Abstract
Background and purpose
Indomethacin is a non-steroidal anti-inflammatory drug (NSAID) that is limited in its enteral or parenteral use by side effects of gastroduodenal bleeding and ulceration. We have investigated the ability of phosphatidylcholine associated with indomethacin to form a therapeutically effective drug (INDO-PC) with reduced gastrointestinal (GI) toxicity for parenteral use.
Experimental approach
Rats were treated acutely by intravenous or chronically with subcutaneous injection of vehicle, indomethacin or INDO-PC using three related protocols. We then evaluated the following properties of these parenterally administered test drugs: (i) GI toxicity (luminal and faecal haemoglobin; intestinal perforations and adhesions; and haematocrit); (ii) bioavailability (plasma indomethacin); and (iii) therapeutic efficacy (analgesia from sensitivity to pressure; anti-inflammatory from ankle thickness; cyclo-oxygenase (COX) inhibition from synovial fluid prostaglandin E2 concentration) in rats with adjuvant-induced joint inflammation.
Key results
Acute and chronic dosing with INDO-PC produced less GI bleeding and intestinal injury than indomethacin alone, whereas the bioavailability, analgesic, anti-inflammatory and COX inhibitory activity of INDO-PC were comparable to indomethacin.
Conclusions and implications
The chemical association of phosphatidylcholine with indomethacin appears to markedly reduce the GI toxicity of the NSAID while providing equivalent therapeutic efficacy in a parenteral INDO-PC formulation.
Keywords: gastrointestinal, phosphatidylcholine, NSAID, ulcer
Introduction
Indomethacin is a non-selective non-steroidal anti-inflammatory (NSAID) that carries warnings to adults, when prescribed orally for rheumatoid and osteoarthritis, regarding its toxicity to the gastrointestinal (GI) tract, namely, the induction of bleeding, ulcerations or perforation of the stomach or intestines which can be fatal. This serious adverse effect limits the time period over which indomethacin can be safely administered. Yet, indomethacin is a highly effective analgesic and anti-inflammatory agent that is one of only a few NSAIDs that is available for parenteral use. Intravenous (i.v.) indomethacin has Food and Drug Administration approval for use in treating newborn infant patent ductus arteriosus in neonates. Warnings about GI bleeding are present for this use as well. An off-label use for i.v. indomethacin has occurred in prevention of heterotopic ossification following orthopedic surgery (Macfarlane et al., 2008), where GI toxicity of indomethacin is also monitored. Considering the potential demand for an oral or i.v. indomethacin product, there is a great ‘unmet’ need for the development of safer formulations of indomethacin.
Experimentally in rodents, indomethacin induces lesions in the mid to distal small intestine, regardless if the NSAID is administered enterally or parenterally (Hucker et al., 1966; Kent et al., 1969; Brodie et al., 1970). Further, this intestinal injury can be prevented by bile duct ligation (Brodie et al., 1970). It has been shown through direct measurements that indomethacin and several other non-salicylate NSAIDs, such as diclofenac and naproxen, are excreted into the bile and their GI toxicity closely correlates with their appearance in the bile (Duggan et al., 1975; Beck et al., 1990). Our laboratory has recently shown that the bile from rats administered indomethacin is toxic to red blood cells (RBCs) and loops of rodent ileum, and that indomethacin added to normal rat bile is also toxic to RBCs, intestinal mucosa and hepatic HepG2 cells in culture (Barrios and Lichtenberger, 2000; Dial et al., 2008a). This in vitro toxicity of indomethacin is reversed by the addition of phosphatidylcholine, a zwitterionic phospholipid with established GI protective activity.
In the current study, we wished to test the in vivo efficacy of indomethacin that is pre-associated with phosphatidylcholine (INDO-PC) for parenteral use, to evaluate its GI safety and to determine whether the analgesic ability of INDO-PC was comparable to that of indomethacin alone. It was also important to establish whether the protection from toxicity seen in vitro could be translated to GI safety in vivo.
Methods
Animal studies
Approval for all protocols was obtained from the institutional Animal Welfare Committee which follows the guidelines and is accredited by the US Public Health Service. Male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN, USA) of 200–250 g were used in all studies. Animals were maintained on a 12 h light/dark cycle with food and water provided ad libitum, except as noted.
Drug formulation
Indomethacin was obtained from Sigma Chemical Co. (St. Louis, MO, USA). It was dissolved in 1.25% sodium bicarbonate with low heat (40°C), the pH was adjusted to pH 7.4, and it was sterilized by filtration (0.22 microns) prior to dosing. INDO-PC was formulated by a method licensed to PLx Pharma Inc. (Houston TX, USA), as detailed in US Patent Application Serial No. 249051. Briefly, indomethacin and phosphatidylcholine (Phospholipon 90G from American Lecithin, Oxford, CT, USA) at a 1:2 weight ratio were combined in acetone, and then the solvent was removed through evaporation. The INDO-PC complex, present as a homogeneous oil, was resuspended for injection by vortex mixing and sonication in saline or sodium bicarbonate and then titrated to pH 7.4 as outlined above.
Acute intestinal toxicity
The acute toxicity of indomethacin was assessed by modifications of Chen et al. (1993) and Tanaka et al. (1999) using a non-selective nitric oxide synthase (NOS) inhibitor to block endogenous protective cNOS. Male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN, USA) were fasted overnight and given Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME, 20 mg·kg−1, ip), an NOS inhibitor, at 0, 2 and 6 h. One hour after the first dose of L-NAME, the rats were given (via tail vein) 1.25% sodium bicarbonate (control vehicle), indomethacin or INDO-PC at 10 mg indomethacin per kg and were re-fed after the last dose of L-NAME. At 24 h after indomethacin dosing, the rats were killed and the distal half of their small intestine was flushed with saline for analysis of haemoglobin (Hb) content in the lumen as previously described (Lichtenberger et al., 1983 1995).
Subchronic model to assess therapeutic efficacy and GI toxicity
Inflammation was induced by injection of 0.2 mL of complete Freund's adjuvant (CFA) into the left hind paw of rats, anaesthetized with isoflurane. Four days later acute analgesic efficacy was assessed when the pain pressure threshold of the affected paw was measured using a modification of the Randall-Sellito technique as previously described (Lichtenberger et al., 2001), before and 30 min after i.v. injection of sodium bicarbonate, indomethacin or INDO-PC (equivalent to 5 mg indomethacin·kg−1). The rats were killed and plasma was collected for high pressure liquid chromatography (HPLC) analysis of drug levels. The synovial fluid of the inflamed paw was assessed for prostaglandin E2 (PGE2) induction by radioimmunoassay following incubation of the excised paw in 50 mmol·L−1 Tris buffer at pH 7.4 for 24 h at 4°C and centrifugation to obtain a cell-free exudate.
Chronic therapeutic efficacy and GI safety of our parenterally administered test drugs were assessed in a second experimental series, where rats were similarly treated with CFA and were then injected subcutaneously (s.c.) for 4 days with sodium bicarbonate, indomethacin or INDO-PC (4 mg indomethacin per kg). The s.c. route for parenteral dosing was chosen to avoid possible injury to the tail from multiple i.v. dosing. The ankle thickness in the inflamed paw was recorded in these rats as a measure of anti-inflammatory activity, and haematocrit was assessed along with faecal pellets for Hb analysis as measures of GI bleeding. The small intestine was examined for number of perforations and severity of adhesions by removing the small intestines as a mass for further examination. Starting at the duodenum, the intestine was gently pulled to separate it from connecting tissue and reveal the length of the structure. The amount of sticking of the bowel was assessed for adhesions by the following score. 0 = normal; 1 = slight sticking, pull apart without tearing; 2 = sticking, must cut adhesions to pull apart; 3 = numerous adhesions, difficulty in cutting intestines apart; 4 = massive adhesions, cannot be cut apart. Intestinal perforations were counted after filling the distal half with 5 mL of water and noting the number of leaks. Analgesia was not assessed because of the confounding influence of visceral discomfort due to NSAID-induced intestinal injury.
Indomethacin analysis by HPLC
Plasma samples (one part) were mixed with three parts acetonitrile to extract indomethacin and remove protein. The samples were centrifuged and the supernatant was collected for HPLC. Samples (10 µL) were run on a Waters HPLC using an Agilent Zorbax SB-Phenyl column with a mobile phase of acetonitrile and phosphoric acid. Indomethacin was detected at a wavelength of 237 nm and a retention time of 6.4 min.
PGE2 analysis by radioimmunoassay
The PGE2 in synovial fluid from rat paws was assayed directly by radioimmunoassay using antibody from Sigma Chemical Co. (St. Louis, MO, USA), according to the manufacturer's instructions.
Statistical analysis
Experiments were analysed initially by one-way anova, followed by post hoc testing with the Fisher LSD test. The level of significance was set at P < 0.05.
Results
GI toxicity
For acute toxicity testing, indomethacin was injected i.v. at 10 mg·kg−1, a dose that is clearly in the higher end of its therapeutic range. Under these conditions, indomethacin induced significant intestinal bleeding in L-NAME-treated rats (Figure 1). In sharp contrast, injection of INDO-PC at an identical (NSAID) dose to indomethacin alone produced almost no bleeding, which was significantly lower than the GI bleeding of rats which were given indomethacin i.v., and not different from the values of vehicle-treated controls.
Figure 1.
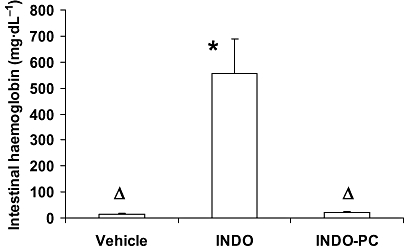
Acute indomethacin toxicity to the GI tract. Rats were treated with L-NAME (20 mg·kg−1, i.p., at 0, 2 and 6 h) and indomethacin (10 mg·kg−1, i.v., at 1 h) and were assessed for intestinal bleeding at 24 h. Indomethacin alone (INDO) induced a significant increase in haemoglobin appearing in the intestinal lumen, whereas INDO-PC did not. *P < 0.05 versus vehicle; ΔP < 0.05 versus INDO. n = 4–5 per group. GI, gastrointestinal; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride.
Acute efficacy
To evaluate the therapeutic efficacy of INDO-PC, both the analgesic response to pressure applied to an inflamed paw and the ability to inhibit PG synthesis of the affected paw were monitored. Figure 2A demonstrates that before dosing (open bars), all the CFA-injected animals had the same level of sensitivity to a pressure stimulus, with the pain pressure threshold being consistently lower than baseline values measured prior to CFA (44 ± 1 mmHg). At 30 min after receiving a single i.v. dose of either sodium bicarbonate, indomethacin or INDO-PC (5 mg·kg−1, i.v.) (solid bars), there was a significant increase in the pressure threshold of both indomethacin and INDO-PC animals, denoting equivalent analgesic efficacy. The level of PGE2 in inflamed paw synovial fluid was elevated in saline-treated CFA rats over controls not injected with CFA [910 ± 111 pg (mg·protein)−1], whereas the PGE2 was significantly reduced to control levels in both the indomethacin and INDO-PC groups (Figure 2B). To confirm that bioavailability of indomethacin was similar between the indomethacin and INDO-PC groups, plasma levels of indomethacin were analysed by HPLC (Figure 3) from blood collected 30 min after i.v. injection and essentially identical concentrations were found.
Figure 2.
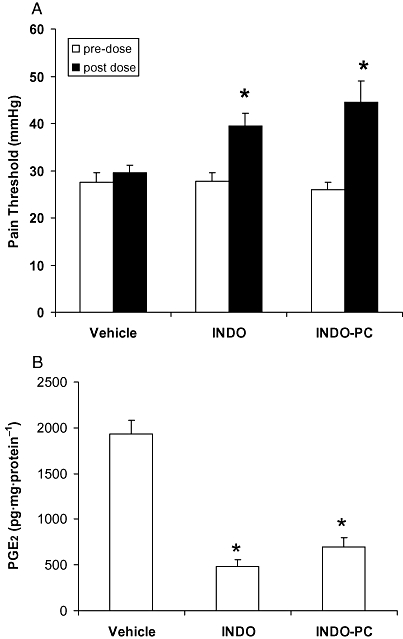
Efficacy of acute indomethacin dosing. Four days after rats were treated with CFA (0.2 mL) to induce paw inflammation, they were dosed with vehicle, indomethacin (INDO) or INDO-PC (5 mg indomethacin per kg, i.v.) and were assessed for:(A) pain pressure threshold and B) PGE2 formation in the inflamed paw. A single dose of both indomethacin or INDO-PC provided a significant increase in (A) analgesic efficacy and (B) COX inhibitory activity. *P < 0.05 versus vehicle. n = 5 per group. CFA, complete Freund's adjuvant; COX, cyclo-oxygenase; PGE2, prostaglandin E2.
Figure 3.
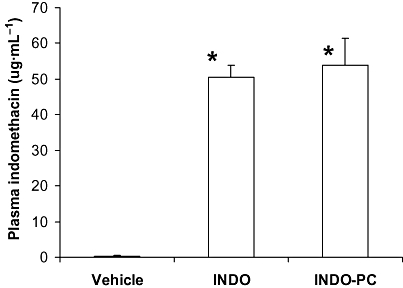
Plasma levels of indomethacin. Blood was collected from rats at 30 min after dosing with either indomethacin (INDO) or INDO-PC (5 mg indomethacin per kg, i.v.). Plasma was used in HPLC analysis of indomethacin content. Nearly identical circulating levels of indomethacin were seen in both indomethacin-and INDO-PC-treated animals. *P < 0.05 versus vehicle. n = 5 per group. HPLC, high pressure liquid chromatography.
Chronic GI safety and therapeutic efficacy
The measurement of inflamed paw ankle thickness is a valid and reproducible measure of anti-inflammatory drug responses. When dosed for 4 days, rats with CFA-induced paw inflammation and treated with vehicle (Figure 4) showed a clear increase in ankle thickness over control rats, not injected with CFA. This inflammation was significantly reduced by chronic treatment with both the indomethacin alone and the INDO-PC treatments.
Figure 4.
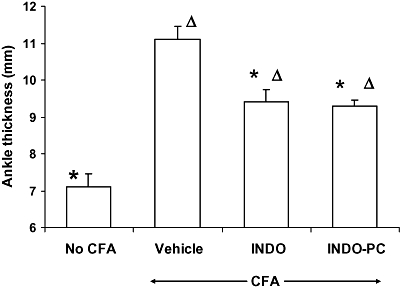
Efficacy of chronic indomethacin dosing. Rats with CFA-induced paw inflammation were assessed for ankle thickness after 4 days of dosing with either vehicle, indomethacin (INDO) or INDO-PC (4 mg indomethacin per kg, s.c.) Both agents produced a significant reduction in swelling suggesting comparable anti-inflammatory activity. *P < 0.05 versus vehicle/CFA; ΔP < 0.05 versus no CFA. n = 5 per group. CFA, complete Freund's adjuvant.
The GI safety of chronic indomethacin treatments was assessed by measures of haematocrit and bleeding into faecal matter, and intestinal perforations/adhesions. Haematocrit was significantly reduced by parenteral indomethacin to values below that of vehicle-treated rats (Figure 5A), whereas this decrease was attenuated in rats receiving INDO-PC. Similarly, faecal pellets from INDO-PC-treated rats showed less bleeding than those from rats given the equivalent dose of indomethacin alone (Figure 5B). It should be noted that the ability of INDO-PC to protect against NSAID-induced GI bleeding appeared to be most pronounced in rats parenterally dosed for 2 days or less. Lastly, after 4 days of indomethacin dosing, there were nearly four intestinal perforations per rat (Figure 5C), whereas dosing with INDO-PC significantly reduced that value by 68%. Adhesions between intestinal segments were also apparent with indomethacin treatment and this effect was significantly reduced by INDO-PC.
Figure 5.
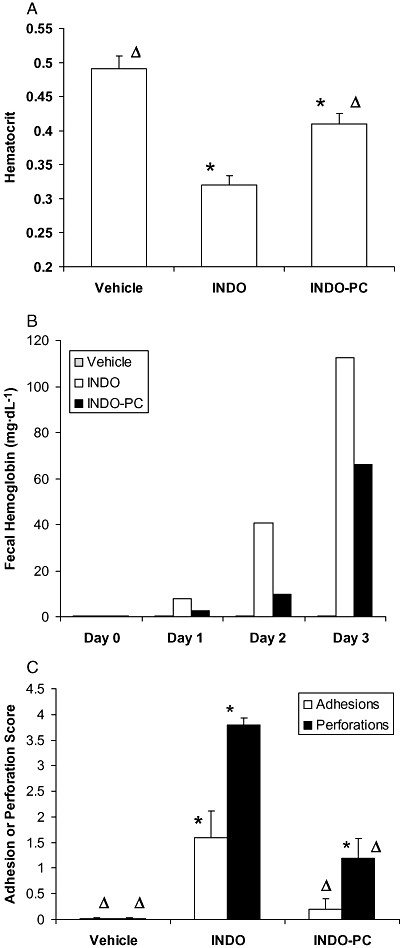
Chronic indomethacin toxicity to the GI tract. Rats treated in Fig. 4 were assessed for GI toxicity by measurements of:(A) haematocrit, (B) haemoglobin in faecal pellets and (C) intestinal adhesions and perforations. CFA treatment alone (vehicle) did not induce bleeding or intestinal injury, but indomethacin (INDO) + CFA caused intestinal injury and a loss of blood that occurred earlier and to a greater extent than that from INDO-PC. *P < 0.05 versus vehicle; ΔP < 0.05 versus INDO. n = 5 per group. CFA, complete Freund's adjuvant; GI, gastrointestinal.
Discussion and conclusions
Our laboratory has demonstrated that phosphatidylcholine is an important component of the surface barrier of the GI mucosa that provides the tissue with hydrophobic, non-wettable characteristics (Hills et al., 1983; Lichtenberger et al., 1983). Disease conditions such as H. pylori infection or inflammatory bowel disease can cause a reduction in surface barrier properties including reduced phosphatidylcholine (Goggin et al., 1992; Go et al., 1993; Tatsumi and Lichtenberger, 1996; Lichtenberger et al., 1999; Ehehalt et al., 2004) and potentially allow back-diffusion of luminal constituents such as acid, bacterial toxins, pancreatic proteases and bile salts that could contribute to inflammation. It was further shown that the use of exogenous phosphatidylcholine could prevent gastric and intestinal injury (Lichtenberger et al., 1983; Lugea et al., 1994).
In addition, the class of drugs known as NSAIDs have a natural affinity to chemically associate with endogenous phosphatidylcholine (Giraud et al., 1999; Lichtenberger et al., 2001). When this occurs in the GI lumen, the surface barrier properties can be attenuated (Lichtenberger et al., 1995 2006; Lugea et al., 1997; Giraud et al., 1999). Indeed, indomethacin is reported to reduce the level of phosphatidylcholine in duodenal mucus over the same dose range (1–5 mg·kg−1) and time course (1–24 h), as it lowered surface hydrophobicity after s.c. administration (Lugea et al., 1997). We have also reported that phosphatidylcholine which represents 20–30% of the organic constituents of bile, plays an important physiological role in protecting the GI/hepatic epithelia against the cytotoxic effects of bile acids (Dial et al., 2008a). Furthermore, this protective activity is compromised by the secretion of certain non-salicylate NSAIDs into the bile (Venneman et al., 2006; Dial et al., 2008b). We have proposed that one potential mechanism by which NSAIDs that are secreted into the bile may induce intestinal injury is by displacing phosphatidylcholine from bile acid/phosphatidylcholine mixed micelles (which have low cytotoxic activity), allowing cytotoxic simple bile acid micelles/monomers to become the predominant species (Barrios and Lichtenberger, 2000; Dial et al., 2008b). Alternatively, indomethacin may form cytotoxic mixed micelles with the bile acid to disrupt biological membranes (Zhou et al., 2008). Interestingly, we have recently reported that the cytotoxic actions of NSAIDs in combination with natural bile and/or synthetic bile acids can be reversed in a dose-dependent fashion by the addition of phosphatidylcholine to the in vivo and in vitro test systems (Barrios and Lichtenberger, 2000; Dial et al., 2008b; Zhou et al., 2008). The finding that a low dose of indomethacin (1–2 mg·kg−1) is capable of reducing surface hydrophobicity and mucus phosphatidylcholine levels also suggests that the phosphatidylcholine in bile is not sufficient to protect against the NSAID within this dose range (Lugea et al., 1997). It, however, is currently uncertain how indomethacin doses of 1–10 mg·kg−1 in rats equates to the human therapeutic dose of <1 mg·kg−1 due to differences in drug metabolism between rodents and humans.
We have shown in rodents and man that by pre-associating synthetic or soy phosphatidylcholine with an NSAID, the GI toxicity of the NSAID is greatly reduced (Lichtenberger et al., 1995; Anand et al., 1999; Barrios and Lichtenberger, 2000). For example, adding phosphatidylcholine to bile from indomethacin-treated rats can prevent bleeding and loss of surface hydrophobicity in an ileal loop preparation (Barrios and Lichtenberger, 2000). Therefore, in the current study, we tested an INDO-PC formulation that had not previously been examined for efficacy and GI safety. Indomethacin is among those NSAIDs that are secreted into the GI tract through the bile (Brodie et al., 1970). We hypothesized that the association of phosphatidylcholine with indomethacin would allow indomethacin to be secreted through the bile and into the GI tract with much less disruption of the GI surface barrier.
In testing of acute GI toxicity, it was found that indomethacin alone at a dose of 10 mg·kg−1 i.v. caused clear bleeding into the small intestine. In contrast, INDO-PC treatment at the same indomethacin dosage produced essentially no bleeding into the GI lumen. Also, the plasma levels of indomethacin were the same after dosing of indomethacin and INDO-PC, suggesting that improved GI safety with INDO-PC was not due to a reduced uptake or lower blood level. Thus, a single dose of INDO-PC was considerably safer for the GI tract than a comparable dose of indomethacin alone, although its bioavailability was similar to the unmodified NSAID.
In addition, the efficacy of INDO-PC after a single dose was the same as that of indomethacin alone as seen by the greater threshold for pain pressure in both treatment groups as compared with vehicle-treated CFA rats, and the identical inhibition of PG formation in inflamed paws. Therefore, acute i.v. dosing with INDO-PC is as effective therapeutically as INDO, but was considerably less toxic to the GI tract.
It is also important to assess the safety and efficacy of INDO-PC after chronic dosing, as that issue is most relevant clinically. The CFA inflammation model is widely used to test for anti-inflammatory activity of test drugs (Billiau and Matthys, 2001). In our laboratory, this model produces a consistent inflammation of the paw that also responds to treatment by a reduction in ankle size. It was found that a 4 day treatment with either indomethacin or INDO-PC significantly lowered paw swelling (as determined by caliper) as a measure of anti-inflammatory activity. In addition, assessments of GI toxicity showed an advantage of INDO-PC over indomethacin alone, as indicated by measures of intestinal injury (perforation/adhesions) and GI blood loss (haematocrit, faecal Hb). Thus, chronic dosing with INDO-PC has similar therapeutic efficacy to indomethacin alone, with superior GI safety over a 3–4 day study period, although the protection appeared to wane with duration of treatment.
These data support the continued development of INDO-PC as a parenteral formulation with anti-inflammatory, analgesic and cyclo-oxygenase inhibitory activity, but with reduced toxicity to the GI tract that is currently associated with indomethacin alone. These data suggest that INDO-PC may be a highly effective and safe parenteral NSAID formulation for the short-term (24–48 h) treatment of conditions such as post-operative pain and inflammation, and patent ductus arteriosus.
Acknowledgments
We would like to thank Mr Charles Dial for his technical assistance in performance of HPLC analysis of plasma indomethacin. Funding was provided by Army grant W81XWH-08-C-0025 and PLx Pharma Inc. (Houston, TX, USA).
Glossary
Abbreviations
- CFA
complete Freund's adjuvant
- COX
cyclo-oxygenase
- Hb
haemoglobin
- HPLC
high pressure liquid chromatography
- NOS
nitric oxide synthase
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- NSAID
non-steroidal anti-inflammatory
- PGE2
prostaglandin E2
- RBCs
red blood cells
Conflicts of interest
The Army grant W81XWH-08-C-0025 was a Small Business Innovation Research grant awarded to PLx Pharma Inc., with a contract to Dr Lichtenberger at The University of Texas Health Science Center at Houston (UTHSCH). PLx Pharma Inc. has licensed the intellectual property related to PC-NSAID formulations from UTHSCH. Drs Lichtenberger and Dial have financial interests in PLx Pharma Inc. and Mr Romero is an employee of this university-based start-up company.
References
- Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM. Phospholipid association reduces the gastric toxicity of aspirin in human subjects. Am J Gastroenterol. 1999;94:1818–1822. doi: 10.1111/j.1572-0241.1999.01211.x. [DOI] [PubMed] [Google Scholar]
- Barrios J, Lichtenberger L. Role of biliary phosphatidylcholine in bile acid protection and NSAID injury of the ileal mucosa in rats. Gastroenterology. 2000;118:1179–1186. doi: 10.1016/s0016-5085(00)70371-4. [DOI] [PubMed] [Google Scholar]
- Beck W, Schneider H, Dietzel K, Nuernberg B, Brune K. Gastrointestinal ulcerations induced by anti-inflammatory drugs in rats. Physicochemical and biochemical factors involved. Arch Toxicol. 1990;64:210–217. doi: 10.1007/BF02010727. [DOI] [PubMed] [Google Scholar]
- Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- Brodie D, Cook P, Bauer B, Dagle G. Indomethacin-induced intestinal lesions in the rat. Toxicol Appl Pharmacol. 1970;17:615–624. doi: 10.1016/0041-008x(70)90036-0. [DOI] [PubMed] [Google Scholar]
- Chen CH, Erickson RA, Lifrak E, Yoshinaka R, Nguyen A. Does nitric oxide affect NSAID injury of the small intestinal mucosa? Gastroenterology. 1993;104:A53. [Google Scholar]
- Dial E, Rooijakkers S, Darling R, Romero J, Lichtenberger L. Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol. 2008a;23:430–436. doi: 10.1111/j.1440-1746.2007.05153.x. [DOI] [PubMed] [Google Scholar]
- Dial E, Darling R, Lichtenberger L. Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J Gastroenterol Hepatol. 2008b;23:e384–e389. doi: 10.1111/j.1440-1746.2007.05266.x. [DOI] [PubMed] [Google Scholar]
- Duggan D, Hooke K, Noll R, Kwan K. Enterohepatic circulation of indomethacin and its role in intestinal irritation. Biochem Pharmacol. 1975;25:1749–1754. doi: 10.1016/0006-2952(75)90450-5. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Wagenblast J, Erben G, Lehmann W, Hinz U, Merle U, et al. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39:737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]
- Giraud M, Motta C, Romero J, Bommelaer G, Lichtenberger L. Interaction of indomethacin and naproxen with gastric surface-active phospholipids:a possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs) Biochem Pharmacol. 1999;57:247–254. doi: 10.1016/s0006-2952(98)00303-7. [DOI] [PubMed] [Google Scholar]
- Go M, Lew G, Lichtenberger L, Genta R, Graham D. Gastric mucosal hydrophobicity and Helicobacter pylori:response to antimicrobial therapy. Am J Gastroenterol. 1993;88:1362–1365. [PubMed] [Google Scholar]
- Goggin P, Marrero J, Spychal R, Jackson P, Corbishley C, Northfield T. Surface hydrophobicity of gastric mucosa in Helicobacter pylori infection:effect of clearance and eradication. Gastroenterology. 1992;103:1486–1490. doi: 10.1016/0016-5085(92)91168-4. [DOI] [PubMed] [Google Scholar]
- Hills B, Butler B, Lichtenberger L. Gastric mucosal barrier:hydrophobic lining to the lumen of the stomach. Am J Physiol Gastrointest Liver Physiol. 1983;244:G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- Hucker H, Zacchei A, Cox S, Brodie D, Cantwell N. Studies on the absorption, distribution and excretion of indomethacin in various species. J Pharmacol Exp Ther. 1966;153:237–249. [Google Scholar]
- Kent T, Cardelli R, Stamler F. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol. 1969;54:237–249. [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger L, Graziani L, Dial E, Butler B, Hills B. Role of surface-active phospholipids in gastric cytoprotection. Science. 1983;219:1327–1329. doi: 10.1126/science.6828859. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L, Wang Z, Romero J, Ulloa C, Perez J, Giraud M, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids:insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L, Dial E, Ottlecz A, Romero J, Lechago J, Fox J. Attenuation of hydrophobic phospholipid barrier is an early event in Helicobacter felis-induced gastritis in mice. Dig Dis Sci. 1999;44:108–115. doi: 10.1023/a:1026610418663. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L, Romero J, De Ruijter W, Behfod F, Darling R, Ashraf A, et al. Phosphatidylcholine association increases the anti-inflammatory and analgesic activity of ibuprofen in acute and chronic rodent models of joint inflammation:relationship to alteration in bioavailability and cyclooxygenase–inhibitory potency. J Pharmacol Exp Ther. 2001;298:279–287. [PubMed] [Google Scholar]
- Lichtenberger L, Zhou Y, Dial E, Raphael R. NSAID injury to the gastrointestinal tract:evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J Pharm Pharmacol. 2006;58:1421–1428. doi: 10.1211/jpp.58.10.0001. [DOI] [PubMed] [Google Scholar]
- Lugea A, Mourelle M, Guarner F, Domingo A, Salas A, Malagelada JR. Phosphatidylcholines as mediators of adaptive cytoprotection of the rat duodenum. Gastroenterology. 1994;107:720–727. doi: 10.1016/0016-5085(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Lugea A, Antolin M, Mourelle M, Guarner F, Malagelada J-R. Deranged hydrophobic barrier of the rat gastroduodenal mucosa after parenteral nonsteroidal anti-inflammatory drugs. Gastroenterology. 1997;112:1931–1939. doi: 10.1053/gast.1997.v112.pm9178685. [DOI] [PubMed] [Google Scholar]
- Macfarlane R, Ng B, Gamie A, El Masry M, Velonis S, Schizas C, et al. Pharmacological treatment of heterotopic ossification following hip and acetabular surgery. Expert Opin Pharmacother. 2008;9:767–786. doi: 10.1517/14656566.9.5.767. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Kunikata T, Mizoguchi H, Kato S, Takeuchi K. Dual action of nitric oxide in pathogenesis of indomethacin-induced small intestinal ulceration in rats. J Physiol Pharmacol. 1999;50:405–417. [PubMed] [Google Scholar]
- Tatsumi R, Lichtenberger L. Molecular association of trinitrobenzenesulfonic acid and surface phospholipids in the development of colitis in rats. Gastroenterology. 1996;110:780–789. doi: 10.1053/gast.1996.v110.pm8608888. [DOI] [PubMed] [Google Scholar]
- Venneman N, Petruzzelli M, van Dijk J, Verheem A, Akkermans M, Kroese A, et al. Indomethacin disrupts the protective effect of phosphatidylcholine against bile salt-induced ileal mucosa injury. Eur J Clin Invest. 2006;36:105–112. doi: 10.1111/j.1365-2362.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Doyen R, Lichtenberger LM. Interaction of bile acids and NSAIDs leads to membrane destabilization:a potential explanation for NSAID-induced small intestinal injury. Gastroenterology. 2008;134:A739. [Google Scholar]


