Abstract
Background and purpose:
Histamine is a modulatory neurotransmitter in the brain. Auto- and hetero-histamine H3 receptors are present in human brain and are potential targets of antipsychotics. These receptors may also display disease-related abnormalities in psychiatric disorders. Here we have assessed how histamine H3 receptors in human brain may be affected in schizophrenia, bipolar disorder, major depression.
Experimental approach:
Histamine H3 receptor radioligand binding assays were applied to frozen post-mortem prefrontal and temporal cortical sections and anterior hippocampal sections from subjects with schizophrenia, bipolar disorder, major depression and matched controls.
Key results:
Compared with the controls, increased H3 receptor radioligand binding was found in dorsolateral prefrontal cortex of schizophrenic subjects (especially the ones who were treated with atypical antipsychotics), and bipolar subjects with psychotic symptoms. No differences in H3 receptor radioligand binding were found in the temporal cortex. In hippocampal formation of control subjects, H3 receptor radioligand binding was prominent in dentate gyrus, subiculum, entorhinal cortex and parasubiculum. Decreased H3 binding was found in the CA4 area of bipolar subjects. Decreased H3 binding in CA2 and presubiculum of medication-free bipolar subjects was also seen.
Conclusions and implications:
The results suggest that histamine H3 receptors in the prefrontal cortex take part in the modulation of cognition, which is impaired in schizophrenic subjects and bipolar subjects with psychotic symptoms. Histamine H3 receptors probably regulate connections between hippocampus and various cortical and subcortical regions and could also be involved in the neuropathology of schizophrenia and bipolar disorder.
Keywords: histamine H3 receptor, schizophrenia, bipolar disorder, major depression, prefrontal cortex, temporal cortex, hippocampal formation, cognition
Introduction
The neurotransmitter histamine regulates several brain functions including cognition and emotions (Frisch et al., 1998; Yanai et al., 1998; Bacciottini et al., 2001; Haas and Panula, 2003). The histaminergic neurons are located exclusively in the tuberomamillary nucleus of the posterior hypothalamus and send projections to almost all parts of the brain. So far, four types of G protein-coupled histamine receptors (H1, H2, H3 and H4 receptors; nomenclature follows Alexander et al., 2008) have been identified, and all have been found in the brain.
Rodents exposed to stressful situations show abnormally high histamine turnover rates in the brain (Taylor and Snyder, 1971; Kobayashi and Kopin, 1974; Mazurkiewickz-Kwilecki, 1979; Mazurkiewickz-Kwilecki and Prell, 1986; Yoshitomi et al., 1986), whereas several anxiolytic drugs decrease this rate (Oishi et al., 1986; 1992; Chikai et al., 1993). On the other hand, drugs that enhance histamine release, either from the histaminergic neurons or from mast cells, induce anxiety-like behaviour in mice (Yuzurihara et al., 2000; Ikarashi and Yuzurihara, 2002). Moreover, a lesion of one of the tuberomamillary subregions has anxiolytic-like effects in rats (Frisch et al., 1998). Studies using different histamine receptor ligands suggest that the anxiogenic-like effect of histamine is mediated by the H1 receptor, whereas H2 receptor activation reduces anxiety-like behaviour and fear (Yuzurihara et al., 2000; Malmberg-Aiello et al., 2002; Santos et al., 2003). Furthermore, activation of H3 receptors might have antidepressant effects (Pérez-García et al., 1999).
Both H1 and H3 receptors seem to take part in the histaminergic modulation of learning and memory (Orsetti et al., 2001; Cangioli et al., 2002; Chen and Shen, 2002). However, the mechanisms are still unclear. It is suggested that the interaction of the histaminergic system with other neurotransmitter systems, most prominently the cholinergic and, to a lesser extent, the dopaminergic systems might be parts of the machinery for cognitive modulation (Ghi et al., 2001; Eidi et al., 2003; Faganello et al., 2003).
Several reports suggest an altered neuronal histaminergic system in the schizophrenic patients and/or beneficial clinical effects of histamine receptor ligands, but the exact role of histamine is still poorly understood in psychiatric diseases (Kaminsky et al., 1990; Nakai et al., 1991; Deutsch et al., 1993; Martinez-Mir et al., 1993; Oyewumi et al., 1994; Prell et al., 1995; Rosse et al., 1995; 1996). The materials selected by the Stanley Foundation provide an opportunity to examine the potential changes of histamine receptor expression in three major psychiatric disorders: schizophrenia, bipolar disorder and major depression. In particular, we focused on the possible alterations of H3 receptor ligand binding in post-mortem brain samples from these subjects, because H3 receptors are involved in regulation of neurotransmitter release in cortical areas and they are a promising target for drug development in cognitive disorders (Passani et al., 2004).
Methods
Post-mortem samples
The samples in the Stanley Foundation have been collected from designated medical examiners in the USA with the permission of the families. A permit for the work on post-mortem human brain samples was obtained from the Office of Medicolegal Affairs. Post-mortem samples obtained from the Stanley Foundation Neuropathology Consortium (Bethesda, MD, USA) consisted of four matched groups of 15 samples each: schizophrenia, bipolar disorder, depression and normal controls. The samples covered three brain areas: lateral dorsal prefrontal cortex (Brodmann's areas 9 and 46), superior temporal cortex (superior temporal gyrus that includes Brodmann's area 22) and anterior hippocampus. Three sets of fresh-frozen sections (14 µm thick) from these areas were obtained for each subject except for two sets of temporal cortex sections missing [one untreated schizophrenic subject, one bipolar subject treated with tricyclic antidepressants (TCA)]. The sections were stored at −70°C until use.
The demographic and clinical data and storage characteristics of all cases have been previously described (Dowlatshahi et al., 1999; Jarskog et al., 2000; Torrey et al., 2000; Knable et al., 2001). Therefore, these data are not provided here in detail except for diagnosis, medication, side of brain and cause of death (Table 1). Shortly, the samples were matched for sex (nine men and six women for each group), age (25–68 years), ethnicity, side of brain, brain pH (6.20 ± 0.03) and post-mortem interval (29.4 ± 13.4 h). There were no significant differences between the four groups in these parameters.
Table 1.
Details of the human brain material obtained from the Stanley Foundation
| Brain No. | Diagnosis | Age/sex | Cause of death | Side of brain | Medications at the time of death | Lifetime fluph. eq (mg) | PMI (h) | pH |
|---|---|---|---|---|---|---|---|---|
| S-49 | N | 52/M | CPD | L | None | – | 28 | 6.5 |
| S-70 | N | 44/F | CPD | R | None | – | 25 | 6.3 |
| S-73 | N | 59/M | CPD | R | None | – | 26 | 6.4 |
| S-85 | N | 52/M | CPD | L | None | – | 8 | 6.5 |
| S-123 | N | 52/M | CPD | R | None | – | 22 | 6.2 |
| S-124 | N | 53/M | CPD | L | None | – | 28 | 6.2 |
| S-126 | N | 44/M | CPD | L | None | – | 10 | 6.4 |
| S-136 | N | 35/F | CPD | R | None | – | 23 | 6.6 |
| S-141 | N | 41/M | CPD | R | None | – | 11 | 6 |
| S-149 | N | 42/M | CPD | R | None | – | 27 | 6.6 |
| S-158 | N | 35/F | CPD | L | None | – | 40 | 5.8 |
| S-162 | N | 68/F | CPD | L | None | – | 13 | 6.3 |
| S-165 | N | 58/M | CPD | L | None | – | 27 | 6 |
| S-174 | N | 29/F | Accident | L | None | – | 42 | 6.2 |
| S-179 | N | 57/F | Accident | R | None | – | 26 | 6 |
| S-13 | S, DO, 295.10 | 30/F | Suicide | R | Thx, Des | 6 000 | 60 | 6.2 |
| S-18 | S, UD, 295.92 | 52/M | CPD | L | None (>20 years) | 9 000 | 61 | 6 |
| S-30 | S, UD, 295.92 | 30/M | CPD | L | Ris, Tdz | 50 000 | 32 | 5.8 |
| S-41 | S, P, 295.30 | 62/F | Accident | L | None (7 months) | 50 000 | 26 | 6.1 |
| S-43 | S, UD, 295.92 | 60/F | CPD | L | None (never treated) | 0 | 40 | 6.2 |
| S-64 | S, UD, 295.92 | 60/M | Accident | R | Tdz, Ami | 80 000 | 31 | 6.2 |
| S-66 | S, UD, 295.92 | 32/M | Other | L | Cloz | 15 000 | 19 | 6.1 |
| S-81 | S, UD, 295.92 | 31/M | Suicide | L | Cloz | 4 000 | 14 | 5.8 |
| S-82 | S, P, 295.30 | 58/F | CPD | R | Hal, Dph | 35 000 | 26 | 5.9 |
| S-93 | S, UD, 295.92 | 25/M | Suicide | L | Ris, Par | 4 000 | 32 | 6.6 |
| S-100 | S, UD, 295.92 | 44/M | CPD | R | Hal, Cbz, Fx, Cz, Bz | 100 000 | 50 | 6.5 |
| S-116 | S, P, 295.30 | 44/M | CPD | L | Cloz, Cpz, Li | 130 000 | 29 | 5.9 |
| S-118 | S, UD, 295.92 | 56/F | Suicide | R | Hal, Li, Dph, CH | 150 000 | 12 | 6.4 |
| S-120 | S, P, 295.30 | 35/M | CPD | R | Cloz, Cpz, Ma, Bz, Dph | 50 000 | 35 | 6.5 |
| S-173 | S, UD, 295.92 | 49/F | CPD | L | Hal, Cloz, Cz | >200 000 | 38 | 6.2 |
| S-33 | B, wP, 296.54 | 25/F | Suicide | R | Thx, Cbz, Li, Trz | 7 500 | 24 | 6.4 |
| S-34 | B, wP, 296.64 | 48/F | CPD | L | Val, Ser, Cpx, Cbz | 32 000 | 22 | 5.8 |
| S-47 | B, wP, 296.64 | 37/F | Suicide | R | Li, Bup, Cz, Lz | 1 200 | 29 | 6.5 |
| S-48 | B, woP, 296.45 | 54/M | Other | R | Li, Cbz | 2 500 | 39 | 5.8 |
| S-60 | B, wP, 296.64 | 30/M | CPD | R | Li, Cloz | 60 000 | 31 | 6.1 |
| S-68 | B, woP, 296.53 | 30/M | Suicide | R | None | 0 | 56 | 5.8 |
| S-72 | B, wP, 296.44 | 57/M | CPD | L | Hal, Dph | 60 000 | 19 | 6.2 |
| S-75 | B, wP, 296.54 | 34/M | Suicide | R | Ris, Val, Vfx | 7 000 | 23 | 6.3 |
| S-83 | B, wP, 296.44 | 48/M | Suicide | R | None (>20 years) | <200 | 13 | 6.1 |
| S-88 | B, wP, 296.54 | 31/M | Suicide | R | Hal, Trz, Trx | 30 000 | 28 | 6.3 |
| S-89 | B, II/H, 296.89 | 30/M | Suicide | L | Val, Bup | 0 | 45 | 6.3 |
| S-91 | B, wP, 296.44 | 50/F | Other | L | several months | 12 000 | 18 | 6.1 |
| S-103 | B, wP, 296.54 | 61/F | Suicide | L | Fx, Val | 40 000 | 60 | 6.5 |
| S-128 | B, wP, 296.44 | 50/M | Suicide | L | Val, Cloz, Fz, Bz | 60 000 | 19 | 6.2 |
| S-147 | B, woP, 296.53 | 50/F | CPD | L | Val, Cmi | 0 | 62 | 6.3 |
| S-16 | D, 296.23 | 32/F | Suicide | L | Imi, Ami, Ntp, Cz | 0 | 47 | 6 |
| S-38 | D, 296.33 | 53/F | Other | R | Li, Trz | 0 | 40 | 6.3 |
| S-46 | D, 296.33 | 44/F | Suicide | L | Fx, Imi, Lz | 0 | 32 | 6.2 |
| S-59 | D, 296.33 | 65/M | CPD | R | Pht (single seizure) | 0 | 19 | 6.2 |
| S-92 | D, NOS:311, woP | 52/M | CPD | R | None (6 years) | 0 | 12 | 6.5 |
| S-99 | D, 296.33 | 46/M | Suicide | R | Dph, Cz | 0 | 26 | 6.1 |
| S-101 | D, 296.22 | 42/F | CPD | R | Fx, Li | 0 | 25 | 6.3 |
| S-104 | D, 296.23 | 51/M | Suicide | R | Nef, Hxz | 0 | 26 | 6.3 |
| S-135 | D, 296.33 | 39/M | Suicide | L | None | 0 | 23 | 6 |
| S-138 | D, 296.31 | 42/M | Suicide | L | None (>2 weeks) | 0 | 7 | – |
| S-156 | D, 296.32 | 56/M | CPD | L | Ser | 0 | 23 | 6.5 |
| S-163 | D, 296.33 | 56/F | CPD | L | Vfx, Bus, Az | 0 | 28 | 5.8 |
| S-168 | D, 296.33 | 30/F | Suicide | L | Ntp, Az, Cmi | 0 | 33 | 6 |
| S-171 | D, 296.33 | 43/M | CPD | L | Tri | 0 | 43 | 5.9 |
| S-172 | D, 296.33 | 47/M | CPD | L | Fx, Nef | 0 | 28 | 6.4 |
Ami, amitriptyline; Az, alprazolan; B, II/H, bipolar II disorder, hypomaniac; B, woP, bipolar disorder without psychotic features; B, wP, bipolar disorder with psychotic features; Bup, bupropion; Bus, buspirone; Bz, benzotropine; Cbz, carbamazepine; CH, chloral hydrate; Cloz, clozapine; Cmi, clomipramine; CPD, cardiopulmonary diseases; Cpx, chlorprothixene; Cpz, chlorpromazine; Cz, clonazepam; D, major depression; Des, desipramine; Dph, diphenhydramine; F, female; Fx, fluoxetine; Fz, flurazepam; Hal, haloperidol; Hxz, hydroxyzine; Imi, imipramine; L, left hemisphere; Li, lithium; Lifetime fluph. eq, estimated lifetime antipsychotics in fluphenazine equivalents; Lz, lorazepam; M, male; Ma, maprotiline; N, normal control; Nef, nefazadone; Ntp, nortriptyline; Par, paroxetine; Pht, phenytoin; PMI, post-mortem interval; R, right hemisphere; Ris, risperidone; S, DO, schizophrenia, disorganized; S, P, schizophrenia, paranoid; S, UD, schizophrenia, undifferentiated; Ser, sertraline; Tdz, thioridazine; Thx, thiothixene; Tri, trimipramine; Trz, trazadone; Trx, trihexphenidyl; Val, valproate; Vfx, venlafaxine.
All 60 subjects were treated simultaneously for each of the three binding experiments (H3 receptor radioligand binding in frontal cortex, temporal cortex and hippocampal formation) to avoid inter-assay variations.
H3 receptor radioligand binding
Slides were equilibrated to room temperature and dried for 45 min, then incubated at room temperature for 45 min in 150 mmol·L−1 Na/K phosphate buffer (pH 7.4) containing 100 µmol·L−1 dithiothreitol (DTT), 2 mmol·L−1 MgCl2 and 4 nmol·L−1[3H]-Nα-methylhistamine [[3H]-NAMH, 82 Ci·mmol−1, DuPont NEN Research products, Boston, MA, USA; Kd about 1.4 nmol·L−1 according to West et al. (1999) and Anichtchik et al. (2001)]. For detecting the non-specific binding, an adjacent section from the same set was incubated in the same buffer containing both 4 nmol·L−1[3H]-NAMH and 5 µmol·L−1 clobenpropit (pA2= 9.9, Van der Goot and Timmerman, 2000). After incubation, the sections were washed 4 × 30 s in the same buffer without ligands at 0°C, rinsed in the ice-cold water, then dried under cold air stream. The dried sections were exposed to Kodak BioMax MR-films together with 3H-standards for 14 or 22 weeks.
Image analysis
Film images were analysed by a computer-based MCID image analysis system (Imaging Research, St. Catherines, Ontario, Canada), as described before (Jin and Panula, 2005). The optical density was converted to the linear grey-scale value by a 3H-standard-derived curve. All grey scales in this study were obtained from linear portions of the 3H-standard curves. The average ligand binding density in the cortical grey matter was obtained from the parts of sections that cross all cortical layers evenly and perpendicularly. For each pair of sections (i.e. reaction and control sections from the same subject for each receptor binding experiment), the non-specific binding measured from the control section was subtracted from the binding density measured from the reaction section to yield the specific binding level for this subject. The diagnoses of the subjects were not revealed during the image analyses. The code was opened after the image analyses had been completed.
Statistical analysis
The original design of the experiment was to examine the possible alterations in histamine receptor radioligand binding in the normal and diseased brains. Thus, the measured receptor radioligand binding levels of samples from various brain areas of 60 subjects were first grouped according to diagnoses and brain area, and the distribution of data was analysed by the Sapiro–Wilk test. If the data passed the test of distribution of normality, they were subjected to anovas (followed by Tukey's HSD post hoc test) to examine the overall effects of diagnosis and medication on H3 receptor radioligand binding levels. Medication effects within each diagnostic group were examined by using one-way anova (with Bonferroni's multiple comparison post hoc test). Data were also further grouped according to diagnosis and medication profile, Student's t-test was used to examine the significance of difference between two subgroups [e.g. control and unmedicated schizophrenic, control and schizophrenic cases treated with atypical antipsychotics (ATA), etc.]. We defined the subgroups based on the published pharmacological profiles of the medications that were used by the subjects included in this study (information on ‘medication at death’ is provided by the Stanley Foundation Neuropathology Consortium, see Table 1). The untreated subjects were either drug-naïve or had been off-medication for at least a few months before death. As both ATA (including clozapine and risperidone in this study) and TCAs have been shown to affect H3 receptors (Kathmann et al., 1994; Ghi et al., 1995; Rodrigues et al., 1995; Alves-Rodrigues et al., 1996), subjects who were treated with any of the ATAs or TCAs were included into the ‘ATA/TCA’ groups, while subjects who were treated with typical antipsychotics, mood stabilizers or non-TCA antidepressants were included in the ‘other medication’ groups. The numbers of subjects in each subgroup are listed in Table 2.
Table 2.
Numbers of subjects in each subgroup (based on diagnosis and medication)
| Untreated | ATA/TCA | Other medication | |
|---|---|---|---|
| Normal | 15 | – | – |
| Schizophrenia | 3 | 7 | 5 |
| Bipolar disorder | 3 | 4 | 8 |
| Major depression | 3 | 4 | 8 |
ATA, atypical antipsychotics, includes clozapine and risperidone; potent anti-H1 medication includes clozapine, imipramine, amitriptyline, nortriptyline, clomipramine, maprotiline, diphenhydramine and hydroxyzine; TCA, tricyclic antidepressants, includes imipramine, amitriptyline, nortriptyline, clomipramine, maprotiline and desipramine; untreated, drug-naïve or had been off-medication for at least 2 weeks before death.
Because we obtained only two sets of temporal cortical samples from two schizophrenic subjects who were off-medication before death, this subgroup (untreated schizophrenic subjects) was not included in the analysis of H3 receptor radioligand binding data in the temporal cortex. The SAS8.2 statistical software and spss for Windows were used for the analyses.
Pearson's test was used to examine the correlation between H3 receptor radioligand binding level and brain pH or post-mortem time. Kendall's tau-b test was used to examine the correlation between H3 receptor radioligand binding level and psychotic symptoms in various brain areas. If a significant correlation was suggested, one-way anova (followed by Tukey's HSD post hoc test) was used to examine the significance of difference in H3 receptor radioligand binding level between various groups (controls, schizophrenic subjects and bipolar subjects with psychotic symptoms, depressed subjects and bipolar subjects without psychotic symptoms).
In addition, the analysis of covariance was performed to analyse the effects of age, side of the brain, drug abuse and alcohol consumption on H3 receptor radioligand binding levels.
Results
General aspects
No correlations were found between H3 receptor radioligand binding levels and post-mortem time or brain pH (Pearson's linear correlation test, Figure 1). The analysis of primary data of this study did not find any significant differences in the cortical laminar distribution patterns of H3 receptor radioligand binding between subjects with psychiatric disorders (schizophrenia, bipolar disorder, major depression) and the normal controls. Therefore, the average densities of H3 receptor radioligand binding in the cortical grey matter (all laminae) were taken into account and are presented here. The analysis of covariance showed that the possible effects of age, drug abuse, side of brain and alcohol consumption on H3 receptor radioligand binding levels were not significant (F < 1.5, P > 0.05). No interactions between alcohol use and medication, drug abuse and medication were found (F < 1.6, P > 0.05).
Figure 1.
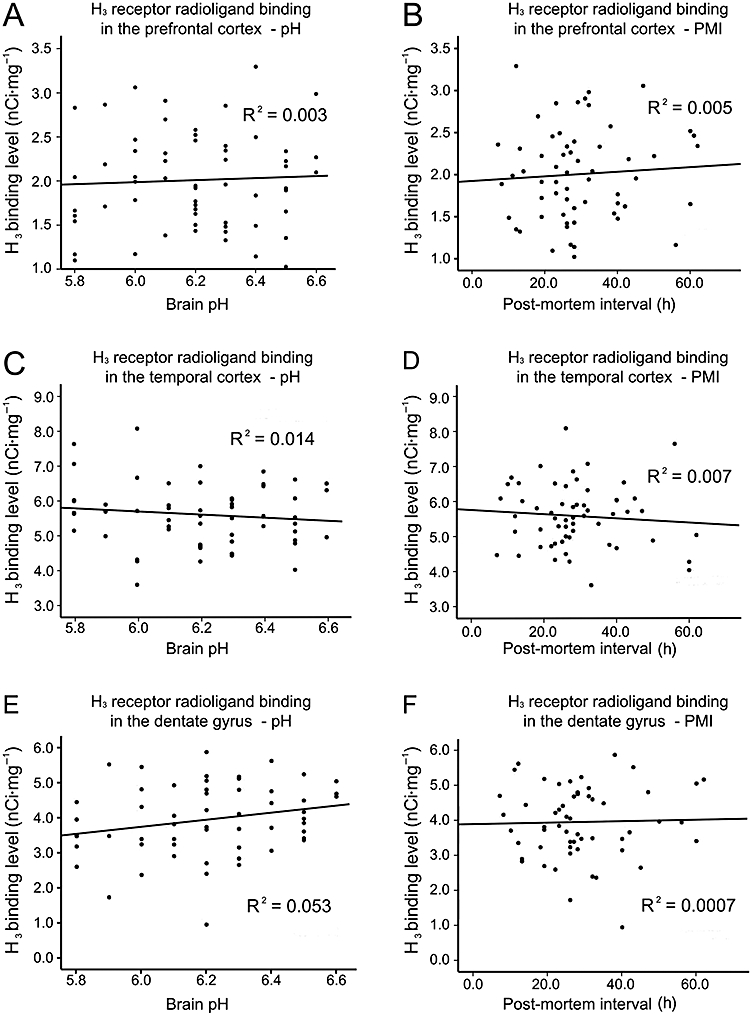
Histamine H3 receptor radioligand binding in prefrontal cortex, temporal cortex and dentate gyrus of all subjects, with respect to brain pH and post-mortem interval.
H3 receptor radioligand binding in prefrontal and temporal cortices
H3 receptor radioligand binding in the prefrontal cortex
In the prefrontal cortex, the average H3 receptor radioligand binding level of the schizophrenic group was significantly higher than those of the control and depressed groups (one-way anova with HSD post hoc test, P < 0.01 and 0.05, respectively; Figures 2A–K), whereas no significant differences in H3 receptor radioligand binding levels were found between the normal controls and the depressed or bipolar subjects. Typical images with appropriate controls are shown (Figure 2A–J).
Figure 2.
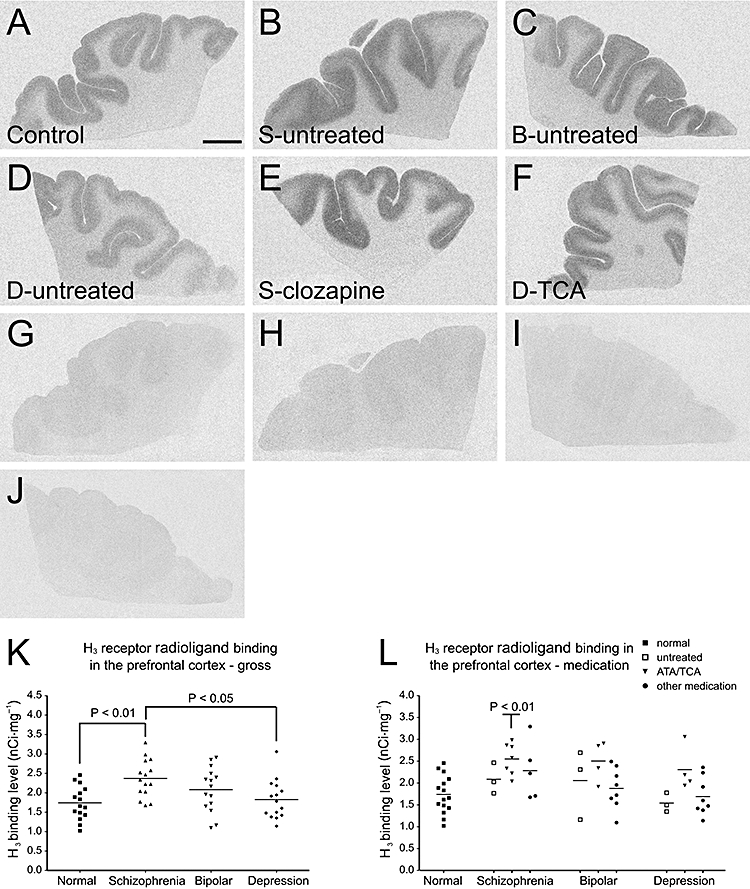
Histamine H3 receptor radioligand [3H]-NAMH binding patterns in the prefrontal cortex of (A) control subject, (B) medication-free schizophrenic subject, (C) medication-free bipolar subject, (D) medication-free depressed subject, (E) schizophrenic subject treated with clozapine and (F) depressed subject treated with TCA. (G–J) Non-specific (5 µmol·L−1 clobenpropit present) binding patterns correspond to those in (A–D). (K) Densities of [3H]-NAMH binding sites in the prefrontal cortices across all diagnostic groups. Mean ± SD binding levels (nCi·mg−1) were: control, 1.74 ± 0.43; schizophrenic subjects, 2.37 ± 0.49; subjects with bipolar disorder, 2.08 ± 0.57; depressed subjects, 1.83 ± 0.50. P-values (one-way anova, HSD post hoc test) are indicated in the groups having significantly different binding levels, compared with the controls and depressed subjects. (L) Densities of [3H]-NAMH binding sites in the prefrontal cortices of all subjects (grouped according to both diagnoses and medication profiles). P-value is indicated in the subgroup that is significantly different from the controls (one-way anova with Bonferroni's post hoc test). No significant differences were found within the schizophrenic, bipolar and depressive groups. The H3 receptor binding level of the depressed subjects who were treated with TCA shows a tendency to increase as compared with the medication-free ones (P= 0.051). The ‘other medication’ category includes typical antipsychotics, mood stabilizers and non-TCA antidepressants. Scale bar: 1 cm. [3H]-NAMH, [3H]-Nα-methylhistamine; ATA, atypical antipsychotic; B, bipolar disorder; D, depression; S, schizophrenia; TCA, tricyclic antidepressant.
A significant overall effect of medication on H3 receptor radioligand binding in the prefrontal cortex was suggested (F= 6.743, P= 0.012). H3 receptor radioligand binding levels were significantly higher in subjects treated with ATAs (clozapine and risperidone) and/or TCAs relative to either untreated subjects (including control and untreated diseased subjects, P < 0.001) or those treated with other medications (including typical antipsychotics, mood stabilizers and antidepressants other than TCAs, P < 0.01). However, no significant differences were found inside each disease (schizophrenic, bipolar, depressive) group comparing the ones that were either off-medication, treated with ATAs or TCAs, or treated with other medications (Figure 2B–F and L). Further comparisons of subgroups (grouped according to both diagnosis and medication) with the control group found significantly higher average H3 receptor radioligand binding levels in the prefrontal cortices of schizophrenic subjects treated with ATAs (clozapine and risperidone) and/or TCAs as compared with the controls (P < 0.05, Figure 2E and L). A similar tendency was found in the depressive subjects who were treated with TCAs but without statistical significance (Figure 2F and L).
In 45 diseased subjects, significant correlation was found between psychotic symptoms and H3 receptor radioligand binding levels in the prefrontal cortex (Kendall's tau-b non-parameter correlation test, correlation coefficiency = 0.366, P= 0.003). H3 receptor radioligand binding levels in the prefrontal cortex were significantly higher in subjects with psychotic symptoms (i.e. all schizophrenic subjects and those bipolar subjects with psychotic symptoms, see Table 1) as compared with those without psychotic symptoms (i.e. bipolar subjects without psychotic symptoms and all depressed subjects) or controls (one-way anova with HSD post hoc test, P < 0.01, Figure 3).
Figure 3.
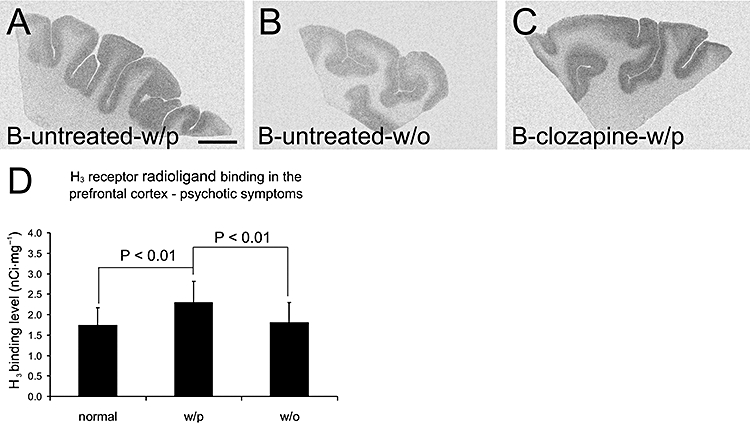
Histamine H3 receptor radioligand [3H]-NAMH binding patterns in the prefrontal cortex of (A) medication-free bipolar subject with psychotic symptoms, (B) medication-free bipolar subject without psychotic symptoms and (C) bipolar subject with psychotic symptoms that were treated with clozapine. (D) Density of [3H]-NAMH binding sites (mean ± SD) is significantly higher (one-way anova with Tukey's HSD post hoc test) in subjects with psychotic symptoms (n= 26, 15 schizophrenic and 11 bipolar subjects) as compared with controls (n= 15) or subjects without psychotic symptoms (n= 19, 4 bipolar subjects and 15 depressed subjects). Scale bar: 1 cm. [3H]-NAMH, [3H]-Nα-methylhistamine; B, bipolar disorder; w/o, without psychotic symptoms; w/p, with psychotic symptoms.
H3 receptor radioligand binding in the temporal cortex
In the temporal cortex, overall effects of diagnosis and medication on H3 receptor radioligand binding levels were not significant (two-way anova, F < 1.121, P > 0.35). No significant differences in H3 receptor radioligand binding levels were found among the four groups of subjects (one-way anova, P > 0.05; Figure 4A–K). The H3 receptor radioligand binding level of schizophrenic subjects who were treated with ATAs and/or TCAs was not different from that of the controls (one-way anova, P > 0.05, Figure 4A–L). No significant difference was found within each diagnostic group (Figure 4A–L).
Figure 4.
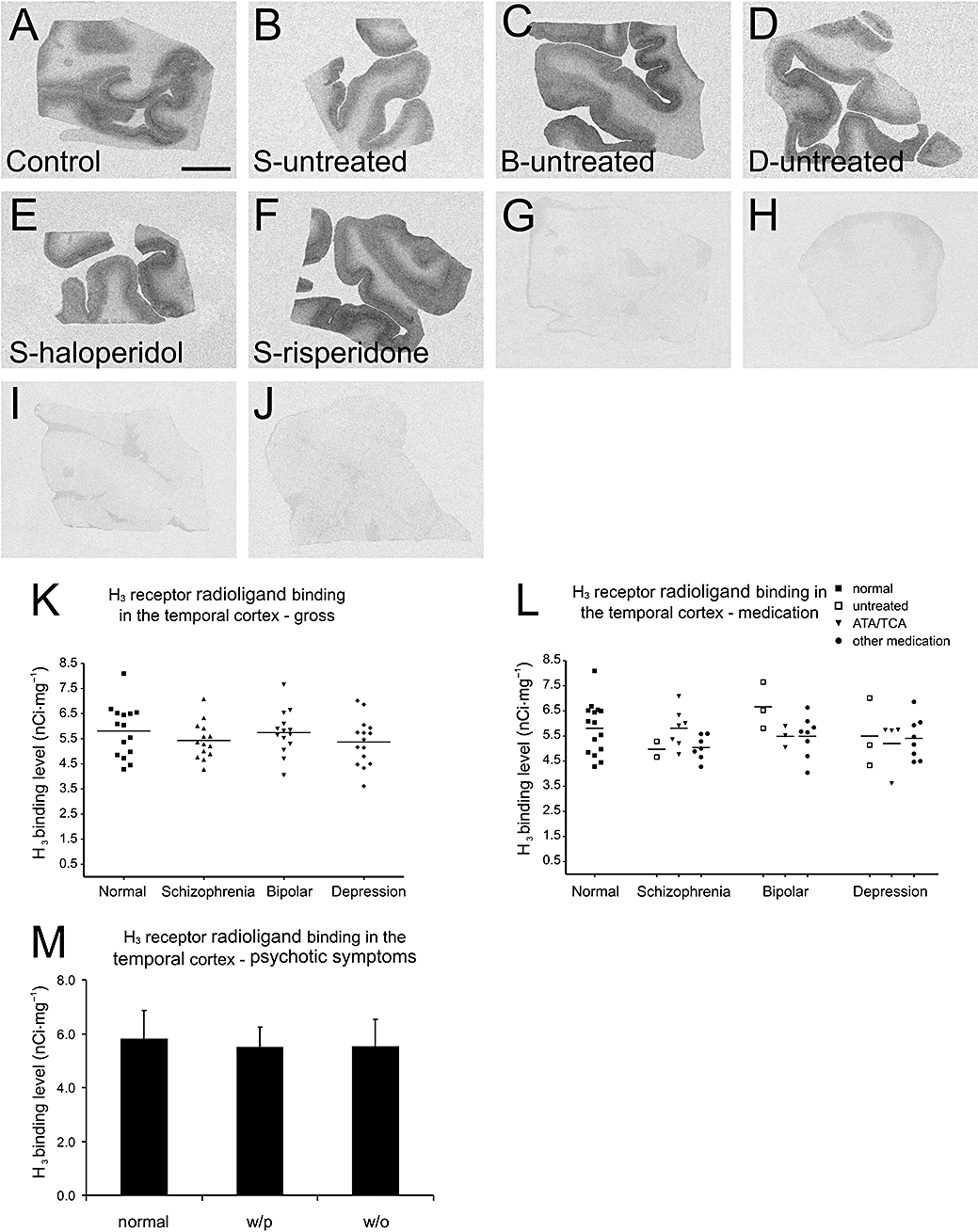
Histamine H3 receptor radioligand [3H]-NAMH binding patterns in the temporal cortex of (A) control subject, (B) medication-free schizophrenic subject, (C) medication-free bipolar subject, (D) medication-free depressed subject, (E) schizophrenic subject treated with haloperidol and (F) schizophrenic subject treated with risperidone. (G–J) Non-specific (5 µmol·L−1 clobenpropit present) binding patterns correspond to those in (A–D). (K) Densities of [3H]-NAMH binding sites in the temporal cortices across all diagnostic groups. Mean ± SD binding levels (nCi·mg−1) are: control, 5.81 ± 1.04; schizophrenic subjects, 5.43 ± 0.74; subjects with bipolar disorder, 5.74 ± 0.87; depressed subjects, 5.37 ± 0.94. No significant differences were found among different groups. (L) Densities of [3H]-NAMH binding sites in the temporal cortices of all subjects (grouped according to both diagnoses and medication profiles). No significant differences were found among different groups, or within each group. The ‘other medication’ category includes typical antipsychotics, mood stabilizers and non-TCA antidepressants. (M) No significant differences in H3 receptor binding levels were found between controls (n= 15), subjects with psychotic symptoms (n= 26, 15 schizophrenic and 11 bipolar subjects) and subjects without psychotic symptoms (n= 19, 4 bipolar subjects and 15 depressed subjects). Scale bar: 1 cm. [3H]-NAMH, [3H]-Nα-methylhistamine; ATA, atypical antipsychotic; B, bipolar disorder; D, depression; S, schizophrenia; TCA, tricyclic antidepressant; w/o, without psychotic symptoms; w/p, with psychotic symptoms.
In the diseased subjects, no significant correlation was found between psychotic symptoms and H3 receptor radioligand binding levels in the temporal cortex (Kendall's tau-b non-parameter correlation test, correlation coefficiency =−0.009, P= 0.942). No significant differences in H3 receptor radioligand binding levels were found between subjects with psychotic symptoms, diseased subjects without psychotic symptoms and controls (one-way anova, P > 0.05, Figure 4M)
H3 receptor radioligand binding in the hippocampal formation
H3 receptor radioligand binding in hippocampal areas of normal subjects
In the hippocampal formation of normal subjects, average H3 receptor radioligand binding levels were highest in dentate gyrus, subiculum, entorhinal cortex and parasubiculum, medium in presubiculum (PrS) and CA1, low in CA2, CA3 and CA4 regions (Figure 5A and C). A layer preference was observed in the subicular complex and entorhinal cortex (Figure 5B). In the subicular complex, the binding density is higher in the deep layer of subiculum, the middle and superficial layers of PrS, and the deep and superficial layers of parasubiculum. In the entorhinal cortex, the binding density was relatively higher in the superficial and deep layers.
Figure 5.
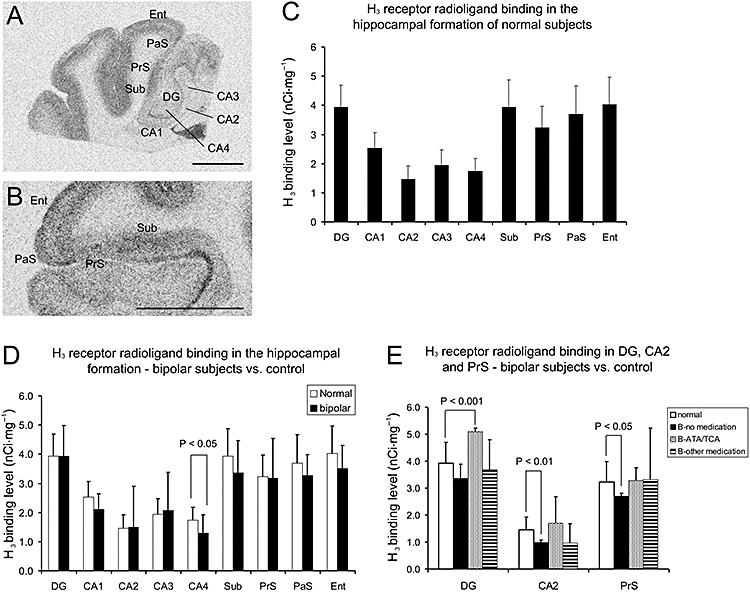
(A) Histamine H3 receptor radioligand [3H]-NAMH binding pattern in the hippocampal formation of a normal subject. (B) The layer preference of H3 receptor binding distribution in the subicular complex and entorhinal cortex of a normal subject. In the subicular complex, the binding density is higher in the deep layer of subiculum, the middle and superficial layers of PrS and the deep and superficial layers of PaS. In the entorhinal cortex, the binding density is prominent in the superficial and deep layers. (C) Densities of [3H]-NAMH binding sites in various parts of hippocampal formation of normal subjects (mean ± SD, n= 15). (D) Densities of [3H]-NAMH binding sites in various parts of hippocampal formation of normal and bipolar subjects (mean ± SD, n= 15 for each group). H3 receptor binding level is significantly decreased in CA4 of bipolar subjects. (E) Densities of [3H]-NAMH binding sites (mean ± SD) in DG, CA2 and PrS of normal (n= 15) and bipolar subjects (n= 15 in total, grouped according to medication profile). H3 receptor radioligand binding levels are significantly increased in DG of bipolar subjects treated with ATA and/or TCA (n= 4, student's t-test, P < 0.001), but significantly decreased in CA2 and PrS of medication-free bipolar subjects (n= 3, Student's t-test, P < 0.01 and 0.05 respectively). Scale bars: 1 cm. [3H]-NAMH, [3H]-Nα-methylhistamine; ATA, atypical antipsychotic; B, bipolar disorder; DG, dentate gyrus; Ent, entorhinal cortex; PaS, parasubiculum; PrS, presubiculum; Sub, subiculum. TCA, tricyclic antidepressant.
H3 receptor radioligand binding in hippocampal areas of diseased subjects
Between diseased and normal subjects, no significant differences were found in most of the hippocampal areas (one-way anova, P > 0.05) except for the significantly lower H3 receptor radioligand binding level in CA4 of bipolar subjects (one-way anova with HSD post hoc test, P < 0.05, Figure 5D). A trend towards lower binding level was also found in CA1 of bipolar subjects but without statistical significance (one-way anova, P= 0.062). The overall effect of medication on H3 receptor radioligand binding level was significant in dentate gyrus (F= 3.499, P= 0.038), but not in any other hippocampal areas. The bipolar subjects treated with ATAs or TCAs had significantly higher H3 receptor radioligand binding levels in dentate gyrus as compared with controls (Student's t-test, P < 0.001, Figure 5E). Significantly lower binding levels were also found in CA2 and PrS of three medication-free bipolar subjects as compared with the normal subjects (Student's t-test, P < 0.01 and 0.05, respectively, Figure 5E).
In the diseased subjects, no correlation was found between psychotic symptoms and H3 receptor radioligand binding levels in any of the hippocampal areas (Kendall's tau-b test, correlation coefficiency < 0.196, P > 0.252). No correlations were found between suicide behaviour and H3 receptor radioligand binding levels (Kendall's tau-b test, correlation coefficiency < 0.152, P > 0.240), or the use of mood stabilizer and H3 receptor radioligand binding levels (Kendall's tau-b test, correlation coefficiency < 0.187, P > 0.182) in any of the hippocampal areas.
Discussion
Significantly higher histamine H3 receptor radioligand binding was found in the prefrontal cortex of the schizophrenic group than in the control group, in particular in those patients who received ATAs. However, no significant changes were found in the temporal cortex and most of the hippocampal regions of these subjects. If we look at the ratio of frontal H3 receptor binding level to temporal H3 receptor binding level in each individual, it is significantly higher (Student's t-test, P < 0.001) in schizophrenic subjects (0.438 ± 0.09) as compared with the controls (0.304 ± 0.08). Moreover, the same schizophrenic subjects treated with ATAs did not show increased H3 receptor binding in the hippocampal region either, and the binding level in CA2 region of these subjects was significantly lower than that of the controls (Student's t-test, P < 0.05, data not shown). Thus, the increase of [3H]-NAMH binding sites in the frontal cortex is probably not directly caused by the medication per se, but is rather related to some factors that are linked to the medication profile, such as symptoms. In none of disease groups, did we find significant differences in prefrontal cortical H3 receptor radioligand binding levels between subjects who had committed suicide and those who died from accidents or other diseases (data not shown). However, we did find a correlation between H3 receptor radioligand binding level in the prefrontal cortex and psychotic symptoms in 45 diseased subjects. H3 receptor radioligand binding levels in the prefrontal cortex were significantly higher in subjects with psychotic symptoms, compared with those without psychotic symptoms or controls. This correlation was not found in the temporal cortex or the hippocampal formation. These data suggest that the H3 receptor in the human prefrontal cortex is likely to be involved in the modulation of cognition, and this is supported by findings in animals that H3 receptor antagonists enhance prepulse inhibition and cognition (Fox et al., 2002; 2005; Browman et al., 2004).
In the hippocampal formation, minor changes in H3 receptor radioligand binding levels were found in subjects suffered from bipolar disorder. These findings include significantly decreased H3 receptor radioligand binding level in CA4, a non-significant trend towards decreased binding level in CA1, significantly decreased binding levels in CA2 and PrS of three medication-free bipolar subjects and significantly increased binding levels in dentate gyrus of bipolar subjects treated with ATA and TCAs. We could not link the findings to either severity of the symptoms (such as suicide) or the spectrum of symptoms (such as psychotic behaviours). Although ATAs and TCAs are H3 receptor ligands, increased H3 receptor binding levels were found in dentate gyrus of bipolar subjects treated with these drugs, and significantly decreased binding levels were found in CA2 of schizophrenic subjects treated with ATAs and TCAs (data not shown). Therefore, the observed changes cannot be directly linked to medication. The change in H3 receptor binding level might reflect cytohistological abnormalities in hippocampal formation of bipolar subjects. Although no significant cytoarchitectural changes were found in the hippocampus of these subjects (Knable et al., 2004) and no difference in hippocampal neuronal density was observed (Oliveira et al., 2008), some alterations have indeed been reported in the same material, such as a significant 12% reduction in hippocampal CA1 neuronal size (Liu et al., 2007), and decreased mRNA expression of complexin I and II in CA4, subiculum and parahippocampal gyrus in bipolar subjects (Eastwood and Harrison, 2000). Thus, the change in H3 receptor binding level might be related to synaptic abnormalities in these subjects, but not to loss of hippocampal neurons. One of the downstream effects following H3 receptor activation is the inhibition of cAMP formation, which is also the major target of antidepressants. Changes in cAMP pathway, such as increased BDNF level were found in dentate gyrus, CA4 and supragranular regions in subjects treated with antidepressants in the same material. The decrease in H3 receptor density might also enhance the cAMP signalling in the hippocampal formation. Our data suggest potential changes in H3 receptor regulation of the hippocampal circuit in bipolar subjects, but more materials need to be examined and physiological functions of the H3 receptor in hippocampal formation should be studied before we can draw a conclusion.
Contrary to the findings in the prefrontal cortex, no significant differences in H3 receptor binding levels were found in the temporal cortex among four groups. The discrepancy suggests a possible functional difference of H3 receptors in these two regions. Furthermore, the existence of H3 receptor isoforms in the human brain needs to be taken into account in the case of different H3 receptor binding profiles in the prefrontal and temporal cortices. So far, 10 isoforms have been described (Cogéet al., 2001; Wellendorph et al., 2002). Among them, at least three are functional: the full length 445-aa protein, the 365-aa isoform with 80-aa deletion in the third intracellular loop and another isoform having both 80-aa deletion in the third intracellular loop and a novel short 8-aa C terminus. In the rat, isoforms with an alternative putative extracellular C terminus act as dominant-negative isoforms (Bakker et al., 2006). The agonist [3H]-NAMH shows different affinity to different human H3 receptor isoforms (Wellendorph et al., 2002). Thus, the different binding profiles of H3 receptors in the two cortical areas of schizophrenic subjects obtained in this study might indicate different compositions of H3 receptor isoforms in these two regions. It is possible that in these schizophrenic subjects, the ratio of full-length H3 receptor isoforms versus the isoforms with third intracellular loop deletion and the ones with different C terminus were different from the normal controls and/or between the cortical areas. However, no information is available on the distribution patterns of all H3 receptor isoforms and their regulation in the human brain. Furthermore, a mutation that alters H3 receptor ligand binding properties remains a possible explanation. Despite the difficulties in assessing the extent and nature of neuropathological alterations in schizophrenia (see Shenton et al., 2001), altered volumes of dorsolateral prefrontal cortex have been reported (Gur et al., 2000; Tregellas et al., 2007). Differences in H3 receptor ligand binding pattern may be a consequence of differences in the density of neurons and neuropil. However, no changes were found in cases of density and somal size of lamina V pyramidal neurons in dorsal lateral prefrontal cortices of these subjects (Law and Harrison, 2003). It would be important to understand the mechanism that leads to altered H3 receptor ligand binding in schizophrenic brains, as this receptor is an important target for drug development for CNS disorders.
In this study we also describe, to our knowledge for the first time, the distributional pattern of H3 receptor radioligand binding in the human hippocampal formation. Components of the cortical input pathway (i.e. layers II and III of the entorhinal cortex, dentate gyrus) and the feedback efferent pathway (i.e. subiculum and layer V of entorhinal cortex) to diverse cortical and subcortical regions show more abundant H3 receptor radioligand binding sites than other hippocampal areas. This pattern suggests that the H3 receptor, most likely located on the axonal terminals of neurons, regulates the strength of inputs from association cortices that converge on neurons in the upper layers of entorhinal cortex, and the further entorhinal projection to dentate gyrus. The entorhinal cortex – dentate gyrus perforant pathway is the main stream for conveying sensory experience information to hippocampus, and the H3 receptor may interact with this inflow. Similarly, the prominent H3 receptor binding in subiculum and deep layers of entorhinal cortex suggests that the hetero-H3 receptor is also involved in regulating the strength of CA1 input to subiculum. The subicular input to layer 5 of the entorhinal cortex, therefore, modulates the hippocampal outflow to sensory specific and multimodal association areas such as frontal and temporal cortices, amygdala and hypothalamus. Thus, the major role of H3 receptors in the hippocampal formation might be tuning the feedforward and feedback connections to various cortical and subcortical regions. Hence, they take part in the hippocampal modulation of higher cognition and emotional processes (Damasio, 1989). We previously described H3 receptor expression patterns in human prefrontal cortex and thalamus (Jin et al., 2002; Jin and Panula, 2005) and hypothesized that the H3 receptor is involved in the regulation of thalamo-cortical and cortico-cortical connections. Data from the hippocampal formation provide further evidence that brain histamine, via H3 receptors, modulates brain network activities and plays important roles in higher brain functions such as cognition and emotion.
In conclusion, the up-regulation of histamine H3 receptor radioligand binding in prefrontal cortices of schizophrenic subjects and bipolar subjects with psychotic symptoms, but not in temporal cortices of these subjects suggests that H3 receptors in the prefrontal cortex take part in the modulation of cognition. It is also possible that an isoform-specific regulation of H3 receptors exists in these two cortical areas. Histamine H3 receptors in hippocampal formation probably regulates both feedforward and feedback pathways of the hippocampus and might be involved in the neuropathology of bipolar disorder.
Acknowledgments
The materials for this study were donated by the Stanley Foundation Neuropathology Consortium courtesy of Dr E Fuller Torrey, Dr Llewellyn B Bigelow, Dr Mary M Herman, Dr Thomas M Hyde, Dr Joel E Kleinman, Dr Robert M Post, Dr Maree J Webster and Dr Robert H Yolken. We are grateful to Professors H Timmerman and R Leurs for providing us with clobenpropit. We thank Heikki Hiekkanen for the help and advice on statistical analysis, and Professor Jarmo Hietala for helpful discussions and suggestions. This study was supported by the Academy of Finland.
Glossary
Abbreviations:
- [3H]-NAMH
[3H]-Nα-methylhistamine
- ATA
atypical antipsychotics
- DTT
dithiothreitol
- MCID
microcomputer imaging device
- TCA
tricyclic antidepressant
Conflict of interest
The authors declare no conflicting interests.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Rodrigues A, Leurs R, Willems E, Timmerman H. Binding of clozapine metabolites and analogues to the histamine H3 receptor in rat brain cortex. Arch Pharm (Weinheim) 1996;329:413–416. doi: 10.1002/ardp.19963290808. [DOI] [PubMed] [Google Scholar]
- Anichtchik OV, Peitsaro N, Rinne JO, Kalimo H, Panula P. Distribution and modulation of histamine H3 receptors in basal ganglia and frontal cortex of healthy controls and patients with Parkinson's disease. Neurobiol Dis. 2001;8:707–716. doi: 10.1006/nbdi.2001.0413. [DOI] [PubMed] [Google Scholar]
- Bacciottini L, Passani B, Mannaioni P, Blandina P. Interactions between histaminergic and cholinergic systems in learning and memory. Behav Brain Res. 2001;124:183–194. doi: 10.1016/s0166-4328(01)00230-3. [DOI] [PubMed] [Google Scholar]
- Bakker RA, Lozada AL, van Marie A, Shenton FC, Drutel G, Karlstedt K, et al. Discovery of naturally occurring splice variants of the rat histamine H3 receptor that act as dominant-negative isoforms. Mol Pharmacol. 2006;69:1194–1206. doi: 10.1124/mol.105.019299. [DOI] [PubMed] [Google Scholar]
- Browman KE, Komater VA, Curzon P, Rueter LE, Hancock AA, Decker MW, et al. Enhancement of prepulse inhibition of startle in mice by the H3 receptor antagonists thioperamide and ciproxifan. Behave Brain Res. 2004;153:69–76. doi: 10.1016/j.bbr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Cangioli I, Baldi E, Mannaioni PF, Bucherelli C, Blandina P, Passani MB. Activation of histaminergic H3 receptors in the rat basolateral amygdala improves expression of fear memory and enhances acetylcholine release. Eur J Neurosci. 2002;16:521–528. doi: 10.1046/j.1460-9568.2002.02092.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Shen YJ. Effects of brain histamine on memory deficit induced by nucleus basalis-lesion in rats. Acta Pharmacol Sin. 2002;23:66–70. [PubMed] [Google Scholar]
- Chikai T, Oishi R, Saeki K. Microdialysis study of sedative drugs on extracellular histamine in the striatum of freely moving rats. J Pharmacol Exp Ther. 1993;266:1277–1281. [PubMed] [Google Scholar]
- Cogé F, Guénin SP, Audinot V, Renouard-Try A, Beauverger P, Macia C, et al. Genomic organization and characterization of splice variants of the human histamine H3 receptor. Biochem J. 2001;355:279–288. doi: 10.1042/0264-6021:3550279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a system level proposal fro the neural substances of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Kendrick KA, Fay-McCarthy M, Collins JP, Jr, Wyatt RJ. Famotidine adjunctive pharmacotherapy for schizophrenia: preliminary data. Clin Neuropharmacol. 1993;16:518–524. [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen GM, Wang JF, Reiach JS, Young LT. G protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. J Neurochem. 1999;73:1121–1126. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol Psychiatry. 2000;5:425–432. doi: 10.1038/sj.mp.4000741. [DOI] [PubMed] [Google Scholar]
- Eidi M, Zarrindast MR, Eidi A, Oryan S, Parivar K. Effects of histamine and cholinergic systems on memory retention of passive avoidance learning in rats. Eur J Pharmacol. 2003;465:91–96. doi: 10.1016/s0014-2999(03)01440-7. [DOI] [PubMed] [Google Scholar]
- Faganello FR, Medalha CC, Mattioli R. Haloperidol and chlorpheniramine interaction in inhibitory avoidance in goldfish. Behav Brain Res. 2003;147:83–88. doi: 10.1016/s0166-4328(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Esbenshade TA, Bennani YL, Black LA, Faghih R, et al. Effects of histamine H3 receptor ligands GT-2331 and ciproxifan in a repeated acquisition avoidance response in the spontaneously hypertensive rat pup. Behav Brain Res. 2002;131:151–161. doi: 10.1016/s0166-4328(01)00379-5. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, et al. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. Pharmacol Exp Ther. 2005;13:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- Frisch C, Hasenöhrl RU, Krauth J, Huston JP. Anxiolytic-like behaviour after lesion of the tuberomammillary nucleus E2-region. Exp Brain Res. 1998;119:260–264. doi: 10.1007/s002210050340. [DOI] [PubMed] [Google Scholar]
- Ghi P, Ferretti C, Blengio M. Effects of different types of stress on histamine-H3 receptors in the rat cortex. Brain Res. 1995;690:104–107. doi: 10.1016/0006-8993(95)00542-x. [DOI] [PubMed] [Google Scholar]
- Ghi P, Ferretti C, Orsetti M. Cognitive enhancing properties of thioperamide infused into the nucleus basalis magnocellularis of the rat. Inflamm Res. 2001;50:S78–S79. doi: 10.1007/PL00022415. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Ltshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Haas HL, Panula P. The role of histamine and the tuberomamillary nucleus in the brain. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Yuzurihara M. Experimental anxiety induced by histaminergics in mast cell-deficient and congenitally normal mice. Pharmacol Bilchem Behav. 2002;72:437–441. doi: 10.1016/s0091-3057(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Gilmore JH, Selinger ES, Lieberman JA. Cortical Bcl-2 protein expression and apoptotic regulation in schizophrenia. Boil Psychiatry. 2000;48:641–650. doi: 10.1016/s0006-3223(00)00988-4. [DOI] [PubMed] [Google Scholar]
- Jin CY, Panula P. The laminar histamine receptor system in human prefrontal cortex suggests multiple levels of histaminergic regulation. Neuroscience. 2005;132:137–149. doi: 10.1016/j.neuroscience.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Jin CY, Kalimo H, Panula P. The histaminergic system in human thalamus: correlation of innervation to receptor expression. Eur J Neurosci. 2002;15:1125–1138. doi: 10.1046/j.1460-9568.2002.01951.x. [DOI] [PubMed] [Google Scholar]
- Kaminsky R, Moriarty TM, Bodine J, Wolf DE, Davidson M. Effect of famotidine on deficit symptoms of schizophrenia. Lancet. 1990;335:1351–1352. doi: 10.1016/0140-6736(90)91237-5. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Schlicker E, Gothert M. Intermediate affinity and potency of clozapine and low affinity of other neuroleptics and of antidepressants at H3 receptors. Psychopharmacology (Berl) 1994;116:464–468. doi: 10.1007/BF02247479. [DOI] [PubMed] [Google Scholar]
- Knable MB, Torrey EF, Webster MJ, Bartko JJ. Multivariate analysis of prefrontal cortical data from the Stanley Foundation Neuropathology Consortium. Brain Res Bull. 2001;55:651–659. doi: 10.1016/s0361-9230(01)00521-4. [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Kobayashi RM, Kopin IJ. The effect of stress and environmental lighting on histamine in the rat brain. Brain Res. 1974;74:356–359. doi: 10.1016/0006-8993(74)90591-5. [DOI] [PubMed] [Google Scholar]
- Law AJ, Harrison PJ. The distribution and morphology of prefrontal cortex pyramidal neurons identified using anti-neurofilament antibodies SMI32, N200 and FNP7. Normative data and a comparison in subjects with schizophrenia, bipolar disorder or major depression. J Psychiatric Res. 2003;37:487–499. doi: 10.1016/s0022-3956(03)00075-x. [DOI] [PubMed] [Google Scholar]
- Liu L, Schulz SC, Lee S, Reutiman TJ, Fatemi SH. Hippocampal CA1 pyramidal cell size is reduced in bipolar disorder. Cell Mol Neurobiol. 2007;27:351–358. doi: 10.1007/s10571-006-9128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg-Aiello P, Ipponi A, Bartolini A, Schunack W. Mouse light/dark box test reveals anxiogenic-like effects by activation of histamine H1 receptors. Pharmacol Biochem Behav. 2002;71:321–326. doi: 10.1016/s0091-3057(01)00691-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Mir MI, Pollard H, Moreau J, Traiffort E, Ruat M, Schwartz JC, et al. Loss of striatal histamine H2 receptors in Huntington's chorea but not in Parkinson's disease: comparison with animal models. Synapse. 1993;15:209–220. doi: 10.1002/syn.890150306. [DOI] [PubMed] [Google Scholar]
- Mazurkiewickz-Kwilecki IM. Single and repeated air blast stress and brain histamine. Pharmacol Biochem Behav. 1979;12:35–39. doi: 10.1016/0091-3057(80)90412-8. [DOI] [PubMed] [Google Scholar]
- Mazurkiewickz-Kwilecki IM, Prell GD. Brain histamine response to stress in 12 month old rats. Life Sci. 1986;38:2339–2345. doi: 10.1016/0024-3205(86)90641-7. [DOI] [PubMed] [Google Scholar]
- Nakai T, Kitamura N, Hashimoto T, Kajimoto Y, Nishino N, Mita T, et al. Decreased histamine H1 receptors in the frontal cortex of brains from patients with chronic schizophrenia. Biol Psychiatry. 1991;30:349–356. doi: 10.1016/0006-3223(91)90290-3. [DOI] [PubMed] [Google Scholar]
- Oishi R, Nishibori M, Itoh Y, Saeki K. Diazepan-induced decrease in histamine turnover in mouse brain. Eur J Pharmacol. 1986;124:337–342. doi: 10.1016/0014-2999(86)90236-0. [DOI] [PubMed] [Google Scholar]
- Oishi R, Itoh Y, Saeki K. Inhibition of histamine turnover by 8-OH-DPAT, buspirone and 5-hydroxytryptophan in the mouse and rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:495–499. doi: 10.1007/BF00168939. [DOI] [PubMed] [Google Scholar]
- Oliveira RMW, Guimarães FS, Deakin JFW. Expression of neuronal nitric oxide synthase in the hippocampal formation in affective disorders. Braz J Med Biol Res. 2008;41:333–341. doi: 10.1590/s0100-879x2008000400012. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Ghi P, Di Carlo G. Histamine H3-receptor antagonism improves memory retention and reverses the cognitive deficit induced by scopolamine in a two-trial place recognition task. Behav Brain Res. 2001;124:235–242. doi: 10.1016/s0166-4328(01)00216-9. [DOI] [PubMed] [Google Scholar]
- Oyewumi LK, Vollick D, Merskey H, Plumb C. Famotidine as an adjunct treatment of resistant schizophrenia. J Psychiatry Neurosci. 1994;19:145–150. [PMC free article] [PubMed] [Google Scholar]
- Passani MB, Lin JS, Hancock A, Crochet S, Blandina P. The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders. Trends Pharmacol Sci. 2004;25:618–625. doi: 10.1016/j.tips.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pérez-García C, Morales L, Cano MV, Sancho I, Alguacil LF. Effects of histamine H3 rceptor ligands in experimental models of anxiety and depression. Psychopharmacology (Berl) 1999;142:215–220. doi: 10.1007/s002130050882. [DOI] [PubMed] [Google Scholar]
- Prell GD, Green JP, Kaufmann CA, Khandelwal JK, Morrishow AM, Kirch DG, et al. Histamine metabolites in cerebrospinal fluid of patients with chronic schizophrenia: their relationships to levels of other aminergic transmitters and ratings of symptoms. Schizophr Res. 1995;14:93–104. doi: 10.1016/0920-9964(94)00034-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues AA, Jansen FP, Leurs R, Timmerman H, Prell GD. Interaction of clozapine with the histamine H3 receptor in rat brain. Br J Pharmacol. 1995;114:1523–1524. doi: 10.1111/j.1476-5381.1995.tb14934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse RB, Kendrick K, Tsui LC, Fay-McCarthy M, Collins JP, Jr., Rosenberg P, et al. Famotidine adjunctive pharmacotherapy of schizophrenia: a case report. Clin Neuropharmacol. 1995;18:369–374. doi: 10.1097/00002826-199508000-00009. [DOI] [PubMed] [Google Scholar]
- Rosse RB, Kendrick K, Fay-McCarthy M, Prell GD, Rosenberg P, Tsui LC, et al. An open-label study of the therapeutic efficacy of high-dose famotidine adjuvant pharmacotherapy in schizophrenia: preliminary evidence for treatment efficacy. Clin Neuropharmacol. 1996;19:341–348. doi: 10.1097/00002826-199619040-00007. [DOI] [PubMed] [Google Scholar]
- Santos NR, Huston JP, Brandão ML. Blockade of histamine H2 receptors of the periaqueductal gray and inferior colliculus induces fear-like behaviors. Pharmacol Biochem Behav. 2003;75:25–33. doi: 10.1016/s0091-3057(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Snyder SH. Brain histamine: rapid apparent turnover altered by restraint and cold stress. Science. 1971;172:1037–1039. doi: 10.1126/science.172.3987.1037. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44:151–155. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Shatti S, Tanabe JL, Martin LF, Gibson L, Wylie K, et al. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophr Res. 2007;97:242–249. doi: 10.1016/j.schres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Van der Goot H, Timmerman H. Selective ligands as tools to study histamine receptors. Eur J Med Chem. 2000;35:5–20. doi: 10.1016/s0223-5234(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Goodman MW, Burstein ES, Nash NR, Brann MR, Weiner DM. Molecular cloning and pharmacology of functionally distinct isoforms of the human histamine H3 receptor. Neuropharmacology. 2002;42:929–940. doi: 10.1016/s0028-3908(02)00041-2. [DOI] [PubMed] [Google Scholar]
- West RE, Jr, Wu RL, Billah MM, Egan RW, Anthes JC. The profiles of human and primate [3H]Nalpha-methylhistamine binding differ from that of rodents. Eur J Pharmacol. 1999;377:233–239. doi: 10.1016/s0014-2999(99)00424-0. [DOI] [PubMed] [Google Scholar]
- Yanai K, Son LZ, Endou M, Sakurai E, Nakagawasai O, Tadano T, et al. Behavioral characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience. 1998;87:479–487. doi: 10.1016/s0306-4522(98)00167-5. [DOI] [PubMed] [Google Scholar]
- Yoshitomi I, Itoh Y, Oishi R, Saeki K. Brain histamine turnover enhanced by foot shock. Brain Res. 1986;362:195–198. doi: 10.1016/0006-8993(86)91418-6. [DOI] [PubMed] [Google Scholar]
- Yuzurihara M, Ikarashi Y, Ishige A, Sasaki H, Kuribara H, Maruyama Y. Effects of drugs acting as histamine releasers or histamine receptor blockers on an experimental anxiety model in mice. Pharmacol Biochem Behav. 2000;67:145–150. doi: 10.1016/s0091-3057(00)00320-8. [DOI] [PubMed] [Google Scholar]


