Abstract
The recent discovery of new potent therapeutic molecules that do not reach the clinic due to poor delivery and low bioavailability have made of delivery a key stone in therapeutic development. Several technologies have been designed to improve cellular uptake of therapeutic molecules, including cell-penetrating peptides (CPPs). CPPs were first discovered based on the potency of several proteins to enter cells. Numerous CPPs have been described so far, which can be grouped into two major classes, the first requiring chemical linkage with the drug for cellular internalization and the second involving formation of stable, non-covalent complexes with drugs. Nowadays, CPPs constitute very promising tools for non-invasive cellular import of cargo and have been successfully applied for in vitro and in vivo delivery of therapeutic molecules varying from small chemical molecule, nucleic acids, proteins, peptides, liposomes and particles. This review will focus on the structure/function and cellular uptake mechanism of CPPs in the general context of drug delivery. We will also highlight the application of peptide carriers for the delivery of therapeutic molecules and provide an update of their clinical evaluation.
This article is part of a themed section on Vector Design and Drug Delivery. For a list of all articles in this section see the end of this paper, or visit: http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Keywords: cell-penetrating peptide, non-covalent delivery system, siRNA, nanoparticle, drug delivery, molecular mechanisms, therapeutics
Introduction: challenges in drug delivery
Over the past 10 years, in order to circumvent limitations of small molecule-and gene-based therapies, we have witnessed a dramatic acceleration in the production of new large therapeutic molecules, which do not follow Lipinski's rules, such as proteins, peptides and nucleic acid therapeutics. However, their development is restricted by very specific issues including poor stability in vivo, lack of cellular uptake and insufficient capability to reach targets. This is associated with the complete loss of pharmaceutical potency or at least with the requirement for high doses and risk of major side effects. Therefore, delivery constitutes a major piece of the therapeutic puzzle, and there is a real demand for new and more efficient drug delivery systems. Major rules have to be satisfied, in particular: (i) delivery efficiency in different and challenging cell lines; (ii) rapid endosomal release; (iii) ability to reach the target; (iv) activity at low doses; (v) lack of toxicity; and (vi) facility of therapeutic application.
Substantial progress has been made in the design of new technologies to improve cellular uptake of therapeutic compounds (Opalinska and Gewirtz, 2002; Järver and Langel, 2004; Glover et al., 2005; Torchilin, 2005; De Fougerolles et al., 2007; Kong and Mooney, 2007). A number of non-viral strategies have been proposed including lipid, polycationic, nanoparticle and peptide-based formulations as reported in this special issue (Morris et al., 2000; Ogris and Wagner, 2002; Järver and Langel, 2004; Torchilin, 2005), but only a subset of these technologies are efficiently applied in vivo at either preclinical or clinical levels. Protein transduction domains (PTDs) or cell-penetrating peptides (CPPs) correspond to short 30 residue synthetic peptides and are part of the most promising strategy to overcome both extracellular and intracellular limitations of various biomolecules of including plasmid DNA, oligonucleotide, siRNA, peptide-nucleic acid (PNA), proteins, peptides as well as liposomes. CPPs can trigger the movement of a cargo across the cell membrane into the cytoplasm of cells and improve its intracellular routing, thereby facilitating interactions with the target (Derossi et al., 1994; Fawell et al., 1994; Pooga et al., 1998; Wender et al., 2000; Deshayes et al., 2005; Meade and Dowdy, 2007; Morris et al., 2008).
Cell-penetrating peptide families
Twenty years ago, the notion of PTD was proposed based on the observation that some proteins, mainly transcription factors, could shuttle within the cell and from one cell to another. Historically, the first observation was made in 1988, by Frankel and Pabo, who showed that the transcription-transactivating (Tat) protein of HIV-1 could enter cells and translocate into the nucleus (Frankel and Pabo, 1988). In 1991, the group of Prochiantz demonstrated that Drosophila Antennapedia homeodomain could be internalized by neuronal cells (Joliot et al., 1991), work which was at the origin of the discovery in 1994 of the first PTD or CPP: a 16-mer-peptide derived form the third helix of the homeodomain of Antennapedia termed penetratin (RQIKIYFQNRRMKWKK) (Derossi et al., 1994). In 1998 the group of Lebleu identified the minimal peptide sequence of Tat required for cellular uptake (47YGRKKRRQRRR57) (Vives et al., 1997). In 1997, the first non-covalent CPP for delivery of nucleic acids MPG was designed by the group of Heitz and Divita (Morris et al., 1997) closely followed by development of Pep-1 for non-covalent cellular delivery of proteins and peptides (Morris et al., 2001). The groups of Wender and of Futaki demonstrated that polyarginine sequences (Arg8) were sufficient to drive molecules into cells and proposed that their uptake mechanism involves a bidentate hydrogen-bonding interaction between guanidinium group of arginine residues and phosphate group in the membrane (Wender et al., 2000; Futaki et al., 2001). A major breakthrough in the CPP field came from the first proofs-of-concept of their in vivo application, by the groups of Dowdy, for the delivery of small peptides and large proteins (Schwarze et al., 1999), and of Langel, for delivery of PNAs using the chimeric peptide Transportan, derived form the N-terminal fragment of the neuropeptide galanin, linked to mastoparan, a wasp venom peptide (Pooga et al., 1998). Ever since many other CPPs able to trigger the movement of a cargo across the cell membrane into the cytoplasm have been designed (Järver and Langel, 2004; Joliot and Prochiantz, 2004; Deshayes et al., 2005; Snyder and Dowdy, 2005). CPPs are generally peptides of less than 30 amino acids, derived from natural or unnatural protein or chimeric sequences and can be subdivided into two main classes, the first requiring chemical linkage with the cargo and the second involving formation of stable, non-covalent complexes. CPPs can also be distinguished from a structural point of view, as either polycationic, essentially containing clusters of polyarginine in their primary sequence or amphipathic. The representative CPPs are reported in Table 1. Although this review mainly focuses on CPPs based on natural amino acids, recent new concepts of CPPs containing unnatural and modified residues have been proposed in order to improve either the stability or the efficiency of the carrier (Farrera-Sinfreu et al., 2007).
Table 1.
Representative CPPs: sequences, applications and major related references
| Peptides | Origin | Sequences | Cargo types | References |
|---|---|---|---|---|
| Peptides deriving from protein transduction domains | ||||
| Tat | HIV-Tat protein | PGRKKRRQRRPPQ | Protein/peptide/siRNA/liposome/nanoparticle | Snyder and Dowdy (2005); Schwarze et al. (1999) |
| Penetratin | Homeodomain | RQIKIWFQNRRMKWKK | peptide/siRNA/liposome | Joliot and Prochiantz (2004) |
| Transportan | Galanin-mastoparan | GWTLNSAGYLLGKINLKALAALAKKIL | Protein/PNA/siRNA | Pooga et al. (1998) |
| VP-22 | HSV-1 structural protein | DAATATRGRSAASRPTERPRAPAR-SASRPRRPVD | Protein | Elliott and O'Hare (1997) |
| Amphipathic peptides | ||||
| MPG | HIV Gp41-SV40 NLS | GALFLGFLGAAGSTMGAWSQPKKKRKV | siRNA/ODN/plasmid | Morris et al. (2008) |
| Pep-1 | Trp-rich motif-SV40 NLS | KETWWETWWTEWSQPKKKRKV | Protein/peptide | Gros et al. (2006) |
| MAP | Chimeric | KALAKALAKALA | Small molecule/plasmid | |
| SAP | Proline-rich motif | VRLPPPVRLPPPVRLPPP | protein/peptide | Pujals et al. (2006) |
| PPTG1 | Chimeric | GLFRALLRLLRSLWRLLLRA | Plasmid | Rittner et al. (2002) |
| Other cell-penetrating peptides: cationic peptides | ||||
| Oligoarginine | Chimeric | Agr8 or Arg9 | Protein/peptide/siRNA/ODN | Wender et al. (2000); Futaki et al. (2001) |
| hCT (9–32) | Human calcitonin | LGTYTQDFNKTFPQTAIGVGAP | Protein/plasmid DNA | Schmidt et al. (1998) |
| SynB | Protegrin | RGGRLSYSRRRFSTSTGR | Doxorubicin | Rousselle et al. (2001) |
| Pvec | Murine VE-cadherin | LLIILRRRIRKQAHAHSK | Protein/peptide | Elmquist et al. (2001) |
CPP, cell-penetrating peptide; NLS, nuclear localization sequence; PNA, peptide-nucleic acid; Tat, transcription-transactivating.
Covalent strategy
Cell-penetrating peptide-based technologies described so far mainly involve the formation of a covalent conjugate between the cargo and the carrier peptide, which is achieved by chemical cross-linking or by cloning followed by expression of a CPP fusion protein (Nagahara et al., 1998; Gait, 2003; Moulton and Moulton, 2004; Zatsepin et al., 2005). Most of the work has been reported for peptides derived from Tat (Fawell et al., 1994; Vives et al., 1997; Frankel and Pabo, 1988), penetratin (Derossi et al., 1994), polyarginine peptide Arg8 sequence (Wender et al., 2000; Futaki et al., 2001) and Transportan, (Pooga et al., 1998). Other protein-derived peptides such as VP22 protein from Herpes Simplex Virus (Elliott and O'Hare, 1997), pVec (Elmquist et al., 2001), calcitonin-derived peptides (Schmidt et al., 1998; Krauss et al., 2004), antimicrobial peptides Buforin I and SynB (Park et al., 1998; Park et al., 2000), as well as polyproline sweet arrow peptide (Pujals et al., 2006) have also been successfully used to improve the delivery of covalently linked cargos (Joliot and Prochiantz, 2004; El-Andaloussi et al., 2005; Murriel and Dowdy, 2006). More recently, new generations of CPPs, combining different transduction motifs (Abes et al., 2007) or transduction domains in tandem with protein or oligonucleotide-binding domains (Meade and Dowdy, 2007) have been proposed. Different chemistries have been proposed for stable or cleavable conjugation involving mainly disulfide or thio-esters linkages. According to the stability and efficiency of the cargo, several parameters need to be considered including the type of linkage chemistry, the nature of the spacer (Gait, 2003; Zatsepin et al., 2005). Covalent strategies have been mainly reported for the delivery of DNA mimic molecules or steric block oligonucleotides, including PNA (Koppelhus et al., 2002; Fabani et al., 2008), phosphorodiamidate morpholino-oligomer (PMO) (Abes et al., 2006; Lebleu et al., 2008; Moulton and Moulton, 2008), peptide and protein (Snyder and Dowdy, 2005). Conjugation methods offer several advantages for in vivo applications including rationalization, reproducibility of the procedure, together with the control of the stoechiometry of the CPP-cargo. However, the covalent CPP technology is limited from a chemical point of view and risks altering the biological activity of the cargo. This is particularly true, in the case of charged oligonucleotide or siRNA, for which CPP coupling has led to restricted biological activities (Juliano et al., 2008), non-covalent strategies thereby appearing more appropriate.
Non-covalent strategy
This strategy is mainly based on short amphipathic peptide carriers consisting of two domains: a hydrophilic (polar) domain and a hydrophobic (non-polar) domain (Table 1). The amphipathic character may arise from either the primary structure or the secondary structure. Primary amphipathic peptides can be defined as the sequential assembly of a domain of hydrophobic residues with a domain of hydrophilic residues. Secondary amphipathic peptides are generated by the conformational state that allows positioning of hydrophobic and hydrophilic residues on opposite sides of the molecule (Deshayes et al., 2005). Several CPPs have been reported to form non-covalent complexes with biomolecules and to improve their delivery into mammalian cells (Morris et al., 2008).
Non-covalent approach was originally developed for gene delivery; several peptides able to condense DNA associated with peptides that favour endosomal escape including fusion peptide of HA2 subunit of influenza hemaglutinin have been described (Lear and Degrado, 1987; Parente et al., 1990). Synthetic peptides analogs GALA, KALA, JTS1 (Gottschalk et al., 1996; Wyman et al., 1997), PPTG1 (Rittner et al., 2002), MPG (Morris et al., 1999) and histidine-rich peptides (Midoux et al., 1998; Kichler et al., 2003) were also reported as potent gene delivery systems. In 2001, we demonstrated that the amphipathic peptide Pep-1 could be successfully applied to the delivery of small peptides and proteins in a non-covalent approach (Morris et al., 2001). In 2003, a non-covalent strategy based on MPG was shown to efficiently deliver siRNA into cultured cell lines (Simeoni et al., 2003). Pep-1 and MPG are primary amphipathic peptides containing a hydrophilic lysine-rich domain derived from the nuclear localization sequence (NLS) of SV40 large T antigen (KKKRKV), and a variable N-terminal hydrophobic moiety derived form the fusion sequence of the HIV protein gp41 (GALFLGFLGAAGSTMGA) for MPG, and from a tryptophan-rich cluster (KETWWETWWTEW) for Pep-1, separated by a linker domain, which improves the flexibility and the integrity of both the hydrophobic and hydrophilic domains (Morris et al., 1997; Morris et al., 1999; Simeoni et al., 2003). MPG and Pep-1 form stable complexes with their respective cargo (oligonucleotide or protein/peptide) through non-covalent electrostatic and hydrophobic interactions (Morris et al., 1997 1999 2001; Simeoni et al., 2003; Gros et al., 2006; Munoz-Morris et al., 2007). Non-covalent strategies for protein and oligonucleotide delivery have been recently been extended to other CPPs, including Tat (Meade and Dowdy, 2007), polyarginine (Kim et al., 2006; Kumar et al., 2007) and Transportan-derived peptides (Pooga et al., 2001; Lundberg et al., 2007).
Cellular uptake mechanism of cell-penetrating peptides
The cellular uptake mechanism of CPPs is an essential piece of the puzzle for the development and optimization of appropriate strategies for in vivo therapeutic applications. Although cellular internalization of CPPs was reported in a wide variety of cell types, their mechanism of internalization remained ‘mysterious’ for a long time, as being independent of endocytosis, of energy and of specific receptor. In the last 5 years, the CPP field has suffered and learnt from technical artifacts. As such, in 2003, Lebleu and colleagues, proposed a revised cellular uptake mechanism for CPPs, essentially associated with the endosomal pathway (Richard et al., 2003). Ever since, the mechanism of many CPPs has re-examined and reported to be mediated by endocytosis (Lundberg and Johansson, 2001; Nakase et al., 2004; Wadia et al., 2004; Fischer et al., 2005; Richard et al., 2005; Murriel and Dowdy, 2006). However, for most CPPs, the cellular uptake mechanism still needs to be confirmed and remains controversial, partly due to the fact that different methods, which are not comparable from one lab to another, have been employed to this aim. Therefore, results should be taken with care as in most of the cases the visualization of CPPs inside the cell is based on fluorescein-labelled CPPs with the risk that fluorescent dyes may alter the uptake mechanism or trigger an unusual cell entry pathway, which does not reflect the biologically active fraction of the CPPs or of the cargo. Evidence for several routes of entry has been reported, some of which are independent of the endosomal pathway and involve the trans-membrane potential (Terrone et al., 2003; Thoren et al., 2003; Rothbard et al., 2004; Deshayes et al., 2005). Therefore, for therapeutic purposes the challenge remains in identifying the route yielding a biological response, which may not be the predominant one and to correlate the uptake pathway with a biological response associated with a specific cargo (Wadia et al., 2004; Gros et al., 2006). For that purpose, several approaches have been described, by using biological reporters (Wadia et al., 2004; Lebleu et al., 2008) or phenotypic (Morris et al., 2007a) assays enabling to follow shuttling and release of the cargo in real time in cultured cells (Lee et al., 2008) or in animal models (Wender et al., 2007)
Although it remains difficult to establish a general scheme for CPP uptake mechanism, there is a consensus that the first contacts between the CPPs and the cell surface take place through electrostatic interactions with proteoglycans, and that the cellular uptake pathway is driven by several parameters including: (i) the nature and secondary structure of the CPP; (ii) its ability to interact with cell surface and membrane lipid components; (iii) the nature, type and active concentration of the cargo; and (iv) the cell type and membrane composition (Figure 1).
Figure 1.
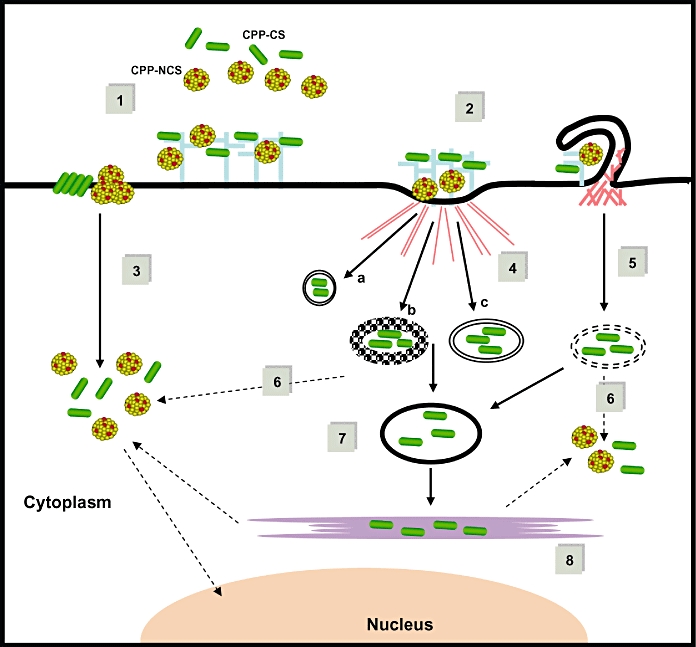
Model of cellular uptake and intracellular trafficking of cell-penetrating peptides (CPPs). Cellular uptake of CPP by the covalent (CPP-CS) and non-covalent (CPP-NCS) strategies. (1) Binding of CPPs or CPP/cargo complexes to extracellular matrix via the cell surface proteoglycan platform, (2) clustering of GlucosAminoGlycan platform triggers selective activation of small GTPase and remodelling of the actin network, (3) increase of membrane fluidity or microdomain dynamic promotes the cell entry and release in the cytosol of CPP-NCS and of CPP-CS (at high concentrations) via membrane fusion or cellular uptake of CPP-CS/CPP-NCS via (4) endocytosis pathway (a: caveolin-dependent, b: clathrin-dependent, c: clathrin-and caveolin-independent) or (5) macropinocytosis. After endocytic capture, CPP-CS can escape from lysosomal degradation and enter the cytosol and the nucleus (6), remain in the early or late endosomes (7), or be delivered in the Golgi apparatus and the endoplasmic reticulum (8).
Role of proteoglycans
Proteoglycans play an essential role in the regulation of cell surface microdomains, and evidence for direct relationships between cytoskeletal organization and activation of small GTPases has been clearly established (Conner and Schmid, 2003; Eitzen, 2003). Heparan sulfate proteoglycans and syndecans are the major components of the extracellular matrix, and their clustering triggers cytoskeletal remodelling upon activation of protein kinase C and Rho/Rac GTPases, which control the dynamics of cholesterol-rich ‘raft’ microdomains, and therefore ligand binding and cellular uptake pathways (Couchman, 2003; Beauvais and Rapraeger, 2004). The first contacts between the CPPs and the cell surface take place through electrostatic binding with cell surface proteoglycans GlucosAminoGlycan (GAG) platform, follow by a remodelling of the actin network and a selective activation of the small GTPase Rho A or Rac1 (Duchardt et al., 2007; Ziegler, 2008). GTPase activation and actin remodelling constitute the ‘onset’ of the internalization mechanism and have a major impact on membrane fluidity, thereby promoting cell entry of Arg8, penetratin and Tat via macropinocytosis (Nakase et al., 2007) or clathrin-dependent endocytosis (Richard et al., 2005), of MPG or Pep-1 particles via membrane perturbation mechanism (Gerbal-Chaloin et al., 2007).
The gates of the cells: cell entry and trafficking pathways
Following binding to the GAG ‘platform’, which facilitates accumulation of the CPP and CPP-cargo complexes at the cell surface, different cell entry gates have been reported depending on the CPPs. Correlation of cellular uptake with a cargo-associated biological response is a major requirement to validate the efficiency of a CPP, as originally established for Tat (Wadia et al., 2004) and has been extended to series of well-known CPPs (Nakase et al., 2004; Padari et al., 2005). One of the major differences between CPPs resides in their mode of interaction with the cellular surface components. The interaction of peptides such as Tat (Console et al., 2003; Murriel and Dowdy, 2006) polyarginine and penetratin (Nakase et al., 2004 2007) with the extracellular matrix has been reported to be primarily electrostatic and to trigger uptake through an energy-dependent endocytotic process (Rusnati et al., 1999; Murriel and Dowdy, 2006). Although, macropinocytosis has been reported as the major route of internalization of cationic CPPs (Wadia et al., 2004; Kaplan et al., 2005), other endocytotic pathways including clathrin-and caveolin-dependent endocytosis (Richard et al., 2005; Ziegler et al., 2005) and trans-Golgi network-mediated internalization (Fischer et al., 2005) have been described for CPPs. Moreover, different mechanisms of membrane translocation and endocytosis may concur simultaneously for most CPPs. This is especially true for amphipathic peptides, which tend to interact with lipids and adopt secondary structures within the membrane that modified membrane integrity. The secondary structure of CPPs and their dynamics constitute major factors in the mechanism of cellular uptake (Magzoub and Gräslund, 2004; Deshayes et al., 2005 2008; Esbjorner et al., 2007). Increasing the local concentration of CPPs at the cell surface favours cellular uptake independently of endocytosis and leads to a more cytoplasmic distribution of CPPs. Indeed, the major route for cell entry of CPP-based nanoparticles, such as Pep-1 and MPG has been shown to be independent of the endosomal pathway. Cellular uptake is associated with the ability of MPG and Pep-1 to interact with membrane lipids, mainly through their hydrophobic domain, and to form transient trans-membrane helical or beta structures that temporarily affect membrane organization, thereby facilitating insertion into the membrane and initiation of the translocation process associated with membrane potential (Deshayes et al., 2004a b). Cellular uptake of biologically active Pep-1 or MPG/cargo complexes is directly correlated with the structure of the nanoparticle that creates a local high concentration of peptides at the cell surface (Gros et al., 2006; Munoz-Morris et al., 2007).
In contrast, to cellular uptake that is well characterized for a subset of CPPs, very little is known about their cellular trafficking, which is important to allow the cargo to reach its target within the cell. Clearly, endosomal escape remains a major limitation and the rate-limiting step of CPP-mediated drug delivery. A small fraction of CPPs is able to escape from the endosome throughout either their endosomal breaker property or to the fact of the poor integrity of the macropinocytosis vesicles. Several studies report that CPPs can traffic through the endoplasmic reticulum and the Golgi network via a ‘retrograde pathway’ that involves cytosolic release (Fischer et al., 2005). Moreover, CPPs harbouring functional NLS motif can directly localize and trigger cargo in the nucleus (Cartier and Reszka, 2002; Simeoni et al., 2003).
Application of CPP strategies to the delivery of therapeutic molecules
The number of applications using CPPs is consciously increasing, and so far more than 300 studies using either covalent or non-covalent CPP-based strategies from in vitro to in vivo have been reported (Dietz and Bähr, 2004; Gros et al., 2006; Moschos et al., 2007; Patel et al., 2007; Foerg and Merkle, 2008). The interest for CPPs is mainly due to their low cytotoxicity and to the fact that there is no limitation for the type of cargo. Although CPPs have been used to improve delivery of cargo that varies greatly in size and nature (small molecules, oligonucleotide, plasmid DNA, peptide, protein, nanoparticle, lipid-based formulation, virus, quantum dots) most of the applications describe the delivery of oligopeptide/protein (Dietz and Bähr, 2004; Gros et al., 2006; Patel et al., 2007) and nucleic acids or analogs (Juliano et al., 2008) (Table 1).
CPP-based strategies for gene delivery
The poor permeability of the plasma membrane of eukaryotic cells to DNA together with the low efficiency of DNA or oligonucleotides to reach their target within cells constitutes the two major barriers for the development of these therapeutic molecules. In the last decade, a number of peptide carriers that combine DNA binding, mainly electrostatic domain (polylysine and polyarginine) and membrane-destabilizing properties have been developed to facilitate gene transfer into cultured cells and living animals (Niidome and Huang, 2002; Glover et al., 2005; Morris et al., 2008). Amphipathic peptides with pH-dependent fusogenic and endosomolytic activities such as the fusion peptide of HA2 subunit of influenza hemaglutinin, or synthetic analogs GALA, KALA, JTS1, and histidine-rich peptides have been shown to increase transfection efficiency when associated with poly-L-lysine/DNA, condensing peptide/DNA, cationic lipids, poly-ethyleneimine or polyamidoamine cascade polymers (for review: Morris et al., 2008). Single peptide chains able to condense DNA and to favour endosomal escape (PPTG1) (Rittner et al., 2002) or prevent endosomal uptake (MPG: Morris et al., 1999) have also been used for gene delivery in cultured cells. However, only a few CPPs have been validated in vivo for gene delivery and so far, the secondary amphipathic peptide PPTG1 constitutes one of the only examples reporting a significant in vivo gene expression response following intravenous injection (Rittner et al., 2002). Tat, Transportan and polyarginine CPPs have been associated with other lipid-based non-viral gene delivery methods, including liposomes, PEI or nanostructures (Branden et al., 1999; Tung et al., 2002; Ignatovich et al., 2003; Rudolph et al., 2003; Kilk et al., 2005). The association of Tat and octa-arginine to pharmaceutical nano-carriers, described as non-viral delivery systems based on new packing concepts ‘Programmed packaging’ Multifunctional Envelope-type NanoDevice (MEND) (Kogure et al., 2004; MacKay et al., 2008), has been shown to improve gene delivery and to offer the advantage of combining delivery, packaging and targeting motifs within the same particle (Torchilin, 2008; Vivès et al., 2008).
The second major barrier of non-viral gene delivery systems is their poor nuclear translocation, which is however essential for transfection of non-dividing cells and gene therapy. In order to improve nuclear delivery of DNA-plasmids, synthetic peptides containing NLS have been extensively applied (Cartier and Reszka, 2002; Escriou et al., 2003). Most of these studies were performed with the sequence derived from SV40 large T antigen NLS (PKKKRKV). This sequence was associated with either membrane-penetrating or cationic peptides, but also directly linked to cargoes or combined with other transfection methods to facilitate delivery into the nucleus. Moreover, NLS sequences have been associated with different hydrophobic CPPs in order to favour nuclear targeting as well as DNA binding and compaction. The NLS domain of MPG has been shown to improve the nuclear translocation of nucleic acids without requiring nuclear membrane breakdown during mitosis. MPG technology has been applied to both plasmid DNA and oligonucleotide delivery with high efficiency into a large number of adherent and suspension cell lines (Simeoni et al., 2003; Morris et al., 2007b).
CPP-based strategies for oligonucleotide analog delivery
Steric block small neutral oligonucleotide including PNAs and phosphorodiamidate morphorodiamidate morpholino-oligomers (PMO) constitute potent molecules for either antisense application or mRNA splicing correction strategies. Several CPPs have been successfully applied for the delivery of uncharged PNA and PMO in vitro and in vivo through covalent coupling (Gait, 2003; Moulton and Moulton, 2004; Zatsepin et al., 2005; Juliano et al., 2008). Originally reported with Transportan for in vivo delivery of an antisense PNA targeting galanine receptors and modifying pain transmission (Pooga et al., 1998), the use of CPPs for steric block oligonucleotide delivery has been extended to Tat, penetratin, TP10 (a short version of Transportan) and arginine-rich peptides. Several CPP-based covalent approaches have been reported for the delivery of antisense PNA (Koppelhus and Nielsen, 2003). However only a few have been used in vivo, and until recently none of them were reported to be active at submicromolar concentrations (Gait, 2003; Abes et al., 2007). A detailed study of CPP-mediated PNA delivery has reported that the major limitation is due to their endosomal sequestration (Koppelhus and Nielsen, 2003), and more recently new CPPs have been described by Lebleu and Gait groups including R6-penetratin and 6-aminohexanoic acid spaced oligoarginine [(R-Ahx-R)4], which exhibit limited endosomal sequestration and lead to submicromolar antisense or splicing correction response (Abes et al., 2006 2007). These CPPs have been validated in vivo for splicing correction on two therapeutic models: Duchenne's muscular dystrophy (Fletcher et al., 2007) and coronavirus replication in mice (Burrer et al., 2007). Non-covalent strategies have also been applied to the delivery of PNA and DNA mimic molecules (Nan et al., 2005). Pep-3 peptide, a variant of Pep-1, was successfully applied to the delivery of PNA and analogs targeting the cell cycle regulatory protein cyclin B1 in vitro and in vivo (Morris et al., 2004b 2007b). Interestingly, the nanoparticle organization of Pep-3/PNA complex allows functionalization of the surface layer of the particle, and PEGylation of the carrier significantly improves the efficacy of the response by stabilizing the complexes. This study shows that such a modification improves Pep-3 for systemic administration into mice, thereby allowing for a significant reduction of the dose required to induce a specific and robust biological response, which consequently limits non-specific cytotoxic effects described upon treatment with high concentrations of CPP-PNA conjugate or non-covalenty complexes (Morris et al., 2007b).
Oligonucleotide and siRNA delivery
Decoy oligonucleotides and short interfering RNAs (siRNA) constitute powerful biomedical tools to control protein activation and/or gene expression post-transcriptionally. (Elbashir et al., 2001; Hannon, 2002). However, the major limitation of siRNA applications, like most antisense or nucleic acid-based strategies remains their poor cellular uptake associated with the poor permeability of the cell membrane to nucleic acids. Several viral and non-viral strategies have been proposed to improve the delivery of either siRNA-expressing vectors or synthetic siRNAs both in cultured cells and in vivo (De Fougerolles et al., 2007; Juliano et al., 2008). CPP-based strategies have been developed to improve the delivery of oligonucleotides both in vitro and in vivo. Delivery of charged oligonucleotide and siRNA is more challenging as multiple anionic charges of the nucleic acid interact with CPP moiety and inhibit uptakes by steric hindrance. Delivery of charged oligonucleotide was achieved by using either peptide-based non-covalent or PNA-hybridization strategies. In the latter, CPPs are covalently linked to a PNA that is able to hybridize with a double-stranded decoy oligonucleotide containing on one strand a flanking sequence complementary to the PNA. Strategies have been applied with Transportan and TP10 CPP for the delivery of decoy oligonucleotide interacting with NFkB or Myc (Fisher et al., 2004; El-Andaloussi et al., 2005). The MPG peptide-based delivery system has been successfully applied for the delivery of various type of nucleic acid, including phosphodiester-oligonucleotide targeting the protein phosphatase cdc25C (Morris et al., 1999), phosphorothioate-oligonucleotides targeting MDR-1 promoter in human CEM leukaemia cells (Marthinet et al., 2000) and thio-phosphoramidate telomerase template antagonists in cancer cells (Asai et al., 2003; Gryaznov et al., 2003). Several CPP-based strategies have been used for the delivery of siRNA into cultured cells. siRNA covalently linked to Transportan (Muratovska and Eccles, 2004) and penetratin (Davidson et al., 2004) have been associated with a silencing response. Nevertheless, non-covalent strategies appear to be more appropriate for siRNA delivery and yield significant associated biological response (Simeoni et al., 2003; Kim et al., 2006; Veldhoen et al., 2006; Crombez et al., 2007; Kumar et al., 2007; Lundberg et al., 2007; Meade and Dowdy, 2007). MPG peptide has been reported to improve siRNA delivery into a large panel of cell lines including adherent cell lines, cells in suspension, cancer and challenging primary cell lines (Simeoni et al., 2003; Morris et al., 2004a; Nguyen et al., 2006). MPG has been applied for in vivo delivery of siRNA targeting OCT-4 into mouse blastocytes (Zeineddine et al., 2006) and of siRNA targeting an essential cell cycle protein, cyclin B1; intravenous injection of MPG/cyclin B1 siRNA particles has been shown to efficiently block tumour growth (Crombez et al., 2007). A variant of MPG (MPG-alpha) harbouring five mutations in the hydrophobic domain, in order to favour helical conformation of the peptide, has also been shown to improve siRNA delivery (Veldhoen et al., 2006). However, such modifications of MPG increase toxicity and favour endosomal cellular uptake (Deshayes et al., 2004c; Veldhoen et al., 2006). This non-covalent approach has been extended to other CPPs including polyarginine-(Kim et al., 2006; Kumar et al., 2007), penetratin-(Lundberg et al., 2007) and Tat-(Meade and Dowdy, 2007) derived peptides. Tat peptide associated with an RNA-binding motif has been reported to block in vivo epidermal growth factor (EGF) factor, cholesterol-Arg9 has been shown to enhance siRNA delivery in vivo against vascular endothelial growth factors (Kim et al., 2006) and more recently, a small peptide derived from rabies virus glycoprotein associated to polyarginine R9 has been shown to deliver siRNA in the CNS (Kumar et al., 2007).
CPP-based strategies for in vivo delivery of proteins and peptides
In order to circumvent the technological problems of gene delivery an increasing interest has been taken in designing novel strategies to enable delivery of peptides and full-length proteins into a large numbers of cells. The first proof of concept of the in vivo potency of CPPs was provided by Dowdy and colleagues in 1999, showing that Tat-β-galactosidase fusion protein can be delivered into almost all tissues including the brain, following intra-peritoneal injection into mice (Schwarze et al., 1999). Over the last decade, CPP-based delivery has been successfully used to deliver peptides and proteins to target different diseases including cell proliferation/cancer, asthma, apoptosis, ischaemia, stimulating cytotoxic immunity and diabetes (Dietz and Bähr, 2004; Snyder and Dowdy, 2005; Gros et al., 2006). Most of these applications use CPPs (Tat, penetratin, polyarginine, VP22) covalently linked to peptides or as fusion proteins. More recently,in vivo applications of Pep-1 technology have been described including intravenous, intra-tumoural and intra-tracheal injections, as well as transduction into oocytes, sprays for nasal delivery or direct penetration through the skin (Gros et al., 2006; Morris et al., 2008). One of the principal applications of CPPs involves the delivery of peptides and proteins for cancer and anti-proliferation treatments. The tumour suppressor p53 constitutes a choice target, and different p53-derived peptides covalently linked to CPPs have been demonstrated to restore p53 functions in cancer cells. Tat-mediated delivery of a peptide derived form the C-terminus of p53 reduces tumour growth upon intra-peritoneal injection into mice with β-cell lymphoma (Snyder et al., 2004; Tang et al., 2007). Similarly, PNC-28, a peptide derived from the MDM-2-binding domain of p53 linked to penetratin has been described to block tumour growth (Michl et al., 2006; Bowne et al., 2007). Another successful anti-proliferation application has been reported using a peptide derived from the N-terminus of the Smac protein, which inactivates the inhibitor of apoptosis protein (Kim et al., 1999). Smac peptide associated to CPPs sensitizes cells to pro-apoptotic stimulus and a synergetic effect of Smac peptide and TNF-related apoptosis inducing ligand has been shown on intracranial glioblastoma xenografted mice (Fulda et al., 2002). Peptides and protein domains derived from natural protein inhibitors (p16Ink, p21, p15 or p27kip) of cyclin-dependent kinases involved in cell cycle progression have been used to block cancer cell proliferation. A tumour suppressor function in vivo was reported by using p27kip tumour suppressor protein genetically coupled to Tat (Nagahara et al., 1998; Snyder et al., 2004), as well as a p16Ink-derived peptide associated to penetratin (Hosotani et al., 2002). Small peptide inhibitors of cyclin-dependent kinase activation have been delivered by using the non-covalent Pep-1-based approach and shown to block cancer cell proliferation (Gondeau et al., 2005). CPPs have also been used to target B-cell lymphoma oncogene. A peptide for EBV ‘Epstein Barr Virus’ associated to Tat blocks proliferation (Knight et al., 2006). Tat-mediated BCL6 peptide inhibitor delivery has been reported to modulate B-cell phenotype (Polo et al., 2004; Melnick, 2007). Pep-1 strategy was also applied to the evaluation of the antitumoural activity of peptide inhibitors of protein kinases or to repair a defective step in a cellular signalling pathway in vivo (Gros et al., 2006; Morris et al., 2008).
Deregulation of apoptosis has been directly or indirectly associated with many pathologies. Several successful applications of CPP-assisted delivery of proteins or peptides regulating apoptosis have been reported. Tat-FLIP (caspase 8 inhibitor) fusion peptide interferes with the activation of FAS inducing signalling complex, thereby preventing apoptosis in vivo (Krautwald et al., 2004). A peptide issued from the Bcl2 homology domain 4 (BH4) or Bcl2/Bclxl protein associated with Tat can regulate apoptosis and induce cytoprotection in vivo (Sugioka et al., 2003). Survivin mutants associated with Tat facilitate apoptosis in cancer cells (Wadia et al., 2004). Pep-1 strategy has been applied in vivo to the delivery of proteins into the lungs of mice to produce alveolar wall apoptosis or to correct defects in protein kinase A function (Aoshiba et al., 2003; Maron et al., 2005).
The ability of CPPs to cross the blood brain barrier and to favour the delivery of proteins in the brain has been used to improve the outcome of ischaemic events. Death of neuronal cells following cerebral ischaemia is associated with apoptosis, and Tat-Bclxl protein can be delivered into mouse brain to decrease neuronal cell death in the area of ischaemic damages (Cao et al., 2002). Reduction of cerebral ischaemia and protection of ischaemia in brain injury has been reported with Tat-cJNK peptide, Tat-NMR2 and Tat-Bclx protein (Cao et al., 2002). Tat-δV-1 peptide, a selective inhibitor of PKCγ has been reported to attenuate heart ischaemia (Bright et al., 2004). CPPs have also been used for the delivery of small molecules through the blood brain barrier, as such D-penetratin or SynB1 have been reported to significantly increase uptake of doxorubicine into the brain (Rousselle et al., 2001). Pep-1 technology has also been demonstrated to be a potent strategy to deliver therapeutic proteins in vivo and across the blood brain barrier (Gallo et al., 2002; Aoshiba et al., 2003; Gallo, 2003; Maron et al., 2005; Gros et al., 2006).
Cell-penetrating peptides have also been used for the treatment of asthma, by using dominant negative forms of Ras or phopshoinositol 3 kinase (Pi3K) fused to Tat to inhibit the airway inflammatory response by cytokine blockage in a mouse (Myou et al., 2003). Different proteins and peptides coupled with Tat and penetratin have been used for immunization against specific infectious diseases. Tat peptide has also been used for the delivery of modular antigen molecules useful for treatment of allergy and vaccine production (Rhyner et al., 2007). Superoxide dismutase (SOD) fused to Tat or to Pep-1 has been shown to protect pancreatic beta cells against oxidative stress (Eum et al., 2004).
Clinical evaluation of CPP-based delivery strategies
Numerous preclinical and clinical evaluations of CPP-based delivery approaches are currently under evaluation. The first, CPP clinical trial was initiated a few years ago by Cell Gate Inc. for topical delivery of cyclosporine linked to polyarginine and entered phase II trials in 2003 (Rothbard et al., 2004). Ever since, several companies are working on clinical development of CPPs, for topical and systemic administration of different therapeutic molecules. Avi Biopharma for the in vivo steric block splicing correction using 6-aminohexanoic acid spaced oligoarginine [(R-Ahx-R)4] (Lebleu et al., 2008; Moulton and Moulton, 2008). Kai Pharmaceutical (Chen and Harrison, 2007) is currently evaluating a Tat protein kinase C inhibitor peptide modulator of protein kinase C for acute myocardial infraction and cerebral ischaemia, which entered phase II in 2007. Other companies including Traversa Inc., for Tat-based non-covalent siRNA delivery, Panomics Inc., for secondary amphipathic peptide-based non-covalent delivery of siRNA are currently evaluating CPP at preclinical and clinical trials.
Conclusions
The dramatic acceleration in the discovery of new and highly potent therapeutic molecules, which do however not make it to the clinic due to poor delivery, low bioavailability and lack of rational targeting has made it clear that delivery was a key stone in therapeutic development (Kong and Mooney, 2007). Accordingly, carrier peptides represent a new and innovative concept to bypass problems of bioavailability associated with certain drugs such as peptides, proteins and nucleic acids, which are currently rarely considered as therapeutics due to the above-mentioned limitations. Such peptide-based strategies present several advantages, including rapid delivery of cargoes into cells with very high efficiency, stability in physiological buffers, lack of toxicity and of sensitivity to serum. Twenty years after their discovery, CPPs are at the door of the clinic. The success reported on the preclinical evaluation of CPPs during the last decade has revealed a tremendous potential of clinical treatment. Covalent strategy has been validated for protein and peptide delivery, and the recent success of phases I and II clinical trials has open great hope in the used of CPPs for therapy. Moreover, the introduction of the CPP-based non-covalent strategy has allowed the introduction of oligonucleotide and siRNA on preclinical states. The lack of prerequisites for covalent coupling upon formation of carrier/macromolecule particles favours the intracellular routing of the cargo and enables its controlled release into the target cellular compartment. Whatever the nature of the delivery system, a major attention should be paid to the targeting of the carrier/drug in order to mediate drug delivery into specific cell types and to limit its dispersion in the whole body.
 |
Acknowledgments
This work was supported in part by the Centre National de la Recherche Scientifique (CNRS), Active Motif (Carlsbad, CA), Panomics Inc. (Fremont, CA) and by grants from the Agence Nationale de Recherche (ANR: Grant ANR-06-BLAN-0071) and the EU (Grant QLK2-CT-2001-01451 & Grant LSHB-CT-2003-503480/TRIoH). We thank members of the laboratory and our collaborators for fruitful discussions.
Glossary
Abbreviations
- CPP
cell-penetrating peptide
- GAG
GlucosAminoGlycan
- NLS
nuclear localization sequence
- PMO
phosphorodiamidate morpholino-oligomer
- PNA
peptide-nucleic acid
- PTD
protein transduction domains
Conflitct of interest
None.
References
- Abes S, Moulton HM, Clair P, Prevot P, Youngblood DS, Wu RP, et al. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J Control Release. 2006;116:304–313. doi: 10.1016/j.jconrel.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Abes S, Turner J, Ivanova GD, Owen D, Williams D, Arzumanov A, et al. Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic Acids Res. 2007;35:4495–4502. doi: 10.1093/nar/gkm418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and amphysematous changes. Am J Respir Cell Mol Biol. 2003;28:555–561. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res. 2003;63:3931–3939. [PubMed] [Google Scholar]
- Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signalling. Reprod Biol Endocrinol. 2004;2:3–5. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowne WB, Michl J, Bluth MH, Zenilman ME, Pincus MR. Novel peptides from the RAS-p21 and p53 proteins for the treatment of cancer. Cancer Ther. 2007;5B:331–344. [PMC free article] [PubMed] [Google Scholar]
- Branden LJ, Mohamed AJ, Smith CI. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat Biotechnol. 1999;17:784–787. doi: 10.1038/11726. [DOI] [PubMed] [Google Scholar]
- Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, et al. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrer R, Neuman BW, Ting JP, Stein DA, Moulton HM, Iversen PL, et al. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J Virol. 2007;81:5637–5648. doi: 10.1128/JVI.02360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier R, Reszka R. Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Ther. 2002;9:157–163. doi: 10.1038/sj.gt.3301635. [DOI] [PubMed] [Google Scholar]
- Chen L, Harrison SD. Cell-penetrating peptides in drug development: enabling intracellular targets. Biochem Soc Trans. 2007;35:821–825. doi: 10.1042/BST0350821. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) ‘protein transduction domains’ promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- Crombez L, Charnet A, Morris MC, Aldrian-Herrada G, Heitz F, Divita G. A non-covalent peptide-based strategy for siRNA delivery. Biochem Soc Trans. 2007;35:44–46. doi: 10.1042/BST0350044. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;10:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fougerolles A, Vornlocher H-P, Maraganore J, Lieberman J. Interfering with desease: a progress report on siRNA-based therapeutics. Nat Rev Drug Disc. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- Deshayes S, Heitz A, Morris MC, Charnet P, Divita G, Heitz F. Insight into the mechanism of internalization of the cell-penetrating carrier peptide Pep-1 through conformational analysis. Biochemistry. 2004a;43:1449–1457. doi: 10.1021/bi035682s. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G, et al. On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids. Biochim Biophys Acta. 2004b;1667:141–147. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Plénat T, Aldrian-Herrada G, Divita G, Le Grimellec C, Heitz F. Primary amphipathic cell-penetrating peptides: structural requirements and interactions with model membranes. Biochemistry. 2004c;43:7698–7706. doi: 10.1021/bi049298m. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes S, Morris M, Heitz F, Divita G. Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Adv Drug Deliv Rev. 2008;60:537–547. doi: 10.1016/j.addr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Bähr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Eitzen G. Actin remodelling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–181. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- El-Andaloussi S, Holm T, Langel Ü. Cell-penetrating peptides: mechanism and applications. Cur Pharma Design. 2005;11:3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a Herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Elmquist A, Lindgren M, Bartfai T, Langel U. VE-cadherin-derived cell-penetrating peptide, pVEC, with carrier functions. Exp Cell Res. 2001;269:237–244. doi: 10.1006/excr.2001.5316. [DOI] [PubMed] [Google Scholar]
- Esbjorner EK, Gräslung A, Nordën P. Membrane interactions of cell penetrating peptide. In: Langel Ü, editor. Cell-Penetrating Peptides. Boca Raton, FL: CRC press; 2007. pp. 109–138. [Google Scholar]
- Escriou V, Carriere M, Scherman D, Wils P. NLS bioconjugates for targeting therapeutic genes to the nucleus. Adv Drug Deliv Rev. 2003;55:295–306. doi: 10.1016/s0169-409x(02)00184-9. [DOI] [PubMed] [Google Scholar]
- Eum WS, Kim DW, Hwang IK, Yoo KY, Kang TC, Jang SH, et al. In vivo protein transduction: biologically active intact pep-1-superoxide dismutase fusion protein efficiently protects against ischemic insult. Free Radic Biol Med. 2004;37:1656–1669. doi: 10.1016/j.freeradbiomed.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Fabani MM, Ivanova GD, Gait MJ. Peptide-Peptide Nucleic Acid conjugates for modulation of gene expression. In: Kurreck J, editor. Therapeutic Oligonucleotide. Cambridge: Royal Society of Chemistry; 2008. pp. 80–102. [Google Scholar]
- Farrera-Sinfreu J, Giralt E, Royo M, Albericio F. Cell-penetrating proline-rich peptidomimetics. Methods Mol Biol. 2007;386:241–267. doi: 10.1007/978-1-59745-430-8_9. [DOI] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the other side-biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem. 2005;6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- Fisher L, Soomets U, Cortés Toro V, Chilton L, Jiang Y, Langel U, Iverfeldt K. Cellular delivery of a double-stranded oligonucleotide NFkappaB decoy by hybridization to complementary PNA linked to a cell-penetrating peptide. Gene Ther. 2004;11:1264–1272. doi: 10.1038/sj.gt.3302291. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Steinhaus JP, et al. Morpholino oligomer-mediated exon skipping averts the onset of dystrophic pathology in the mdx mouse. Mol Ther. 2007;15:1587–1592. doi: 10.1038/sj.mt.6300245. [DOI] [PubMed] [Google Scholar]
- Foerg C, Merkle HP. On the biomedical promise of cell penetrating peptides: limits versus prospects. J Pharm Sci. 2008;97:144–162. doi: 10.1002/jps.21117. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL-or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Gait MJ. Peptide-mediated cellular delivery of antisense oligonucleotides and their analogues. Cell Mol Life Sci. 2003;60:844–853. doi: 10.1007/s00018-003-3044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. Making proteins into drugs: assisted delivery of proteins and peptides into living neurons. Methods Cell Biol. 2003;71:325–338. doi: 10.1016/s0091-679x(03)01015-x. [DOI] [PubMed] [Google Scholar]
- Gallo G, Yee HF, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbal-Chaloin S, Gondeau C, Aldrian-Herrada G, Heitz F, Gauthier-Rouvière C, Divita G. First step of the cell-penetrating peptide mechanism involves Rac1 GTPase-dependent actin-network Remodelling. Biol Cell. 2007;99:223–238. doi: 10.1042/BC20060123. [DOI] [PubMed] [Google Scholar]
- Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- Gondeau C, Gerbal-Chaloin S, Bello P, Aldrian-Herrada G, Morris MC, Divita G. Design of a novel class of peptide inhibitors of cyclin-dependent kinase/cyclin activation. J Biol Chem. 2005;280:13793–13800. doi: 10.1074/jbc.M413690200. [DOI] [PubMed] [Google Scholar]
- Gottschalk S, Sparrow JT, Hauer J, Mims MP, Leland FE, Woo SL, et al. A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996;3:448–457. [PubMed] [Google Scholar]
- Gros E, Deshayes S, Morris MC, Aldrian-Herrada G, Depollier J, Heitz F, et al. A non-covalent peptide-based strategy for protein and peptide nucleic acid delivery. Biochim Biophys Acta. 2006;1758:384–393. doi: 10.1016/j.bbamem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Gryaznov S, Asai A, Oshima Y, Yamamoto Y, Pongracz K, Pruzan R, et al. Oligonucleotide N3′–> P5′ thio-phosphoramidate telomerase template antagonists as potential anticancer agents. Nucleosides Nucleotides Nucleic Acids. 2003;22:577–581. doi: 10.1081/NCN-120021958. [DOI] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Hosotani R, Miyamoto Y, Fujimoto K, Doi R, Otaka A, Fujii N, et al. Trojan p16 peptide suppresses pancreatic cancer growth and prolongs survival in mice. Clin Cancer Res. 2002;8:1271–1276. [PubMed] [Google Scholar]
- Ignatovich IA, Dizhe EB, Pavlotskaya AV, Akifiev BN, Burov SV, Orlov SV, et al. Complexes of plasmid DNA with basic domain 47–57.of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- Järver P, Langel Ü. The use of cell-penetrating peptides as a toll for gene regulation. Drug Discov Today. 2004;9:395–402. doi: 10.1016/S1359-6446(04)03042-9. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. Cationic Tat peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Kichler A, Leborgne C, März J, Danos O, Bechinger B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc Natl Acad Sci USA. 2003;100:1564–1568. doi: 10.1073/pnas.0337677100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilk K, El-Andaloussi S, Järver P, Meikas A, Valkna A, Bartfai T, et al. Evaluation of transportan 10 in PEI mediated plasmid delivery assay. J Control Release. 2005;103:511–523. doi: 10.1016/j.jconrel.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Kim AL, Raffo AJ, Brandt-Rauf PW, Pincus MR, Monaco R, Abarzua P, et al. Conformational and molecular basis for induction of apoptosis by a p53 C-terminal peptide in human cancer cells. J Biol Chem. 1999;274:34924–34931. doi: 10.1074/jbc.274.49.34924. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Christensen LV, Jo S, Yockman JW, Jeong JH, Kim YH, et al. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol Ther. 2006;14:343–350. doi: 10.1016/j.ymthe.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Knight JS, Lan K, Bajaj B, Sharma N, Tsai DE, Robertson ES. A peptide-based inhibitor for prevention of B cell hyperproliferation induced by Epstein-Barr virus. Virology. 2006;354:207–214. doi: 10.1016/j.virol.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Kogure K, Moriguchi R, Sasaki K, Ueno M, Futaki S, Harashima H. Development of a non-viral multifunctional envelope-type nano device by a novel lipid film hydration method. J Control Release. 2004;98:317–323. doi: 10.1016/j.jconrel.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Kong H, Mooney DJ. Microenvironmental regulation of biomacromolecular therapies. Nat Rev Drug Discov. 2007;6:455–463. doi: 10.1038/nrd2309. [DOI] [PubMed] [Google Scholar]
- Koppelhus U, Nielsen PE. Cellular Delivery of peptide nucleic acid. Adv Drug Del Rev. 2003;55:267–280. doi: 10.1016/s0169-409x(02)00182-5. [DOI] [PubMed] [Google Scholar]
- Koppelhus U, Awasthi SK, Zachar V, Holst HU, Ebbesen P, Nielsen PE. Cell-dependent differential cellular uptake of PNA, peptides, and PNA-peptide conjugates. Antisense Nucleic Acid Drug Dev. 2002;12:51–63. doi: 10.1089/108729002760070795. [DOI] [PubMed] [Google Scholar]
- Krauss U, Muller M, Stahl M, Beck-Sickinger AG. In vitro gene delivery by a novel human calcitonin (hCT)-derived carrier peptide. Bioorg Med Chem Lett. 2004;14:51–65. doi: 10.1016/j.bmcl.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Krautwald S, Ziegler E, Tiede K, Pust R, Kunzendorf U. Transduction of the TAT-FLIP fusion protein results in transient resistance to Fas-induced apoptosis in vivo. J Biol Chem. 2004;277:44005–44011. doi: 10.1074/jbc.M401327200. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;7149:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Lear JD, DeGrado WF. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J Biol Chem. 1987;262:6500–6510. [PubMed] [Google Scholar]
- Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, et al. Cell penetrating peptide conjugates of steric block oligonucleotides. Adv Drug Deliv Rev. 2008;60:517–529. doi: 10.1016/j.addr.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Dubikovskaya EA, Hwang H, Semyonov AN, Wang H, Jones LR, et al. Single-molecule motions of oligoarginine transporter conjugates on the plasma membrane of Chinese hamster ovary cells. J Am Chem Soc. 2008;130:9364–9370. doi: 10.1021/ja710798b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg M, Johansson M. Is VP22 nuclear homing an artefact? Nat Biotechnol. 2001;19:713–714. doi: 10.1038/90741. [DOI] [PubMed] [Google Scholar]
- Lundberg P, El-Andaloussi S, Sutlu T, Johansson H, Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;11:2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- MacKay JA, Li W, Huang Z, Dy EE, Huynh G, Tihan T, et al. HIV TAT peptide modifies the distribution of DNA nanolipoparticles following convection-enhanced delivery. Mol Ther. 2008;16:893–900. doi: 10.1038/mt.2008.36. [DOI] [PubMed] [Google Scholar]
- Magzoub M, Gräslund A. Cell-penetrating peptides: small from inception to application. Q Rev Biophys. 2004;37:147–195. doi: 10.1017/s0033583505004014. [DOI] [PubMed] [Google Scholar]
- Maron MB, Folkesson HG, Stader SM, Walro JM. PKA delivery to the distal lung air spaces increases alveolar liquid clearance after isoproterenol-induced alveolar epithelial PKA desensitization. Am J Physiol Lung Cell Mol Physiol. 2005;289:349–354. doi: 10.1152/ajplung.00134.2004. [DOI] [PubMed] [Google Scholar]
- Marthinet E, Divita G, Bernaud J, Rigal D, Baggetto LG. Modulation of the typical multidrug resistance phenotype by targeting the MED-1 region of human MDR1 promoter. Gene Ther. 2000;7:1224–1233. doi: 10.1038/sj.gt.3301231. [DOI] [PubMed] [Google Scholar]
- Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliv Rev. 2007;59:134–140. doi: 10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Melnick A. Targeting aggressive B-cell lymphomas with cell-penetrating peptides. Biochem Soc Trans. 2007;35:802–806. doi: 10.1042/BST0350802. [DOI] [PubMed] [Google Scholar]
- Michl J, Scharf B, Schmidt A, Huynh C, Hannan R, von Gizycki H, et al. PNC-28, a p53-derived peptide that is cytotoxic to cancer cells, blocks pancreatic cancer cell growth in vivo. Int J Cancer. 2006;119:1577–1585. doi: 10.1002/ijc.22029. [DOI] [PubMed] [Google Scholar]
- Midoux P, Kichler A, Boutin V, Maurizot JC, Monsigny M. Membrane permeabilization and efficient gene transfer by a peptide containing several histidines. Bioconjug Chem. 1998;9:260–267. doi: 10.1021/bc9701611. [DOI] [PubMed] [Google Scholar]
- Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004a;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–2736. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Chaloin L, Mery J, Heitz F, Divita G. A novel potent strategy for gene delivery using a single peptide vector as a carrier. Nucleic Acids Res. 1999;27:3510–3517. doi: 10.1093/nar/27.17.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Chaloin L, Heitz F, Divita G. Translocating peptides and proteins and their use for gene delivery. Curr Opin Biotechnol. 2000;11:461–466. doi: 10.1016/s0958-1669(00)00128-2. [DOI] [PubMed] [Google Scholar]
- Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- Morris MC, Chaloin L, Choob M, Archdeacon J, Heitz F, Divita G. Combination of a new generation of PNAs with a peptide-based carrier enables efficient targeting of cell cycle progression. Gene Ther. 2004b;11:757–764. doi: 10.1038/sj.gt.3302235. [DOI] [PubMed] [Google Scholar]
- Morris MC, Deshayes S, Simeoni F, Aldrian-Herrada G, Heitz F, Divita G. A noncovalent peptide-based strategy for peptide and short interfering RNA delivery. In: Langel Ü, editor. Cell-Penetrating Peptides. Boca Raton, FL: CRC press; 2007a. pp. 387–408. [Google Scholar]
- Morris MC, Gros E, Aldrian-Herrada G, Choob M, Archdeacon J, Heitz F, et al. A non-covalent peptide-based carrier for in vivo delivery of DNA mimics. Nucleic Acids Res. 2007b;35:e49–e59. doi: 10.1093/nar/gkm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- Moschos S, Williams A, Lindsay M. In vivo applications of Cell-Penetrating peptide. In: Langel Ü, editor. Cell-Penetrating Peptides. Boca Raton, FL: CRC press; 2007. pp. 423–438. [Google Scholar]
- Moulton HM, Moulton JD. Arginine-rich cell-penetrating peptides with uncharged antisense oligomers. Drug Discov Today. 2004;9:870–875. doi: 10.1016/S1359-6446(04)03226-X. [DOI] [PubMed] [Google Scholar]
- Moulton HM, Moulton JD. Antisense morpholino oligomers and theirs peptide conjugates. In: Kurreck J, editor. Therapeutic Oligonucleotide. Cambridge: Royal Society of Chemistry; 2008. pp. 43–79. [Google Scholar]
- Munoz-Morris MA, Heitz F, Divita G, Morris MC. The peptide carrier Pep-1 forms biologically efficient nanoparticle complexes. Biochem Biophys Res Commun. 2007;355:877–882. doi: 10.1016/j.bbrc.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558:63–68. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- Murriel CL, Dowdy SF. Influence of protein transduction domains on intracellular delivery of macromolecules. Expert Opin Drug Deliv. 2006;3:739–746. doi: 10.1517/17425247.3.6.739. [DOI] [PubMed] [Google Scholar]
- Myou S, Leff AR, Myo S, Boetticher E, Meliton AY, Lambertino AT, et al. Activation of group IV cytosolic phospholipase A2 in human eosinophils by phosphoinositide 3-kinase through a mitogen-activated protein kinase-independent pathway. J Immunol. 2003;171:4399–4405. doi: 10.4049/jimmunol.171.8.4399. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAp27kip1 induced cell migration. Nat Med. 1998;4:1449–1453. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- Nan L, Wu Y, Bardag-Gorce F, Li J, French BA, Wilson L, et al. RNA interference of VCP/p97 increases Mallory body formation. Exp Mol Pathol. 2005;78:1–9. doi: 10.1016/j.yexmp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Nguyen QN, Chavli RV, Marques JT, Jr, Conrad PG, Wang D, He W, et al. Light controllable siRNAs regulate gene suppression and phenotypes in cells. Biochim Biophys Acta. 2006;1758:394–403. doi: 10.1016/j.bbamem.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Niidome T, Huang L. Gene therapy progress and prospects: non viral vectors. Gene Ther. 2002;10:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- Ogris M, Wagner E. Targeting tumors with non-viral gene delivery systems. Drug Discov Today. 2002;7:479–485. doi: 10.1016/s1359-6446(02)02243-2. [DOI] [PubMed] [Google Scholar]
- Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–516. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- Padari K, Saalik P, Hansen M, Koppel K, Raid R, Langel U, et al. Cell transduction pathways of transportans. Bioconjug Chem. 2005;16:1399–1410. doi: 10.1021/bc050125z. [DOI] [PubMed] [Google Scholar]
- Parente RA, Nadasdi L, Subbarao NK, Szoka FC., Jr Association of a pH-sensitive peptide with membrane vesicles: role of amino acid sequence. Biochemistry. 1990;29:8713–8719. doi: 10.1021/bi00489a030. [DOI] [PubMed] [Google Scholar]
- Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad USA. 2000;97:8245–8250. doi: 10.1073/pnas.150518097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IY, Park CB, Kim MS, Kim SC, Parasin I. An antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 1998;437:258–262. doi: 10.1016/s0014-5793(98)01238-1. [DOI] [PubMed] [Google Scholar]
- Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- Polo JM, Dell'Oso T, Ranuncolo SM, Cerchietti L, Beck D, Da Silva GF, et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004;10:1329–1335. doi: 10.1038/nm1134. [DOI] [PubMed] [Google Scholar]
- Pooga M, Soomets U, Hallbrink M, Valkna A, Saar K, Rezaei K, et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol. 1998;16:857–861. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- Pooga M, Kut C, Kihlmark M, Hallbrink M, Fernaeus S, Raid R, et al. Cellular translocation of proteins by transportan. FASEB J. 2001;8:1451–1453. doi: 10.1096/fj.00-0780fje. [DOI] [PubMed] [Google Scholar]
- Pujals S, Fernandez-Carneado J, Lopez-Iglesias C, Kogan MJ, Giralt E. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim Biophys Acta. 2006;1758:264–279. doi: 10.1016/j.bbamem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rhyner C, Kündig T, Akdis CA, Crameri R. Targeting the MHC II presentation pathway in allergy vaccine development. Biochem Soc Trans. 2007;35:833–834. doi: 10.1042/BST0350833. Pt 4. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik V. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R, et al. New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo. Mol Ther. 2002;5:104–114. doi: 10.1006/mthe.2002.0523. [DOI] [PubMed] [Google Scholar]
- Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- Rousselle C, Smirnova M, Clair P, Lefauconnier JM, Chavanieu A, Calas B, et al. Enhanced delivery of doxorubicin into the brain via a peptide-vector-mediated strategy: saturation kinetics and specificity. J Pharmacol Exp Ther. 2001;296:124–131. [PubMed] [Google Scholar]
- Rudolph C, Plank C, Lausier J, Schillinger U, Muller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278:11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Tulipano G, Spillmann D, Tanghetti E, Oreste P, Zoppetti G, et al. Multiple interactions of HIV-I Tat protein with size-defined heparin oligosaccharides. J Biol Chem. 1999;274:28198–28205. doi: 10.1074/jbc.274.40.28198. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Rothen-Rutishauser B, Rist B, Beck-Sickinger A, Wunderli-Allenspach H, Rubas W, et al. Translocation of human calcitonin in respiratory nasal epithelium is associated with self assembly in lipid membrane. Biochemistry. 1998;37:16582–16590. doi: 10.1021/bi981219h. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31:2717–2724. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EL, Dowdy SF. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert Opin Drug Deliv. 2005;2:43–51. doi: 10.1517/17425247.2.1.43. [DOI] [PubMed] [Google Scholar]
- Snyder EL, Meade BR, Saenz CC, Dowdy SF. Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS Biol. 2004;2:E36. doi: 10.1371/journal.pbio.0020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Funatsu T, Tamagawa H, Sawa Y, Kawakami T, et al. BH4-domain peptide from Bcl-xL exerts anti-apoptotic activity in vivo. Oncogene. 2003;22:8432–8440. doi: 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- Tang X, Molina M, Amar S. p53 short peptide regulateds lipopolysaccharide – induced tumor necrosis factor-a-factor/cytokine expression. Cancer Res. 2007;67:1308–1316. doi: 10.1158/0008-5472.CAN-06-1600. [DOI] [PubMed] [Google Scholar]
- Terrone D, Sang SL, Roudaia L, Silvius JR. Penetratin and related cell-penetrating cationic peptides can translocate across lipid bilayers in the presence of a transbilayer potential. Biochemistry. 2003;42:13787–13799. doi: 10.1021/bi035293y. [DOI] [PubMed] [Google Scholar]
- Thoren PE, Persson D, Isakson P, Goksor M, Onfelt A, Norden B. Uptake of analogs of penetratin, Tat(48–60) and oligoarginine in live cells. Biochem Biophys Res Commun. 2003;307:100–107. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- Thyner C, Kündig T, Akdis CA, Crameri R. Targeting the MHC II presentation pathway in allergy vaccine development. Biochem Soc Trans. 2007;35:833–844. doi: 10.1042/BST0350833. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv Drug Deliv Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Tung CH, Mueller S, Weissleder R. Novel branching membrane translocational peptide as gene delivery vector. Bioorg Med Chem. 2002;10:3609–3614. doi: 10.1016/s0968-0896(02)00248-1. [DOI] [PubMed] [Google Scholar]
- Veldhoen S, Laufer SD, Trampe A, Restle T. Cellular delivery of small interfering RNA by a noncovalenty attached cell-penetrating peptide: quantitative analysis of uptake and biological effect. Nucleic Acids Res. 2006;34:6561–6573. doi: 10.1093/nar/gkl941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008;1786:126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Wadia J, Stan RV, Dowdy S. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Goun EA, Jones LR, Pillow TH, Rothbard JB, Shinde R, et al. Real-time analysis of uptake and bioactivatable cleavage of luciferin-transporter conjugates in transgenic reporter mice. Proc Natl Acad Sci USA. 2007;104:10340–10345. doi: 10.1073/pnas.0703919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC., Jr Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- Zatsepin TS, Turner JJ, Oretskaya TS, Gait MJ. Conjugates of oligonucleotides and analogues with cell penetrating peptides as gene silencing. Curr Pharmaceutical Design. 2005;11:3639–3654. doi: 10.2174/138161205774580769. [DOI] [PubMed] [Google Scholar]
- Zeineddine D, Papadimou E, Chebli K, Gineste M, Liu J, Grey C, et al. Oct-3/4 dose dependently regulates specification of embryonic stem cells toward a cardiac lineage and early heart development. Dev Cell. 2006;11:535–546. doi: 10.1016/j.devcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Ziegler A. Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv Drug Deliv Rev. 2008;60:580–597. doi: 10.1016/j.addr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Nervi P, Dürrenberger M, Seelig J. The cationic cell-penetrating peptide CPP(TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry. 2005;44:138–148. doi: 10.1021/bi0491604. [DOI] [PubMed] [Google Scholar]


