Abstract
Background and purpose
Cyclooxygenase-2 (COX2) and hyaluronic acid (HA) are common in tumours and both independently promote tumour progression. Furthermore, COX2-dependent synthesis of prostaglandins (PGs) stimulates HA synthase-1 (HAS1) and HAS2 mRNA expression, together with HA synthesis via the cAMP/protein kinase A pathway in vascular smooth muscle cells. Therefore, the aim of the present study was to elucidate whether COX2-mediated PGs induce transcription of HAS isoforms in cancer cells as well.
Experimental approach
Human oesophageal squamous cell (OSC) carcinoma specimens were characterized with respect to HA, COX2 and CD44 expression by immunohistochemistry. OSC cell lines (OSC1, OSC2) and HeLa cell lines (D98, H21) were exposed to exogenous PG analoques (100 nmol·L−1), etoricoxib (10 µmol·L−1) and forskolin (10 µmol·L−1). Subsequently, cAMP levels, HA secretion and HAS isoform expression were determined by elisa and real-time RT-PCR (reverse transcriptase polymerase chain reaction) respectively.
Key results
COX2, HA and CD44 were detected immunohistochemically in >90% of human oesophageal tumour samples. Under basal conditions, OSC1 and OSC2 cells express HAS2 and HAS3, COX2 and Gαs-coupled EP2 and EP4 PG receptors. Neither stimulation with the PGI2 analogue, iloprost, addition of exogenous PGE2 nor forskolin induced HAS1 or HAS2 mRNA expression in OSC1 and OSC2 cells. Furthermore, in HeLa cells after induction of COX2 by tumour necrosis factor α and subsequent PGE2 release, inhibition of COX2 by etoricoxib did not affect HAS expression or HA secretion.
Conclusions and implications
We conclude that in oesophageal and HeLa cancer cells, HAS1/2 expression was not responsive to the PG/cAMP pathway.
Keywords: oesophageal cancer, COX2, etoricoxib, prostaglandins, hyaluronic acid
Introduction
Oesophageal cancer is a rare but severe form of gastrointestinal cancer that is differentiated into adenocarcinomas and squamous cell carcinomas of the oesophagus. Squamous cell carcinomas are found in the upper two-thirds of the oesophagus and are mainly induced by alcohol and cigarette smoke. Adenocarcinomas are usually found in the lower third of the oesophagus and are preceded by metaplasia, referred to as Barrett's oesophagus (Enzinger and Mayer, 2003).
Cyclooxygenase (COX) is the key enzyme during synthesis of prostanoids [prostaglandins (PGs), prostacyclin, thromboxane A2]. These important mediators account for a variety of physiological and pathophysiological processes such as inflammation, constriction and dilatation of blood vessels, regulation of platelet aggregation and control of calcium regulation. COX catalyses the production of PGH2 from its precursor arachidonic acid. Downstream enzymes like PGE2 synthase and prostacyclin synthase account for the transformation of PGH2 into the final products of the prostanoid family. The COX enzyme exists in two isoforms, COX1 that is responsible for the production of baseline levels of prostanoids and COX2 that is induced under pathological conditions and sustains inflammation. Prostanoids bind to a set of nine prostanoid receptors (Hata and Breyer, 2004; nomenclature follows Alexander et al., 2008).
Over-expression of COX isoforms, especially COX2, represents a well-known correlate of malignancy in a variety of cancers including those derived from colon (Kargman et al., 1995), stomach (Uefuji et al., 1998), lung (Wolff et al., 1998), oesophagus (Zimmermann et al., 1999), pancreas (Tucker et al., 1999), liver (Shiota et al., 1999), as well as head and neck tumours (Chan et al., 1999). Treatment with COX2 inhibitors leads to decreased proliferation of a variety of tumours such as colorectal (Coffey et al., 1997), oesophageal (Zimmermann et al., 1999), prostate (Liu et al., 1998) and breast cancer (Hong et al., 1999). These effects can be reversed by addition of exogenous PGE2 or PGF2α.
Hyaluronan (hyaluronic acid; HA), an unbranched polysaccharide, is composed of 2000–25 000 disaccharides of glucuronic acid and N-acetylglucosamine. Besides hydration and expansion of extracellular spaces, HA exhibits a plethora of physiological and pathophysiological functions that play a role in diverse processes including angiogenesis and inflammation involving signalling through HA receptors, CD44 and the receptor of HA-mediated motility (RHAMM). HA is produced by three isoforms of transmembrane HA synthase enzymes (HAS1–3) that link the two precursor molecules in an alternating manner and extrude the growing HA strand to the outside of the cell. While HAS1 and HAS2 produce HA up to 107 Da, HAS3 generates HA with an apparent molecular weight of approximately 105 Da. In this context, it is important to differentiate between HA of different molecular weights, because depending on its length, HA serves different functions (Stern et al., 2006). An overproduction of HA in tumour cells such as breast cancer (Auvinen et al., 1997), lung cancer (Horai et al., 1981), colon cancer (Ropponen et al., 1998), mesothelioma (Azumi et al., 1992) or pancreatic cancer (Ringel et al., 1999) results in elevated proliferation, migration, invasion, angiogenesis and resistance to apoptosis and cytostatic treatment. Urinary HA excretion is also an emerging prognostic marker for bladder cancer (Golshani et al., 2007). Interestingly, COX2 and HA are often found in the same cancer cells.
Thus, both COX2 and HA are involved in promoting a variety of malignancies. Recently, it was discovered that PGE2 and prostacyclin induce HAS1 and HAS2 mRNA expression in human vascular smooth muscle cells (hSMCs). This was proposed to be the outcome of an EP2 or IP receptor-mediated activation of protein kinase A (PKA) and an increase in cAMP, that in turn binds to the cAMP-responsive element, a transcription site in the HAS2 promoter region (Monslow et al., 2004; Sussmann et al., 2004; van den Boom et al., 2006). Based on these findings, we proposed, as a working hypothesis, that COX2-dependent PGs induce expression of HAS isoforms that subsequently give rise to HA-rich pericellular matrices, thereby promoting the malignant phenotype of cancer cells. The aim of the present study was to test this working hypothesis in oesophageal cancer cells.
Methods
Cell culture
The oesophageal cancer cell lines OSC1 and OSC2 (oesophageal squamous cell) and the HeLa cell lines H21 and D98 were described previously (Defilippi et al., 1987; Sarbia et al., 1997). The cells were routinely maintained as monolayer cultures in RPMI-1640 supplemented with 10% foetal bovine serum, L-glutamine, penicillin and streptomycin at 37°C and 5% CO2. For experiments, cells were subjected to serum withdrawal for 24 h and subsequently treated with the test compounds.
Immunohistochemical analysis of human tumour samples
The tumour samples were collected for diagnostic purposes and were examined by a senior pathologist (MS). Informed consent was obtained from patients or relatives. The present study was performed according to the Declaration of Helsinki. Immunohistochemical investigations were based on 69 patients (55 male; age range: 35–81 years) with squamous cell carcinoma of the oesophagus that underwent oesophageal resection without prior radio-or chemotherapy between 1978 and 1991 at the Department of Surgery, University of Düsseldorf. Subsequently, the resection specimens were fixed in buffered 4% formaldehyde. Pathological tumour stage was determined at the Department of Pathology, University of Düsseldorf according to standard procedures. For the purpose of this study, all cases were restaged according to the current TNM classification (Wittekind et al., 2002). Accordingly, 26 cases were in pathological stage IIa (37.7%), 8 in stage IIb (11.6%) and 35 in stage III (50.7%).
For each of the tumours, one paraffin block including representative, non-necrotic tumour areas was selected. Three tissue cylinders with a diameter of 0.6 mm per tumour were punched from these areas and brought into a recipient paraffin block by using a tissue arraying instrument (Beecher Instruments, Silver Spring, MD, USA). Sections (4 µm) from the tissue microarrays were mounted on Superfrost-plus slides (Langenbrinck, Teningen, Germany, Order-No 030060) for the subsequent immunohistochemical study.
Sections were immunohistochemically stained by using antibodies against COX2 (Cayman Chemical, Ann Arbor, MI, USA, 1:150) and CD44v (Sigma-Aldrich, Munich, Germany, HPA005785, 1:500). HA was detected by using biotinylated HABP (Seikagaku Corp., Tokyo, Japan; 2 µg·mL−1) as described (Ripellino et al., 1985). This staining procedure on paraffin-embedded sections detects expression of both, intracellular and extracellular HA. The sections were de-paraffinized and rehydrated in graded alcohol. Subsequently, for COX2 and CD44 detection, the sections were subjected to citrate buffer (pH 6.0) in a steamer at 100°C for 20 min. Primary antibody was applied overnight at 4°C. After application of the secondary antibody, antigen detection was performed by using diaminobenzidine as chromogen. Finally, the sections were counterstained with haematoxylin. Negative controls were treated identically to the tumour samples including antigen retrieval procedure with the exception that the primary antibody was replaced by non-immune serum. In negative controls no signals were detectable.
Using light microscopy, expression of HA, COX2 and CD44 was determined by a senior pathologist (MS). Cases were considered positive for expression when cancer cells in at least one out of three tissue cylinders per tumour case showed a distinctive extracellular/cytoplasmic (HA), cytoplasmic (COX2) or membranous expression pattern (CD44). Cases were considered negative if cancer cells in all analysable tissue cylinders per case showed no immunoreactivity.
RNA isolation
Total RNA from treated cells was isolated by using Tri-Reagent (Sigma-Aldrich) according to the manufacturer's protocol. The RNA was quantitated by spectroscopic analysis at 260 nm with an Eppendorf photometer.
Quantification of gene expression
The expression levels of HAS1, HAS2 and HAS3 were analysed by real-time RT-PCR (reverse transcriptase polymerase chain reaction). For real-time RT-PCR quantification, total RNA (1 µg) was used for cDNA synthesis, and specific primers for human HAS isoforms were generated according to the known sequences HAS1 (NM_001523.1), HAS2 (NM_005328.1), HAS3 (NM_005329.2) as follows: HAS-1: fwd 5′-TACAACCAGAAGTTCCTGGG-3′, rev 5′-CTGGAGGTGTACTTGGTAGC-3′; HAS-2: fwd 5′-GTGGATTATGTACAGGTTTGTGA-3′, rev 5′-TCCAACCATGGGATCTTCTT-3′; HAS-3: fwd 5′-GAGATGTCCAGATCCTCAACAA-3′, rev 5′-CCCACTAATACACTGCACAC-3′; GAPDH: fwd 5′-GTGAAGGTCGGAGTCAACG-3′, rev 5-′TGAGGTCAATGAAGGGGTC-3′. The relative mRNA expression levels were determined by using GAPDH as house keeping gene and the 2[−ΔΔC(T)] method. Values were then expressed as fold of respective controls.
Prostaglandin receptors were detected by RT-PCR using the following sequences: EP1: fwd 5′-GCGCTGCCCATCTTCTCC-3′, rev 5′-GGTACTGCAGCTCATAGC-3′; EP2: fwd 5′-GCTGGACTATGGGCAGTACG-3′, rev 5′-AACAGGAGGCCTAAGGATGG-3′; EP3: fwd 5′-TCGGGCTCTCCTCGTTGTTC-3′, rev 5′-AGTGAAGCCAGGCGAACAGC-3′; EP4: fwd 5′-ACTACGTGGACAAGCGATTG-3′, rev 5′-TCACAGAAGCAATTCGGATG-3′; IP: fwd 5′-GCGTCCTCTTCTGCGCGCTGCCCCTGCTGG-3′, rev 5′-GGTCCCCCATCTCACTGCTGCTGGTCAGG-3′.
Determination of the HA concentration
Cells were plated at a density of 105 cells per well in a 6 well plate and allowed to adhere for 24 h. The cells were cultured with or without stimulus for 6–24 h. HA released into the culture medium was quantified by using a commercially available assay based on HA-binding protein according to the manufacturer's instructions (Corgenix, Broomfield, CO, USA). Secreted HA was normalized to total cellular protein.
Quantification of PGI2 and PGE2 levels
Cell culture supernatants were collected at the indicated times and the concentration of 6-oxo-PGF1α, the stable hydrolysis product of PGI2, was determined by radioimmunoassay (RIA) as described previously (Sussmann et al., 2004). PGE2 was quantified by use of a commercially available elisa assay (Prostaglandin E2 EIA Kit – Monoclonal, Cayman Chemical, Ann Arbor, MI, USA).
Measurement of intracellular cAMP concentrations
Cells were seeded in 6 well plates in serum-free medium for 72 h. Then, the cells were washed twice with 2 mL of a balanced salt solution containing 130 mmol·L−1 NaCl, 5.4 mmol·L−1 KCl, 1.8 mmol·L−1 CaCl2, 0.8 mmol·L−1 MgCl2, 5.5 mmol·L−1 glucose and 20 mmol·L−1 HEPES, pH 7.3. Stimuli were added for 10 min. The reaction was stopped by removing the buffer and by addition of ice-cold ethanol (96%). Following evaporation of the ethanol, intracellular cAMP levels were determined by an RIA as described previously (Schröder and Schrör, 1993). All experiments were carried out in triplicate.
Data analysis
Data are presented as means ± SEM of the indicated number of independent experiments. Statistical comparisons between groups were performed by using one-way anova followed by the Bonferroni post test. A P-value <0.05 was considered significant.
Materials
Reagents for RT-PCR and real-time RT-PCR were obtained from Qiagen (Hilden, Germany) and Invitrogen (Karlsruhe, Germany). All cell culture reagents were obtained from Invitrogen or Sigma-Aldrich (Munich, Germany). Iloprost was kindly provided by Schering AG (Berlin, Germany). Forskolin, PGE2 and human recombinant tumour necrosis factor α (TNF-α) were purchased from Sigma-Aldrich. Etoricoxib was bought from WITEGA Laboratorien Berlin-Adlershof GmbH (Berlin, Germany).
Results
Expression of HA, COX2 and CD44 in human oesophageal cancer sections
Due to technical reasons, that is, detachment of tissue sections during immunohistochemical staining, only a subset of the 69 cases could be evaluated for expression of HA (61/69), COX2 (61/69) and CD44 (58/69). Expression of COX2 and CD44 was found in 100% of informative tumour samples, whereas accumulation of HA was found in 57 out of 61 samples (Figure 1A–C). HA was detected in tumour parenchyma (Figure 1A, inset, arrowheads) and even more pronounced in stromal cells (Figure 1A, asterisks). Because there is evidence that cancer cells tend to express large CD44 variants (Ponta et al., 1994 2003), an antibody detecting long CD44 variants was used. In consecutive sections CD44 and COX2 staining was observed in the cancer cells, whereas the stroma was predominantly negative (Figure 1B,C). Taken together, in the majority of cases co-localization of HA, COX2 and CD44 was observed in the cancer cells. Therefore, we analysed in vitro whether COX2-dependent PGs would induce HA production in human oesophageal cancer cells.
Figure 1.
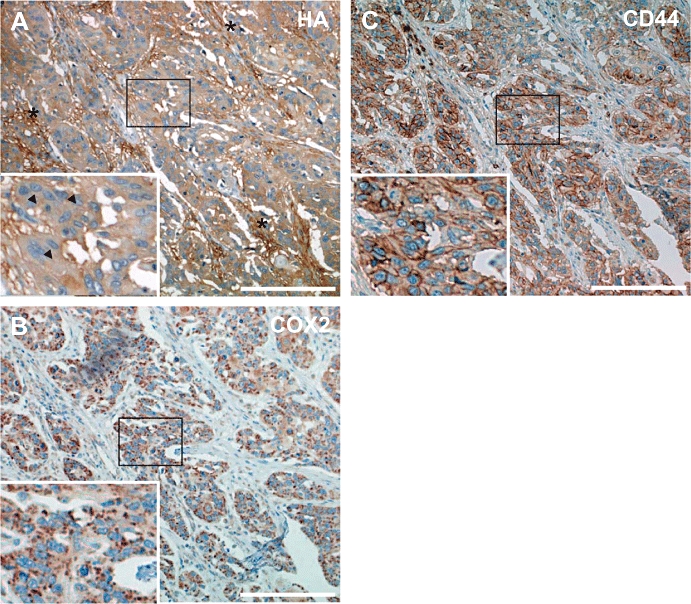
Immunohistochemical detection of hyaluronic acid (HA), cyclooxygenase-2 (COX2) and CD44 in human oesophageal cancer tissue. Shown are sections of human oesophageal tumours, representative for 69 cases. To assess the spatial relationships of HA, COX2 and CD44, consecutive sections were stained. HA (A) was detected in both tumour cell parenchyma and stromal cells; asterisks indicate accumulation of HA in tumour stroma; inset, magnification of the indicated area of a tumour cell cluster demonstrating HA within the cancer cells as well (arrowheads). COX2 (B) and the HA receptor CD44 (C) were detected mainly in tumour cell islands. Taken together, the majority (>90%) of the specimens displayed a strong expression of all three proteins. The micrographs show representative sections (scale bar marks 200 µm).
Characterization of the HA system in the oesophageal cancer cell lines OSC1 and OSC2
Two oesophageal cancer cell lines exhibiting a different expression pattern of HAS isoforms were used in the subsequent experiments. In OSC1 cells, HAS2 and HAS3 mRNA were highly expressed at a ratio of about 1:2, while HAS1 mRNA was undetectable (Figure 2A). OSC2 cells, in contrast, only expressed HAS3 (not shown). HA was regularly found in both cell lines as pericellular HA as well as secreted HA in their supernatants. As shown by affinity cytochemistry, pericellular HA was distributed in both cell types as membrane-bound patches (Figure 2B), and both cell lines produced also approximately the same amount of HA (Figure 2C).
Figure 2.
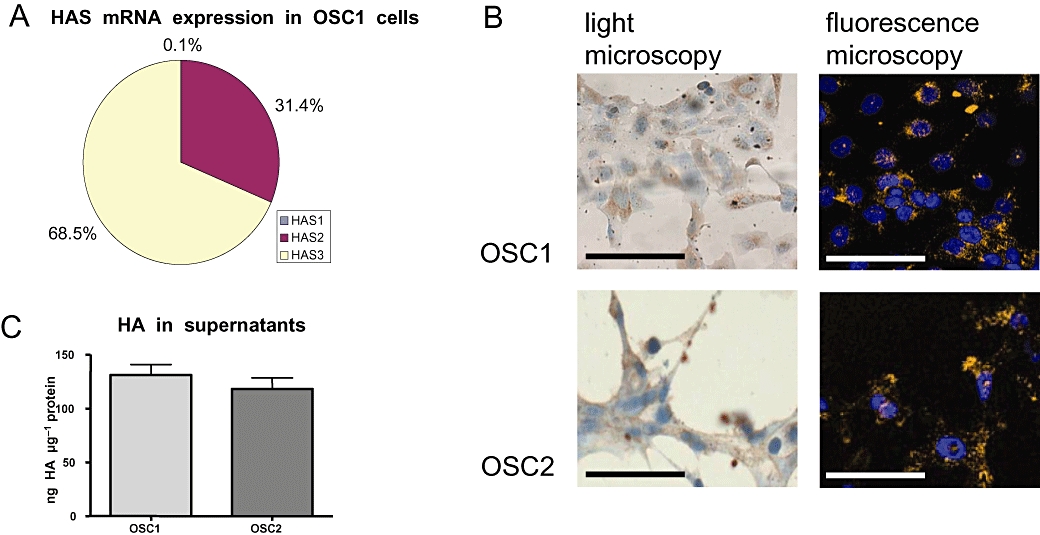
Characterization of the hyaluronan system of oesophageal cancer cell lines (OSC1 and OSC2). (A) Expression of HAS2 and HAS3 in OSC1 and OSC2 cells was determined by quantitative real-time RT-PCR. In both cell lines, HAS1 mRNA expression was only barely detectable, whereas HAS2 and HAS3 could be detected in OSC1 cells at a ratio of about 1:2. In OSC2 cells, only HAS3 mRNA was detected (data not shown). (B) Affinity cytochemical staining showed membrane-bound patches of HA. Detection was performed by light microscopy (left column) and fluorescence staining (right column). The micrographs show representative reactions (scale bar marks 50 µm). (C) The quantification of HA in the supernatants of OSC1 and OSC2 cells revealed approximately equal amounts of secreted HA normalized to protein in both cell lines. Representative data from experiments are shown. HA, hyaluronic acid, hyaluronan; HAS, hyaluronic acid synthase; OSC, oesophageal squamous cell; RT-PCR, reverse transcriptase polymerase chain reaction.
COX2 expression, PG production and PG receptor expression in OSC cells
Cyclooxygenase expression and activity in OSC1 and OSC2 cells were measured on protein and product level respectively. As shown by immunoblotting, COX was found to be constitutively expressed in both cell lines with OSC2 cells displaying a stronger protein expression, which is in accordance with a previous study (Zimmermann et al., 1999) (Figure 3A). As an indicator for COX2 activity, the secretion of PGI2 was quantified. Consistent with the COX2 protein expression levels, PGI2 levels were approximately 12 times higher in OSC2 cells compared with OSC1 cells (Figure 3B). Also the mRNAs of the corresponding prostacyclin (IP) and PGE receptors (EP1, EP2, EP4) were differentially expressed in both cell lines. Whereas OSC1 cells were found to abundantly express only the Gαs-coupled EP2, EP4 and IP receptors, OSC2 cells expressed mRNA of all EP receptors and the IP receptor (Figure 3C).
Figure 3.
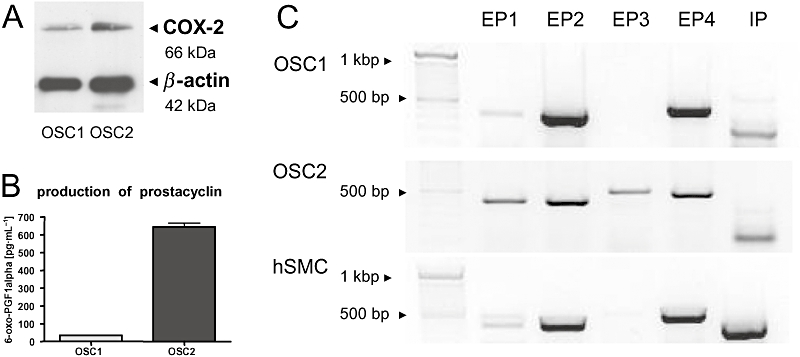
Characterization of the COX2/PG system in OSC1 and OSC2 cells. (A) As shown by immunoblotting, COX2 was more abundantly expressed in OSC2 cells than in OSC1 cells. One representative immunoblot out of three is shown. (B) To determine the amount of PGs secreted by OSC1 and OSC2 cells, the prostacyclin metabolite, 6-oxo-PGF1α, indicative of PG synthesis was determined in the supernatant. OSC2 cells produced approximately 12-fold more prostacyclin than OSC1 cells (n = 3, mean ± SEM). (C) Expression of PG receptors by OSC1 and OSC2 cells was assessed by RT-PCR. Human smooth muscle cells were examined as control. All cells expressed EP1, EP2, EP4 and IP receptors. Only OSC2 cells additionally expressed marked amounts of EP3 receptor. Receptor bands were found at the expected sizes, EP1/2 (450 bp), EP3/4 (580 bp), IP (380/520 bp). Shown are the results of one representative experiment out of three. COX2, cyclooxygenase-2; hSMC, human vascular smooth muscle cell; OSC, oesophageal squamous cell; PG, prostaglandin; RT-PCR, reverse transcriptase polymerase chain reaction.
Stimulation of OSC1 and OSC2 cells with PGs did not alter HAS isoform expression
Next we stimulated the cells with the prostacyclin (PGI2) analogue iloprost or with PGE2 to assess their influence on HAS mRNA expression. Surprisingly, neither PGE2 nor iloprost induced HAS1 mRNA or HAS2 mRNA in either OSC1 or OSC2 cells despite the fact that both cell lines abundantly express the IP, EP2 and EP4 receptors (see Figure 3C). To preclude the possibility that this deficiency is caused by a lack of cAMP induction due to inactive PG receptors, forskolin, an adenylate cyclase activator was used to raise cAMP levels independently of PG receptor activity. However, forskolin had also no effect on the expression of the HAS2 mRNA (Figure 4A). Only phorbol myristate acetate (PMA), an activator of PKC that was used as a positive control, resulted in a strong increase in HAS2 mRNA transcription (Figure 4A). As, with the exception for PMA, none of the stimuli used was able to induce HAS2 mRNA expression, we hypothesized that HAS transcription might be uncoupled from the cAMP system in oesophageal cancer cells. In addition, HAS3 mRNA levels were not altered by any stimulus in either cell line (Figure 4B,C).
Figure 4.
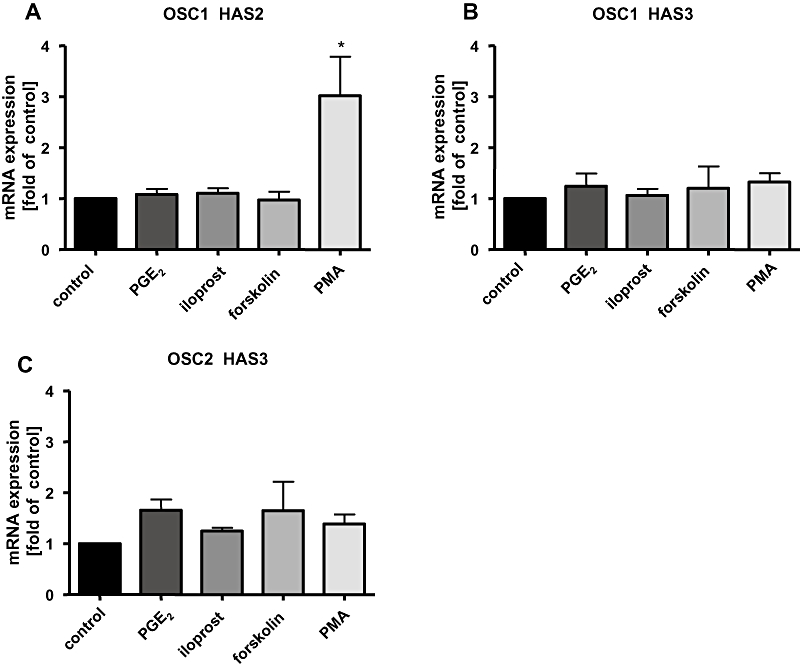
HAS isoform expression in OSC1 and OSC2 cells. Cells were stimulated for 6 h with the prostacyclin (PGI2) analogue, iloprost (100 nmol·L−1), PGE2 (100 nmol·L−1), forskolin (10 µmol·L−1) or with PMA (100 nmol·L−1) as a positive control. Shown are the results of one real-time RT-PCR out of three to five independent experiments (mean ± SEM, *P < 0.05). The HAS2/3 mRNA expression was normalized to GAPDH. Surprisingly, neither PGE2 nor iloprost or forskolin induced HAS2 mRNA in either cell line [OSC1, (A); OSC2, not shown]. Only PMA led to a strong increase of HAS2 mRNA transcription in OSC1 cells (A). HAS3 mRNA levels were not altered by any stimulus (B,C), HAS1 mRNA was not detectable (not shown). HAS, hyaluronic acid synthase; OSC, oesophageal squamous cell; PG, prostaglandin; PMA, phorbol myristate acetate; RT-PCR, reverse transcriptase polymerase chain reaction.
Induction of cAMP levels in response to prostaglandins
To verify that PG receptors were active and coupled to the Gαs subunits in OSC cells, we performed cAMP assays to measure the activation of adenylyl cyclase in response to PGs. In contrast to the positive control in hSMCs (data not shown) and fibroblasts (Figure 5C), activation of the IP receptor by iloprost did not lead to an increase of cAMP in either OSC1 or OSC2 cells, suggesting that IP receptors are inactive (Figure 5A,B). On the other hand, PGE2 stimulation resulted in a strong cAMP response, however, only in OSC1 cells (Figure 5A). The lack of a cAMP response in OSC2 cells might be due to receptor down-regulation because of the high endogenous PG levels in these cells (Figure 3B). Forskolin significantly increased cAMP levels in all examined cell lines proving the presence of functional adenylyl cyclase (Figure 5A–C). Thus, in OSC2 cells, both the IP and the EP receptors (EP2 and EP4) did not induce cAMP formation, which may explain why HAS2 expression was not affected by PGs in these cells. However, in OSC1 cells, PGE2 strongly induced cAMP production, suggesting that cAMP-dependent activation of HAS2 gene transcription was blocked downstream of cAMP in these cells.
Figure 5.
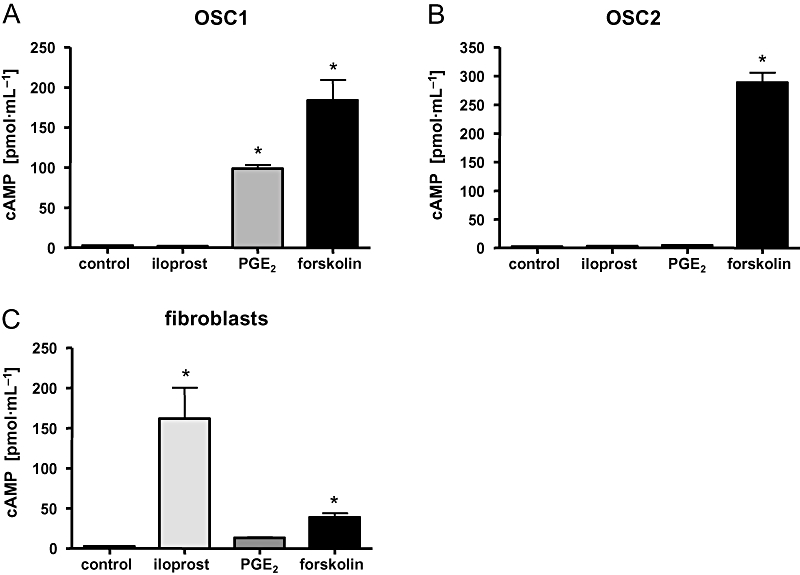
Intracellular cAMP levels after stimulation of OSC1 and OSC2 cells and fibroblasts by iloprost, PGE2 and forskolin. Intracellular cAMP levels were determined by radioimmunoassay. To examine the cAMP response, OSC1 (A) and OSC2 (B) cells were stimulated with agents known to induce cAMP levels. Human skin fibroblasts (C) were used as a positive control. Iloprost failed to induce cAMP increase in OSC1 and OSC2 cells, whereas it was able to raise cAMP levels in fibroblasts (C). PGE2 stimulated cAMP production only in OSC1 cells (A), whereas forskolin led to an increase in cAMP levels in all tested cell types. Representative results from four independent experiments are shown and displayed as mean ± SEM; asterisk indicates a P-value < 0.05. OSC, oesophageal squamous cell; PG, prostaglandin.
Expression of the HAS isoenzymes in HeLa cells
To verify these results also in another cellular model, cancer cells known to produce large amounts of PGE2 upon cytokine-induced COX2 expression were investigated. Therefore, two HeLa cells lines (D98, H21) exhibiting a TNF-α-inducible, COX2-dependent PG synthesis (Figure 6A,B) (Jänicke et al., 1994; Totzke et al., 2003) were used to assess the effects of endogenous PGE2 on HAS mRNA expression. TNF-α had no effect on mRNA levels of HAS2 and HAS3 isoforms in both cell lines (Figure 6C,D) despite the dramatic induction of PGE2 release (Figure 6A,B). Compatible with the conclusion that autocrine release of PGE2 in response to TNF-α did not induce HAS2 or HAS3, no effect of the COX2 inhibitor etoricoxib on HAS expression was detected (Figure 6C,D). Interestingly, etoricoxib itself caused a slight induction of HAS2 in HeLa D98 cells that was, however, not significant. Taken together, these data further substantiate our conclusion that HAS mRNA expression is not regulated by PGs in cancer cells.
Figure 6.
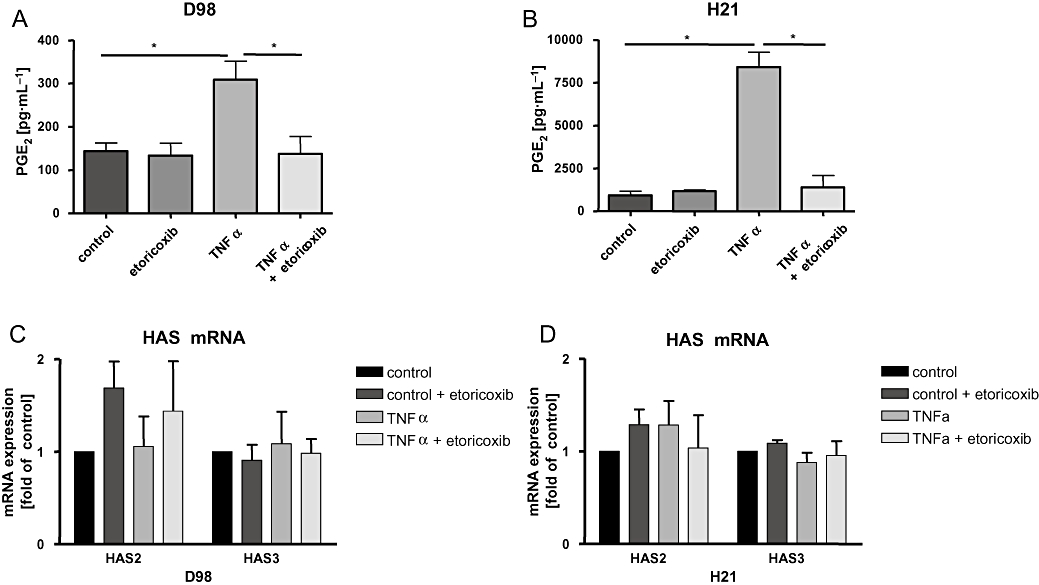
HAS isoenzyme expression in HeLa cells. The HeLa cell lines D98 and H21 were stimulated with TNF-α (30 ng·mL−1) for 6 h in the absence or presence of the COX2 inhibitor etoricoxib (10 µmol·L−1). The determination of PGE2 levels revealed high endogenous PGE2 synthesis in response to TNF-α that was responsive to etoricoxib (A,B). Real-time RT-PCR revealed that HAS2 and HAS3 mRNA levels were not regulated in response to COX2 and PG induction (C,D). HAS1 was not detectable (data not shown). The diagrams show representative results from three experiments (mean ± SEM, *P < 0.05). COX2, cyclooxygenase-2; HAS, hyaluronic acid synthase; PG, prostaglandin; RT-PCR, reverse transcriptase polymerase chain reaction; TNF-α, tumour necrosis factor α.
Discussion and conclusions
Previously it has been shown in hSMC that HAS1 and HAS2 are strongly induced by stimulation of the Gαs-coupled IP and EP2 receptors (Sussmann et al., 2004; van den Boom et al., 2006). Subsequently, it was reported that closure of the ductus arteriosus is also dependent on COX2-mediated PGE2 synthesis and subsequent stimulation of HAS2-mediated HA synthesis via stimulation of the EP4 receptor (Yokoyama et al., 2006). Furthermore, it has been known for a long time that forskolin stimulates HA synthesis in oocytes thereby stimulating the rapid expansion of the pericellular matrix of oocytes (Fischer and Schrör, 2007). These findings suggest that the PG-mediated activation of HAS expression is a regulatory pathway of general importance. The fact that the majority of human oesophageal tumour samples were characterized by the presence of HA, COX2 and CD44 was compatible with the working hypothesis that COX2-mediated PG synthesis stimulates HA production in human tumours. However, in vitro in OSC1 and OSC2 cells, prostacyclin and PGE2 did not induce HAS1 or HAS2 mRNA expression. In contrast, PMA, known to induce HAS2 transcription via activation of PKC (Feusi et al., 1999; Pienimaki et al., 2001; Anggiansah et al., 2003) did up-regulate HAS transcription in both cell lines.
To investigate the reason for the absence of PG-mediated activation of HAS1/2 transcription, we analysed the prostanoid signalling pathway in more detail. RT-PCR analyses revealed that all the necessary receptors including the EP1–4 and IP receptors are expressed in both OSC cell lines. Next, we pursued the question, if a loss of PG receptor-dependent induction of cAMP levels could be the reason for the lack of HAS2 induction in response to exogenous PGs. Forskolin, a direct cAMP inductor was used as positive control. Interestingly, in contrast to hSMC and fibroblasts, iloprost failed to stimulate cAMP production in either cell line. This might indicate that the IP receptor is either not expressed at the protein level or desensitized. PGE2 was able to raise cAMP levels in OSC1, but not in OSC2 cells, which are characterized by an abundant native overproduction of PGI2 and PGE2 (Zimmermann et al., 1999) (Figure 3B), that in turn could cause a down-regulation of active EP receptors. Forskolin led to a strong increase of cAMP levels in both cell lines showing the presence of activatable adenylyl cyclase. However, forskolin failed to induce HAS2 mRNA as revealed by quantitative real-time RT-PCR (Figure 4). In conclusion, in OSC1 and OSC2 cells, the cAMP-mediated HAS2 mRNA induction might be inhibited downstream of cAMP generation. A possibility that might be considered in future studies is that epigenetic changes such as methylation of HAS2 promoter may occur. Another possibility for aberrant HAS2 mRNA expression was pointed out by Chao and Spicer who described a naturally occurring HAS2 antisense RNA (HASNT) that leads to inactivation of HAS2 gene expression (Chao and Spicer, 2005).
To further investigate the possibility that a highly elevated endogenous PG production could induce HAS2 mRNA in an autocrine manner and in turn permanently activate the HAS system, we used the two HeLa cell lines D98 and H21 that display strong COX2 induction in response to TNF-α (Jänicke et al., 1994; Totzke et al., 2003) and abundant production of PGE2. However, also in these cells no correlation between HAS mRNA expression and PGE2 production was observed as indicated by the use of the COX2 inhibitor etoricoxib. These experiments show that the observed lack of response towards PG-mediated HAS expression is not restricted to oesophageal cancer cells and might therefore be relevant for cancer cells of varying origins.
Tofuku et al. showed that metastasis-associated properties of cancer cells such as invasion and proliferation are enhanced by lower molecular weight forms of HA that are characteristic of HAS3 activity (Itano and Kimata, 2002). In contrast, HAS2 is believed to deliver higher molecular weight HA that supports pericellular coat formation and thus inhibits a malignant tumour cell phenotype (Tofuku et al., 2006) unless it is further processed by for example hyaluronidases (Stern, 2008). This hypothesis was corroborated by Bullard et al. (2003) who compared the primary colon carcinoma cell line SW480 and the corresponding lymph node metastasis cell line SW620. The expression levels of HAS3 mRNA were much higher in the metastasizing cells (Bullard et al., 2003). Therefore, it is tempting to speculate that the observed absence of PG-and cAMP-dependent HAS2 mRNA induction is leading to a higher relative contribution of HAS3 to HA synthesis, which might support the malignant phenotype of OSC1 and OSC2 cells.
Taken together, the present data did not support our working hypothesis that COX2-dependent PGs induce HA synthesis in oesophageal cancer (Figure 7). In OSC2 cells, this is likely to have been caused by desensitized PG receptors, which did not lead to raised cAMP levels after stimulation. In OSC1 cells, the EP receptors induce cAMP levels in response to PGE2; however, HAS1 and HAS2 were not induced. Instead, we showed that in OSC1 and OSC2 cells, PG-and cAMP-dependent induction of HAS1/2 mRNA expression was not active. In future studies it should therefore be investigated whether epigenetic changes or natural occurring antisense RNA against HAS2 suppress the expression of HAS1 and HAS2 in these cancer cells.
Figure 7.
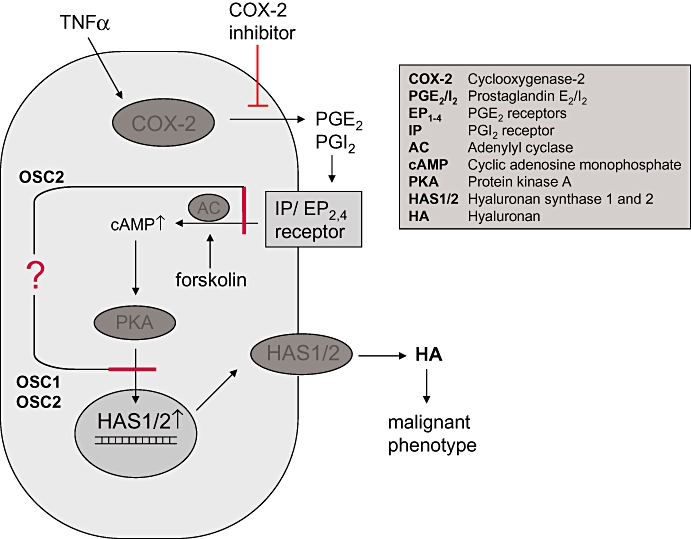
Schematic illustration of the proposed stimulation of HAS isoform regulation by prostaglandins in cancer cells. According to our working hypothesis COX2-dependent prostaglandins (PGI2 and PGE2) activate the Gαs-coupled IP and EP2/4 receptors, raise intracellular cAMP levels and in turn activate HAS1 and HAS2 expression via PKA-mediated signals. The increased HA secretion stimulates a malignant tumour cell phenotype through signalling via HA receptors CD44 or receptor of HA-mediated motility (RHAMM). The present findings indicate that in oesophageal squamous cancer cells, OSC1 and OSC2, stimulation of HAS1 and HAS2 expression by prostaglandins is inhibited either at the level of prostaglandin receptor-stimulated cAMP induction (OSC2) and/or downstream of cAMP (OSC1, OSC2).
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft, SFB 612 (TP B9) and SFB728 (TP C6 and B1) and a fellowship of the Gründerstiftung zur Förderung von Forschung und wissenschaftlichen Nachwuchs an der Heinrich Heine Universität Düsseldorf.
Glossary
Abbreviations
- COX2
cyclooxygenase-2
- HA
hyaluronic acid, hyaluronan
- HAS
hyaluronic acid synthase
- hSMC
human vascular smooth muscle cell
- OSC
oesophageal squamous cell
- PG
prostaglandin
- PMA
phorbol myristate acetate
- TNF-α
tumour necrosis factor α
Conflict of interest
The authors state no conflict of interest.
References
- Anggiansah CL, Scott D, Poli A, Coleman PJ, Badrick E, Mason RM, et al. Regulation of hyaluronan secretion into rabbit synovial joints in vivo by protein kinase C. J Physiol. 2003;550:631–640. doi: 10.1113/jphysiol.2003.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen PK, Parkkinen JJ, Johansson RT, Agren UM, Tammi RH, Eskelinen MJ, et al. Expression of hyaluronan in benign and malignant breast lesions. Int J Cancer. 1997;74:477–481. doi: 10.1002/(sici)1097-0215(19971021)74:5<477::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Azumi N, Underhill CB, Kagan E, Sheibani K. A novel biotinylated probe specific for hyaluronate. Its diagnostic value in diffuse malignant mesothelioma. Am J Surg Pathol. 1992;16:116–121. doi: 10.1097/00000478-199202000-00003. [DOI] [PubMed] [Google Scholar]
- van den Boom M, Sarbia M, von Wnuck Lipinski K, Mann P, Meyer-Kirchrath J, Rauch BH, et al. Differential regulation of hyaluronic acid synthase isoforms in human saphenous vein smooth muscle cells: possible implications for vein graft stenosis. Circ Res. 2006;98:36–44. doi: 10.1161/01.RES.0000199263.67107.c0. [DOI] [PubMed] [Google Scholar]
- Bullard KM, Kim HR, Wheeler MA, Wilson CM, Neudauer CL, Simpson MA, et al. Hyaluronan synthase-3 is upregulated in metastatic colon carcinoma cells and manipulation of expression alters matrix retention and cellular growth. Int J Cancer. 2003;107:739–746. doi: 10.1002/ijc.11475. [DOI] [PubMed] [Google Scholar]
- Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- Chao H, Spicer AP. Natural antisense mRNAs to hyaluronan synthase 2 inhibit hyaluronan biosynthesis and cell proliferation. J Biol Chem. 2005;280:27513–27522. doi: 10.1074/jbc.M411544200. [DOI] [PubMed] [Google Scholar]
- Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P, Poupart P, Tavernier J, Fiers W, Content J. Induction and regulation of mRNA encoding 26-kDa protein in human cell lines treated with recombinant human tumor necrosis factor. Proc Natl Acad Sci USA. 1987;84:4557–4561. doi: 10.1073/pnas.84.13.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- Feusi E, Sun L, Sibalic A, Beck-Schimmer B, Oertli B, Wuthrich RP. Enhanced hyaluronan synthesis in the MRL-Fas(lpr) kidney: role of cytokines. Nephron. 1999;83:66–73. doi: 10.1159/000045475. [DOI] [PubMed] [Google Scholar]
- Fischer JW, Schrör K. Regulation of hyaluronan synthesis by vasodilatory prostaglandins. Implications for atherosclerosis. Thromb Haemost. 2007;98:287–295. [PubMed] [Google Scholar]
- Golshani R, Hautmann SH, Estrella V, Cohen BL, Kyle CC, Manoharan M, et al. HAS1 expression in bladder cancer and its relation to urinary HA test. Int J Cancer. 2007;120:1712–1720. doi: 10.1002/ijc.22222. [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hong SH, Avis I, Vos MD, Martinez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer Res. 1999;59:2223–2228. [PubMed] [Google Scholar]
- Horai T, Nakamura N, Tateishi R, Hattori S. Glycosaminoglycans in human lung cancer. Cancer. 1981;48:2016–21. doi: 10.1002/1097-0142(19811101)48:9<2016::aid-cncr2820480918>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- Jänicke RU, Lee FH, Porter AG. Nuclear c-Myc plays an important role in the cytotoxicity of tumor necrosis factor alpha in tumor cells. Mol Cell Biol. 1994;14:5661–5670. doi: 10.1128/mcb.14.9.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargman SL, O'Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S. Expression of prostaglandin G/H synthase-1 and-2 protein in human colon cancer. Cancer Res. 1995;55:2556–2559. [PubMed] [Google Scholar]
- Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- Monslow J, Williams JD, Guy CA, Price IK, Craig KJ, Williams HJ, et al. Identification and analysis of the promoter region of the human hyaluronan synthase 2 gene. J Biol Chem. 2004;279:20576–20581. doi: 10.1074/jbc.M312666200. [DOI] [PubMed] [Google Scholar]
- Pienimaki JP, Rilla K, Fulop C, Sironen RK, Karvinen S, Pasonen S, et al. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem. 2001;276:20428–20435. doi: 10.1074/jbc.M007601200. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sleeman J, Dall P, Moll J, Sherman L, Herrlich P. CD44 isoforms in metastatic cancer. Invasion Metastasis. 1994;14:82–86. [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Ringel J, Rychly J, Nebe B, Schmidt C, Muller P, Emmrich J, et al. CD44, bFGF and hyaluronan in human pancreatic cancer cell lines. Ann N Y Acad Sci. 1999;880:238–242. doi: 10.1111/j.1749-6632.1999.tb09528.x. [DOI] [PubMed] [Google Scholar]
- Ripellino JA, Klinger MM, Margolis RU, Margolis RK. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J Histochem Cytochem. 1985;33:1060–1066. doi: 10.1177/33.10.4045184. [DOI] [PubMed] [Google Scholar]
- Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, et al. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 1998;58:342–347. [PubMed] [Google Scholar]
- Sarbia M, Bosing N, Hildebrandt B, Koldovsky P, Gerharz CD, Gabbert HE. Characterization of two newly established cell lines derived from squamous cell carcinomas of the oesophagus. Anticancer Res. 1997;17:2185–2192. [PubMed] [Google Scholar]
- Schröder H, Schrör K. Prostacyclin-dependent cyclic AMP formation in endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:101–104. doi: 10.1007/BF00168779. [DOI] [PubMed] [Google Scholar]
- Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407–412. [PubMed] [Google Scholar]
- Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Sussmann M, Sarbia M, Meyer-Kirchrath J, Nusing RM, Schror K, Fischer JW. Induction of hyaluronic acid synthase 2 (HAS2) in human vascular smooth muscle cells by vasodilatory prostaglandins. Circ Res. 2004;94:592–600. doi: 10.1161/01.RES.0000119169.87429.A0. [DOI] [PubMed] [Google Scholar]
- Tofuku K, Yokouchi M, Murayama T, Minami S, Komiya S. HAS3-related hyaluronan enhances biological activities necessary for metastasis of osteosarcoma cells. Int J Oncol. 2006;29:175–183. [PubMed] [Google Scholar]
- Totzke G, Schulze-Osthoff K, Janicke RU. Cyclooxygenase-2 (COX-2) inhibitors sensitize tumor cells specifically to death receptor-induced apoptosis independently of COX-2 inhibition. Oncogene. 2003;22:8021–8030. doi: 10.1038/sj.onc.1206837. [DOI] [PubMed] [Google Scholar]
- Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- Uefuji K, Ichikura T, Mochizuki H, Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168–172. doi: 10.1002/(sici)1096-9098(199811)69:3<168::aid-jso9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511–2516. doi: 10.1002/cncr.10492. [DOI] [PubMed] [Google Scholar]
- Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006;116:3026–3034. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]


