Abstract
γδ T cells contribute uniquely to host immune defense, but how they do so remains unclear. Recent work suggests that thymic selection does little to constrain γδ T cell antigen specificities, but instead determines their effector fate. When triggered through the T cell receptor, ligand-experienced cells make IFNγ, whereas ligand-naïve γδ T cells produce IL-17, a major initiator of inflammation. These advances warrant a fresh look at how γδ T cells may function in the immune system.
Introduction
γδ T cells, like αβ T cells, develop in the thymus before entering the periphery. In the case of αβ T cells, thymic development entails endogenous ligand driven positive and negative selection, which determine what αβ T cell can recognize and whether these T cells will develop into CD4+ helper or CD8+ cytolytic T cells. However, the role of ligand-mediated selection in γδ T cell development and function has been less clear. In the past year this issue has been reexamined, and the emerging picture is fundamentally different from what we know about αβ T cell development and differentiation. Not only is encountering ligand in the thymus not required for γδ T cells to mature and exit to the periphery, but antigen naïve γδ T cells appear to constitute a large fraction of the peripheral repertoire. Furthermore, regardless of antigen experience, γδ T cells can be triggered to produce cytokines without requirement for antigen specific priming. Importantly, antigen naive γδ T cells make IL-17. In this review, we will discuss these recent findings and their implications.
The development of γδ T cells with invariant TCRs
Murine γδ T cells can be divided into two categories based on their receptor diversity, ontogeny and anatomical location. The first group, the invariant γδ T cells, are generated from the first two waves of T cells in the fetal thymus and later found in either the epidermis of the skin, or the epithelium of the reproductive tract. The first wave produces the skin resident dendritic epidermal T cells (DETCs), which express Vγ5 and Vδ1 TCRs [1], whereas the second wave produces the γδ T cells that populate the vaginal epithelium and express Vγ6 and Vδ1 TCRs [2]. The corresponding TCR chains essentially lack N regions or other types of junctional diversity [3], and the particular timing and choice of Vγ gene rearrangement correlates with its location 5′ to the Cγ region [4]. All experimental results suggest that ligand driven positive selection is required for DETCs to migrate to the skin and to acquire their ability to react to keratinocytes [5–7]. In general, it is believed that these invariant γδ T cells recognize host antigens and play a role in epithelial cell maintenance.
Recently, the expression of an immunoglobulin-like transmembrane protein, encoded by Skint1 (selection and upkeep of intraepithelial T cells 1), on fetal thymic stromal cells was identified as necessary for the positive selection of the DETCs [8]. In the absence of proper Skint1 expression, the epidermal layer lacks the invariant Vδ1+Vγ5+ DETCs (iDETCs) found in normal mice and is instead populated by γδ T cells that express diverse TCR V genes. However, Skint1 mutant mice develop spontaneous ear inflammation, suggesting that the ‘replacement’ DETCs function differently than the iDETCs [7]. Although neither the invariant TCR-antigen, nor the molecular entity on the iDETC which interacts with Skint1 has been identified, several indirect evidences indicate that the ‘replacement’ DETCs do not recognize the same ligand as the iDETCs. Given the observations made by Jensen et al. (discussed below), it would be interesting to determine whether or not these ‘replacement’ DETCs have encountered ligand during development in the thymus and if these cells function as antigen naïve γδ T cells.
Encountering thymic ligand is neither required, nor inhibitory for the generation of antigen specific γδ T cells in adult animals
The second group of γδ T cells appears postnatally after the first two waves of fetal γδ thymocyte development. Unlike the invariant γδ T cells, this group expresses TCRs with various Vδs and Vγs, and diverse CDR3 regions. In adult mice, these cells are found in all secondary lymphoid organs and below the epithelium or mucosal surfaces of many tissues, including the small intestine and lung. Only a few ligands have been identified for these cells, which include both host- and pathogen- derived antigens. However, it is clear that MHC molecules are not obligatory components of γδ T cell ligands, and there is no apparent common structural motif shared by defined γδ T cell antigens (reviewed in [9]).
The impact of thymic ligand expression on the development of this second group of γδ T cells was first examined in transgenic mice expressing the TCRs of two independently derived γδ T cell clones, KN6 and G8, which recognize the closely related β2-microglobulin associated MHC class Ib molecules, T10 and T22. It was reported that development of KN6 and G8 transgenic T cells was inhibited in both C57BL6 (B6) (which express both T10 and T22) and β2-microglobulin deficient mice (B2m−/−, which do not have cell surface T10 or T22 expression), but occurred normally in BALB/c mice (which only express T10). These observations lead to the conclusion that γδ T cells, similar to αβ T cells, undergo ligand driven positive and negative selection in the thymus [10–13]. However, analyzing the same G8 transgenic mice, Schweighoffer and Fowlkes found that G8 T cells were able to mature in B2m−/− mice, contradicting the conclusion that positive selection is required [14].
Subsequently, T10/T22 were found to be natural ligands for a sizable population (~0.2–2%) of γδ T cells in normal mice [15]. Surprisingly, using a T22 tetrameric staining reagent, a comparable frequency (~0.2–1%) of T10/T22 specific γδ T cells were found in B6, BALB/c, and B2m−/− mice [16]. A similar frequency of T10/T22 specific γδ T cells was also found in the thymuses and spleens of mice lacking both β2m and class II MHC molecules, and in the thymuses of these mice treated with cyclosporin A [16]. In addition, normal numbers of γδ thymocytes are found in calcineurin deficient animals [17]. Thus, the ligands and signaling pathways required for positive selection of γδ T cells are not required for γδ thymocyte development.
Furthermore, T10/T22 specific γδ thymocytes from different genetic backgrounds have similar levels of phosphorylated ERK1/2 (extracellular signal-regulated kinase) and CD5, both of which are downstream indicators of TCR-signaling and/or signaling-threshold in the thymus. While sphingosine-1-phosphate receptor 1 (S1P1) up-regulation is necessary for mature αβ thymocytes to exit the thymus [18] and is highly expressed in positively selected fetal thymocyte DETC-precursors [6], there was no observable difference in the S1P1 surface expression between tetramer-positive and tetramer-negative γδ thymocytes from B6 and B2m−/− mice. Also, T10/T22 specific γδ thymocytes are lineage committed, as evidenced by remaining CD4-CD8- [16] (Figure 1). Taken together, these results indicate that endogenous thymic ligand expression does very little to constrain γδ thymocyte development and thymic exit. Indeed, 0.85% of ‘non-selected’ TCRδ sequences from CD3ε-deficient murine thymocytes and from out-of-frame VDJ recombination events contain the T10/T22 recognition motif [19] (Figure 2). This frequency is well within the range of the 0.2–2% T10/T22 specific γδ T cells observed in normal mice. These observations represent a significant departure from what has been known for αβ T cell development and what has been thought previously about γδ T cell selection through the analysis of γδ TCR transgenic mice. Skewed thymocyte development by prearranged TCR transgenes [20], and extrapolating results from the analysis of a single transgenic TCR to populations of T cells with a range of affinity for the same ligand may have contributed to this discrepancy.
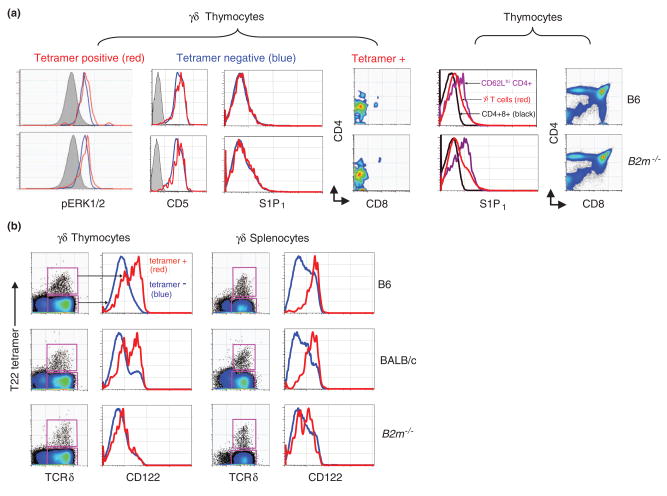
Figure 1. Ligand expression is not required for the development of antigen specific γδ thymocytes.
a) T10/T22 specific γδ thymocytes that developed in the presence (B6, top row) or absence of ligand (B2m−/− bottom row) were analyzed for basal (i.e. non-stimulated) intracellular levels of phosphorylated-ERK1/2 (basal level of pERK1/2 in CD8+CD4+ DP thymocytes for comparison in grey), or the surface expression of CD5 (isotype staining control in grey), S1P1, CD4 and CD8. T22-tetramer negative thymocytes are also shown (blue). For comparison, the S1P1 surface expression on DP (black) and mature αβ thymocytes (CD4+ CD62Lhi, purple); as well as the CD4/8 surface expression profiles on thymocytes from B6 and B2m−/− mice are plotted. Data is from Jensen et al. [16]. b) The CD122 surface expression on T10/T22 specific γδ thymocytes and splenocytes (red) that developed in the presence (B6, BALB/c) or absence (B2m−/−) of ligand. Tetramer-negative γδ T cells are shown in blue. Data is from Jensen et al.
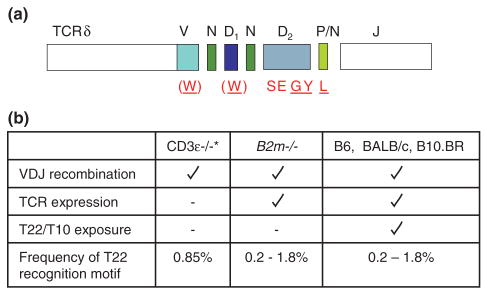
Figure 2. Frequency of the T10/T22 recognition motif in γδ T cells from different genetic backgrounds.
a) The T10/T22 recognition motif (amino acids in red, W SEGYE L) expressed by T10/T22 specific γδ T cells from B10.BR mice, are also found in T10/T22 specific γδ T cells isolated from B6, B2m−/− and BALB/c mice (analyzed at a single cell level). Underlined, are the amino acids found in all T10/T22 specific γδ T cells from B10.BR mice as reported by Shin, et al. [19]. The recognition motif is largely encoded by an intact Dδ2 (SEGYE) gene segment with contributions from a V-, Dδ1- or N-nucleotide encoded tryptophan (W) and a P-nucleotide encoded leucine (L). Diverse TCR Vγ and Vδ genes are associated T10/T22 specific TCRs. b) Frequency of the T10/T22 recognition motif observed in γδ T cell populations derived from different genetic backgrounds that vary in TCR expression and/or ligand recognition. *CDR3 sequences are from CD3ε−/− thymocytes and out of frame VDJ δ TCR rearrangements, both of which undergo gene rearrangement but for different reasons fail to express TCRs on the cell surface. Presumably, these thymocytes are not subjected to endogenous ligand selection events. B2m−/− mice fail to express T10/T22 on the cell surface and consequently, γδ TCR expressing cells are not exposed to T10/T22. The frequency of the recognition motif in wild type and B2m−/− mice was confirmed by tetramer staining and direct single cell sequence analysis [16,19].
In search of a mechanism that allows γδ thymocytes to signal through the TCR without encountering ligand, Jensen et al. used a cellular dimerization assay worked out for the preTα receptor and found that dimerization is a general feature of many Vδ’s. Whether or not γδ TCRs expressed on T cells do dimerize or multimerize, and its biological consequences remains to be determined. However, this provides an attractive hypothesis as to why γδ thymocytes do not need a ‘pre-Tγδ receptor’ to sense properly folded and surface expressed γδ TCRs, and can signal without ligand recognition (Figure 3). In the periphery, γδ TCR dimerization might also serve to enhance antigen specific stimulation, even though most of these cells do not express either CD4 or CD8 co-receptors and do not recognize MHC molecules.
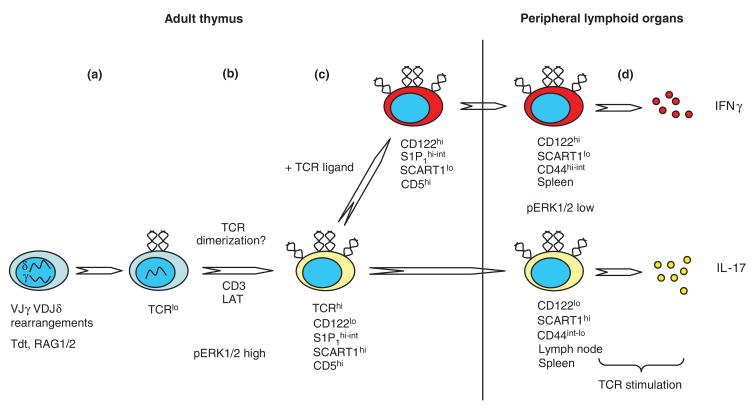
Figure 3. A model for the development of a functional γδ T cell repertoire.
a) Vδ2, 4, 5, 6, 7, 8, 10, etc. (not Vδ1)-DJδ and Vγ1, 2, 4, 7-Jγ rearrangements occur in DN2/3 thymocytes. The Terminal Deoxynucleotidyl Transferase (Tdt) is expressed in adult thymocytes and thus, the resulting TCRs have considerable CDR3 diversity. b) TCR expression is initially low (TCRlo), but progression to a TCRhi phenotype requires the CD3 and LAT signaling subunits [32]. We hypothesize that γδ TCR dimerization may drive this progression, and possibly serves as a checkpoint for correctly folded and surface expressed TCR units. The high pERK1/2 levels may lower the signaling threshold and allow thymocytes to sense dimerization events mediated through the TCR. c) γδ T cells that bind self-ligand through their TCR (+ TCR ligand) in the thymus up-regulate the expression of CD122, and down-regulate SCART1 (extrapolated from Kisielow and colleagues data [23]). d) In peripheral lymphoid organs, antigen naïve and experienced cells, when stimulated through their TCR, secrete IL-17 and IFNγ respectively; Tγδ-IFNγs are preferentially found in the spleen, whereas Tγδ-17s are abundantly found in both the spleen and lymph nodes. In the periphery, the pERK1/2 levels in un-stimulated γδ T cells are low [16]. Thus, TCR-ligand engagement is required to activate the cytokine production program. However, in the periphery, TCR-ligand interactions could also be augmented by TCRδ dimerization events.
A large fraction of lymphoid γδ T cells may not have encountered ligand during development or in the periphery
T10/T22 specific γδ T cells that developed in B6, BALB/c, and B2m−/− mice are similar in number, but phenotypically different, leading to several insights. First, it was obvious that the majority of T10/T22 specific γδ T cells from B6 and BALB/c mice have encountered antigen whereas those from the B2m−/− mice have not. In particular, T10/T22 specific cells from B6 and BALB/c mice expressed higher levels of the IL-2 and IL-15 receptor common β chain (CD122) than those from B2m−/− mice (Figure 1). The upregulation of CD122 has been used as an indicator of self-ligand recognition for αβ thymocytes [21] and during DETC development [6].
Secondly, the surface expression pattern of a variety of markers (e.g. CD122, CD25, CD44, CD127, NK1.1, HSA, DX5, etc.) on tetramer-negative γδ T cells (i.e. >99% of the total γδ T cell population) in all strains of mice were more similar to tetramer-positive cells from B2m−/− mice than from B6 and BALB/c mice, suggesting that a large fraction of lymphoid γδ T cells may not have encountered ligand during development or in the periphery. Finally, analysis of the turnover rates of these γδ T cell populations was consistent with this supposition [16]. However, these differences did not substantially bias the repertoire, as similar frequencies of T10/T22 specific cells can be found in the periphery of B2m−/− and wild type mice.
Antigen naïve γδ T cells make IL-17 without requirement for antigen specific priming
Jensen et al. found that upon stimulation through the TCR, CD122lo cells make IL-17, and CD122hi γδ T cells make IFNγ. The ability to differentially make IL-17 versus IFNγ can be observed in γδ T cells from the spleen, lymph nodes [16], and peritoneal cavity [22]. Importantly, CD122lo γδ T cells have already gained the ability to make IL-17 in the thymus [16,22]. One exception is in the inter-epithelial lymphocyte (IEL) compartment of the small intestine, where γδ IELs are mainly CD122lo, constitutively cytolytic, and were found to secrete IFNγ but not IL-17 when stimulated. Whether or not CD122 expression on γδ T cells is induced only by TCR-ligand engagement will require more extensive analysis. Nevertheless, these results are consistent with the hypothesis that at least for γδ T cells of the lymphoid organs, antigen recognition drives effector function development, where antigen naïve cells make IL-17 and antigen experienced cells make IFNγ (Figure 3).
Kisielow and colleagues used a cDNA subtraction screening approach to determine genes that are involved in γδ thymocyte development. They identified two novel genes, which belong to the scavenger receptor superfamily B, SCART1 and 2, to be highly expressed in adult γδ thymocytes and lymph node γδ T cells [23]. Interestingly, when triggered through the TCR, SCARThi but not SCART2-, γδ T cells make IL-17 without previous priming. Furthermore, activating SCART2hiγδ T cells through the TCR, and in the presence of IL-2, down-regulates the expression of SCART2. Therefore, it appears that SCART2 expression marksγδ T cells which have not encountered ligand (Figure 3). However, this possibility remains to be tested.
Conclusion
Whether or not the development of T10/T22 specific γδ T cells is typical of the entire adult γδ T cell repertoire will require further studies when additional γδ T cell antigens are identified. Until then, these recent developments seem to indicate that γδ T cells differ from αβ T cells in how thymic development influences their TCR specificities and effector-fate development. In particular, the absence of positive selection, and the lack of antigen specific priming, seems ideal for γδ T cells to function in the first line of defense. Importantly, antigen naïve γδ T cells do so by mounting a robust IL-17 response. One of the main functions of IL-17 is to promote the expansion and maturation of neutrophils in the bone marrow [24,25]. In order to replenish circulating neutrophils that deploy within hours to the site of infection, a swift IL-17 response must be elicited, days before the development of CD4+ Th17 αβ T cells. Therefore, the rapid IL-17 response mounted by antigen-inexperienced γδ T cells would play a critical role at the onset of an acute inflammatory response to pathogens that the host encounters for the first time, or to host antigens that are only revealed by injury. Indeed, γδ T cells are the major early producers of IL-17 after CFA immunization [16] and in several murine models of infection and autoimmunity (reviewed in [26]).
Consistent with a role for γδ T cells in the regulation of neutrophil homeostasis, mice lacking γδ T cells fare worse in neutrophil-dominated inflammatory responses, such as in heat-, ozone- or chlorine-induced injuries [27–29] and in bacterial infections (Nocardia asteroids [27], Klebsiella pneumonia [30]). In these cases, fewer infiltrating neutrophils, increased bacterial load, early dissemination and higher mortality rates are noted. These observations may explain reports showing increased numbers of γδ T cells in the peripheral blood of patients with acute bacterial and viral infections (from <5% in healthy individuals up to >45% in patients with these diseases), and the abundance of infiltrating γδ T cells in primary lesions of the central nervous system in Multiple Sclerosis patients (up to 20–30% of the total number of T cells) [31].
In addition, by acting early in the inflammatory response, γδ T cells may affect the development of antigen specific αβT cell and B cell responses during the priming phase. Thus, γδ T cells may play a much larger role in the adaptive immune response than previously recognized. This may be the key to understanding how γδ T cells contribute to host immune competence and why these cells have been maintained throughout vertebrate evolution, wherever αβ T cells and B cells are found.
Acknowledgments
The authors thank our numerous collaborators, and past and present laboratory members for contributions and stimulating discussions on this subject. We also thank M. M. Davis for critically reading this manuscript. K.J. was supported by the Stanford Graduate Fellowship (SGF) and the National Institutes of Health (NIH) Cell and Molecular Biology (CMB) training grant. This work was supported by grants A1 33431 and U19 AI 057229 from the NIH (Y.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• of outstanding interest
- 1.Allison JP, Havran WL. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 2.Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;59:859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- 3.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 4•.Xiong N, Zhang L, Kang C, Raulet DH. Gene placement and competition control T cell receptor gamma variable region gene rearrangement. J Exp Med. 2008;205:929–938. doi: 10.1084/jem.20071275. This study analyzes the mechanism by which Vγ genes preferentially rearrange during γδ T cell development. The Vγ genes that rearrange in the early fetal thymus are because of their closer proximity to the Jγ1 Cγ1 gene cluster, independent of promoter or recombination signal sequences and unrelated to the extent of germline transcription. Using gene deletion studies, the authors show that the downstream Vγ genes located closer to the Jγ1 Cγ1 locus competitively inhibit upstream Vγ rearrangements at the fetal stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal gammadelta cells with disrupted primary Vgamma gene usage. Science. 1998;279:1729–1733. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 6.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 7••.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. This report describes an FVB substrain of mice in which the DETCs express a collection of heterogeneous TCRγ and δ chains instead of the invariant Vγ5Vδ1 TCR. These mice develop spontaneous ear inflammation, and had an exaggerated irritant contact dermatitis response. In the mutant fetal thymus, the Vγ5Vδ1 cells fail to mature, express CD122 and proper skin homing receptors. This phenotype is attributable to a defect in thymic stromal cells from the FVB substrain and can be ameliorated by TCR cross-linking. These results lend further support to the supposition that the Vγ5Vδ1 DETCs need ligand driven positive selection to mature and populate the epidermis of the skin. [DOI] [PubMed] [Google Scholar]
- 8•.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. A follow-up to the study described in Lewis et al. By mapping and genetic complementation, this study determined that the defect in the FVB substrain, which resulted in a loss of epidermal Vγ5+Vδ1+ cells due to a failure of thymic selection, was caused by a mutation in Skint1, a newly identified gene expressed in the thymus and skin that encodes a protein with immunoglobulin-like and trans-membrane domains. The mechanism by which Skint1 mediates positive selection of the invariant Vγ5Vδ1 DETCs is not known. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konigshofer Y, Chien YH. Gammadelta T cells - innate immune lymphocytes? Curr Opin Immunol. 2006;18:527–533. doi: 10.1016/j.coi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Bonneville M, Ishida I, Itohara S, Verbeek S, Berns A, Kanagawa O, Haas W, Tonegawa S. Self-tolerance to transgenic gamma delta T cells by intrathymic inactivation. Nature. 1990;344:163–165. doi: 10.1038/344163a0. [DOI] [PubMed] [Google Scholar]
- 11.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 12.Wells FB, Gahm SJ, Hedrick SM, Bluestone JA, Dent A, Matis LA. Requirement for positive selection of gamma delta receptor-bearing T cells. Science. 1991;253:903–905. doi: 10.1126/science.1831565. [DOI] [PubMed] [Google Scholar]
- 13.Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, Tonegawa S. Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. Embo J. 1992;11:25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic gamma delta T cells. J Exp Med. 1996;183:2033–2041. doi: 10.1084/jem.183.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 16••.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. This study shows that encountering antigen in the thymus is neither required for nor inhibitory to the development of T10/T22 specific γδ T cells. γδ TCRs mediate autonomous signaling, suggesting a mechanism to drive γδ thymocyte development without ligand engagement. A sizable number of γδ T cells in normal mice are phenotypically and functionally similar to B2m−/− T10/T22 specific cells, suggesting that most γδ T cells in the periphery have yet to encounter antigen. When activated through their TCRs, cells with prior antigen exposure produce IFNγ while cells that develop in the absence of ligand make IL-17. γδ T cells are the major IL-17 producers in the draining lymph nodes immediately after immunization with Complete Freund’s Adjuvant (CFA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo EM, Ho L, Winslow MM, Staton TL, Crabtree GR. Selective role of calcineurin in haematopoiesis and lymphopoiesis. EMBO Rep. 2008;9:1141–1148. doi: 10.1038/embor.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 19.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 20.Serwold T, Hochedlinger K, Inlay MA, Jaenisch R, Weissman IL. Early TCR expression and aberrant T cell development in mice with endogenous prerearranged T cell receptor genes. J Immunol. 2007;179:928–938. doi: 10.4049/jimmunol.179.2.928. [DOI] [PubMed] [Google Scholar]
- 21.Hanke T, Mitnacht R, Boyd R, Hunig T. Induction of interleukin 2 receptor beta chain expression by self-recognition in the thymus. J Exp Med. 1994;180:1629–1636. doi: 10.1084/jem.180.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ gammadelta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. This report shows that IL-17 and IFNγ are produced by different populations of peritoneal γδ T cells. While Tγδ 17s are CD122−CD25+, Tγδ IFNγs are CD122+CD25−. During fetal thymic ontogeny, intracellular IL-17+ γδ T cells from day 15 fetal thymus can be identified after PMA ionomycin stimulation. The frequency of these IL-17+ thymic γδ T cells peaks around birth, and decline postnatally. All IL-17+ γδ thymocytes are CD122−. [DOI] [PubMed] [Google Scholar]
- 23••.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult gammadelta T cells. J Immunol. 2008;181:1710–1716. doi: 10.4049/jimmunol.181.3.1710. This study describes the discovery of SCART1 and SCART2, members of the scavenger receptor family that are primarily expressed on developing and mature γδ T cells. SCART2hi γδ T cells are preferentially found in the adult thymus and lymph nodes. αβTCR transgenic cells which have been diverted to the ‘γδ T cell lineage’ also express high levels of SCART2 on their cell surface. SCART2hi, but not SCART2-, γδ T cells from peripheral lymph nodes of naïve mice can be induced to make IL-17 by anti-CD3, anti-CD28 stimulation. [DOI] [PubMed] [Google Scholar]
- 24.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 26.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, Stovall MY, Van Winkle LS, Beaman BL, Ferrick DA. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 28.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of gammadelta T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76:545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 29.Koohsari H, Tamaoka M, Campbell HR, Martin JG. The role of gamma delta T cells in airway epithelial injury and bronchial responsiveness after chlorine gas exposure in mice. Respir Res. 2007;8:21. doi: 10.1186/1465-9921-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 31.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci U S A. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]