [3H]-mepyramine, [3H]-tiotidine and [3H]-N-α-methylhistamine respectively. All binding reactions were terminated by filtration under vacuum onto polyethylenimine (0.3%) pre-soaked Unifilters (Perkin Elmer Life Sciences, Boston, MA, USA) or Whatman GF/B filters (for histamine H4 receptors) followed by three brief washes with 2 mL of ice-cold TE buffer. Bound radiolabel was determined by liquid scintillation counting.
For all of the radioligand competition binding assays, IC50 values and Hill slopes were determined by Hill transformation of the data as previously described (Strakhova and Esbenshade, 2006) and pKi values were determined by the Cheng–Prusoff equation (Cheng and Prusoff, 1973) using Kd values derived from saturation studies.
[35S]-GTPγS binding assays
For assays to determine antagonist activity, membranes expressing rat H4-FLAG receptors were diluted in GTPγS assay buffer (25 mmol·L−1 HEPES, 2.5 mmol·L−1 MgCl2 and 75 mmol·L−1 NaCl, pH 7.4) and 10 µg of membrane protein was incubated in a 96-well deep-well block in the presence of 5.0 mmol·L−1 unlabelled GDP, approximately 0.5 nmol·L−1 of [35S]-GTPγS, histamine (10 µmol·L−1) and increasing concentrations of H4 receptor antagonists. Samples were incubated at 37°C for 5 min. The assays were terminated by the addition of cold buffer (50 mmol·L−1 Tris-HCl, 75 mmol·L−1 NaCl and 2.5 mmol·L−1 MgCl2, pH 7.6) and subsequent harvesting onto a Packard Unifilter 96-well GF/B plate from Perkin Elmer Life Sciences. After extensive washing, the plates were dried, Microscint 20 was added to the samples and the amount of bound [35S]-GTPγS was determined utilizing the Topcount (Perkin Elmer Life Sciences). The percentage of [35S]-GTPγS bound in each sample was calculated as a percentage of that bound to control samples (basal or histamine-stimulated) incubated in the absence of histamine H4 receptor ligands. Triplicate determinations were obtained at each concentration and the data were analysed using GraphPad Prism (San Diego, CA, USA) to obtain IC50 values and Hill slopes. Kb values were calculated from IC50 values using the generalized Cheng–Prusoff equation (Cheng and Prusoff, 1973; Leff and Dougall, 1993). The mean with 95% confidence limits of at least three independent experiments is reported.
Measurement of intracellular calcium levels
Intracellular calcium responses were measured in cell lines co-expressing Gαqi5 and either human, rat or mouse H4 receptors, using FLIPR methods as previously described, with modifications (Esbenshade et al., 2003). Briefly, the FLIPR assay was modified by setting the FLIPR temperature to 37°C, with cell plates and drug plates pre-warmed in a 37°C incubator for 10 min before placement in the FLIPR. Chlorpheniramine (10 µmol·L−1) was added to the incubation buffer to mask any H1 receptor-mediated responses. Antagonist potencies (Kb) were obtained by measuring inhibition of a reference response (1000, 300 and 100 nmol·L−1 histamine for rat, mouse and human H4 cell lines respectively). Antagonists were added 5 min prior to addition of histamine. Peak response values for the antagonist wells were expressed as a percentage of the reference peak response for histamine in the absence of H4 receptor antagonists. Experiments were performed in duplicate wells, and data were analysed using GraphPad Prism to obtain IC50 values. The generalized Cheng–Prusoff equation (Cheng and Prusoff, 1973; Leff and Dougall, 1993) was used to determine Kb values, which are presented as the mean with 95% confidence limits.
Eosinophil chemotaxis assay
Venous blood from healthy volunteers was sampled according to local ethics committee-approved protocol. To determine the ability of compounds to block histamine H4 receptor-mediated chemotaxis of human eosinophils, eosinophils from individual subjects were isolated and processed separately following the isolation as outlined below. Aliquots of heparin-treated whole venous blood were slowly layered over 20 mL of Polymorphprep (Accurate Chemical and Scientific Corp., Westbury, NY, USA) and centrifuged at 870×g for 25 min at 4°C. The upper platelet rich fraction and the upper mononuclear cell band were discarded, while the polymorphonuclear cell band and the lower ‘red zone’ were collected. The collected cells were washed in cold autoMACS® rinsing solution (Miltenyi Biotec, Auburn, CA, USA) containing 0.5% BSA and centrifuged at 1150×g for 5 min at 4°C. Following centrifugation, supernatants were decanted and the red cells were lysed in 500 µL H2O for 30 s. Immediately following lysis, cells were diluted in cold rinsing solution and lysis was repeated as above until all red cells were depleted. After the final lysis, cells were resuspended in cold rinsing solution with 0.5% BSA at a final concentration of 1 × 106 cells·µL−1 and labelled for at least 30 min with an equal volume of cold CD16 magnetic microbeads (Miltenyi Biotec) at 4°C. After labelling, cells were washed and resuspended in 500 µL cold rinsing solution with 0.5% BSA. Eosinophils were purified through MACS® CS separation columns (Miltenyi Biotec) by negative selection. Eosinophils (which do not bind to the magnetic CD16 microbeads) were eluted with 30 mL of cold RPMI 1640 media (Invitrogen) with 0.5% BSA and centrifuged at 300×g for 10 min at 4°C. Cell pellet was resuspended at 1.2 × 106–2 × 106 cell·mL−1 in warm RPMI 1640 with 0.5% BSA. In order to verify purity, cell aliquots were stained using an eosinophil stain kit (ENG Scientific, Inc. Clifton, NJ, USA).
To measure eosinophil chemotaxis, the bottom chamber of the chemotaxis plate was filled, in quintuplicate, with 300 µL of RPMI 1640 with 0.5% BSA (vehicle), 1 µmol·L−1 histamine alone or test compounds in 1 µmol·L−1 histamine, and was separated from the top chamber by a 5 µm filter (Neuro Probe, Gaithersburg, MD, USA) coated with 100 ng·mL−1 fibronectin. Purified eosinophils were resuspended at 60 000–100 000 cells in 50 µL of RPMI 1640 with 0.5% BSA with or without test compounds and added to the top of the chamber. Chemotaxis plates were incubated for 90 min in a 37°C 5% CO2 incubator. Eosinophil migration from the top chamber to the bottom chamber was quantified using Cell Titer-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA). Data were analysed using Prism GraphPad software.
Zymosan-induced peritonitis
Female BALB/c mice were used to establish zymosan-induced peritonitis and study the effects of histamine H4 receptor antagonists. JNJ7777120 and A-940894 were given at 1–100 mg·kg−1 s.c. (n= 5 per group) in saline 30 min prior to i.p. administration of 2 mg zymosan (Sigma-Aldrich) in saline (0.5 mL per mouse). For analysis of histamine and cytokine content, animals were killed 1.5 h after the challenge, and the lavage fluids were collected by washing peritoneal cavities with 3 mL of phosphate-buffered saline (pH 7.2) with 10 units heparin·mL−1. The samples were centrifuged at ∼100×g, 4°C for 5 min and supernatants were removed and frozen for future analysis. Dexamethasone at a dose of 10 mg·kg−1 (dissolved in water) was also included in the same study. For analysis of neutrophil influx and RNA, animals were killed at various time points after the zymosan challenge, lavages were conducted as described above and cell pellets were preserved. Dexamethasone-induced inhibition of myeloperoxidase (MPO) and prostaglandin (PG)D2 levels was considered a full response.
Assay of MPO activity
The lavage samples were centrifuged at ∼100×g, 4°C for 5 min. The cell pellets were brought up in 1 mL of 50 mmol·L−1 potassium phosphate buffer (pH 6.0) containing 0.5% (hexadecyltrimethylammonium bromide), freeze-thawed three times, and centrifuged at ∼100×g for 10 min at room temperature. At this point the pellets were discarded and supernatants were frozen and stored at −70°C until future use. MPO assays were performed as described previously (Bradley et al., 1982) with some modifications to accommodate a 96-well format. The reaction was initiated by the addition of assay buffer containing tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO), allowed to proceed for 15 min at room temperature, then stopped with 1 mol·L−1 H2SO4. The absorbance was read at 450 nmol·L−1.
Determination of histamine and prostaglandin D2 content
Concentration of histamine in the peritoneal lavages was determined using histamine EIA kit (IBL, Minneapolis, MN, USA) according to the manufacturer's recommendations. Prostaglandin D2 (PGD2) was assayed using EIA kits from Cayman Chemicals (Ann Arbor, MI, USA) following the manufacturer's recommendations.
RNA isolation and real-time quantitative RT-PCR
For analysis of H4 mRNA expression, lavages were conducted as described above and were flash-frozen and stored at −70°C. Samples were crushed and homogenized in 5 mL Trizol and 5 µL RNaseout (Invitrogen). RNA was isolated using Trizol according to the manufacturer's protocol. Quantitative determination of RNA expression levels was performed by quantitative RT-PCR (qRT-PCR) TaqMan® technology. Gene Expression Assays (Applied Biosystems, Foster City, CA, USA), containing primers and FAM™ dye-labelled TaqMan® MGB probe were used to detect mouse histamine H4 receptor (Mm00467633_ml) and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mm99999915_g1). For qRT-PCR analysis, RNA was converted into cDNA using the ABI high-capacity cDNA reverse transcription kit. cDNA was run in a 20 µL reaction using Taqman Universal PCR Master Mix (Invitrogen). Cycling parameters: 10 min at 95°C, and then 45 cycles of 15 s at 95°C, 1 min at 60°C. Data were collected during each extension phase of the PCR reaction and analysed with the ABI-7000 SDS software package (Applied Biosystems). Each sample was run in triplicate and normalized using the GAPDH data.
Bone marrow mast cell culture
The femurs from BALB/c female mice (aged 8–10 weeks) were flushed with cold RPMI 1640. Cells were collected, filtered through a 100 µm filter and centrifuged (∼250×g, 5 min). After treatment with PharmLyse (Pharmingen, San Jose, CA, USA) to lyse red blood cells, the cells were washed twice with cold RPMI 1640 and plated at 1 × 105–106 cells·mL−1 in complete RPMI medium (RPMI 1640 containing 5 mmol·L−1 glutamine, 0.1 mmol·L−1 non-essential amino acids, 160 units·mL−1 penicillin, 160 µg·mL−1 streptomycin, 50 µmol·L−1β-mercaptoethanol and 10 ng·mL−1 recombinant murine IL-3). After each week, non-adherent cells were removed, centrifuged (∼250×g, 5 min), fresh media added and transferred to a new dish for further culture. After 4 weeks, cells were analysed by flow cytometry for FcεRI and c-kit (CD117) expression. Mast cells from 4–7 week cultures were >99% positive for both FcεRI and c-kit and were used in gated autofluorescence forward scatter experiments.
Shape change (gated autofluorescence forward scatter) assay
Cells prepared as described above were centrifuged (∼250×g, 5 min) and resuspended at 2 × 106 cells·mL−1 in RPMI 1640 + 0.1% BSA. A total of 140 µL of cells were incubated in the presence or absence of antagonists for 5 min at 37°C. Cells were stimulated with histamine (10 µmol·L−1 final concentration) for 10 min at 37°C, then fixed with 1.5% paraformaldehyde on ice to maintain cell shape. Fluorescence was measured by flow cytometry (Becton Dickinson FACSCaliber, Becton Dickinson, San Jose, CA, USA). Bone marrow mast cells were gated by their autofluorescence and 10000 events were acquired for each sample. Results were calculated as % increase in forward scatter compared with unstimulated cells. Raw data were analysed using GraphPad Prism® 4 software. IC50 values for antagonists were calculated using non-linear regression and Kb values calculated using a modified Cheng–Prusoff equation (Leff and Dougall, 1993).
Pharmacokinetics
Male Sprague-Dawley-derived rats, weighing 250–400 g, were obtained from Charles Rivers Laboratories. The animals were fasted overnight prior to dosing and throughout the first 8 h of the study period. Dosing solutions were prepared as a 10 mg·mL−1 solution in 10% EtOH: 5% dextrose in water. All animals received a 10 mg·kg−1 (1 mL·kg−1) dose by i.v. or oral administration. Samples were analysed in our Exploratory Kinetics and Analysis Department. Briefly, compounds were selectively removed from the plasma using protein precipitation with acetonitrile at neutral pH. The samples were vortexed vigorously followed by centrifugation. The supernatant was evaporated to dryness with a gentle stream of nitrogen over low heat (∼35°C). The samples were reconstituted by vortexing with mobile phase. A-940894 and its internal standard were separated from co-extracted contaminants on a 50 mm × 3 mm 5 µ Luna CN column with an acetonitrile/1% acetic acid/0.1% trifluoroacetic acid (47.6:46.0:6.4, by volume) mobile phase at a flow rate of 0.4 mL·min−1 with a 25 µL injection. Compounds were quantified using MRM detection with a turbo ionspray source on a mass spectrometer (PE Sciex Applied Biosystems, Foster City, CA). The limit of quantification was approximately 13 ng·mL−1 for rat plasma samples. Plasma binding was determined using rat whole plasma.
Materials
A-940894 (4-piperazin-1-yl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine) was synthesized at Abbott Laboratories as previously described (Altenbach et al., 2008). ABT-239 ([4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile) was synthesized at Abbott Laboratories (Abbott Park, IL, USA). JNJ7777120 was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). [3H]-histamine (30–60 Ci·mmol−1) was from Amersham Biosciences Inc. (Piscataway, NJ, USA). [3H]-N-(-methylhistamine (45–90 Ci·mmol−1), [3H]-pyrilamine (20–30 Ci·mmol−1), [3H]-tiotidine (70–90 Ci·mmol−1), [3H]-histidine (40–60 Ci·mmol−1) and [35S]-GTP(S (1250 Ci·mmol−1) were obtained from PerkinElmer Life and Analytical Sciences (Boston, MA, USA). Thioperamide was purchased from Tocris Cookson Inc. (Ellisville, MO, USA); histamine, loratadine, terfenadine and cimetidine were from Sigma-Aldrich.
Results
Radioligand binding competition studies
The histamine H4 receptor antagonist A-940894 (Figure 1) exhibited high affinity for recombinant human and rat H4 receptors with respective Ki values of 71 and 7.6 nmol·L−1 (Table 1). This compound is highly selective over the other histamine receptor subtypes; for example, it exhibits nearly 70-fold selectivity for the human H4 receptor versus human H1 and H2 receptors (Ki > 5000 nmol·L−1) and also exhibits lower affinity for human (Ki= 3900 nmol·L−1) and rat (Ki= 483 nmol·L−1) H3 receptors. Additional selectivity of A-940894 was evaluated across a panel of over 85 receptors, kinases and ion channels (CEREP and in-house). At 10 µmol·L−1, A-940894 inhibited specific binding at the following human receptors: β1-adrenoceptors (77%), M4 muscarinic (83%), µ-opioid (90%), several 5 HT receptors, namely 5-HT1A (92%, Ki= 890 nmol·L−1), 5-HT1B (99%), 5-HT3 (98%) receptors and the 5-HT transporter (93%). JNJ7777120 displayed high affinity towards recombinant human and rat histamine H4 receptors with Ki values of 12 and 3.1 nmol·L−1 respectively, but low affinity towards H1, H2 and H3 receptors (Ki > 1000 nmol·L−1).
Figure 1.

Chemical structure of A-940894 (4-piperazin-1-yl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine).
Table 1.
Binding affinities of A-940894 and JNJ7777120 at recombinant human or rat histamine H1, H2, H3 and H4 receptors
| Receptor subtype | Ki (nmol·L−1) | |
|---|---|---|
| A-940894 | JNJ7777120 | |
| Human H4 | 71a | 12 |
| (26–196)b | (2–63) | |
| Rat H4 | 7.6 | 3.1 |
| (5.1–11.4) | (1.6–6.0) | |
| Human H1 | >5000 | 2767 |
| (1087–7048) | ||
| Human H2 | >5000 | >5000 |
| Human H3 | 3899 | 2210 |
| (2582–5902) | (1696–2882) | |
| Rat H3 | 483 | 1382 |
| (333–702) | (1083–1764) | |
n≥ 3.
95% confidence interval.
Recombinant H4 receptor functional antagonism
The antagonist profile of A-940894 was assessed by measuring intracellular calcium mobilization in cell lines co-expressing human, rat and mouse H4 receptors along with the chimeric G-protein, Gαqi5. Histamine gave robust dose-dependent responses with EC50 values of 17 nmol·L−1 (pEC50= 7.77 ± 0.01) for human, 328 nmol·L−1 (pEC50=6.49 ± 0.01) for rat and 50 nmol·L−1 (pEC50=7.23 ± 0.04) for mouse H4 receptor cell lines respectively. A-940894 blocked histamine-evoked intracellular calcium responses at human, rat and mouse H4 receptor cell lines with Kb values of 74, 15 and 18 nmol·L−1 respectively (Table 2) while JNJ7777120 provided Kb values ranging from 2.7 to 4.7 nmol·L−1 at the three species. The functional character of A-940894 was also determined in a [35S]-GTPγS binding assay using membranes from cells expressing the rat H4-FLAG receptor. Histamine stimulated basal [35S]-GTPγS binding in a dose-dependent manner, with a mean EC50 value of 2.6 µmol·L−1, producing maximal efficacy of 208% of basal. A-940894 had no effect on basal [35S]-GTPγS binding up to a concentration of 100 µmol·L−1 and as such, did not display agonist or inverse agonist activity in this assay, but did display dose-dependent inhibition (Kb= 60 nmol·L−1) of histamine-stimulated [35S]-GTPγS binding (Table 2). JNJ7777120 exhibited dose-dependent inhibition of histamine-stimulated [35S]-GTPγS binding (Kb= 62 nmol·L−1).
Table 2.
Functional properties of A-940894 and JNJ7777120
| Receptor and assay | Kb (nmol·L−1) |
|
|---|---|---|
| A-940894 | JNJ7777120 | |
| Human H4– FLIPR | 74a | 4.7 |
| (60–92)b | (4.4–5.0) | |
| Rat H4– FLIPR | 15 | 2.8 |
| (10–22) | (2.6–3.1) | |
| Mouse H4– FLIPR | 18 | 2.7 |
| (15–23) | (2.0–3.5) | |
| Rat H4– GTPγS | 60 | 66 |
| (32–112) | (64–68) | |
| Mouse mast cells – shape change | 316 | 594 |
| (210–476) | (336–1054) | |
n≥ 3.
95% confidence interval.
FLIPR, fluorometric imaging plate reader.
Functional antagonism at native histamine H4 receptors in vitro
The ability of A-940894 to inhibit native rodent and human H4 receptor-mediated responses in immune cells was also evaluated. First, we established a histamine-induced shape change using mouse bone marrow-derived mast cells. In these experiments, histamine-induced shape change in a dose-dependent manner giving an average EC50 value of 3.7 µmol·L−1 (pEC50= 5.42 ± 0.01, n= 7). A-940894 blocked this response in a concentration-dependent manner with a resultant IC50 value of 1.3 ± 0.1 µmol·L−1 (Kb value of 316 nmol·L−1, see Table 2) and complete blockade at 10 µmol·L−1 (Figure 2). Similarly, histamine response was blocked by JNJ7777120 with a mean IC50 value of 4.0 ± 1.0 µmol·L−1 (Kb= 594 nmol·L−1, Table 2, Figure 2). A-940894 also behaved as an antagonist at the native human H4 receptors, inhibiting chemotaxis of human eosinophils towards 1 µmol·L−1 histamine (Figure 3). JNJ7777120 behaved as an antagonist in this assay as well, displaying dose-dependent inhibition of histamine-stimulated chemotaxis (Figure 3), in good agreement with the previously reported data (Ling et al., 2004). Chemotaxis of purified eosinophils towards different concentrations of histamine in these experiments was observed with a mean EC50 value of 36 nmol·L−1 (pEC50= 7.54 ± 0.13, n= 6), in line with data previously reported by Ling et al. (2004).
Figure 2.

Representative dose–response curves for inhibition of histamine-induced shape change of mouse bone marrow-derived mast cells by A-940894 and JNJ7777120. Changes in shape change were determined as described in the Methods and are presented as % shape change. Data represent the mean ± SEM for each concentration and are representative of three individual experiments.
Figure 3.

Representative dose–response curves for inhibition of histamine-evoked eosinophil chemotaxis by A-940894 and JNJ7777120. Chemotaxis experiments were performed as described in the Methods. Cell migration was analysed in quintuplicate for each concentration point. Data represent the means ± SEM of the percent control response (stimulation with 1 µmol·L−1 histamine) and from one of two individual experiments.
Pharmacokinetics
The pharmacokinetic parameters for A-940894 are summarized in Table 3. A-940894 had a range of t1/2 values across multiple administration routes in rats and mice (Table 3). Oral bioavailability for A-940894 after oral dosing was 37% and 33% in rat and mouse respectively, but considerably higher after i.p. dosing (10 mg·kg−1) in rat. Following s.c. administration of A-940894 (10 mg·kg−1) and JNJ7777120 (20 mg·kg−1), maximum plasma concentration (Cmax) levels were 2.52 and 2.56 µmol·L−1, respectively, in mouse and rat. Serum protein binding for A-940894 was 66% and 50.6% in rat and human respectively.
Table 3.
Pharmacokinetic properties of A-940894
| Species | Route of administration and dose (mg·kg−1) | t1/2(h) | Cmax(µmol·L−1) | Vβ(L·kg−1) | F (%) |
|---|---|---|---|---|---|
| Mouse | i.v. (5) | 4.9 | 0.71 | 88.9 | – |
| p.o. (10) | 6.8 | 0.13 | – | 33 | |
| s.c. (10) | 3.3 | 2.52 | – | – | |
| Rat | i.v. (10) | 2.2 | 4.16 | 20 | – |
| p.o. (10) | 3.4 | 0.51 | – | 37 | |
| i.p. (10) | 3.1 | 1.63 | – | 78 |
Zymosan-induced peritonitis
The relevance of the zymosan-induced peritonitis model to histamine H4 receptor biology was first established by determining the levels of H4 receptor in peritoneal lavage of wild-type mice. qRT-PCR analysis of peritoneal exudate cells showed that there was expression of H4 receptor mRNA in cells derived from peritoneal lavage of naïve BALB/c. This level of expression was significantly lower in cells derived from WBB6F1/J-KitW/KitW-v (W/Wv mice) that are devoid of mast cells, whereas H4 receptor expression levels in littermate control WBB6F1/J-KitW wild type were similar to that found in BALB/c (Figure 4A). These data indicate that histamine H4 receptors were associated predominantly with the mast cells in peritoneal lavage.
Figure 4.
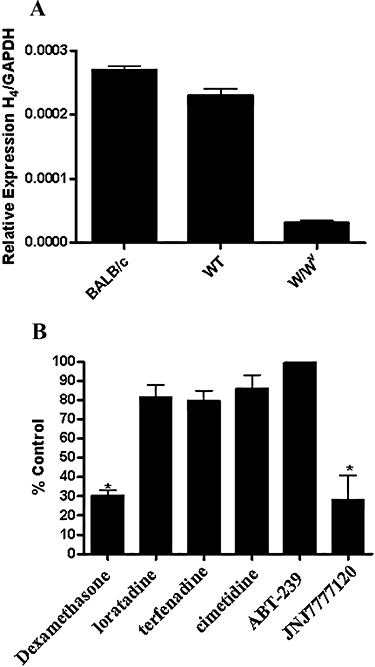
(A) Expression of histamine H4 receptor mRNA in lavaged peritoneal cells from naïve BALB/c, wild-type WBB6F1/J-KitWand mast cell-deficient WBB6F1/J-KitW/KitW-v (W/Wv) mice. Levels of mRNA were determined as described in the Methods and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels for each mRNA sample. Data represent the mean ± SEM for n= 5 animals for each group. (B) Effect of histamine receptor subtype antagonists on zymosan-induced neutrophil influx in BALB/c mice. Animals were given the drugs [H1 antagonists, loratadine and terfenadine (30 mg·kg−1 p.o.); H2 antagonist, cimetidine (30 mg·kg−1 p.o.); H3 antagonist, ABT-239 (10 mg·kg−1 i.p.); H4 antagonist, JNJ7777120 (30 mg·kg−1 s.c.) and dexamethasone (10 mg·kg−1 p.o.)] 30 min prior to zymosan (2 mg per animal). Lavage fluid was collected 4 h later and myeloperoxidase (MPO) activity measured as described in the Methods. Data represent the mean ± SEM of the per cent control MPO activity for five to six animals for each group.
Intraperitoneal challenge with zymosan-induced leukocyte accumulation, predominantly of neutrophils (>80% of total leukocytes) in the mouse peritoneum, consistent with previous descriptions of the model (Doherty et al., 1985). The presence of inflammatory mediators in the peritoneal lavage fluid was evaluated at 15, 30, 45, 90, 180, 240 and 360 min after zymosan administration (Figure 5). Both histamine and PGD2 levels continued to increase in a time-dependent manner through 6 h time point, reaching values corresponding to 10- and 17-fold increases over basal levels (7.8 ng·mL−1 for histamine and 3.8 ng·mL−1 for PGD2). Additionally, the increases in both histamine and PGD2 appeared to occur in two phases with the first phase peaking at 90 min for histamine and 30 min for PGD2.
Figure 5.
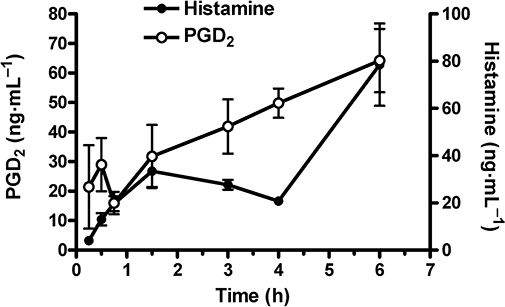
Time course for increases in histamine and prostaglandin D2 (PGD2) content of peritoneal lavage fluid obtained from mice given zymosan (2 mg per animal i.p.). Levels of histamine and PGD2 were determined as described in Methods. Basal levels of histamine and PGD2 were 7.8 ± 0.8 ng·mL−1 and 3.8 ± 1.7 ng·mL−1 respectively. Data represent the mean ± SEM for five animals per time point.
Profiling of the histamine receptor subtypes capable of mediating zymosan-induced neutrophil influx in BALB/c mice revealed that the H1 receptor antagonists loratadine and terfenadine (30 mg·kg−1 p.o.), the H2 receptor antagonist cimetidine (30 mg·kg−1 p.o.) and the H3 receptor antagonist ABT-239 (10 mg·kg−1 i.p.) had no effect on neutrophil influx (Figure 4B). Only the H4 receptor antagonist JNJ7777120 (30 mg·kg−1 s.c.) significantly reversed neutrophil influx (72% inhibition), to levels comparable to those obtained with dexamethasone (10 mg·kg−1 p.o.).
A-940894, given s.c., 30 min prior to injection of zymosan, resulted in an almost complete inhibition of neutrophil MPO activity in lavages collected 4 h after zymosan administration. With responses to dexamethasone set to 100% efficacy, A-940894 reduced MPO activity by 111%, 120% and 111% (P < 0.01) at 1, 3 and 10 mg·kg−1 respectively (Figure 6A) relative to the dexamethasone control, while JNJ7777120, at 10, 30 and 100 mg·kg−1 s.c., showed significant inhibition of 88%, 98% and 86% (P < 0.01) (Figure 6B). PGD2 levels were also determined in lavage fluid collected 1.5 h after zymosan administration. A-940894 dose-dependently reduced PGD2 levels by 65% and 114% (P < 0.01) at 3 and 10 mg·kg−1, relative to the dexamethasone control, while no reduction was observed at 1 mg·kg−1 (Figure 6C). JNJ7777120, given at 10, 30 and 100 mg·kg−1 s.c., significantly decreased PGD2 levels at all doses tested by 87%, 91% and 936% (P < 0.01) respectively (Figure 6D). Similar results were observed for PGE2 levels (data not shown). This inhibition was selective for PGs, as no alteration in levels of other inflammatory mediators, including cysteinyl leukotrienes, leukotriene B4 or the chemokine CXCL1, was observed (data not shown). A selective reduction in PGD2 was also observed in the mast cell-deficient W/Wv mice.
Figure 6.

Inhibitory effects of increasing i.p. doses of A-940894 and JNJ7777120 on zymosan-induced, mouse peritoneal neutrophil myeloperoxidase (MPO) activity and prostaglandin D2 (PGD2) levels in mice. MPO activity (units·mL−1 lavage) and levels of PGD2 (ng·mL−1) were determined as described in Methods. Data represent the means ± SEM for five animals per group. Significant differences compared with vehicle group are indicated as *P < 0.05.
Discussion and conclusions
The newly identified, non-imidazole histamine H4 receptor antagonist A-940894 was found to bind with high affinity to rat and human recombinant H4 receptors and is over 50-fold selective for the human histamine H4 receptor versus other histamine receptor subtypes, an in vitro profile similar to previously described histamine H4 receptor antagonists A-943931, A-987306 and JNJ7777120 (Jablonowski et al., 2003; Cowart et al., 2008; Liu et al., 2008). A broad selectivity binding screen at high concentration of the compound revealed interactions with several additional binding sites (e.g. β1-adrenoceptor, M4 muscarinic, µ opioid, 5-HT1A, 5-HT1B, 5-HT3 receptors and the 5-HT transporter). However, because these sites are not usually associated with inflammatory responses discussed in this report, their involvement in the A-940894-induced responses is unlikely. In cells co-expressing the chimeric Gαqi5 protein with human, rat or mouse histamine H4 receptors, A-940894 potently and dose-dependently blocked histamine-evoked intracellular calcium responses. Comparable potencies at the rat and mouse H4 receptors (15–18 nmol·L−1) and somewhat lower potency (74 nmol·L−1) for the human receptor were demonstrated, reflecting the 10-fold lower binding affinity of A-940894 for the human H4 receptor when compared with the rat receptor. Pharmacological species differences have been observed for other histamine H4 receptor ligands, including histamine itself (Liu et al., 2001b). A-940894 also displayed potent antagonism in [35S]-GTPγS binding in cell membranes expressing rat H4 receptors, dose-dependently inhibiting histamine-induced [35S]-GTPγS binding (Kb= 60 nmol·L−1). Interestingly, in the absence of histamine, this compound did not affect basal GTPγS binding activity (data not shown); thus it is not an H4 receptor inverse agonist in this assay, nor a partial agonist at the rat H4 receptor.
We further characterized the ability of A-940894 to antagonize histamine-induced responses using native H4 receptor systems. A-940894 was able to inhibit both histamine-mediated shape change of the bone marrow-derived mouse mast cells and histamine-induced chemotaxis of purified human eosinophils. Interestingly, in the shape change assay with mouse mast cells, A-940894 displayed in vitro potencies that were ∼17-fold lower than those determined in the mouse recombinant H4 receptor-mediated intracellular calcium assay. The prototypic H4 receptor antagonist JNJ7777120 (Jablonowski et al., 2003) also exhibits substantially lower potency in antagonizing native histamine H4 receptor responses in comparison with recombinant receptors. Whether these lower potencies seen at the native H4 receptor are reflective of differences in signalling responses between the native and recombinant receptors, conformational differences between the receptors, or other factors, remains to be determined. Mast cells are known to be one of the main sources of histamine in the body, so it is conceivable that continuous exposure of H4 receptors to histamine over the period of time that is required for generation of mature mast cells in culture could lead to H4 receptor desensitization. Nevertheless, A-940894 effectively blocks H4 receptor-mediated immune cell responses in a concentration-dependent manner in vitro, including mouse mast cell shape change and human eosinophil chemotactic responses. These findings are consistent with the known expression of histamine H4 receptors in differentiated mast cells derived from mouse bone marrow (Hofstra et al., 2003) and in human eosinophils (Buckland et al., 2003; Ling et al., 2004) as well as the findings that JNJ7777120 inhibits calcium mobilization and chemotaxis in these cells (Hofstra et al., 2003; Ling et al., 2004; Thurmond et al., 2004). A variety of inflammatory disease states, such as asthma and inflammatory bowel disease, are linked to infiltration of eosinophils; thus blockade of this response via H4 receptor antagonism may offer a novel means of treating these ailments (Varga et al., 2005; Dunford et al., 2006). Overall, the in vitro findings demonstrate that A-940894 is a potent and selective histamine H4 receptor antagonist at recombinant human, rat and mouse H4 receptors as well as at native histamine H4 receptors expressed in mouse mast cells and human eosinophils.
A commonly used model of acute inflammation is the mouse model of zymosan-induced peritonitis that culminates in peritoneal neutrophil influx (Doherty et al., 1985; Ajuebor et al., 1998; Ajuebor et al., 1999). This model has previously been shown to be both mast cell- (Kolaczkowska et al., 2001a) and histamine H4 receptor-dependent (Thurmond et al., 2004). Further characterization of this model in our laboratories revealed that along with an increase in peritoneal neutrophils upon exposure to zymosan, a concomitant increase in inflammatory mediators including histamine and PGD2 was also seen. Basal levels of histamine increased 10-fold from 7.8 to 80 ng·mL−1 over a 6 h time course in a time-dependent manner while PGD2 levels increased 17-fold from 3.7 to over 60 ng·mL−1. Interestingly, the increases in these inflammatory mediators appeared to be biphasic. Histamine showed an initial early spike in levels at 1.5 h after zymosan administration followed by a decrease in levels to 4 h and another large increase by 6 h. In contrast, PGD2 levels showed an earlier initial spike in levels, by 30 min following zymosan administration, a decline in levels at 45 min, followed by a steady increase in PGD2 levels up to 6 h. Whether these time-dependent fluctuations in the levels of these inflammatory mediators reflect the time-dependent recruitment and activation of mast cells, neutrophils or other immune cells and the subsequent release from these cells remains to be determined. Additional evidence supporting the critical role of the histamine H4 receptor in mediating mast cell-dependent inflammatory events was provided by the finding that the level of H4 receptor mRNA obtained from lavaged peritoneal cells from naïve animals was more than sevenfold lower in mast cell-deficient W/Wv mice when compared with their normal WBB6F1/J-KitW controls or with BALB/c mice. This suggests that resident mast cells may be the predominant H4 receptor-expressing cell type in the peritoneum that is responsible for mediating inflammatory reactions to substances such as zymosan. Additionally, further characterization of the histamine receptor subtypes responsible for mediating zymosan-induced influx of neutrophils into the peritoneum confirmed previous findings that the H4 receptor mediated this response. Our results also demonstrated that the H4 receptor was the only histamine receptor subtype involved as antagonists of H1, H2 and H3 receptors did not block this inflammatory response.
We have shown here that in addition to the direct inhibitory effect on H4-mediated mast cell effects in vitro, the H4 antagonist A-940894 has potent in vivo anti-inflammatory effects, completely blocking neutrophil influx in the mouse zymosan-induced peritonitis model at doses as low as 1 mg·kg−1 s.c. Interestingly, A-940894 also diminished PGD2 levels in a dose-dependent fashion with complete reversal of the increased levels at 10 mg·kg−1, although now inhibition of PDG2 responses was observed at 1 mg·kg−1. A-940894 also inhibited increases in PGE2 levels that were seen in this model (data not shown), but had no effect on leukotrienes or inflammatory chemokine CXCL1, suggesting that H4 receptors may play a role in regulating PG production in mast cells. This hypothesis is further supported by the finding that PGD2 levels are reduced in mast cell-deficient mice. Similarly, JNJ7777120 blocked peritoneal neutrophil influx induced by zymosan at 10 mg·kg−1 (as has been previously demonstrated by Thurmond et al., 2004) and also blocked the increases in PGD2 levels. The inhibition of PGD2 by both A-940894 and JNJ7777120 suggests that histamine H4 receptors may play a role in regulating PG production in mast cells. However, as A-940894 appears to be 3–10 times less potent as an inhibitor of PGD2 output, compared with its inhibition of neutrophil influx, it is also possible that this effect is due to secondary pharmacology of the antagonists towards an unknown target. The favourable pharmacokinetic properties of A-940894 across multiple routes of administration in mouse and rat (t1/2 from 2.2 to 6.8 h in rat and mouse dosed p.o. good bioavailability) predicted that this compound would be useful in vivo for determining the H4 receptor-dependent anti-inflammatory properties of this compound. The longer half-life of A-940894 in rats and especially mice, a commonly used animal model of inflammation, in comparison with other H4 receptor antagonists such as JNJ7777120 (mouse t1/2= 0.5–1.4 h, data not shown), suggests that this compound would be potentially more useful to determine the effects of long-term blockade of H4 receptors by chronic dosing in inflammatory disease states than previously reported H4 receptor antagonists.
Our current study as well as previously reported findings (Ajuebor et al., 1999; Kolaczkowska et al., 2001a,b; Hofstra et al., 2003; Thurmond et al., 2004; Godot et al., 2007) strongly suggest several common participants with respect to the zymosan-induced peritonitis model of inflammation. These include mast cells, the H4 receptor, histamine and other inflammatory mediators. It has been hypothesized, and findings to date seem to indicate, that histamine H4 receptors expressed on mast cells may regulate mast cell chemotaxis as well the synthesis and release of pro-inflammatory mediators, including PGs, chemoattractants (i.e. leukotriene B4) and possibly other mediators (chemokines and cytokines). These mediators can then modulate the activity of other local immune cells, such as macrophages, as well as recruit infiltrating cell types, such as neutrophils and eosinophils, to further mediate the inflammatory response. The findings that H4 receptor antagonists such as A-940894 can inhibit immune cell chemotaxis in vitro and can reduce inflammatory responses in vivo by decreasing the influx of neutrophils and local levels of inflammatory mediators such as PGD2 following an inflammatory response suggest that the H4 receptor plays an important role in mediating inflammatory processes.
In conclusion, we have demonstrated that A-940894 is a potent histamine H4 receptor antagonist with at least a 50-fold selectivity over other histamine receptor subtypes. Additionally, A-940894 blocks the activation of histamine-stimulated effects in cells involved in inflammatory processes, including H4 receptor-mediated shape change of mouse bone marrow-derived mast cells as well as human eosinophil chemotaxis. This compound also exhibits potent in vivo anti-inflammatory activity, including antagonism of H4 receptor-mediated zymosan-induced peritonitis and concomitant inhibition of increases in the intraperitoneal levels of the inflammatory mediator PGD2. A-940894 exhibits favourable pharmacokinetic properties with a longer half-life in mouse than that for other described H4 receptor antagonists that should allow coverage to determine the role of H4 receptors in chronic inflammation models. Together with other H4 receptor antagonists, A-940894 will serve as a useful tool compound to further investigate the role of histamine H4 receptors in inflammatory responses and the potential utility of this target for drug treatment of human inflammatory disease states.
Glossary
Abbreviations:
- A-940894
(4-piperazin-1-yl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine)
- ABT-239
[4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile
- JNJ7777120
1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine
- Cmax
maximum concentration
- FLIPR
fluorometric imaging plate reader
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HEK293
human embryonic kidney cells
- MPO
myeloperoxidase
- PGD2
prostaglandin D2
- qRT-PCR
quantitative RT-PCR
- Vβ
volume of distribution
Statement of conflict of interest
All authors are employees of Abbott Laboratories, which funded the research.
References
- Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, et al. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63:108–116. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- Altenbach RJ, Adair RM, Bettencourt BM, Black LA, Fix-Stenzel SR, Gopalakrishnan SM, et al. Structure-activity studies on a series of a 2-aminopyrimidine-containing histamine H4 Receptor ligands. J Med Chem. 2008;51:6547–6557. doi: 10.1021/jm8005959. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Buckland KF, Williams TJ, Conroy DM. Histamine induces cytoskeletal changes in human eosinophils via the H4 receptor. Br J Pharmacol. 2003;140:1117–1127. doi: 10.1038/sj.bjp.0705530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Adami M, Guaita E, de Esch IJ, Leurs R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur J Pharmacol. 2007;563:240–244. doi: 10.1016/j.ejphar.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Cowart MD, Altenbach RJ, Liu H, Hsieh GC, Drizin I, Milicic I, et al. Rotationally constrained 2,4-diamino-5,6-disubstituted pyrimidines: a new class of histamine H4 receptor antagonists with improved druglikeness and in vivo efficacy in pain and inflammation models. J Med Chem. 2008;51:6571–6580. doi: 10.1021/jm800670r. [DOI] [PubMed] [Google Scholar]
- Damaj BB, Becerra CB, Esber HJ, Wen Y, Maghazachi AA. Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. J Immunol. 2007;179:7907–7915. doi: 10.4049/jimmunol.179.11.7907. [DOI] [PubMed] [Google Scholar]
- Daugherty BL. Histamine H4 antagonism: a therapy for chronic allergy? Br J Pharmacol. 2004;142:5–7. doi: 10.1038/sj.bjp.0705730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty NS, Poubelle P, Borgeat P, Beaver TH, Westrich GL, Schrader NL. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins. 1985;30:769–789. doi: 10.1016/0090-6980(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Krueger KM, Miller TR, Kang CH, Denny LI, Witte DG, et al. Two novel and selective nonimidazole histamine H3 receptor antagonists A-304121 and A-317920: I. In vitro pharmacological effects. J Pharmacol Exp Ther. 2003;305:887–896. doi: 10.1124/jpet.102.047183. [DOI] [PubMed] [Google Scholar]
- Fokkens WJ, Godthelp T, Holm AF, Blom H, Mulder PG, Vroom TM, et al. Dynamics of mast cells in the nasal mucosa of patients with allergic rhinitis and non-allergic controls: a biopsy study. Clin Exp Allergy. 1992;22:701–710. doi: 10.1111/j.1365-2222.1992.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W-P, Thurmond RL, Ling P, Karlsson L. Histamine H4 receptor antagonists: the new antihistamines? Curr Opin Investig Drugs. 2004;5:1174–1183. [PubMed] [Google Scholar]
- Godot V, Arock M, Garcia G, Capel F, Flys C, Dy M, et al. H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. J Allergy Clin Immunol. 2007;120:827–834. doi: 10.1016/j.jaci.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung W-P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- Jablonowski JA, Grice CA, Chai W, Dvorak CA, Venable JD, Kwok AK, et al. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Seljelid R, Plytycz B. Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1 mice. J Leukoc Biol. 2001a;69:33–42. [PubMed] [Google Scholar]
- Kolaczkowska E, Seljelid R, Plytycz B. Critical role of mast cells in morphine-mediated impairment of zymosan-induced peritonitis in mice. Inflamm Res. 2001b;50:415–421. doi: 10.1007/PL00000264. [DOI] [PubMed] [Google Scholar]
- Leff P, Dougall IG. Further concerns over Cheng-Prusoff analysis. Trends Pharmacol Sci. 1993;14:110–112. doi: 10.1016/0165-6147(93)90080-4. [DOI] [PubMed] [Google Scholar]
- Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004;142:161–171. doi: 10.1038/sj.bjp.0705729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ma X, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, et al. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol Pharmacol. 2001a;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- Liu C, Wilson SJ, Kuei C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001b;299:121–130. [PubMed] [Google Scholar]
- Liu H, Altenbach RJ, Carr TL, Chandran P, Hsieh GC, Lewis LGR, et al. cis-4-(piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A–987306), a new histamine H4 antagonist that blocks pain responses against carrageenan-induced hyperalgesia. J Med Chem. 2008;51:7094–7098. doi: 10.1021/jm8007618. [DOI] [PubMed] [Google Scholar]
- Morse KL, Behan J, Laz TM, West RE, Greenfeder SA, Anthes JC, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther. 2001;296:1058–1066. [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Slater A, Smallman LA, Drake-Lee AB. Increase in epithelial mast cell numbers in the nasal mucosa of patients with perennial allergic rhinitis. J Laryngol Otol. 1996;110:929–933. doi: 10.1017/s0022215100135388. [DOI] [PubMed] [Google Scholar]
- Strakhova M, Esbenshade TA. Characterization of histaminergic receptors. In: Enna SJ, editor. Current Protocols in Pharmacology. New Jersey: John Wiley and Sons; 2006. pp. 1.19.11–11.19.23. [Google Scholar]
- Takeshita K, Bacon KB, Gantner F. Critical role of L-selectin and histamine H4 receptor in zymosan-induced neutrophil recruitment from the bone marrow: comparison with carrageenan. J Pharmacol Exp Ther. 2004;310:272–280. doi: 10.1124/jpet.103.063776. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung W-P, Hofstra CL, Jiang W, et al. A potent and selective histamine H4 receptor antagonist with anti- inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- Varga C, Horvath K, Berko A, Thurmond RL, Dunford PJ, Whittle BJ. Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat. Eur J Pharmacol. 2005;522:130–138. doi: 10.1016/j.ejphar.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Yao BB, Sharma R, Cassar S, Esbenshade TA, Hancock AA. Cloning and pharmacological characterization of the monkey histamine H3 receptor. Eur J Pharmacol. 2003;482:49–60. doi: 10.1016/j.ejphar.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Zhang M, Venable JD, Thurmond RL. The histamine H4 receptor in autoimmune disease. Expert Opin Investig Drugs. 2006;15:1443–1452. doi: 10.1517/13543784.15.11.1443. [DOI] [PubMed] [Google Scholar]


