Abstract
Antisense oligonucleotides and small interfering RNA have enormous potential for the treatment of a number of diseases, including cancer. However, several impediments to their widespread use as drugs still have to be overcome: in particular their lack of stability in physiological fluids and their poor penetration into cells. Association with or encapsulation within nano-and microsized drug delivery systems could help to solve these problems. In this review, we describe the progress that has been made using delivery systems composed of natural or synthetic polymers in the form of complexes, nanoparticles or microparticles.
This article is part of a themed section on Vector Design and Drug Delivery. For a list of all articles in this section see the end of this paper, or visit: http://www3.interscience.wiley.com/journal/121548564/issueyear?year=200
Keywords: antisense oligonucleotides; siRNA; pharmacokinetics; cell penetration, nanoparticles; microparticles, controlled release
Introduction
The aim of this review is to describe advances that have been made in the delivery of antisense oligonucleotides (AS-ODN) and small interfering RNA (siRNA) by the use of natural and synthetic polymers. Over recent decades, much hope, and research effort, has been invested in nucleic acids as therapeutic agents. In particular, AS-ODN and siRNA, which are able to inhibit expression of specific genes by interfering with transcription or, more usually, translation, have enormous potential for the treatment of many diseases, including cancer, in which they can be used to silence oncogene expression. However, several impediments to their widespread use as drugs still have to be overcome. The most serious of these are their lack of stability in physiological fluids and their poor penetration into cells. Association with or encapsulation within nano-and microsized drug delivery systems could help to solve these problems but, as we attempt to describe below, the design of the carrier is important to achieve the correct tissular, cellular and subcellular localization of the AS-ODN or siRNA.
Mechanism of action of AS-ODN and siRNA
An understanding of the mechanisms of action of AS-ODN and siRNA is a prerequisite for designing efficient therapeutic systems. Although the activity of both types of molecule is due to their base-pairing capacity with complementary cellular nucleic acids, the method by which they inhibit gene expression is different.
Antisense oligonucleotides are single-stranded DNA sequences of 13–25 bases, which are complementary to specific messenger RNA molecules (mRNA). Their binding to this mRNA by Watson-Crick base-pairing stops translation and thereby reduces synthesis of the encoded protein (Loke et al., 1989). The first explanation of this was a steric blockage of the process of translation, in which the presence of the bound AS-ODN prevents the binding of translation factors and modulators and impedes the movement of the ribosome along the mRNA strand (Baker et al., 1997; Dias et al., 1999) (Figure 1). A second mechanism that comes into play is the action of ribonuclease H (RNase H). This enzyme specifically cleaves the RNA strand of RNA-DNA duplexes (Figure 1). This releases the AS-ODN, which can then bind to a new mRNA strand; this mechanism is therefore catalytic (Walder and Walder, 1988). However, chemically modified AS-ODN may not be recognized by the enzyme (see below).
Figure 1.
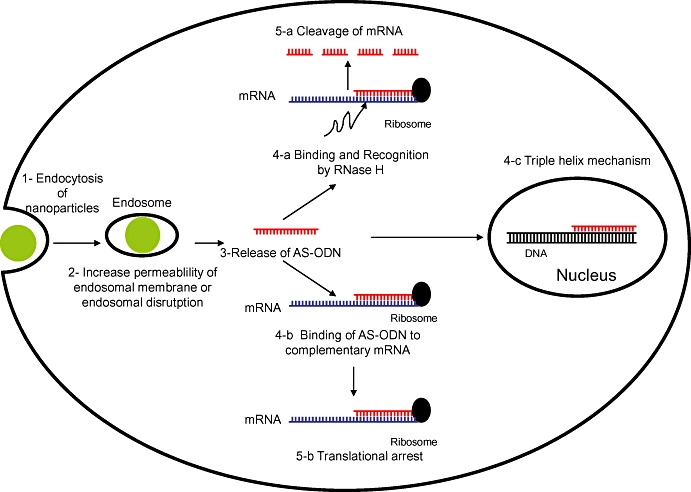
Intracellular penetration of nanoparticulate systems containing antisense oligonucleotides and their subsequent mechanisms of action after release in the cytoplasm. AS-ODN, antisense oligonucleotides; mRNA, messenger RNA; RNase H, ribonuclease H.
The mechanisms described above involve mRNA and occur in the cytoplasm. However, AS-ODN can also exert activity in the nucleus, where they are able to form triple helixes with DNA, inserting into the major groove of the double helix. This occurs preferentially when a sequence composed entirely of purines is base-paired with one composed of pyrimidines (Moser and Dervan, 1987). The formation of a triple helix leads to cleavage of the DNA and will therefore inhibit gene expression at the transcription stage.
Small interfering RNAs differ from AS-ODN in being made up of ribonucleotides rather than deoxyribonucleotides and in being double-stranded rather than single-stranded. These double-stranded RNAs of 21–23 base pairs bind to a multi-protein complex in the cytoplasm, known as RISC for RNA-induced silencing complex (Figure 2). During assembly of RISC, one of the two siRNA strands, referred to as the passenger strand, is released, whereas the other strand (guide strand) binds to the argonaute protein, Ago2 (Meister et al., 2004). This strand then guides RISC to its complementary target RNA, which is finally cleaved by the RNase activity located in the Ago2 protein, triggering its destruction (Song et al., 2004). The mechanism is therefore an antisense, catalytic one. The RISC contains elements that search for homology, which means that siRNA is more efficient than AS-ODN and a smaller number of molecules need to be delivered to the cell (Corey, 2007). The site of action of siRNA is always the cytoplasm. As they are double-stranded, they have better in vivo stability than AS-ODN and therefore give more prolonged gene-silencing activity; however, they share with AS-ODN the drawback of poor penetration into cells, being negatively charged hydrophilic molecules (Table 1).
Figure 2.
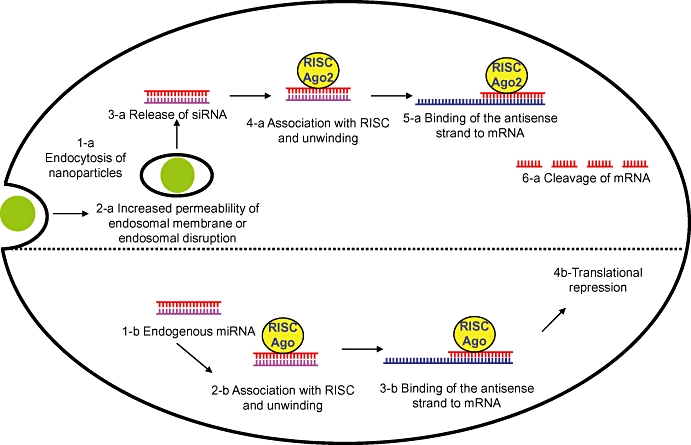
Intracellular penetration of nanoparticulate systems containing siRNA and their subsequent mechanism of action after release in the cytoplasm. The mechanism of action of endogenous miRNA is presented here for comparison purposes. miRNA, microRNA; mRNA, messenger RNA; RISC, RNA-induced silencing complex; siRNA, small interfering RNA.
Table 1.
Main differences between unmodified AS-ODN and siRNA
| ODN | siRNA |
|---|---|
| Single-stranded | Double-stranded |
| Short length | Short length |
| Negatively charged | Negatively charged |
| Hydrophilic | Hydrophilic |
| Poorly stable | Stable |
| Low intracellular penetration | Low intracellular penetration |
| Target is cytoplasm or nucleus | Target is cytoplasm |
| Cleavage or blockage of mRNA | Cleavage of mRNA |
| Short-term activity | Long-term activity |
AS-ODN, antisense oligonucleotides; mRNA, messenger RNA; siRNA, small interfering RNA.
Similar to siRNA, miRNA, which stands for microRNA (Lee et al., 1993) constitutes a class of evolutionarily conserved, endogenously expressed, non-coding RNAs that are 19–24 nucleotides long and which bind with only a partial complementarity to the 3′ untranslated region of mRNA and negatively regulate target mRNAs (Ambros, 2004; Bartel, 2004). All four mammalian argonaute proteins, Ago1 to Ago4, seem to participate in miRNA-mediated repression (Pillai et al., 2004). Because of partial complementarity, in most cases cleavage of mRNA does not occur, as noted for siRNA. miRNAs are therefore important post-transcriptional regulators of gene inhibition and appears to play an important role in a wide range of diseases, including cancer. Indeed a number of miRNAs have been identified to function as oncogenes. These so-called ‘oncomirs’ are usually found to be aberrantly expressed in various malignancies of different cellular origin (Wiemer, 2007). The use of strategies such as AS-ODN as inhibitors of miRNA has been reviewed recently (Esau and Monia, 2007).
Barriers to the use of AS-ODN and siRNA in clinical practice
Despite the obvious promise shown by nucleic acids as therapeutic agents, a number of problems need to be solved before their use can become routine. These are essentially their instability in biological media and their poor penetration into cells. The phosphodiester backbone of natural ODN is readily hydrolysed by nucleases present in serum. Thus, when pharmacokinetic studies were first undertaken with an AS-ODN (a 25-mer) by Agrawal et al. (1995), the circulating half-life in the monkey was found to be only 5 min. It was shown by PAGE analysis of blood samples that no intact AS-ODN could be detected after 15 min. A similarly short half-life was observed by Sands et al. (1994) after administration of a 20-mer to rats. AS-ODN that is not degraded in the plasma is distributed into peripheral tissues, principally the liver (Agrawal et al., 1995).
On a cellular level, AS-ODN, which are large negatively charged hydrophilic molecules, cannot penetrate the plasma membrane of cells by simple diffusion. However, their mechanism of action requires that they reach the cytoplasm. The ways in which AS-ODN are internalized by cells have not been completely elucidated. Given their physicochemical nature, the available pathways would be receptor-mediated uptake and endocytosis. This is consistent with the observations that uptake depends on temperature (Yakubov et al., 1989) and the AS-ODN concentration (Vlassov et al., 1994). There is some evidence that uptake by a membrane-bound receptor occurs at relatively low ODN concentrations (Yakubov et al., 1989). Whether uptake is by a specific receptor or by absorptive or fluid-phase pinocytosis, the outcome for the ODN will be the same; it will be delivered to a lysosome where low pH and the presence of hydrolytic enzymes will combine to degrade it. Lysosomal escape is therefore an important factor for therapeutic effect.
Small interfering RNAs face similar pharmacokinetic and cell penetration limitations. As a result of their small size, they are rapidly eliminated by the kidneys and show circulating half-lives of the order of seconds to minutes (Soutschek et al., 2004). They are also susceptible to degradation by nucleases in the plasma. A study of the biodistribution of radiolabelled siRNA indicated accumulation mainly in the liver and spleen and to a lesser extent in the heart, spleen and lung, in a similar pattern to AS-ODN (Braasch et al., 2004). Within the tissues, they do not cross cell membranes readily because of their negative charge, hydrophilicity and molecular size. Although uptake into cells of invertebrates such as Caenorhabditis elegans has been observed, siRNAs are not taken up by most mammalian cells in a way that preserves their activity (Aagaard and Rossi, 2007) and therefore an effective delivery system is a prerequisite for effective gene silencing (Dykxhoorn and Lieberman, 2006).
Beside the barriers related to stability and bioavailability, several classes of off-target effects have been reported for AS-ODNs and siRNA, some of which are related to specific sequence motifs. Unmethylated CpG motifs have been shown to stimulate the immune system (Krieg et al., 1995). In fact, the mechanism occurs through binding to the Toll-like receptor (TLR) 9 in the endosomal compartment and further activation of this receptor (Bauer et al., 2001; Ishii et al., 2002; Rutz et al., 2004). Although CpG motifs have beneficial effects in stimulating host defence, it can also induce inflammation and may promote tissue injury during infection or local administration (Schwartz et al., 1999). However, these effects generally appear at higher doses of nucleic acids than those at which therapeutic effects are seen. At present, CpG oligonucleotides are considered as effective and well-tolerated vaccine adjuvants. Nevertheless, the effects of CpG on the immunity are strictly sequence-dependent (Krieg, 2006) and are not enhanced by polymeric or lipid non-viral carriers (Bonnet et al., 2008). Other off-target effects can result from guanine quartet sequences, which form planar structures that stack together to make a four-stranded helical structure have been shown to bind many cellular proteins, resulting in non-antisense effects (Dapic et al., 2002).
Two, not mutually exclusive, approaches can be adopted to improve the therapeutic potential of AS-ODN and siRNA. One is a chemical approach that consists in synthesizing nucleic acids with variations in the natural structure, which give them better resistance in physiological media while conserving the specificity of binding to the target. The second is a pharmaceutical technology approach that aims to associate the nucleic acid with appropriate delivery systems, which can protect it from degradation and modify its tissue and cellular distribution to reach the target. This review will concentrate on the second approach; however, a brief overview of modified nucleic acid structures will first be given, as these non-natural ODN and siRNA also benefit from a targeting strategy and the most efficient systems are most likely to be those that use a combination of the two approaches.
Modification of AS-ODN and siRNA structure
Modification of phosphate groups
The first type of modification to be attempted was replacement of the non-bridging oxygen of the phosphodiester backbone by a sulphur atom, resulting in phosphorothioate AS-ODN (Figure 3). These are more resistant to degradation by nucleases while retaining susceptibility to RNase H. This results in a prolongation of their circulating half-life to 30–60 min (Agrawal et al., 1991; Cossum et al., 1993; Zhang et al., 1995; Geary et al., 2001), although some variations between species were seen, with the clearance in mice being twice as rapid as that in monkeys in one study (Yu et al., 2001). They are characterized by high kidney uptake (Lendvai et al., 2005). Accumulation of phosphorothioate AS-ODN within cells is observed 24 h after administration, but the mechanism of uptake has not yet been elucidated (Butler et al., 1997; Butler et al., 2000). The efficacy of the modified AS-ODN, compared with the native structure, is still a matter for debate. For siRNA, two studies found that the silencing efficiency was not diminished (Braasch et al., 2003; Harborth et al., 2003), whereas Chiu and Rana (2003) clearly observed reduced activity.
Figure 3.
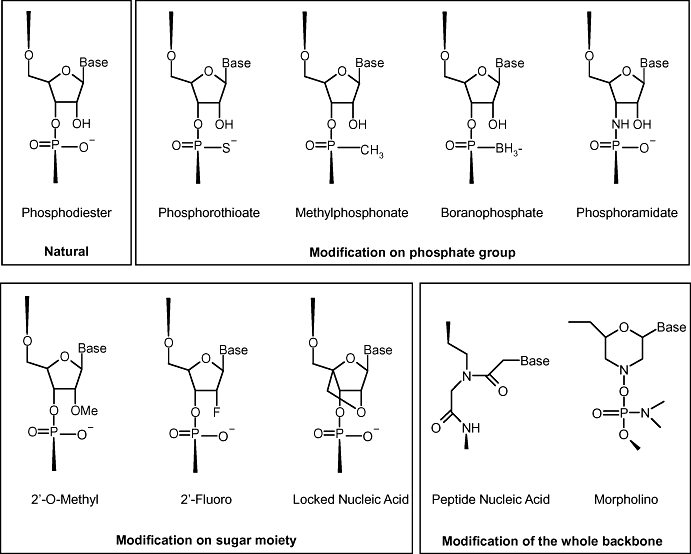
Structure of the main chemically modified antisense oligonucleotides and small interfering RNA.
The non-bridging oxygen in AS-ODN can also be replaced by a methyl group (yielding a methylphosphonate) or a boron atom (resulting in a boranophosphonate) (Figure 3). These structures are more hydrophobic than the natural molecule and show reduced activation of RNase H (Agrawal, 1999). Boranophosphonate derivatives of siRNA have also been prepared. These are much more resistant to nucleases than phosphodiester or phosphorothioate siRNA and consequently show higher efficacy (Hall et al., 2004; Hall et al., 2006). Nevertheless, the features of the phosphorothioate backbone modification can also be associated with non-antisense effects through binding of cellular proteins (Levin, 1999).
A more profound modification of the backbone is to substitute every 3′ oxygen by a 3′ amino group, leading to the synthesis of phosphoramidates (Figure 3). These are very stable, nuclease-resistant molecules, which form stable triplexes with double-stranded DNA under near physiological conditions (Gryaznov et al., 1995; Gryaznov et al., 1996).
Modification of the sugar moiety
Attempts have been made to improve the stability of AS-ODN and siRNAs by making substitutions at the 2′ position of the sugar. O-methyl, O-methyoxy-ethyl and fluoro substitutions have been made (Figure 3). These modifications increase the nuclease resistance and the stability of the complexes formed with native RNA. However, the modifications also have consequences for activity, as the substitutions prevent RNase H cleavage of the target RNA in the antisense strategy. Therefore, it can be assumed that the antisense effects observed with these analogues (Kurreck, 2003; Urban and Noe, 2003) are due to steric hindrance of transcription. Increased potency has been claimed for sugar-substituted siRNA (Allerson et al., 2005); however, in another study O-methoxy derivatives were found to have the same activity as the unmodified sequence (Kraynack and Baker, 2006). According to Layzer et al. (2004), fluoro-substituted siRNA did not show increased in vivo activity, despite better stability in plasma.
The ribose moiety can also be used to produce locked nucleic acid, in which a covalent bridge is formed between the 2′ oxygen and the 4′ carbon of the ribose, fixing it in the 3′-endo configuration (Figure 3). These constructs, which are also referred to as inaccessible RNA, are extremely stable in biological medium and are able to activate RNase H and to form tight hybrids with complementary RNA and DNA (Wahlestedt et al., 2000; Vester and Wengel, 2004). Antisense activity has been obtained in experimental murine tumours (Jepsen et al., 2004), but these molecules have also been observed to produce severe hepatotoxicity (Swayze et al., 2007). Locked nucleic acid also undergo intracellular processing characteristic of siRNA, and therefore represent a useful advance in this technology (Braasch et al., 2003; Elmen et al., 2005).
Modification of the whole backbone
A further style of nucleic acid modification is that in which the entire backbone structure is changed. For example, the phosphodiester backbone can be completely replaced by a peptide one consisting of (2-aminoethyl)-glycine residues. These are known as peptide nucleic acids (Hanvey et al., 1992). As AS-ODN, they have the advantages of strong hybridization, low toxicity and good biological stability with respect to nucleases, but also the disadvantages of low solubility in water, poor intracellular penetration and a lack of ability to activate RNase H. Another completely modified structure is the morpholino AS-ODN, in which the ribose is replaced by a morpholino moiety and phosphoroamidate intersubunit linkages are used as a substitute for phosphodiester bonds. They have low toxicity and show affinity for their targets similar to that of unmodified AS-ODN (Summerton, 1999; Summerton and Weller, 1997).
Drug delivery systems for AS-ODN and siRNA
Despite the advances provided by the modification of nucleic acid structure, these molecules require effective delivery systems in order to become useful therapeutic agents. Different formulation strategies have been developed to overcome the problems mentioned above: the susceptibility of these molecules to degradation by extracellular and intracellular nucleases and their inability to cross the cell plasma membrane by diffusion. Drug delivery systems are therefore designed to protect the nucleic acid and to allow it to enter the cell and reach its site of action: the cytoplasm. This review deals only with systems that are based on synthetic or natural polymers. Lipid-based systems have also made an enormous contribution to the field and are covered by other articles in this issue, as well as a recent review by Li and Szoka (2007).
The different polymer-based strategies that have been adopted are complexation with cationic molecules, adsorption onto preformed nanoparticles and encapsulation within nanoparticles (Figure 4). In formulating such systems, it is important to consider their biocompatibility, as most of the polymers are exogenous to the organism. In particular, interactions with plasma proteins at the particle surface may lead to rapid uptake by the mononuclear phagocyte system (MPS). For example, complement activation may be a problem (Plank et al., 1996). Cationic particles are particularly susceptible to forming aggregates after binding of negatively charged plasma proteins, which are then either entrapped in the pulmonary capillary bed or taken up by the MPS (Opanasopit et al., 2002). The use of poly(ethylene glycol) (PEG) to provide a hydrophilic shield over the surface of the particle is the most usual means of minimizing these interactions with plasma proteins, as will be seen in the specific examples below. These particles known as stealth® or long circulating particles have the ability to escape the opsonization process that leads to MPS uptake. As a result, they will be able to circulate in the vascular compartment for a long time without being cleared (Owens and Peppas, 2006). Their main advantage is their ability to pass through leaky endothelium if their size allows (typically 200 nm or less). This phenomenon is called ‘EPR’ (enhanced permeability and retention effect). It is due to defective capillary endothelium and poor lymphatic drainage in solid tumours (Hashizume et al., 2000). Small particles are able to leave the circulation through the spaces between endothelial cells in the tumour vasculature and reach the interstitial fluid. To reach the tumour cells, they have to cross the tight network constituted by the extracellular matrix composed of a variety of polysaccharides and proteins overlying the surface of the cells that produce them (Li and Szoka, 2007). Some systems incorporate a specific ligand recognizing the target cell. Because the EPR effect is limited to tumour targeting only AS-ODNs used for cancer treatment can take advantage of this specific tissue distribution.
Figure 4.
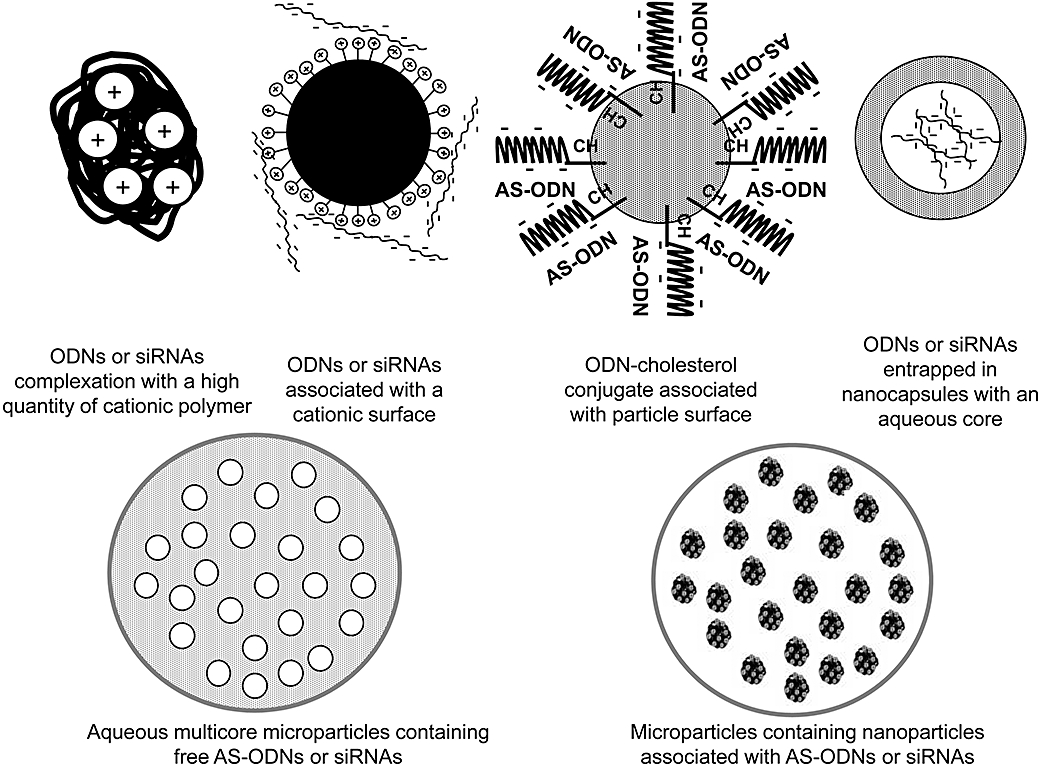
Different types of nano-and microtechnologies for the delivery of antisense oligonucleotides and siRNA. AS-ODN, antisense oligonucleotides; siRNA, small interfering RNA.
Nanoparticles obtained by complexation with cationic molecules
Nucleic acids have a high density of negative charge at physiological pH, which means that they can be readily associated with positively charged polymers forming nanoparticles (Figure 4). One such polymer that has been extensively used is poly(ethyleneimine) (PEI). This synthetic polymer is available with a range of molecular weights and in linear and branched forms. The complexes formed between nucleic acids, and this polymer have a net positive charge, which allows interaction with negatively charged cell membranes, followed by internalization by endocytosis. Delivery of the nucleic acid to the cytoplasm occurs by the ‘proton sponge effect’, in which the protonation of the numerous amino groups in the polymer causes osmotic rupturing of the endosome and release of the nucleic acid (Boussif et al., 1996; Akinc et al., 2005). The ability of nucleic acid to escape from the endosome is an important factor in its efficacy, because it would otherwise be destroyed. However, PEI poses some problems of toxicity (Roques et al., 2007), and biodistribution in vivo is restricted to organs of the MPS (Chemin et al., 1998). As explained above, the rapid adsorption of plasma proteins onto the positively charged polymers can lead to aggregation. Toxicity can be reduced by using lower molecular weight PEI, and by modifying the surface with PEG or by glycosylation. Specific targeting groups such as the Arg–Gly–Asp peptide can also be coupled to PEI. PEI has been shown to deliver AS-ODN and siRNA efficiently to cells in culture including non-dividing cells that are notoriously difficult to transfect (Boussif et al., 1995; Dheur et al., 1999; Gomes dos Santos et al., 2006b). As far as the siRNA strategy is concerned, a very narrow window of conditions and PEI structure (25 kDa branched) are required for successful delivery (Grayson et al., 2006). It has been shown by atomic force microscopy that PEI forms complexes that condense and completely cover chemically unmodified siRNA (Grzelinski et al., 2006). After complexation, siRNA are efficiently protected against nucleolytic degradation both in vitro in the presence of RNase and in vivo in the presence of serum nucleases (Urban-Klein et al., 2005).
Tumour cells internalized these PEI/siRNA complexes rapidly in cell culture and, as full biological activity of the siRNA molecules was preserved, it was assumed that they can be released intact from the complexes. One practical application that has been investigated in vitro and in vivo is the knock-down of the growth factor, pleiotrophin (Grzelinski et al., 2006). Anti-tumour effects have also been observed in vivo with siRNA directed against the HER-2 receptor complexed with PEI (Urban-Klein et al., 2005). Ligands can be added to the particles to improve targeting. For example the Arg–Gly–Asp peptide ligand was attached at the distal end of the PEG, which was itself attached to PEI (Schiffelers et al., 2004). The resulting nanoparticles had affinity for tumour neovasculature expressing integrins and were able to deliver siRNA inhibiting vascular endothelial growth factor (VEGF) receptor-2 expression and thereby block tumour angiogenesis.
However, the addition of PEG has consequences for the stability of siRNA/PEI complexes, as shown by Mao et al. (2006)). A high degree of PEG modification increased the size of the complexes to 300–400 nm, and condensation of siRNA only occurred at high polymer to nucleotide ratios. However, PEGylation increased the stability of the resulting complexes against displacement of the nucleic acid by heparin and degradation by nucleases. Furthermore, polyplexes with PEG showed a better gene-silencing effect than those without PEG in an experimental systems involving NIH/3T3 cells transfected with β-galactosidase, with an effect of the PEG chain length. According to the authors, one hypothesis to explain this observation might be that the looser complexes formed in the presence of PEG facilitated the escape of the siRNA into the cytoplasm (Mao et al., 2006).
Dendrimers (highly branched synthetic polymers) consisting of poly(amidoamine) (PAMAM) also have a high density of positive charge on their surface and are able to condense nucleic acids, thus protecting them from nuclease degradation. Although they have often been used for DNA, their use for AS-ODN and siRNA is less well documented. The size-to-charge ratio and the dendrimer generation have been found to be an important factor in the preparation of stable, homogeneous nanoparticles (Zhou et al., 2006; Shen et al., 2007). With short RNA sequences, only higher generation dendrimers provide sufficient electrostatic interactions to form stable particles, whereas with longer RNA, dendrimers from generation 1 to 7 readily form nanoparticles. Dendrimers have been used to deliver AS-ODN to clones of D5 mouse melanoma and Rat2 embryonal fibroblasts expressing luciferase cDNA (Bielinska et al., 1996). Gene expression was inhibited in a specific and dose-dependent fashion. Similar results were obtained by Yoo et al. (1999)). According to Helin et al. (1999) the intracellular distribution of a fluorescein isothiocyanate-labelled ODN delivered by PAMAM was dependent on the phase of the cell cycle, with a nuclear localization predominantly in the G2/M phase.
Dendrimer technology can also be applied to siRNA. Nanosized particles are produced, which protect the siRNA from nuclease activity and allow sustained release, leading to efficient gene silencing in A549Luc cells stably expressing the GL3 luciferase gene (Zhou et al., 2006). The dendrimer was effective when loaded with the specific nucleotide sequence, whereas free siRNA and the G7-dendrimer carrying a non-specific siRNA had no effect on gene expression. Kang et al. (2005) have attached the Tat peptide to the surface of PAMAM dendrimers, in an attempt to improve cell penetration, producing particles carrying both AS-ODN and siRNA. However, the Tat peptide did not improve the delivery of the nucleic acid to NIH 3T3 MDR cells. Although MDR1 gene expression was partially inhibited by the antisense complex and weakly inhibited by the siRNA complex when both were tested at non-toxic levels of dendrimer, this effect was not increased by conjugation with the cell-penetrating peptide (Kang et al., 2005). More recently, Pan et al. (2007) have grown PAMAM dendrimers on the surface of magnetic iron oxide nanoparticles, to combine the advantages of the nucleic acid binding properties of the polymer and the possibility of magnetic guidance of the particles, as well as their use as contrast agents. The initial nanoparticle diameter was 8 nm, and after growth of 5 generations of dendrimer they remained small and individualized. They were able to adsorb AS-ODN, as shown by zeta potential measurements, had minimal cytotoxicity and were rapidly internalized by cells.
Recently, chemical modifications have been made to dendrimers in order to reduce both interactions with plasma proteins and their toxicity. For example, water-soluble carbosilane dendrimers have been used to complex nucleic acids (Shcharbin et al., 2007 2008). The polymers formed complexes with AS-ODN and reduced their interactions with serum albumin. In another study, dendrimers were coupled to the amino acid lysine by using its α and ε amino groups. Generation 5 dendrimers were able to complex AS-ODN, changing in size from 12 to 73 nm in AFM images. The dendrimers had slightly lower cytotoxicity than PAMAM dendrimers and linear poly(lysine) and showed moderate efficiency for AS-ODN delivery to HeLa-Luc cells (Eom et al., 2007).
Other polymers used to form complexes with nucleic acids are natural polypeptides. Protamine is a cationic, non-toxic, naturally occurring polypeptide, rich in arginine residues that confer positive charge and the ability to bind nucleic acids. Complexes between this small polypeptide as the free base and AS-ODN also form spontaneously on mixing. Junghans et al. (2000) called these assemblies ‘proticles’. They were formed at protamine to AS-ODN ratios of 0.5–5. At the ratio of 5, the particles reached a size of 150–170 nm after 30 min of co-incubation and rose to about 300 nm after 3 days. Particles with higher protamine content were stable in phosphate buffer and cell culture medium, with very low AS-ODN release. However, particle formation required a minimum AS-ODN length of nine bases. In such conditions, phosphodiester AS-ODN were protected against nuclease degradation (Junghans et al., 2001). Similar particles have been prepared by Dinauer et al. (2004). Protamine with a molecular weight of about 6000 was labelled with a fluorescent marker to follow the uptake of the particles by mononuclear cells. Again, protamine to AS-ODN ratios of 0.5–5 yielded particles of 150–180 min diameter, with a zeta potential that varied according to the ratio and become positive at ratios above 1.5. Proticles significantly decreased cellular growth in a cell proliferation assay by using AS-ODN against the c-myc proto-oncogene (Junghans et al., 2000) as well as specific inhibition of tat mediated HIV-1 transactivation. In Vero cells, the internalized AS-ODN were localized in the cytoplasm and in the nucleus of the cells, and particle uptake was shown to be an energy-dependent endocytotic mechanism (Junghans et al., 2001). However, the transfection ability of these complexes is lower than that of PEI or polylysine complexes (Mok et al., 2007) because of weaker links between the guanidino residues and the nucleic acid.
Other disadvantages of protamine–nucleic acid systems are their tendency to aggregate at physiological salt concentrations and poor intracellular release of nucleic acid from the complex, particularly when phosphorothioates are used. In an attempt to solve these two problems, Lochmann et al. (2005) added human serum albumin to the preparation. This was mixed with protamine before adding AS-ODN. Nanoparticles of 230–320 nm are formed in this way. These particles showed low cytotoxicity and good penetration into mouse fibroblasts (Weyermann et al., 2005). Recently, an attempt has been made to target these protein/AS-ODN complexes to specific cells by coating them with the apolipoprotein A-1, to promote transcytosis across the blood–brain barrier (Kratzer et al., 2007).
A similar approach was applied to siRNA. The system is which is slightly more complex than those described above, is composed of nucleic acids, protamine and cationic liposomes. The nanoparticles are then modified by PEG-lipid containing a targeting ligand, anisamide for targeting sigma receptor-expressing B16F10 tumours, which were stably transduced with a luciferase gene (Li et al., 2008). Targeting siRNA for silencing luciferase activity was significantly higher than the other formulations, lasted for 4 days and was shown to be due to a specific targeting effect (Li et al., 2008).
Natural and synthetic polysaccharides have also been used to complex nucleic acids. In particular, chitosan, a positively charged polysaccharide from the exoskeleton of invertebrates, has often been used alone or in combination with other polymers (Liu and Yao, 2002). To form complexes, the nucleic acid is added to an acidic solution of low molecular weight chitosan and stirred to allow nanoparticle formation. A modified galactosylated chitosan has also been synthesized and used to form particles with AS-ODN designed to be taken up by liver cells (Dong et al., 2008). A detailed study (Liu et al., 2007) has shown the importance of various physicochemical parameters: particularly polymer molecular weight and degree of deacetylation, on particle formation and gene silencing. Indeed, high values of both parameters were necessary to obtain small, stable particles with siRNA (Liu et al., 2007). When incubated with H1299 human lung carcinoma cells, silencing of endogenous green fluorescent protein (GFP) was obtained (Howard et al., 2006) and shown to vary according to the type of complex. Indeed the higher the molecular weight and degree of deacetylation, the more efficient the silencing effect (Liu et al., 2007). Force spectroscopy studies have shown the importance of low pH for strong siRNA–chitosan interactions (Xu et al., 2007). Moreover, it is important to stress that these particles can be freeze-dried without loss of activity (Andersen et al., 2008).
A versatile system for complexing AS-ODN has been developed by using a polymer based on cyclodextrin molecules linked by short sequences of a cationic polymer, which forms particles when mixed with nucleic acid. PEG or PEG bearing a targeting moiety (transferrin) can be added to the system by modifying them with adamantine at one extremity of the PEG, giving an affinity for the central cavity of the cyclodextrin. ‘Stealth’ and targeted particles can then be prepared by mixing water-soluble components (Bartlett and Davis, 2007). The PEG-based molecules can be added before or after complexing the cyclodextrin-containing polymer with nucleic acid. Functional efficacy of the delivered siRNA was demonstrated through luciferase reporter protein knock-down in Hela cells (Bartlett and Davis, 2007) and in vivo, after systemic administration through the inhibition of EWS-Fli-1 gene in a murine model of metastatic Ewing's sarcoma (Hu-Lieskovan et al., 2005). In this study, the targeted formulation displayed the highest efficacy through recognition of transferrin by the transferrin-receptor expressing cells (Hu-Lieskovan et al., 2005). The safety of this system after repeated administration to non-human primates has also been demonstrated (Heidel et al., 2007), and tumour-specific targeting has been shown by in vivo imaging in mice bearing subcutaneous Neuro2A tumours. In this model, although both non-targeted and transferrin-targeted siRNA nanoparticles exhibit similar biodistribution and tumour localization by positron emission tomography, transferrin-targeted siRNA nanoparticles reduce tumour luciferase activity by about 50% compared with non-targeted siRNA nanoparticles 1 day after injection (Bartlett et al., 2007). Mathematical model calculations of siRNA-mediated target protein knock-down and tumour growth inhibition were used to provide guidelines for designing more effective siRNA-based treatment regimens by adjustment of the dose and dosing schedule (Bartlett and Davis, 2008).
Micelle-forming copolymers have also been used to complex nucleic acids. For example, PEG-polycation di-block polymers with diamine side-chains can be used as endosome-escaping carriers for siRNA (Kataoka et al., 2005). Several types of smart polymeric micelles have been designed for nucleic acid delivery. These include PEG-poly(aspartic acid) copolymers (Kakizawa et al., 2004) that can exert a ‘proton sponge’ effect in endosomes, lactosylated PEG that can form complexes with poly(lysine) and AS-ODN and allow targeting to galactose receptors on liver cells (Oishi et al., 2005 2007) and PEG-poly(methylmethacrylate) block copolymers (Kakizawa et al., 2006) that can be used to produce hybrid organic-inorganic nanoparticles.
Adsorption onto preformed nanoparticles
Matrix nanoparticles, called nanospheres (NS), prepared from poly(alkylcyanoacrylates) (PACA) (Fattal et al., 1998) or poly(lactide-co-glycolide) (PLGA) (Singh et al., 2003; Oster et al., 2005) have been used to adsorb AS-ODN, by the intermediary action of cationic surfactant (Figure 4). More recently, block copolymers of poly(isobutylcyanoacrylate) (PIBCA) and chitosan have been used to adsorb siRNA (de Martimprey et al., 2008). Although in these systems the nucleic acid is not truly encapsulated within the polymer, its close association with the particle surface is able to protect it from degradation by nucleases. The PACA NS are produced by an emulsion polymerization process, in which droplets of water-insoluble monomers are emulsified in an aqueous phase (Couvreur et al., 1979). Anionic polymerization takes place in micelles after diffusion of monomer molecules through the water phase and is initiated by the water itself. The pH of the medium determines both the polymerization rate and the adsorption of the drug when the latter is ionizable (Couvreur et al., 1979). Drugs can be combined with NS after dissolution in the polymerization medium either before the introduction of the monomer or after its polymerization. Because only non-toxic additives are used, no further purification is needed and freshly prepared NS may be freeze-dried with their drug content. Nucleic acids have little affinity for the polymer; therefore their adsorption on the NS surface is obtained by ion pairing with a cationic surfactant, cetyltrimethylammonium bromide (CTAB) (Fattal et al., 1998). The adsorption of the negatively charged AS-ODN onto the positively charged NS can be monitored by measuring the zeta potential (Lambert et al., 1998). Another way of adsorbing nucleic acids onto PACA nanoparticles is to use cationic diethylaminoethyl (DEAE)-dextran as a surfactant during nanoparticle preparation (Schneider et al., 2008).
When AS-ODN were bound to PACA NS by means of cationic surfactant they were protected from nucleases in vitro (Chavany et al., 1992; Lambert et al., 1998) and their intracellular uptake was increased (Chavany et al., 1994). A study of their distribution in vivo showed accumulation of intact AS-ODN in the liver and in the spleen (Nakada et al., 1996). AS-ODN within this sort of formulation were able to specifically inhibit mutated Ha-ras-mediated cell proliferation and tumorigenicity in nude mice after injection into tumours (Schwab et al., 1994). Other hydrophobic compounds such as cationic lipids were also employed as adjuvants for loading AS-ODN to nanoparticles (Figure 4) (Balland et al., 1996). However, using compounds such as polyamines [dioctadecylamidoglycyl-spermine (DOGS)], the absorption was lower than with CTAB in similar conditions of adsorption (Balland et al., 1996). AS-ODN coupled to cholesterol has also been adsorbed onto poly(isohexylcyanoacrylate) nanoparticles (Godard et al., 1995). However, because this conjugate was negatively charged, it was partially repulsed by the negative charges displayed by the polymer surface leading to a low loading efficiency of AS-ODN in the absence of CTAB.
A novel nanoparticulate system proposed recently by (de Martimprey et al., 2008) consists of a biodegradable core of PIBCA surrounded by a shell of chitosan. The preparation method involves a first step where the polysaccharide is hydrolysed as described by Bertholon et al. (2006) and used to initiate redox radical polymerization of the isobutylcyanoacrylate monomer in emulsion in the presence of cerium ions. NS formed spontaneously from the resulting amphiphilic block copolymer. The siRNA complementary sequence to the ret/PTC1 junction oncogene was adsorbed to such NS at a NS:siRNA ratio of 50 leading to 90% siRNA adsorption (de Martimprey et al., 2008). These NS were tested against subcutaneous tumours in mice made up of papillary thyroid carcinoma cells carrying this particular oncogene. The expression of ret/PTC1 was inhibited, and the tumour size was reduced to a greater extent than with free siRNA.
Poly(lactide-co-glycolide) NS containing a range of cationic material: PEI, N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTMA), 3β[N-(N′, N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol), CTAB, have been prepared by a spray-drying technique (Takashima et al., 2007). Chitosan-coated PLGA NS prepared by an emulsion-solvent evaporation technique using poly(vinyl alcohol) as a stabilizer have also been developed as a platform to carry nucleic acids (Nafee et al., 2007). They were shown to increase cell uptake of AS-ODN in A549 lung cells (Nafee et al., 2007).
Nanospheres based on poly(methylmethacrylate) have also been used as a platform to adsorb AS-ODN. In one study, methylmethacrylate and aminoalkylmethacrylate monomers were copolymerized by free radical polymerization to form NS of undisclosed size with positive zeta potential (Zobel et al., 2000). Oligonucleotides were adsorbed by co-incubation for 2 h at 20–25°C, and the whole complex was tested on PC12 cells for the inhibition of ecto-5′-nucleotidase expression. As a result of enhanced intracellular penetration, these NS led to around 75% inhibition of enzyme expression. Using this technical platform, Tondelli et al. (1998) included a reactive cationic monomer in the emulsion polymerization medium to produce core-shell NS with a mean diameter of 480 nm, onto which AS-ODN could be adsorbed by an ionic mechanism. They demonstrated a strong inhibition of proliferation in HL60 cells when delivering an AS-ODN targeted to the codons 2–7 of c-myb (Tondelli et al., 1998). In later work (Tondelli et al., 2003), PEG was included in the shell to generate ‘Stealth’ NS.
Encapsulation within nanoparticles
True encapsulation of nucleic acids in polymeric systems encounters the problem that nucleic acids are hydrophilic while the polymers used to form nanoparticles are hydrophobic (Figure 4). As one solution, Lambert et al. (2000b) developed a method of producing nanocapsules (NC) with an aqueous core and a PIBCA shell, which could encapsulate hydrophilic molecules such as nucleic acids. In this procedure, an aqueous solution of the drug to be encapsulated is emulsified in an oily phase containing surfactant, after which the isobutylcyanoacrylate monomer is added and polymerizes at the surface of the aqueous droplets (Lambert et al., 1998; Lambert et al., 2000b 2001). The NC are recovered from the oily phase by centrifugation over an aqueous phase containing a suitable surfactant. This method has proved to be efficient for the encapsulation of both AS-ODN (Lambert et al., 2000b) and siRNA (Toub et al., 2006b). The stability of AS-ODN in serum was improved compared with both the free molecule and molecules adsorbed onto NS. The kinetics of degradation suggested that only released AS-ODN were available to nucleases. The release from NC was biphasic, with a high initial burst followed by a slower, linear release, which was probably a reflection of heterogeneity in the NC population (Lambert et al., 2000b).
Poly(isobutylcyanoacrylate) NC containing a radiolabelled 20-mer model oligonucleotide were able to deliver their cargo to cultured vascular smooth muscle cells, a difficult cell type to transfect (Toub et al., 2005). Using an original technique based on selective solubilization of membranes with different detergents, these authors were able to determine the subcellular distribution of the AS-ODN. While the most of the small amount of free AS-ODN associated with the cells was found to be in the plasma membrane fraction, most of that delivered by NC was found to be internalized. The intracellular fraction of free AS-ODN was mainly in the membrane fraction, whereas with the NC formulation about 50% was found in the nuclear fraction, and less than 40% was in the vesicular fraction. The use of a fluorescently labelled AS-ODN revealed a similar distribution by confocal microscopy. The amount of AS-ODN in the nucleus increased with time between 2 and 15 h (Toub et al., 2006a).
Similar results were obtained for siRNA encapsulated in PIBCA NC with an aqueous core (Toub et al., 2006b). Confocal microscopy studies showed that when a fluorescently labelled naked siRNA was added to the culture medium, only a small amount of fluorescence localized in the extracellular matrix was found. In contrast, when siRNA was encapsulated within NC, punctuate fluorescence was observed within the cells, suggesting an endosomal localization of the siRNA (Toub et al., 2006b).
In vivo, AS-ODN-containing NC were tested on a murine model of Ewing sarcoma-related tumours targeting EWS-Fli-1, a fusion gene resulting from a translocation that is found in 90% of both Ewing sarcoma and primitive neuroectodermal tumour (Lambert et al., 2000a). All treatments were given by the intratumoural route. At a cumulative dose of 14.4 nmol, only AS-ODN encapsulated within NC led to a significant inhibition of tumour growth, while a slight effect was observed with naked AS-ODN at the same dose. These results can be compared with those of Tanaka et al. (1997) in the same tumour model, in which a significant reduction of tumour growth was observed only after injection of 500 nmol naked AS-ODN. This suggests that encapsulation in NC renders the AS-ODN 35 times more effective. A number of different nanoparticles formulations have been tested in the EWS-Fli-1 model. The NC containing encapsulated AS-ODN were compared with poly(isohexylcyanoacrylate) NS onto which the same AS-ODN had been adsorbed with the aid of CTAB, as described above (Maksimenko et al., 2003). Furthermore, both a completely phosphorothioate and a chimeric phosphorothioate/phosphodiester AS-ODN were tested. Both nanoparticulate systems led to a down-regulation of EWS-Fli-1 mRNA and significant reduction of the tumour volume from about 66–82%. CTAB may also play a role in endosomal escape and efficient delivery of AS-ODN to the cytoplasm. siRNA within PIBCA NC is also very effective in this in vivo experimental model, in contrast to free siRNA (Toub et al., 2006b). In this case, substantial inhibition of tumour growth, up to 80%, was observed, especially with a high cumulative dose.
Nucleic acids can also be encapsulated within NS prepared from preformed polymers. The polymers most frequently employed for this purpose are poly(lactide) and PLGA, and the particulate systems are prepared by water-in-oil-in-water multiple emulsion/solvent evaporation or extraction technique, which allows the nucleic acid to be introduced into the preparation in aqueous solution, minimizing contact with organic solvent (Berton et al., 2001; Delie et al., 2001). Anti-VEGF AS-ODN were successfully encapsulated within PLGA NS prepared by this method and tested for VEGF inhibition in a human retinal pigment epithelial cell line (ARPE-19) (Aukunuru et al., 2003). The AS-ODN inhibited VEGF mRNA and protein secretion when delivered by using NS but not in its free form. This was consistent with the ability of NS to increase AS-ODN cell uptake fourfold (Aukunuru et al., 2003). In a rat carotid model in vivo, PLGA NS containing an AS-ODN against the platelet-derived growth factor receptor showed an anti-restenosis effect 14 days after the treatment (Cohen-Sacks et al., 2002). In this case, however, the same effect was observed with naked AS-ODN administered at the same dose. It is noteworthy that in the case of NS, only a small proportion of the loaded AS-ODN would be released over 14 days; consequently, the naked AS-ODN was effective compared with the encapsulated AS-ODN, but at higher available doses (Cohen-Sacks et al., 2002). For siRNA, only one recent report has used PLGA NS containing a GFP gene silencing siRNA with GFP-expressing 293T cells (Yuan et al., 2006). A significant silencing effect was observed after 1 day incubation and was still persistent 1 week later (Yuan et al., 2006).
Chitosan nanoparticles for RNA delivery can also be formed by ionic gelation with tripolyphosphate. Katas and Alpar (2006) compared encapsulation within and adsorption of siRNA onto chitosan particles formed in this way with simple complexation. Nanoparticles entrapping siRNA in the matrix were found to be the best carriers for silencing the transfected luciferase gene when incubated with the CHO K1 and HEK 293 cell lines, possibly because of their high binding capacity and loading efficiency. The interaction between the negatively charged polysaccharide alginate and poly(lysine) has also been exploited to prepare nanoparticles. These systems can be described as ‘nanosponges’, as AS-ODN added to the preparation after formation of nanoparticles diffuse progressively into them over a period of a few days. Once inside the nanoparticles, the AS-ODN are protected from nucleases (Aynie et al., 1999). However, these particles distribute essentially into the lungs, probably because of an interaction with serum proteins leading to an aggregation and subsequent filtration by lung capillaries.
The fate of most of these systems is endosomal entrapment, and but the effects observed with AS-ODNs and siRNAs make them likely to escape from the endosomes into the cytoplasm. The mechanism by which this occurs is not yet fully understood. According to Torchilin et al. (1993), one possible explanation is that detergents such as sorbitan monooleate, used in colloid formulations as a particle stabilizer, may induce endosomal membrane disruption.
Microspheres for controlled delivery of AS-ODN and siRNA
Controlled release systems are mainly employed in regions of the body where the administration of AS-ODN and siRNA cannot be repeated frequently. Their purpose is to assure long-term delivery in the proximity of the target tissue and cells. Most of the studies have been carried out by using PLGA microspheres, which are degradable and allow drug release for weeks or even months. The nucleic acid is usually introduced in the microspheres during the preparation process by a water-in-oil-in-water multiple emulsion/solvent evaporation or extraction technique (Figure 4). Preliminary studies have demonstrated that length and chemistry (phosphodiester or phosphorothioate), AS-ODN loading as well as the size of the microspheres strongly affect encapsulation efficiency and release properties (Lewis et al., 1998). Higher drug loading is generally associated with a larger size (Akhtar and Lewis, 1997) but is independent of ODN length (Lewis et al., 1998). The in vitro release profile of AS-ODN from particles was found to be affected by microsphere size, drug loading, AS-ODN length and polymer molecular weight. Small microspheres (1–2 µm) released most of the entrapped AS-ODN within the first 4 days, compared with 40 days for larger particles (10–20 µm) (Akhtar and Lewis, 1997). In the case of larger particles, a triphasic release was observed: a burst release in the first 48 h, attributed to the rapid release of the AS-ODN present on or close to the microsphere surface; a sustained release phase (about 25 days) in which an AS-ODN diffusion through pores present in the polymer matrix should occur; a final more rapid release phase (up to a complete release at about 60 days) due to the polymer degradation that would accelerate the ODN release rate (Lewis et al., 1998). In the case of smaller particles, the increased surface area to volume ratio results in a greater concentration of AS-ODN at or near to the surface with the consequent more rapid release (Lewis et al., 1998). An increase in AS-ODN loading also results in a higher burst effect (Zhu et al., 2002; Kilic et al., 2005) and a more rapid release rate (Lewis et al., 1998). The release profile is only slightly dependent on nucleic acid length, with a release rate that increases as the AS-ODN molecular weight decreases (Khan et al., 2004). Finally, a high burst effect and a rapid in vitro release rate have been attributed to microsphere porosity. Pores on the microsphere surface occur when an osmotically active agent, such as AS-ODN, is added to the internal aqueous phase. The use of an osmotic agent, such as NaCl, into the external aqueous phase allows to reduce microsphere surface porosity, thus reducing the initial ODN burst effect as well as the in vitro release rate (Freytag et al., 2000; De Rosa et al., 2003b; Gomes dos Santos et al., 2006a).
Besides single-stranded AS-ODN, double-stranded ODN have also been successfully encapsulated within PLGA microspheres (De Rosa et al., 2005). In particular, microspheres encapsulating a decoy ODN against the transcription factor κB (NF-κB) showed a negligible burst effect and a very gradual release profile, with the decoy ODN completely released after about 40 days (De Rosa et al., 2005). A double-stranded siRNA was also encapsulated within PLGA microspheres (Khan et al., 2004). For the same formulation, the in vitro release of siRNA was found to be much slower and with a very limited burst effect, compared with that obtained with a single-stranded AS-ODN. The difference between the release profiles was ascribed to the different chemical nature of the two nucleic acids, while only a modest effect was attributed to the different molecular weight (Khan et al., 2004). Increase in AS-ODN encapsulation efficiency within PLGA or poly(lactide) microspheres, as well as optimization of their in vitro release properties, has been achieved by using cationic additives able to complex the nucleic acid within the matrix. The in vitro release rate of AS-ODN from PLGA microspheres was slowed by complexing with zinc acetate (Putney et al., 1999). A limited burst effect together with a linear release was observed (70% of the loaded ODN released over 9 days).
Most of the studies dealing with controlled release systems have been conducted in animal experimental models. However a few of them have been focused on the in vitro fate of AS-ODN when delivered by large particles that cannot to be taken up by cells. Very surprisingly, the release rate can play an important role in cellular uptake. In recent studies, it was demonstrated that, at a fixed concentration, AS-ODN cell uptake is enhanced by slow release, compared with AS-ODN administered in naked form (De Rosa et al., 2002 2003b 2005). Confocal microscopy studies showed a significant higher uptake of a single-stranded AS-ODN when slowly released form microspheres of about 30 µm, compared with naked AS-ODN (De Rosa et al., 2002). Encapsulation in 25 µm PLGA microspheres also increased the cellular uptake of double-stranded decoy ODN against NF-κB (De Rosa et al., 2005). This was accompanied by an increased inhibition of NF-κB activation (80-fold higher than with naked decoy ODN), as suggested by decreased NO production, inducible nitric oxide synthase and TNF, tumour necrosis factor-α (TNF-α) expression as well as reduced NF-κB/DNA binding (De Rosa et al., 2005). All these results could be explained by the fact that AS-ODN entry into cells occurs principally by a pinocytotic process (Lebedeva et al., 2000) that is saturated at high concentrations of naked AS-ODN, exposing a high dose fraction to degradation by exonucleases. When encapsulated within PLGA microspheres, AS-ODN is efficiently protected from the enzymic activity; then, when the AS-ODN is released gradually from microspheres, pinocytosis would not be expected to be saturated and a higher percentage of ODN can enter into cells.
In vivo, PLGA microspheres loaded with AS-ODN against rat tenascin mRNA were tested on smooth muscle cell proliferation and migration (Cleek et al., 1997). The study demonstrated a dose-dependent growth inhibition with the AS-ODN-loaded microspheres. Different AS-ODN distribution in neostriatum of rat brain was observed by comparing a fluorescently labelled AS-ODN administered in naked or in microencapsulated form (Khan et al., 2000). In particular, in the case of naked AS-ODN, a punctuate fluorescence was found in the cell cytosol at 24 h post injection, while a weak signal was observed at 48 h. In contrast, when administered in microencapsulated form, fluorescently labelled AS-ODN were still clearly visible in the neuronal tissue (Khan et al., 2000). Biodistribution of tritium-labelled AS-ODN, administered in naked form or encapsulated into microspheres, was investigated after subcutaneous administration to Balb-c mice (Khan et al., 2004). In the case of naked AS-ODN, the majority of the radioactivity associated to the nucleic acid was eliminated within 24 h. The use of AS-ODN entrapped within microspheres resulted in high levels of radioactivity at the injection site and a lower level throughout the body, 24 h after inoculation. After 7 days, the level of radioactivity remained high at the administration site and was considerably lower in the liver, kidney and spleen. Micro-autoradiography analysis of liver and kidney showed a similar biodistribution for naked and microencapsulated AS-ODN (Khan et al., 2004). The in vivo potential of PLGA microspheres as a delivery system for a decoy ODN to NF-κB has been recently tested in a rat model of chronic inflammation. Subcutaneous injection of PLGA microspheres releasing decoy ODN inhibited λ-carrageenan-induced leukocyte infiltration and formation of granulation tissue for up to 15 days, whereas naked AS-ODN at the same administered dose showed a similar effect only from 1 to 5 days (G. De Rosa, pers. comm.).
Our group has designed a ‘Trojan’ delivery system that combines the advantages of microspheres (sustained release) and of PEI/AS-ODN nanoparticulate complexes: that is an enhanced ODN intracellular penetration (Figure 4) (De Rosa et al., 2002 2003a b; Gomes dos Santos et al., 2006a). The rationale behind these systems is to provide controlled release of nucleic acids in a form that can penetrate cells. Thus, AS-ODN/PEI complexes have been encapsulated within porous PLGA (De Rosa et al., 2002 2003a b; Gomes dos Santos et al., 2006a). The simultaneous release of AS-ODN and PEI resulted in a further improvement of ODN cell uptake with accumulation into the nucleus (De Rosa et al., 2002 2003b). Several different types of AS-ODN were entrapped with the microspheres, and depending on the ODN properties and on the experimental conditions in which ODN and PEI are mixed, soluble (De Rosa et al., 2002) or insoluble complexes can be encapsulated (De Rosa et al., 2003a; Gomes dos Santos et al., 2006a). AS-ODN complexation with PEI improved its encapsulation efficiency (De Rosa et al., 2002 2003a; Gomes dos Santos et al., 2006a), probably by an electrostatic interaction between the cationic ODN/PEI complex and the anionic PLGA (De Rosa et al., 2002). The release rate was shown to be dependent on the porosity of the microspheres; the higher the porosity, the faster the release and the higher the burst effect. Using an analytical solution of Fick's second law of diffusion, it was shown that the early phase of phosphorothioate AS-ODN and phosphorothioate AS-ODN/PEI complex release was primarily controlled by pure diffusion, irrespective of the type of microspheres (Gomes dos Santos et al., 2006a). This system was tested in vivo in an experimental model involving an AS-ODN against the cytokine TGF-β2 intended to promote wound healing after filtration surgery in a model of glaucoma in the rabbit (Gomes dos Santos et al., 2006a). Twenty-four hours after the subconjunctival injection of microspheres containing AS-ODN/PEI complexes, the AS-ODN was observed to be in the conjunctival stroma. Six days after the injection, the AS-ODN was located in conjunctival cells, with accumulation within the nucleus. No conjunctival hyperaemia was observed for 6 weeks after the injection. Treatment of rabbits with PLGA microspheres allowing slow release of anti TGF-β2 AS-ODN prolonged bleb survival for 28 days (50% of the treated eyes) following trabeculectomy. The effect was significantly higher in the case of PLGA microspheres releasing AS-ODN/PEI complexes, with a bleb survival of 42 days in 100% of the treated eyes (Gomes dos Santos et al., 2006a).
Conclusion
This review highlights the large diversity of particulate systems that have been designed for the delivery of AS-ODN and siRNA. There are still many difficulties to be overcome before any of them can proceed into clinical trials. The development of ‘smart’ nanotechnologies able to control the interactions with biological fluids and to be recognized by target cells should be pursued. The research on alternative routes of administration and on local controlled delivery systems should also be extended.
 |
Glossary
Abbreviations
- AS-ODN
antisense oligonucleotides
- CTAB
cetyltrimethylammonium bromide
- DC-Chol
3β[N-(N′, N′-dimethylaminoethane)-carbamoyl] cholesterol
- DOTMA
N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride
- EPR
enhanced permeability and retention effect
- GFP
green fluorescent protein
- MPS
mononuclear phagocyte system
- mRNA
messenger RNA
- NS
nanospheres
- PACA
poly(alkylcyanoacrylates)
- PAMAM
poly(amidoamine)
- PEG
poly(ethylene glycol)
- PEI
poly(ethyleneimine)
- PIBCA
poly(isobutylcyanoacrylate)
- PLGA
poly(lactide-co-glycolide)
- RISC
RNA-induced silencing complex
- RNase H
ribonuclease H
- siRNA
small interfering RNA
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor
Conflict of interest
None.
References
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S. Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:53–68. doi: 10.1016/s0167-4781(99)00141-4. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Temsamani J, Tang JY. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci USA. 1991;88:7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Temsamani J, Galbraith W, Tang J. Pharmacokinetics of antisense oligonucleotides. Clin Pharmacokinet. 1995;28:7–16. doi: 10.2165/00003088-199528010-00002. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Lewis KJ. Antisense oligonucleotide delivery to cultured macrophages is improved by incorporation into sustained-release biodegradable polymer microspheres. Int J Pharm. 1997;151:57–67. [Google Scholar]
- Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, et al. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem. 2005;48:901–904. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Andersen MO, Howard KA, Paludan SR, Besenbacher F, Kjems J. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials. 2008;29:506–512. doi: 10.1016/j.biomaterials.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Aukunuru JV, Ayalasomayajula SP, Kompella UB. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. J Pharm Pharmacol. 2003;55:1199–1206. doi: 10.1211/0022357021701. [DOI] [PubMed] [Google Scholar]
- Aynie I, Vauthier C, Chacun H, Fattal E, Couvreur P. Spongelike alginate nanoparticles as a new potential system for the delivery of antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 1999;9:301–312. doi: 10.1089/oli.1.1999.9.301. [DOI] [PubMed] [Google Scholar]
- Baker BF, Lot SS, Condon TP, Cheng-Flournoy S, Lesnik EA, Sasmor HM, et al. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- Balland O, Saison-Behmoaras T, Garestier T. Nanoparticles as carriers for antisense oligonucleotides. In: Gregoriadis G, McCormack B, editors. Targeting of Drugs 5: Strategies for Oligonucleotide and Gene Delivery in Therapy. New York: Plenum Press; 1996. pp. 131–142. [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug Chem. 2007;18:456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DW, Davis ME. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol Bioeng. 2008;99:975–985. doi: 10.1002/bit.21668. [DOI] [PubMed] [Google Scholar]
- Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholon I, Hommel H, Labarre D, Vauthier C. Properties of polysaccharides grafted on nanoparticles investigated by EPR. Langmuir. 2006;22:5485–5490. doi: 10.1021/la060570y. [DOI] [PubMed] [Google Scholar]
- Berton M, Turelli P, Trono D, Stein CA, Allemann E, Gurny R. Inhibition of HIV-1 in cell culture by oligonucleotide-loaded nanoparticles. Pharm Res. 2001;18:1096–1101. doi: 10.1023/a:1010962507273. [DOI] [PubMed] [Google Scholar]
- Bielinska A, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR., Jr Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res. 1996;24:2176–2182. doi: 10.1093/nar/24.11.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet ME, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm Res. 2008;25:2972–2982. doi: 10.1007/s11095-008-9693-1. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Zanta MA, Behr JP. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther. 1996;3:1074–1080. [PubMed] [Google Scholar]
- Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, et al. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42:7967–7975. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- Braasch DA, Paroo Z, Constantinescu A, Ren G, Oz OK, Mason RP, et al. Biodistribution of phosphodiester and phosphorothioate siRNA. Bioorg Med Chem Lett. 2004;14:1139–1143. doi: 10.1016/j.bmcl.2003.12.074. [DOI] [PubMed] [Google Scholar]
- Butler M, Stecker K, Bennett CF. Cellular distribution of phosphorothioate oligodeoxynucleotides in normal rodent tissues. Lab Invest. 1997;77:379–388. [PubMed] [Google Scholar]
- Butler M, Crooke RM, Graham MJ, Lemonidis KM, Lougheed M, Murray SF, et al. Phosphorothioate oligodeoxynucleotides distribute similarly in class A scavenger receptor knockout and wild-type mice. J Pharmacol Exp Ther. 2000;292:489–496. [PubMed] [Google Scholar]
- Chavany C, Le Doan T, Couvreur P, Puisieux F, Helene C. Polyalkylcyanoacrylate nanoparticles as polymeric carriers for antisense oligonucleotides. Pharm Res. 1992;9:441–449. doi: 10.1023/a:1015871809313. [DOI] [PubMed] [Google Scholar]
- Chavany C, Saison-Behmoaras T, Le Doan T, Puisieux F, Couvreur P, Helene C. Adsorption of oligonucleotides onto polyisohexylcyanoacrylate nanoparticles protects them against nucleases and increases their cellular uptake. Pharm Res. 1994;11:1370–1378. doi: 10.1023/a:1018923301967. [DOI] [PubMed] [Google Scholar]
- Chemin I, Moradpour D, Wieland S, Offensperger WB, Walter E, Behr JP, et al. Liver-directed gene transfer: a linear polyethlenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo. J Viral Hepat. 1998;5:369–375. doi: 10.1046/j.1365-2893.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleek RL, Rege AA, Denner LA, Eskin SG, Mikos AG. Inhibition of smooth muscle cell growth in vitro by an antisense oligodeoxynucleotide released from poly(DL-lactic-co-glycolic acid) microparticles. J Biomed Mater Res. 1997;35:525–530. doi: 10.1002/(sici)1097-4636(19970615)35:4<525::aid-jbm12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cohen-Sacks H, Najajreh Y, Tchaikovski V, Gao G, Elazer V, Dahan R, et al. Novel PDGFbetaR antisense encapsulated in polymeric nanospheres for the treatment of restenosis. Gene Ther. 2002;9:1607–1616. doi: 10.1038/sj.gt.3301830. [DOI] [PubMed] [Google Scholar]
- Corey DR. RNA learns from antisense. Nat Chem Biol. 2007;3:8–11. doi: 10.1038/nchembio0107-8. [DOI] [PubMed] [Google Scholar]
- Cossum PA, Sasmor H, Dellinger D, Truong L, Cummins L, Owens SR, et al. Disposition of the 14C-labeled phosphorothioate oligonucleotide ISIS 2105 after intravenous administration to rats. J Pharmacol Exp Ther. 1993;267:1181–1190. [PubMed] [Google Scholar]
- Couvreur P, Kante B, Roland M, Guiot P, Bauduin P, Speiser P. Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: preparation, morphological and sorptive properties. J Pharm Pharmacol. 1979;31:331–332. doi: 10.1111/j.2042-7158.1979.tb13510.x. [DOI] [PubMed] [Google Scholar]
- Dapic V, Bates PJ, Trent JO, Rodger A, Thomas SD, Miller DM. Antiproliferative activity of G-quartet-forming oligonucleotides with backbone and sugar modifications. Biochemistry. 2002;41:3676–3685. doi: 10.1021/bi0119520. [DOI] [PubMed] [Google Scholar]
- De Rosa G, Quaglia F, La Rotonda MI, Appel M, Alphandary H, Fattal E. Poly(lactide-co-glycolide) microspheres for the controlled release of oligonucleotide/polyethylenimine complexes. J Pharm Sci. 2002;91:790–799. doi: 10.1002/jps.10063. [DOI] [PubMed] [Google Scholar]
- De Rosa G, Bochot A, Quaglia F, Besnard M, Fattal E. A new delivery system for antisense therapy: PLGA microspheres encapsulating oligonucleotide/polyethyleneimine solid complexes. Int J Pharm. 2003a;254:89–93. doi: 10.1016/s0378-5173(02)00689-0. [DOI] [PubMed] [Google Scholar]
- De Rosa G, Quaglia F, Bochot A, Ungaro F, Fattal E. Long-term release and improved intracellular penetration of oligonucleotide-polyethylenimine complexes entrapped in biodegradable microspheres. Biomacromolecules. 2003b;4:529–536. doi: 10.1021/bm025684c. [DOI] [PubMed] [Google Scholar]
- De Rosa G, Maiuri MC, Ungaro F, De Stefano D, Quaglia F, La Rotonda MI, et al. Enhanced intracellular uptake and inhibition of NF-kappaB activation by decoy oligonucleotide released from PLGA microspheres. J Gene Med. 2005;7:771–781. doi: 10.1002/jgm.724. [DOI] [PubMed] [Google Scholar]
- Delie F, Berton M, Allemann E, Gurny R. Comparison of two methods of encapsulation of an oligonucleotide into poly(D,L-lactic acid) particles. Int J Pharm. 2001;214:25–30. doi: 10.1016/s0378-5173(00)00627-x. [DOI] [PubMed] [Google Scholar]
- Dheur S, Dias N, van Aerschot A, Herdewijn P, Bettinger T, Remy JS, et al. Polyethylenimine but not cationic lipid improves antisense activity of 3′-capped phosphodiester oligonucleotides. Antisense Nucleic Acid Drug Dev. 1999;9:515–525. doi: 10.1089/oli.1.1999.9.515. [DOI] [PubMed] [Google Scholar]
- Dias N, Dheur S, Nielsen PE, Gryaznov S, Van Aerschot A, Herdewijn P, et al. Antisense PNA tridecamers targeted to the coding region of Ha-ras mRNA arrest polypeptide chain elongation. J Mol Biol. 1999;294:403–416. doi: 10.1006/jmbi.1999.3277. [DOI] [PubMed] [Google Scholar]
- Dinauer N, Lochmann D, Demirhan I, Bouazzaoui A, Zimmer A, Chandra A, et al. Intracellular tracking of protamine/antisense oligonucleotide nanoparticles and their inhibitory effect on HIV-1 transactivation. J Control Release. 2004;96:497–507. doi: 10.1016/j.jconrel.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Dong L, Gao S, Diao H, Chen J, Zhang J. Galactosylated low molecular weight chitosan as a carrier delivering oligonucleotides to Kupffer cells instead of hepatocytes in vivo. J Biomed Mater Res A. 2008;84:777–784. doi: 10.1002/jbm.a.31328. [DOI] [PubMed] [Google Scholar]
- Dykxhoorn DM, Lieberman J. Knocking down disease with siRNAs. Cell. 2006;126:231–235. doi: 10.1016/j.cell.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmen J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005;33:439–447. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom KD, Park SM, Tran HD, Kim MS, Yu RN, Yoo H. Dendritic alpha,epsilon-poly(L-lysine)s as delivery agents for antisense oligonucleotides. Pharm Res. 2007;24:1581–1589. doi: 10.1007/s11095-006-9231-y. [DOI] [PubMed] [Google Scholar]
- Esau CC, Monia BP. Therapeutic potential for microRNAs. Adv Drug Deliv Rev. 2007;59:101–114. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Fattal E, Vauthier C, Aynie I, Nakada Y, Lambert G, Malvy C, et al. Biodegradable polyalkylcyanoacrylate nanoparticles for the delivery of oligonucleotides. J Control Release. 1998;53:137–143. doi: 10.1016/s0168-3659(97)00246-0. [DOI] [PubMed] [Google Scholar]
- Freytag T, Dashevsky A, Tillman L, Hardee GE, Bodmeier R. Improvement of the encapsulation efficiency of oligonucleotide-containing biodegradable microspheres. J Control Release. 2000;69:197–207. doi: 10.1016/s0168-3659(00)00299-6. [DOI] [PubMed] [Google Scholar]
- Geary RS, Watanabe TA, Truong L, Freier S, Lesnik EA, Sioufi NB, et al. Pharmacokinetic properties of 2′-O-(2-methoxyethyl)-modified oligonucleotide analogs in rats. J Pharmacol Exp Ther. 2001;296:890–897. [PubMed] [Google Scholar]
- Godard G, Boutorine AS, Saison-Behmoaras E, Helene C. Antisense effects of cholesterol-oligodeoxynucleotide conjugates associated with poly(alkylcyanoacrylate) nanoparticles. Eur J Biochem. 1995;232:404–410. [PubMed] [Google Scholar]
- Gomes dos Santos AL, Bochot A, Doyle A, Tsapis N, Siepmann J, Siepmann F, et al. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release. 2006a;112:369–381. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Gomes dos Santos AL, Bochot A, Tsapis N, Artzner F, Bejjani RA, Thillaye-Goldenberg B, et al. Oligonucleotide-polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm Res. 2006b;23:770–781. doi: 10.1007/s11095-006-9748-0. [DOI] [PubMed] [Google Scholar]
- Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23:1868–1876. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- Gryaznov S, Skorski T, Cucco C, Nieborowska-Skorska M, Chiu CY, Lloyd D, et al. Oligonucleotide N3′–>P5′ phosphoramidates as antisense agents. Nucleic Acids Res. 1996;24:1508–1514. doi: 10.1093/nar/24.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryaznov SM, Lloyd DH, Chen JK, Schultz RG, DeDionisio LA, Ratmeyer L, et al. Oligonucleotide N3′–>P5′ phosphoramidates. Proc Natl Acad Sci USA. 1995;92:5798–5802. doi: 10.1073/pnas.92.13.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Hobel S, et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–766. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- Hall AH, Wan J, Shaughnessy EE, Ramsay Shaw B, Alexander KA. RNA interference using boranophosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 2004;32:5991–6000. doi: 10.1093/nar/gkh936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AH, Wan J, Spesock A, Sergueeva Z, Shaw BR, Alexander KA. High potency silencing by single-stranded boranophosphate siRNA. Nucleic Acids Res. 2006;34:2773–2781. doi: 10.1093/nar/gkl339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvey JC, Peffer NJ, Bisi JE, Thomson SA, Cadilla R, Josey JA, et al. Antisense and antigene properties of peptide nucleic acids. Science. 1992;258:1481–1485. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, et al. Sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing. Antisense Nucleic Acid Drug Dev. 2003;13:83–105. doi: 10.1089/108729003321629638. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci USA. 2007;104:5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin V, Gottikh M, Mishal Z, Subra F, Malvy C, Lavignon M. Cell cycle-dependent distribution and specific inhibitory effect of vectorized antisense oligonucleotides in cell culture. Biochem Pharmacol. 1999;58:95–107. doi: 10.1016/s0006-2952(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A, et al. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J Exp Med. 2002;196:269–274. doi: 10.1084/jem.20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen JS, Pfundheller HM, Lykkesfeldt AE. Downregulation of p21(WAF1/CIP1) and estrogen receptor alpha in MCF-7 cells by antisense oligonucleotides containing locked nucleic acid (LNA) Oligonucleotides. 2004;14:147–156. doi: 10.1089/1545457041526281. [DOI] [PubMed] [Google Scholar]
- Junghans M, Kreuter J, Zimmer A. Antisense delivery using protamine-oligonucleotide particles. Nucleic Acids Res. 2000;28:E45. doi: 10.1093/nar/28.10.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans M, Kreuter J, Zimmer A. Phosphodiester and phosphorothioate oligonucleotide condensation and preparation of antisense nanoparticles. Biochim Biophys Acta. 2001;1544:177–188. doi: 10.1016/s0167-4838(00)00219-3. [DOI] [PubMed] [Google Scholar]
- Kakizawa Y, Furukawa S, Kataoka K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery. J Control Release. 2004;97:345–356. doi: 10.1016/j.jconrel.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Kakizawa Y, Furukawa S, Ishii A, Kataoka K. Organic-inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. J Control Release. 2006;111:368–370. doi: 10.1016/j.jconrel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kang H, DeLong R, Fisher MH, Juliano RL. Tat-conjugated PAMAM dendrimers as delivery agents for antisense and siRNA oligonucleotides. Pharm Res. 2005;22:2099–2106. doi: 10.1007/s11095-005-8330-5. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Itaka K, Nishiyama N, Yamasaki Y, Oishi M, Nagasaki Y. Smart polymeric micelles as nanocarriers for oligonucleotides and siRNA delivery. Nucleic Acids Symp Ser (Oxf) 2005;49:17–18. doi: 10.1093/nass/49.1.17. [DOI] [PubMed] [Google Scholar]
- Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115:216–225. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Khan A, Sommer W, Fuxe K, Akhtar S. Site-specific administration of antisense oligonucleotides using biodegradable polymer microspheres provides sustained delivery and improved subcellular biodistribution in the neostriatum of the rat brain. J Drug Target. 2000;8:319–334. doi: 10.3109/10611860008997909. [DOI] [PubMed] [Google Scholar]
- Khan A, Benboubetra M, Sayyed PZ, Ng KW, Fox S, Beck G, et al. Sustained polymeric delivery of gene silencing antisense ODNs, siRNA, DNAzymes and ribozymes: in vitro and in vivo studies. J Drug Target. 2004;12:393–404. doi: 10.1080/10611860400003858. [DOI] [PubMed] [Google Scholar]
- Kilic AC, Capan Y, Vural I, Gursoy RN, Dalkara T, Cuine A, et al. Preparation and characterization of PLGA nanospheres for the targeted delivery of NR2B-specific antisense oligonucleotides to the NMDA receptors in the brain. J Microencapsul. 2005;22:633–641. doi: 10.1080/02652040500162766. [DOI] [PubMed] [Google Scholar]
- Kratzer I, Wernig K, Panzenboeck U, Bernhart E, Reicher H, Wronski R, et al. Apolipoprotein A-I coating of protamine-oligonucleotide nanoparticles increases particle uptake and transcytosis in an in vitro model of the blood-brain barrier. J Control Release. 2007;117:301–311. doi: 10.1016/j.jconrel.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynack BA, Baker BF. Small interfering RNAs containing full 2′-O-methylribonucleotide-modified sense strands display Argonaute2/eIF2C2-dependent activity. RNA. 2006;12:163–176. doi: 10.1261/rna.2150806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628–1644. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- Lambert G, Fattal E, Brehier A, Feger J, Couvreur P. Effect of polyisobutylcyanoacrylate nanoparticles and lipofectin loaded with oligonucleotides on cell viability and PKC alpha neosynthesis in HepG2 cells. Biochimie. 1998;80:969–976. doi: 10.1016/s0300-9084(99)80002-9. [DOI] [PubMed] [Google Scholar]
- Lambert G, Bertrand JR, Fattal E, Subra F, Pinto-Alphandary H, Malvy C, et al. EWS fli-1 antisense nanocapsules inhibits Ewing sarcoma-related tumor in mice. Biochem Biophys Res Commun. 2000a;279:401–406. doi: 10.1006/bbrc.2000.3963. [DOI] [PubMed] [Google Scholar]
- Lambert G, Fattal E, Pinto-Alphandary H, Gulik A, Couvreur P. Polyisobutylcyanoacrylate nanocapsules containing an aqueous core as a novel colloidal carrier for the delivery of oligonucleotides. Pharm Res. 2000b;17:707–714. doi: 10.1023/a:1007582332491. [DOI] [PubMed] [Google Scholar]
- Lambert G, Fattal E, Pinto-Alphandary H, Gulik A, Couvreur P. Polyisobutylcyanoacrylate nanocapsules containing an aqueous core for the delivery of oligonucleotides. Int J Pharm. 2001;214:13–16. doi: 10.1016/s0378-5173(00)00624-4. [DOI] [PubMed] [Google Scholar]
- Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva I, Benimetskaya L, Stein CA, Vilenchik M. Cellular delivery of antisense oligonucleotides. Eur J Pharm Biopharm. 2000;50:101–119. doi: 10.1016/s0939-6411(00)00088-6. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lendvai G, Velikyan I, Bergstrom M, Estrada S, Laryea D, Valila M, et al. Biodistribution of 68Ga-labelled phosphodiester, phosphorothioate, and 2′-O-methyl phosphodiester oligonucleotides in normal rats. Eur J Pharm Sci. 2005;26:26–38. doi: 10.1016/j.ejps.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Levin AA. A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:69–84. doi: 10.1016/s0167-4781(99)00140-2. [DOI] [PubMed] [Google Scholar]
- Lewis KJ, Irwin WJ, Akhtar S. Development of a sustained-release biodegradable polymer delivery system for site-specific delivery of oligonucleotides: characterization of P(LA-GA) copolymer microspheres in vitro. J Drug Target. 1998;5:291–302. doi: 10.3109/10611869808995882. [DOI] [PubMed] [Google Scholar]
- Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16:163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- Liu W, Yao K. Chitosan and its derivatives-a promising nonviral vector for gene transfection. J Control Release. 2002;83:1–11. doi: 10.1016/s0168-3659(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Howard KA, Dong M, Andersen MO, Rahbek UL, Johnsen MG, et al. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28:1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lochmann D, Weyermann J, Georgens C, Prassl R, Zimmer A. Albumin-protamine-oligonucleotide nanoparticles as a new antisense delivery system. Part 1: physicochemical characterization. Eur J Pharm Biopharm. 2005;59:419–429. doi: 10.1016/j.ejpb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Loke SL, Stein CA, Zhang XH, Mori K, Nakanishi M, Subasinghe C, et al. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci USA. 1989;86:3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimenko A, Malvy C, Lambert G, Bertrand JR, Fattal E, Maccario J, et al. Oligonucleotides targeted against a junction oncogene are made efficient by nanotechnologies. Pharm Res. 2003;20:1565–1567. doi: 10.1023/a:1026122914852. [DOI] [PubMed] [Google Scholar]
- Mao S, Neu M, Germershaus O, Merkel O, Sitterberg J, Bakowsky U, et al. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- de Martimprey H, Bertrand JR, Fusco A, Santoro M, Couvreur P, Vauthier C, et al. siRNA nanoformulation against the ret/PTC1 junction oncogene is efficient in an in vivo model of papillary thyroid carcinoma. Nucleic Acids Res. 2008;36:e2. doi: 10.1093/nar/gkm1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mok H, Park JW, Park TG. Antisense oligodeoxynucleotide-conjugated hyaluronic acid/protamine nanocomplexes for intracellular gene inhibition. Bioconjug Chem. 2007;18:1483–1489. doi: 10.1021/bc070111o. [DOI] [PubMed] [Google Scholar]
- Moser HE, Dervan PB. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987;238:645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine. 2007;3:173–183. doi: 10.1016/j.nano.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Fattal E, Foulquier M, Couvreur P. Pharmacokinetics and biodistribution of oligonucleotide adsorbed onto poly(isobutylcyanoacrylate) nanoparticles after intravenous administration in mice. Pharm Res. 1996;13:38–43. doi: 10.1023/a:1016017014573. [DOI] [PubMed] [Google Scholar]
- Oishi M, Nagasaki Y, Itaka K, Nishiyama N, Kataoka K. Lactosylated poly(ethylene glycol)-siRNA conjugate through acid-labile beta-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells. J Am Chem Soc. 2005;127:1624–1625. doi: 10.1021/ja044941d. [DOI] [PubMed] [Google Scholar]
- Oishi M, Nagasaki Y, Nishiyama N, Itaka K, Takagi M, Shimamoto A, et al. Enhanced growth inhibition of hepatic multicellular tumor spheroids by lactosylated poly(ethylene glycol)-siRNA conjugate formulated in PEGylated polyplexes. Chem Med Chem. 2007;2:1290–1297. doi: 10.1002/cmdc.200700076. [DOI] [PubMed] [Google Scholar]
- Opanasopit P, Nishikawa M, Hashida M. Factors affecting drug and gene delivery: effects of interaction with blood components. Crit Rev Ther Drug Carrier Syst. 2002;19:191–233. doi: 10.1615/critrevtherdrugcarriersyst.v19.i3.10. [DOI] [PubMed] [Google Scholar]
- Oster CG, Kim N, Grode L, Barbu-Tudoran L, Schaper AK, Kaufmann SH, et al. Cationic microparticles consisting of poly(lactide-co-glycolide) and polyethylenimine as carriers systems for parental DNA vaccination. J Control Release. 2005;104:359–377. doi: 10.1016/j.jconrel.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Pan B, Cui D, Sheng Y, Ozkan C, Gao F, He R, et al. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007;67:8156–8163. doi: 10.1158/0008-5472.CAN-06-4762. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- Putney SD, Brown J, Cucco C, Lee R, Skorski T, Leonetti C, et al. Enhanced anti-tumor effects with microencapsulated c-myc antisense oligonucleotide. Antisense Nucleic Acid Drug Dev. 1999;9:451–458. doi: 10.1089/oli.1.1999.9.451. [DOI] [PubMed] [Google Scholar]
- Roques C, Salmon A, Fiszman MY, Fattal E, Fromes Y. Intrapericardial administration of novel DNA formulations based on thermosensitive Poloxamer 407 gel. Int J Pharm. 2007;331:220–223. doi: 10.1016/j.ijpharm.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H, et al. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence-and pH-dependent manner. Eur J Immunol. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- Sands H, Gorey-Feret LJ, Cocuzza AJ, Hobbs FW, Chidester D, Trainor GL. Biodistribution and metabolism of internally 3H-labeled oligonucleotides. I. Comparison of a phosphodiester and a phosphorothioate. Mol Pharmacol. 1994;45:932–943. [PubMed] [Google Scholar]
- Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Becker A, Ringe K, Reinhold A, Firsching R, Sabel BA. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol. 2008;195:21–27. doi: 10.1016/j.jneuroim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Schwab G, Chavany C, Duroux I, Goubin G, Lebeau J, Helene C, et al. Antisense oligonucleotides adsorbed to polyalkylcyanoacrylate nanoparticles specifically inhibit mutated Ha-ras-mediated cell proliferation and tumorigenicity in nude mice. Proc Natl Acad Sci USA. 1994;91:10460–10464. doi: 10.1073/pnas.91.22.10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA, Wohlford-Lenane CL, Quinn TJ, Krieg AM. Bacterial DNA or oligonucleotides containing unmethylated CpG motifs can minimize lipopolysaccharide-induced inflammation in the lower respiratory tract through an IL-12-dependent pathway. J Immunol. 1999;163:224–231. [PubMed] [Google Scholar]
- Shcharbin D, Pedziwiatr E, Chonco L, Bermejo-Martin JF, Ortega P, de la Mata FJ, et al. Analysis of interaction between dendriplexes and bovine serum albumin. Biomacromolecules. 2007;8:2059–2062. doi: 10.1021/bm070333p. [DOI] [PubMed] [Google Scholar]
- Shcharbin D, Ottaviani MF, Cangiotti M, Przybyszewska M, Zaborski M, Bryszewska M. Impact of PAMAM G2 and G6 dendrimers on bovine serum albumin (fatty acids free and loaded with different fatty acids) Colloids Surf B Biointerfaces. 2008;63:27–33. doi: 10.1016/j.colsurfb.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Shen XC, Zhou J, Liu X, Wu J, Qu F, Zhang ZL, et al. Importance of size-to-charge ratio in construction of stable and uniform nanoscale RNA/dendrimer complexes. Org Biomol Chem. 2007;5:3674–3681. doi: 10.1039/b711242d. [DOI] [PubMed] [Google Scholar]
- Singh M, Ugozzoli M, Briones M, Kazzaz J, Soenawan E, O'Hagan DT. The effect of CTAB concentration in cationic PLG microparticles on DNA adsorption and in vivo performance. Pharm Res. 2003;20:247–251. doi: 10.1023/a:1022327305369. [DOI] [PubMed] [Google Scholar]
- Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- Swayze EE, Siwkowski AM, Wancewicz EV, Migawa MT, Wyrzykiewicz TK, Hung G, et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35:687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Saito R, Nakajima A, Oda M, Kimura A, Kanazawa T, et al. Spray-drying preparation of microparticles containing cationic PLGA nanospheres as gene carriers for avoiding aggregation of nanospheres. Int J Pharm. 2007;343:262–269. doi: 10.1016/j.ijpharm.2007.05.042. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Iwakuma T, Harimaya K, Sato H, Iwamoto Y. EWS-Fli1 antisense oligodeoxynucleotide inhibits proliferation of human Ewing's sarcoma and primitive neuroectodermal tumor cells. J Clin Invest. 1997;99:239–247. doi: 10.1172/JCI119152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondelli L, Ricca A, Laus M, Lelli M, Citro G. Highly efficient cellular uptake of c-myb antisense oligonucleotides through specifically designed polymeric nanospheres. Nucleic Acids Res. 1998;26:5425–5431. doi: 10.1093/nar/26.23.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondelli L, Ballestri M, Magnani L, Vivarelli D, Fini A, Cerasi A, et al. Core-shell nanospheres for oligonucleotide delivery. V: adsorption/release behavior of ‘stealth’ nanospheres. J Biomater Sci Polym Ed. 2003;14:1209–1227. doi: 10.1163/156856203322553446. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Zhou F, Huang L. pHsensitive liposomes. J. Liposome Res. 1993;3:201–255. [Google Scholar]
- Toub N, Angiari C, Eboue D, Fattal E, Tenu JP, Le Doan T, et al. Cellular fate of oligonucleotides when delivered by nanocapsules of poly(isobutylcyanoacrylate) J Control Release. 2005;106:209–213. doi: 10.1016/j.jconrel.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Toub N, Bertrand JR, Malvy C, Fattal E, Couvreur P. Antisense oligonucleotide nanocapsules efficiently inhibit EWS-Fli1 expression in a Ewing's sarcoma model. Oligonucleotides. 2006a;16:158–168. doi: 10.1089/oli.2006.16.158. [DOI] [PubMed] [Google Scholar]
- Toub N, Bertrand JR, Tamaddon A, Elhamess H, Hillaireau H, Maksimenko A, et al. Efficacy of siRNA nanocapsules targeted against the EWS-Fli1 oncogene in Ewing sarcoma. Pharm Res. 2006b;23:892–900. doi: 10.1007/s11095-006-9901-9. [DOI] [PubMed] [Google Scholar]
- Urban E, Noe CR. Structural modifications of antisense oligonucleotides. Farmaco. 2003;58:243–258. doi: 10.1016/S0014-827X(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- Vester B, Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 2004;43:13233–13241. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- Vlassov VV, Balakireva LA, Yakubov LA. Transport of oligonucleotides across natural and model membranes. Biochim Biophys Acta. 1994;1197:95–108. doi: 10.1016/0304-4157(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci USA. 2000;97:5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Walder JA. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci USA. 1988;85:5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyermann J, Lochmann D, Georgens C, Zimmer A. Albumin-protamine-oligonucleotide-nanoparticles as a new antisense delivery system. Part 2: cellular uptake and effect. Eur J Pharm Biopharm. 2005;59:431–438. doi: 10.1016/j.ejpb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43:1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Xu S, Dong M, Liu X, Howard KA, Kjems J, Besenbacher F. Direct force measurements between siRNA and chitosan molecules using force spectroscopy. Biophys J. 2007;93:952–959. doi: 10.1529/biophysj.106.093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov LA, Deeva EA, Zarytova VF, Ivanova EM, Ryte AS, Yurchenko LV, et al. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci USA. 1989;86:6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Sazani P, Juliano RL. PAMAM dendrimers as delivery agents for antisense oligonucleotides. Pharm Res. 1999;16:1799–1804. doi: 10.1023/a:1018926605871. [DOI] [PubMed] [Google Scholar]
- Yu RZ, Geary RS, Leeds JM, Watanabe T, Moore M, Fitchett J, et al. Comparison of pharmacokinetics and tissue disposition of an antisense phosphorothioate oligonucleotide targeting human Ha-ras mRNA in mouse and monkey. J Pharm Sci. 2001;90:182–193. doi: 10.1002/1520-6017(200102)90:2<182::aid-jps9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yuan X, Li L, Rathinavelu A, Hao J, Narasimhan M, He M, et al. SiRNA drug delivery by biodegradable polymeric nanoparticles. J Nanosci Nanotechnol. 2006;6:2821–2828. doi: 10.1166/jnn.2006.436. [DOI] [PubMed] [Google Scholar]
- Zhang R, Diasio RB, Lu Z, Liu T, Jiang Z, Galbraith WM, et al. Pharmacokinetics and tissue distribution in rats of an oligodeoxynucleotide phosphorothioate (GEM 91) developed as a therapeutic agent for human immunodeficiency virus type-1. Biochem Pharmacol. 1995;49:929–939. doi: 10.1016/0006-2952(95)00010-w. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wu J, Hafdi N, Behr JP, Erbacher P, Peng L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem Commun (Camb) 2006;22:2362–2364. doi: 10.1039/b601381c. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lu L, Currier BL, Windebank AJ, Yaszemski MJ. Controlled release of NFkappaB decoy oligonucleotides from biodegradable polymer microparticles. Biomaterials. 2002;23:2683–2692. doi: 10.1016/s0142-9612(01)00409-4. [DOI] [PubMed] [Google Scholar]
- Zobel HP, Junghans M, Maienschein V, Werner D, Gilbert M, Zimmermann H, et al. Enhanced antisense efficacy of oligonucleotides adsorbed to monomethylaminoethylmethacrylate methylmethacrylate copolymer nanoparticles. Eur J Pharm Biopharm. 2000;49:203–210. doi: 10.1016/s0939-6411(00)00080-1. [DOI] [PubMed] [Google Scholar]


