Abstract
With the advent of the cancer stem cell hypothesis, the field of cancer research has experienced a revolution in how we think of and approach cancer. The discovery of “brain tumor stem cells” has offered an explanation for several long-standing conundrums on why brain tumors behave the way they do to treatment. Despite the great amount of research that has been done in order to understand the molecular aspects of malignant gliomas, the prognosis of brain tumors remains dismal. The slow progress in extending the survival of patients with malignant CNS neoplasms is very likely due to poor understanding of the cell of origin in these tumors. This review article discusses the progress in our understanding of brain tumor stem cells as the cell of origin in brain cancers. We review the different proposed mechanisms of how brain tumor stem cells may originate, the intracellular pathways disrupted in the pathogenesis of BTSCs, the molecular markers used to identify BTSCs, the molecular mechanisms of cancer initiation and progression, and finally the clinical implications of this research.
Keywords: BTSC, Tumorigenesis, Neuro-oncology, Cancer, Treatment
Introduction
Over the last decade, strong evidence has supported the theory that human tumors are composed of a heterogeneous collection of cells with varying tumor initiation potential. Based on evidence gathered from the study of leukemic cells, it was discovered that only a subset of cells isolated from various solid tumors, such as breast [1], pancreas [2], prostate [3], head and neck [4], and colon [5] cancers, retained the ability to initiate tumor formation upon transplantation into mice. An investigation of human brain tumors revealed a similar trend, where a small collection of cells, called Brain Tumor Stem Cells (BTSC), were distinguished from other cells in the tumor stroma in that they (1) exhibited the properties of traditional neural stem cells, namely the ability to self-renew over an extended period of time in addition to exhibiting mutipotent potential (neurons, astroglia) [6, 7] and (2) these BTSCs possessed the ability to initiate brain tumors upon injection into immunocompromised mice, with a histological and invasive pattern similar to the original tumor [8]. There is mounting evidence to suggest that BTSCs are responsible for the highly invasive [9] and resistance [10] potential of a large majority of human brain tumors. Several groups report that this unique group of cells has the ability to migrate long distances in the brain parenchyma [11], and exhibit profound resistance to both chemotherapy and radiotherapy [12]. Radical resection of the tumor bed has failed to remove these rapidly proliferating cells, with Walter Dandy as far back as the 1930s reported recurrence of contralateral gliomas even after hemispherectomy [13]. Extent of surgery is often limited by the fact that gliomas can infiltrate surrounding tissues well beyond the MR-demarcated contrast-enhancing boundaries via perivascular spaces and myelinated fiber tracts. In an autopsy series by Selazar and Rubin, nearly half of GBMs extended beyond one lobe, 25% involved an entire hemisphere, and 25% crossed to the other hemisphere [14]. Thus, complete resection of the tumor often is impossible without significant risk to quality of life. Considering the marked resistance of BTSCs to radiotherapy and chemotherapy, there is now a new emphasis on specifically targeting BTSCs in order to treat human brain tumors. A number of potential therapeutic modalities have come from the BTSC theory: inducing the differentiation of BTSCs into more committed lines with less proliferative capacity [15], or by inhibiting intracellular pathways which promote BTSC proliferation [16], or disrupting the niche upon which BTSCs rely on to survive[17]. The objective of this article is to review the origins of the BTSC hypothesis, adult NSCs and their relevance to the study of BTSCs, the intracellular pathways disrupted in the pathogenesis of BTSCs, the markers involved in identifying these stem cells, and finally the clinical application of the brain tumor stem cell hypothesis to the field of neuro-oncology.
Discovery of adult neural stem cells and the hierarchy of adult neurogenesis
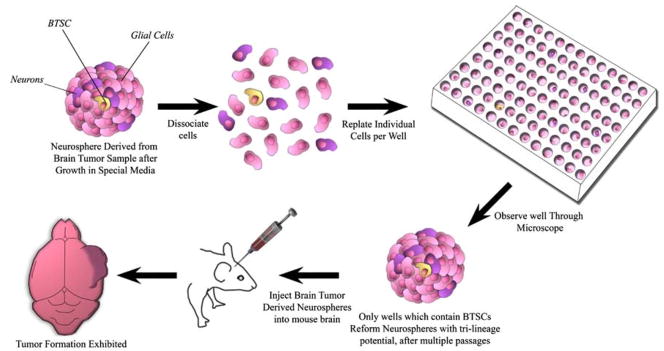
The majority of cells in the adult CNS consist of an assortment of terminally differentiated cells with dedicated phenotypic properties as neurons, astrocytes, oligodendrocytes, and ependymal cells. Until a few years ago, it was believed that the adult brain was a static organ with no potential for regeneration. Almost one hundred years ago, Santiago Ramon y Cajal, whose impact on the field of neuroscience resonates to this day, insisted that “in adult centres, the nerve paths are something fixed, ended, immutable. Everything may die, nothing may be regenerated” [18]. This rule was first challenged when Altman et al. [19] in 1962 noted that there were dividing cells in the adult rat brain, supported by Goldman and Nottebohm [20, 21] in female canaries, and subsequently confirmed in the adult human brain [22–26]. The in vitro isolation of these NSCs was not possible until the advent of the clonal neurosphere assay, where astrocytes that have been purified from tissue are grown in serum-free media supplemented with mitogens, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) [27]. These conditions promote the formation of spherical aggregations termed “neurospheres,” which have the potential to differentiate and stain positive for all three major neural lineages: neurons, oligodendrocytes, and astrocytes. Only a sub-population of cells within the neurosphere are actual stem cells. In order to identify a neural stem cell, the original neurosphere is dissociated and individual cells are sorted into separate wells. Wells that once again reform a neurosphere are thought to have contained a NSC. The clonal neurosphere assay has been instrumental in the identification and isolation of NSCs from the adult brain [27] as well as to prove the existence of BTSCs [8] (Fig. 1).
Fig. 1.
Neurosphere assay was originally developed in 1992 to help isolate and identify normal NSCs from neural tissue. This assay was subsequently utilized 13 years later to identify BTSCs from human tumor samples
The brain represents a complex hierarchy of cells, with NSCs adopting increasingly more dedicated functions and concurrently becoming more limited in differentiation potential. There are three different phases of proliferation during the fetal development of the mammalian brain. During the embryonic phase, NSCs of the central nervous system undergo symmetrical division to form identical copies, accumulating cells with massive proliferative potential. During the neurogenic phase, this potential is exploited where NSCs undergo multiple successions of asymmetrical division to (1) regenerate new NSCs and (2) differentiate into neural precursor cells. These precursor cells are lower on the hierarchy, and terminally differentiate after a limited set of divisions. In the final post-neurogenesis stage, NSCs begin to terminally differentiate in all but three recognized areas of the adult mammalian brain: (1) the subventricular zone (SVZ) of the lateral ventricles, (2) the subgranular layer of the hippocampal dentate gyrus, and (3) the olfactory bulbs.
The largest germinal center of the adult brain, the SVZ, maintains a hierarchy of cells, with NSCs at the top differentiating into more dedicated neural progenitor cells. The SVZ niche of stem cells in the rodent brain has been the most extensively studied and is the best understood to date [28–30]. In the adult brain, primary neural progenitors reside in the subventricular zone, displaced by ependymal cells. This is in contrast to the developing brain in which primary neural progenitors reside in the ventricular zone (VZ) as a pseudostraified epithelium in direct contact with the embryonic ventricles through an apical process, which maintains the germinal activity of the primary neural progenitors [23, 31–33]. The primary progenitors in the embryonic VZ are radial glia [34]. However, shortly after birth, radial glia transform into ependymal cells [35] and astrocytes [36].
In the adult rodent SVZ, NSCs have been identified as the slow-growing type B1 cells [29], which are derived from radial glia [37]. In the hierarchy, SVZ astrocytes (type B cells) function as stem cells and divide to give rise to rapidly dividing precursors (type C cells) that in turn generate neuroblasts (type A cells), which migrate to the olfactory bulb [28, 29, 38, 39]. These type A cells divide to directly give rise to new neurons in the adult brain [29]. The basal processes of type B1 cells wrap around chains of neuroblasts (type A cells), which migrate through these tube-like structures throughout the wall of the lateral ventricle forming a pathway from the SVZ to the olfactory bulb known as the rostral migratory stream (RMS) [28, 38, 39].
The basal processes of type B1 cells are derived from radial glial fibers that terminate directly on blood vessels [21, 30, 40]. Focal aggregations of type C cells are scattered throughout the network of chains and rapidly divide to produce type A cells. The presence of stem cells in the adult brain has been implicated to be involved in the formation of a multitude of new neuronal connections and memory formation in the normal adult brain [41, 42]. More importantly for our discussion, these cells are also thought to contribute to the formation and progression of human central nervous system cancers [21, 43, 44].
The discovery of cancer stem cells
The prevalent theory in cancer biology for years maintained that all cells within the tumor stroma were relatively homogeneous in their potential to regenerate tumors. It was first recognized by Bruce et al. [45], however, that only a few blood cells isolated from leukemia patients had the ability to proliferate and differentiate in vivo. Further advancement in molecular biology allowed for the realization by researchers that the progeny of these cells were clonal in origin, suggesting that a small group of precursor cells were responsible for giving rise to the bulk of non-regenerating leukemic cells [46]. It was proposed that, much like during normal organogenesis, a hierarchy exists in tumors where tumor stem cells proliferate to give rise to tumor stromal cells that have significantly less or no proliferative potential [46, 47]. This concept spread to other cancer types, where it was found that only a subgroup of cells in human breast [1], pancreas [2], prostate [3], head and neck [4], and colon [5] cancer tissue were able to generate a new malignancy upon transplantation.
Ignatova et al. utilized the neurosphere assay, normally employed for the isolation of NSCs, to demonstrate that glioblastoma tissue also had the uncanny ability to form neurospheres [6], with a bi-potential differentiating capacity, having the ability to generate neurons and astrocytes. Galli et al. [8] established through a series of experiments that neurospheres-forming cells isolated from human GBMs had the capacity to differentiate into the three lineages of the brain tissue (neurons, astrocytes and oligodendrocytes) therefore qualifying as bona fide stem cells. In addition, those GBM derived stem cells had the unique ability to recapitulate the typical histologic, cytologic, and architectural features of the original human tumor when injected into an immunocompromised mouse, even after multiple serial passages of these cells in vitro [8]. Moreover, stem cells isolated with the stem cell marker CD133 could differentiate in vitro into tumor cells that phenotypically resembled those of the original tumor [48], and these CD133 labeled cells had a robust tumor initiating capacity upon injection into mice [44]. Unlike neurospheres isolated from normal adult tissue, neurospheres isolated from human tumors contained genetic derangements and undergo aberrant proliferation and differentiation [7, 48]. Additionally, the number of neurospheres isolated in vitro correlated directly with the growth rate and invasive pattern of the tumor formed when injected into immunocompromised mice [49]. These findings suggests that the tumor environment is not a collection of a homogenous population of cells, but rather is composed of only a few “cancer stem cells” that have substantial proliferative potential to reform the original tumor upon transplantation in mice. Thus, similar to the differentiation potential hierarchy of the brain during fetal neurogenesis, only the BTSCs, which comprise a small portion of the tumor, are responsible for forming the bulk of the non-proliferative tumor stroma. The more of these cells found in the tumor, presumably, the greater the potential for the growth and spread of malignant tissue throughout the brain.
Markers in the identification of BTSCs
BTSCs share many of the molecular markers once thought to be exclusively attributed only to NSCs [50]. Two cell surface markers, Nestin and CD133 (prominin-1), have been of particular interest to groups attempting to unravel the mystery of brain tumor organization. It has been observed that these markers are associated with grade of malignancy [3, 51, 52] and are likely prognostic markers for brain tumor patients [51, 53], and may identify brain tumor proliferating stem cells in a given sample. Intracellular markers, such as Musashi-1, have also been established as markers of normal NSCs [54], and are known to be correlated with grade of malignancy [55], suggesting that it may also be a marker of BTSCs. Recent evidence suggests that no individual marker may adequately characterize all BTSCs within a given tumor sample [56], but rather, a collection of markers may be necessary to identify all such cells.
Nestin is an intermediate filament (IF) protein, which is produced in both NSCs/neural progenitor cells during both embryogenesis [50] and in adult tissues [27, 57], and is thought to mediate the morphological and adhesive properties of NSCs. Evidence has suggested that nestin expression is highly correlated with “stemness,” whereby NSCs which take on more committed roles result in a decrease in nestin expression and a concomitant rise in expression of neuronal [58] and glial specific cell markers [59]. Cells expressing this marker have been reported in multiple brain tumor samples isolated from human patients, including ependymomas [60], pilocytic astrocytomas [61], malignant astrocytomas [60], and oligodendrogliomas [60], and gliomas [3, 61]. These nestin positive brain tumor cells share some unique properties with NSCs: these cells have an enhanced invasive and migratory capacity as compared to nestin negative tumor stromal cells [62], and experimental depletion of these cells in tumors correlate with decreased tumor growth [17]. Studies have also suggested that nestin may be used as an appropriate clinical marker for tumor aggressiveness, with some groups showing that tissues with increased nestin staining (suggesting greater concentration of BTSCs) are correlated with poorer outcomes [3].
CD133 is a marker originally identified in human hematopoietic stem cells, but has now been shown to be an effective marker for stem cells from several organs [63, 64]. It was first noted that CD133 marked cells were prominent in post-natal murine tissue [64]. Upon further investigation, cells from normal neural tissue sorted specifically for CD133 had the unique ability to form neurospheres in vitro and gave rise to cells with both neuronal and glial markers, demonstrating the multilineage potential necessary to define a NSC [64]. When it was recognized that NSCs and BTSCs share many phenotypic properties, researchers began to sort for cells with the CD133 marker from tissue samples of brain tumor patients. It was noted that CD133-positive cells from human glioblastomas and medulloblastomas had the ability to also form neurospheres in vitro, and as few as 100 cells were required to successfully form tumors in immunodeficient mice [44]. Conversely, more than one million CD133-negative cells failed to form tumors upon identical injection in mice [44]. Although there has been overwhelming evidence to support this issue, more recent evidence call into question the reliability of CD133 as a marker for BTSCs. Wang et al. demonstrate that CD133-negative cells isolated from human brain tissue also have the ability to form tumors when transplanted in mice, and can also give rise to CD133-positive cells [65], suggesting that multiple different cell surface markers are likely necessary to more consistently characterize BTSCs [65, 66]. Additionally, studies into the natural function of CD133 call into question traditional theories on the origins of BTSCs. For example, Griguer et al. notes that alternations in mitochondrial function among glioma cells can induce CD133 antigen production. These CD133 positive cells exhibit the traditional properities of BTSCs, namely multi-lineage potential and formation of tumor “spheriods” in neuro-sphere media. Conversely, replacement of dysfunctional mitochondrial genes can reverse CD133 expression [67]. This suggests that the expression of CD133 among glioma cells may in fact reflect an environmental stress response, further questioning the reliability of CD133 as a marker for stem cells.
Musashi-1 is an RNA-binding protein [68, 69], which is expressed in the SVZ of numerous vertebrates [54, 68]. In rodents, Musashi-1 expression occurs predominantly in proliferating, multipotent neural precursor cells [54, 69, 70] and is progressively downregulated as neurogenesis proceeds [68]. Expression of this gene is finally lost in terminally differentiated, post-mitotic neurons [68]. Musashi-1 is thought to repress the translation of mRNAs believed to be involved in the differentiation of NSCs, and increasing evidence points towards this molecule’s involvement in tumorigenesis [69]. The expression of Musashi-1 has been consistently correlated with both the grade of the malignancy and proliferative activity in gliomas [55] and also in neurospheres derived from brain tumors [7], thus suggesting that this molecule may mark tumor proliferating brain tumor stem cells. However, more information needs to be gathered before a conclusion can be made about the true function of the Musashi protein and its relation to BTSCs.
Origins of brain cancer
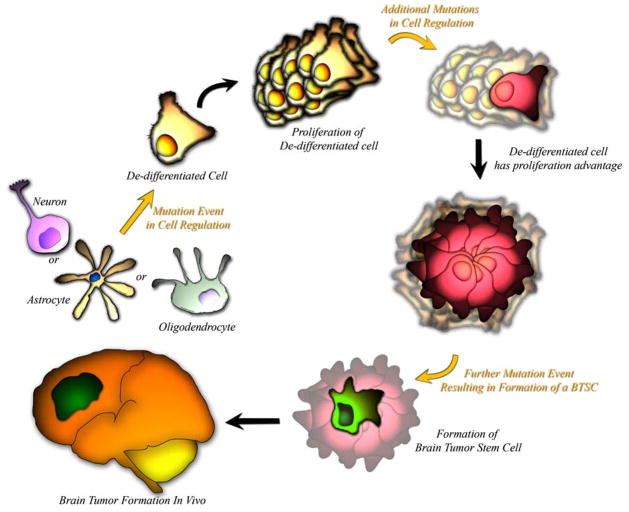
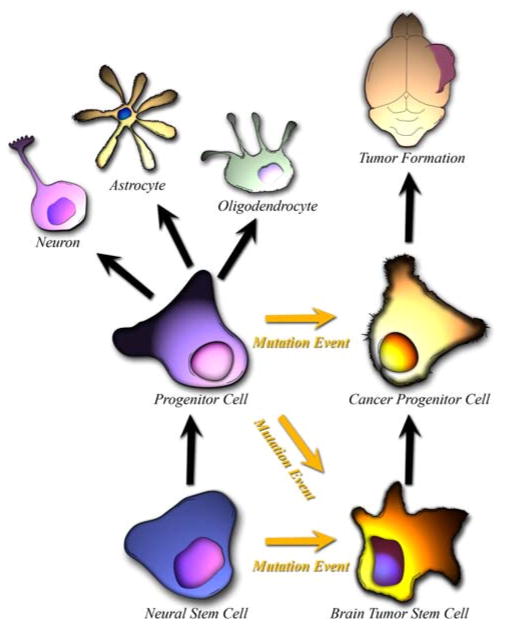
It is believed that the deadly nature of a majority of human brain tumors can be attributed to tumor initiating BTSCs, which are incredibly resistant to chemo- and radiotherapy treatments [10], and have an uncanny ability to invade the local brain parenchyma. The origin of these BTSCs is controversial, with three underlying theories on how these tumor initiating cells come about: (1) mature glia acquire mutations that endow them with unregulated “stem cell” like properties (Fig. 2), (2) restricted neural progenitors, which normally terminally differentiate after successive divisions, acquire mutations which give them unregulated “stem cell” like properties (Fig. 3), and (3) adult NSCs which normally have internal regulations on division and proliferation, acquire mutations which render them tumorigenic (Fig. 3) [6, 43].
Fig. 2.
BTSCs have been theorized to come from terminally differentiated cells, which acquire genetic mutations which endow them with a proliferative advantage. A slow accumulation of critical mutations results in the eventual formation of a BTSC
Fig. 3.
Normal NSCs give rise to progenitors with limited proliferative and self-replicative capacity. BTSCs have been theorized to form as a result of abnormal differentiation of a Normal NSC or Neural Progenitor cell
A significant amount of evidence points towards committed glial cells as the precursors of tumor initiating BTSCs (Fig. 2). Reprogramming of adult skin cells to pluripotent embryonic stem cells by transfection of a small number of transcription factors [71–74] suggest that cells once believed to be permanently quiescent can be experimentally endowed with “stem cell” like properties, necessitating the question of whether a similar process occurs during brain tumor pathogenesis. Several studies have demonstrated that early cortical astrocytes can be targeted in vitro or in vivo with oncogenes or activated signaling proteins to produce tumors in animal models, resulting in masses with glioma-like histology [75–82]. Knocking out the cell cycle regulator genes Ink-4a-Arf from mature glial cells in rodents while concurrently upregulating the EGF Receptor, results in the formation of gliomas [75]. Additionally, the over-expression of the transcription factor gene, c-myc, in astrocytes results in the downregulation of astrocytic cell type marker GFAP and the upregulation of BTSC marker nestin [83]. Finally, introduction of a gene into astrocytes resulting in the overexpression of PDGF, a molecule which maintains glial precursors in an undifferentiated state, the increased the number of rodents that formed gliomas [84]. These observations suggest that even differentiated brain cells can acquire not only stem cell like abilities but also the ability to initiate tumor formation.
Another possible source of BTSCs are restricted neural progenitors (Fig. 3), which are lower on the differentiation potential hierarchy and have a limited self-renewal potential. In order to become tumorigenic, these cells would have to acquire a mutation which would allow for increased self-renewal potential. Studies have shown that committed oligodendroglial progenitors can be induced through the modification of extracellular signals to gain stem-like properties [85], resulting in chromatin remodeling and reactivation of the primitive neural epithelial marker, Sox2 [86]. As another example, in the postnatal brain, the NG2 progenitor cell population are the most actively cycling cells in the adult brain, and they have been reported to be multipotent [87, 88]. The majority of NG2 cells express the oligodendroglial marker Olig2, which is associated with oligodendroglial lineage development [89], suggesting that NG2 cells are similar to or give rise to oligodendroglial precursors [90]. NG2 cells can be influenced in culture to produce neurons and astrocytes via epigenetic mechanisms [88].
There are innumerable similarities between BTSCs and adult NSCs. Both sets of cells share multiple cell surface markers, exhibit a marked ability to migrate through the brain parenchyma, are able to form neurospheres when cultured in vitro, and have multipotent progeny. One prevalent theory on the origin of brain cancers asserts that they are a result of NSCs gone awry [48] (Fig. 3). It has been observed that many gliomas tend to be adjacent to germinal areas [91, 92], usually periventricular or contiguous with the SVZ [43, 93]. Evidence from animal models have shown that gliomas arising from the periventricular region can grow out into the deep white matter tracts and lose their apparent connection to the SVZ [94]. Additionally, experimental exposure of the adult CNS germinal centers with viral or chemical mutagens has been directly linked with the formation of gliomas in rats, with greater tendency to form gliomas if the area around the ventricles is exposed to mutagens as compared to the cerebral cortex [95, 96]. Additionally, younger rats with a more plastic central nervous system and presumably more NSCs have higher rates of glioma formation when exposed to mutagens than older rats [97]. More sophisticated tools in the recent decades have further supported this origin of BTSCs. Oncogenes that are under a nestin promoter, and therefore expressed in only NSCs, are extremely efficient in forming gliomas [77].
Molecular pathways disrupted in BTSCs
Multiple intracellular pathways have been implicated to be disrupted in BTSCs. The most likely candidates are those pathways which serve to regulate the self-renewal and proliferative properties of normal stem cells. Sonic hedgehog (SHH) is a secreted protein important in controlling the fate of ventral cell types in the CNS during embryogenesis. Normally, this molecule is thought to control the number of stem cells in the CNS by activating cell surface receptors which activate the GLI family transcription factors [98]. These transcription factors induce the eventual translation of genes which encode proteins necessary for maintaining the “stemness” of NSCs in the adult CNS. Numerous brain tumor lines are known to over express GLI genes. Constitutive activation of the SHH pathway has been experimentally shown to induce the formation of medulloblastomas in mice [99]. Conversely, selective inhibitors of the SHH pathway (i.e. cyclopamine) have been shown to reduce the proliferation of human gliomas [16, 100]. Evidence suggests that the SHH pathway plays an important role in the formation of CNS tumors, although most likely it is not the only pathway disrupted [100].
Like SHH, notch signaling is thought to be involved in the maintenance of stem cells in the adult CNS. When this secreted molecule activates the notch receptor, intracellular changes activate the HLH family of transcription factors leading to the expression of proteins which maintain “stemness” in the adult CNS [101]. Notch deficient mice exhibit enhanced glial and neuronal differentiation, and blockade of notch signaling among medulloblastoma cells results in the increased differentiation of these cells into neurons with a significantly reduced proliferative potential [101]. It was also observed that the blocking the notch pathway in medulloblastoma cells results in a significant reduction in the frequency of tumor formation [102], with a concurrently reduced number of CD133-positive cells. This suggests that notch signaling may be involved in tumorigenesis by inducing BTSCs to maintain an undifferentiated state.
Mitogens and their respective tyrosine kinase receptors, such as EGFR, PDGFR, FGFR, and Met-receptor, have been studied extensively in gliomas. There is increased expression and/or activating mutations of the EGF and PDGF receptors in adult high-grade gliomas [103], and autocrine loops of PDGF ligand and receptor have been shown to be common among malignant gliomas [104–106]. The receptors for EGF, FGF, PDGF, and LIF activate the Ras/Raf/MAPK signaling pathway, which has a strong influence on cell proliferation [107].
In a recent genomic analysis of 22 human GBM samples, all patients had mutations in genes encoding components of one of three pathways, TP53, RB1, PI3K, suggesting that these pathways are critical in the pathogenesis of human gliomas [108]. A critical downstream component of the PI3K pathway is AKT, which is critical to the survival and growth of gliomas [109]. Activating mutations of PI3K are associated with reduced apoptosis, suggesting that this molecule may serve to enhance survival of malignant cells [110, 111]. The RB signaling axis plays a very important role in governing cell cycle progression in many type of cells [108]. Bmi1 lies genetically upstream of the RB signaling axis and plays an important role in sustaining the replication-competent state of normal neural progenitors. Knockout of Bmi1 in the postnatal stages dramatically reduces forebrain SVZ neural progenitors [112]. In the postnatal brain, Bmi1 plays an important role in repressing gene products of p16INK4a/p19IARF [113], even though the cell cycle target of Bmi1 in the developing brain is actually p21 rather than p16INK4a/p19IARF [114]. Bmi1 plays an important role in the neural stem cell self-renewal and function. p16INK4a, p21, and p53 are important regulator elements in the RB signaling axis which have function in regulating the proliferation of neural progenitor cells. Deletion of p16INK4a can partially oppose the age-related decline in the number and self-renal potential of neural progenitors in the SVZ [115]. Loss of function mutations of the p16INK4a/p19IARF negative regulators of the RB signaling axis are the most common genetic mutations found in high-grade gliomas. Experiments utilizing p21 null phenotype in mice have demonstrated that p21 contributes to the relative quiescence of neural progenitors, which is necessary for the neural stem cell self-renewal. p53 is another very important tumor suppressor associated with the RB signaling axis’ control of the cell cycle that plays an important role in the regulation of growth and proliferation of NSCs. In mice lacking p53, there is an increased rate of cell proliferation in the adult SVZ as well as increase in the number of cells capable of forming neurospheres in vitro, characteristics similar to BTSCs [116].
Clinical implications
The majority of human brain tumors exhibit exquisite resistance to both radio- and chemotherapy, and recur despite complete resection of the tumor. This recurrence may be attributable to BTSCs, which migrate throughout the brain parenchyma, and retain the ability to form tumors in adjacent tissue. The BTSC theory maintains that even with resection of the bulk of the tumor stroma, if a tumor initiating stem cell remains in the brain tissue, the malignancy will recur. BTSCs are resistant to many forms of chemotherapeutic agents, and can reconstitute their DNA even after high dose radiotherapy. BTSCs have been shown to exhibit an enhanced ability to repair DNA damage after radiotherapy, in stark contrast to the remainder of the tumor stromal cells which remain sensitive to radiation [10]. It has been observed that the number of CD133 positive cells increases after ionizing radiation therapy despite a decrease in the total number of cells, suggesting that radiation therapy may select for BTSCs, which are more resistance to the DNA damaging effects of radiation treatment [10].
Multiple strategies can be used to selectively target and destroy the cells responsible for the formation and regeneration of CNS malignancies: (1) Target the intra-cellular pathways BTSCs rely on for proliferation and migration. The SHH pathway has recently been the focus of intense study in glioma therapy, with the SHH pathway inhibitor cyclopamine showing promise as a clinically effective inhibitor gliomas proliferation [16]. (2) BTSCs can be induced to differentiate into a more committed cell line, losing the ability to self-renew and proliferate into tumor stromal cells. BMP-4 is a strong candidate for this modality of treatment, and already has been shown to reduce the number of CD133-positive cells while enhancing the survival of mice in experimental brain tumor models [15]. (3) Destroying the delicate microvascular niche BTSCs rely on to survive is also being considered as a possible therapeutic target [17]. (4) Finally, recent experimental evidence has shown that neuromodulators have the ability to inhibit the growth and proliferation of tumor neurospheres in vitro. Clinical evidence suggests that Parkinsonian patients matched for age have a lower incidence of CNS tumors, suggesting that the neuromodulatory anti-parkinsonian drugs may deplete the BTSC pool. If found to be clinically anti-tumorigenic, these neuroactive agents would represent an important new class of therapy for brain tumors [117].
Conclusions
The BTSC hypothesis represents an important advancement in our understanding of the origins of human CNS malignancies, and opens up a new array of potential therapeutic avenues for patients. The BTSC hypothesis suggests that perhaps there is a cell population with stem-like properties that we are inadequately targeting with our treatments. Understanding the mechanisms by which these cells form in the normal pathogenesis of human brain tumors is largely unknown, but a better understanding of these origins will help us create targeted therapy for patients in the future.
Acknowledgments
This work was supported by grants from the Maryland Stem Cell Foundation and NIH KO8. Moreover, the Howard Hughes Medical Institute has generously supported the work of Thomas Kosztowski, Hasan Zaidi, and Dr. Alfredo Quinones-Hinojosa. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito I, Kleeff J, Bischoff SC, Fischer L, Collecchi P, Iorio M, Bevilacqua G, Buchler MW, Friess H. The stem cell factor-c-kit system and mast cells in human pancreatic cancer. Lab Invest. 2002;82:1481–1492. doi: 10.1097/01.lab.0000036875.21209.f9. [DOI] [PubMed] [Google Scholar]
- 3.Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334–5341. [PubMed] [Google Scholar]
- 4.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 6.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 7.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036 535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 9.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3:508–513. doi: 10.1016/S1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 10.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05 236. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Chin LS. Molecular and cell biology of brain tumor stem cells: lessons from neural progenitor/stem cells. Neurosurg Focus. 2008;24:E25. doi: 10.3171/FOC/2008/24/3-4/E24. [DOI] [PubMed] [Google Scholar]
- 12.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandy W. Removal of right cerebral hemispheres for certain tumors with hemiplegia: preliminary report. JAMA. 1928;90:823–825. [Google Scholar]
- 14.Salazar OM, Rubin P. The spread of glioblastoma multiforme as a determining factor in the radiation treated volume. Int J Radiat Oncol Biol Phys. 1976;1:627–637. doi: 10.1016/0360-3016(76)90144-9. [DOI] [PubMed] [Google Scholar]
- 15.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 16.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Ramon y Cajal S. Degeration and regeneration of the nervous system. Oxford University Press; New York: 1928. [Google Scholar]
- 19.Altman J. Autoradiographic study of degenerative and regenerative proliferation of neuroglia cells with tritiated thymidine. Exp Neurol. 1962;5:302–318. doi: 10.1016/0014-4886(62) 90040-7. [DOI] [PubMed] [Google Scholar]
- 20.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinones-Hinojosa A, Chaichana K. The human sub-ventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–324. doi: 10.1016/j.expneu rol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 23.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- 25.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 26.Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science. 1553558. [DOI] [PubMed] [Google Scholar]
- 28.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 30.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bittman K, Owens DF, Kriegstein AR, LoTurco JJ. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadarajah B, Jones AM, Evans WH, Parnavelas JG. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj. emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Neural stem and progenitor cells in cortical development. Novartis Found Symp. 2007;288:59–73. (discussion 73–58, 96–58) [PubMed] [Google Scholar]
- 35.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEURO SCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902 890106. [DOI] [PubMed] [Google Scholar]
- 37.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 39.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 40.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 42.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 43.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 44.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 45.Bruce WR, Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm07 97-730. [DOI] [PubMed] [Google Scholar]
- 47.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 48.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 49.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 51.Strojnik T, Rosland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050 (discussion 143-134). [DOI] [PubMed] [Google Scholar]
- 52.Thon N, Damianoff K, Hegermann J, Grau S, Krebs B, Schnell O, Tonn JC, Goldbrunner R. Presence of pluripotent CD133(+) cells correlates with malignancy of gliomas. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, Beier CP. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 2008;18:370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 55.Toda M, Iizuka Y, Yu W, Imai T, Ikeda E, Yoshida K, Kawase T, Kawakami Y, Okano H, Uyemura K. Expression of the neural RNA-binding protein Musashi1 in human gliomas. Glia. 2001;34:1–7. doi: 10.1002/glia.1034. [DOI] [PubMed] [Google Scholar]
- 56.Woodward WA, Sulman EP. Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev. 2008;27:459–470. doi: 10.1007/s10555-008-9130-2. [DOI] [PubMed] [Google Scholar]
- 57.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 58.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 59.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bi-potent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-A. [DOI] [PubMed] [Google Scholar]
- 60.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- 61.Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. J Histochem Cytochem. 2002;50:147–158. doi: 10.1177/002215540205000203. [DOI] [PubMed] [Google Scholar]
- 62.Rutka JT, Ivanchuk S, Mondal S, Taylor M, Sakai K, Dirks P, Jun P, Jung S, Becker LE, Ackerley C. Co-expression of nestin and vimentin intermediate filaments in invasive human astrocytoma cells. Int J Dev Neurosci. 1999;17:503–515. doi: 10.1016/S0736-5748(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 63.Kania G, Corbeil D, Fuchs J, Tarasov KV, Blyszczuk P, Huttner WB, Boheler KR, Wobus AM. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells. 2005;23:791–804. doi: 10.1634/stemcells. 2004-0232. [DOI] [PubMed] [Google Scholar]
- 64.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 66.Sulman E, Aldape K, Colman H. Brain tumor stem cells. Curr Probl Cancer. 2008;32:124–142. doi: 10.1016/j.currproblcancer. 2008.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR, Jr, Gillespie GY. CD133 is a marker of bioenergetic stress in human glioma. PLoS ONE. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J Neurosci. 1997;17:8300–8312. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okano H, Imai T, Okabe M. Musashi: a translational regulator of cell fate. J Cell Sci. 2002;115:1355–1359. doi: 10.1242/jcs.115.7.1355. [DOI] [PubMed] [Google Scholar]
- 70.Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 71.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 72.Okita K, Ichisaka T, Yamanaka S. Generation of germ-line-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 74.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 75.Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/S1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 76.Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, Gutmann DH, Squire JA, Nagy A, Guha A. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 2001;61:3826–3836. [PubMed] [Google Scholar]
- 77.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Res. 2001;61:3556–3560. [PubMed] [Google Scholar]
- 79.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 80.Uhrbom L, Kastemar M, Johansson FK, Westermark B, Holland EC. Cell type-specific tumor suppression by Ink4a and Arf in Kras-induced mouse gliomagenesis. Cancer Res. 2005;65:2065–2069. doi: 10.1158/0008-5472.CAN-04-3588. [DOI] [PubMed] [Google Scholar]
- 81.Weiss WA, Burns MJ, Hackett C, Aldape K, Hill JR, Kuriyama H, Kuriyama N, Milshteyn N, Roberts T, Wendland MF, DePinho R, Israel MA. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 2003;63:1589–1595. [PubMed] [Google Scholar]
- 82.Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- 83.Lassman AB, Dai C, Fuller GN, Vickers AJ, Holland EC. Overexpression of c-MYC promotes an undifferentiated phenotype in cultured astrocytes and allows elevated Ras and Akt signaling to induce gliomas from GFAP-expressing cells in mice. Neuron Glia Biol. 2004;1:157–163. doi: 10.1017/S174092 5X04000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004;18:2963–2972. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 87.Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu A, Han YR, Li J, Sun D, Ouyang M, Plummer MR, Casaccia-Bonnefil P. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J Neurosci. 2007;27:7339–7343. doi: 10.1523/JNEUROSCI.1226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA. 2006;103:7853–7858. doi: 10.1073/pnas.0511 001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baracskay KL, Kidd GJ, Miller RH, Trapp BD. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55:1001–1010. doi: 10.1002/glia.20519. [DOI] [PubMed] [Google Scholar]
- 91.Globus J, Kuhlenbeck H. Tumors of the striatothalmaic and related regions: their probable source of origin and more common forms. Arch Pathol (Chic) 1942;24:674–734. [Google Scholar]
- 92.Globus J, Kuhlenbeck H. Idem: the subependymal cell plate (matrix) and its relationship to brain tumors of the ependymal type. J Neuropathol Exp Neurol. 1944;3:1–5. doi: 10.1016/0014-4886(61)90003-6. [DOI] [Google Scholar]
- 93.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncology. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005. 07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hopewell JW, Wright EA. The importance of implantation site in cerebral carcinogenesis in rats. Cancer Res. 1969;29:1927–1931. [PubMed] [Google Scholar]
- 96.Hopewell JW. The subependymal plate and the genesis of gliomas. J Pathol. 1975;117:101–103. doi: 10.1002/path.1711170208. [DOI] [PubMed] [Google Scholar]
- 97.Copeland DD, Bigner DD. Influence of age at inoculation on avian oncornavirus-induced brain tumor incidence, tumor morphology, and postinoculation survival in F344 rats. Cancer Res. 1977;37:1657–1661. [PubMed] [Google Scholar]
- 98.Stecca B, Ruiz i Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64:476–490. doi: 10.1002/neu.20160. [DOI] [PubMed] [Google Scholar]
- 99.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 100.Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz i Altaba A. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 101.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, Russell TL, Ellenbogen RG, Bernstein ID, Beachy PA, Olson JM. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 103.Kesari S, Ramakrishna N, Sauvageot C, Stiles CD, Wen PY. Targeted molecular therapy of malignant gliomas. Curr Oncol Rep. 2006;8:58–70. doi: 10.1007/s11912-006-0011-y. [DOI] [PubMed] [Google Scholar]
- 104.Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60:168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 105.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 106.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729–3735. [PubMed] [Google Scholar]
- 107.Weinberg R. Garland Science. Taylor & Francis Group; New York: 2007. The biology of cancer. [Google Scholar]
- 108.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma-animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004. 07.193. [DOI] [PubMed] [Google Scholar]
- 111.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 112.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nat ure05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 117.Diamandis P, Wildenhain J, Clarke ID, Sacher AG, Graham J, Bellows DS, Ling EK, Ward RJ, Jamieson LG, Tyers M, Dirks PB. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat Chem Biol. 2007;3:268–273. doi: 10.1038/nchembio873. [DOI] [PubMed] [Google Scholar]