Abstract
In the last three decades gemcitabine has progressed from the status of a laboratory cytotoxic drug to a standard clinical chemotherapeutic agent and a potent radiation sensitizer. In an effort to improve the efficacy of gemcitabine, additional chemotherapeutic agents have been combined with gemcitabine (both with and without radiation) but with toxicity proving to be a major limitation. Therefore, the integration of molecularly targeted agents, which potentially produce less toxicity than standard chemotherapy, with gemcitabine-radiation is a promising strategy for improving chemoradiation. Two of the most promising targets, described in this review, for improving the efficacy of gemcitabine-radiation are EGFR and Chk1.
Keywords: gemcitabine, radiation, EGFR, Chk1, molecularly targeted therapy
Introduction
Gemcitabine was first introduced into the clinic as a chemotherapeutic agent nearly 3 decades ago. Since then, both laboratory and clinical investigations have shown gemcitabine to be a potent radiation sensitizer. In this review we will begin with a discussion of gemcitabine biochemistry and its mechanisms of interaction with radiation, highlighting observations which may lead to improving the design of clinical trials combining gemcitabine with radiation. Previous attempts to improve the efficacy of gemcitabine-radiotherapy have included the addition of other chemotherapeutic agents (1-3) such as cisplatin (4) and oxaliplatin (5). More recent studies have focused on the addition of molecularly targeted therapies, to gemcitabine and radiation (6, 7). In this review we will present our rationale for integrating checkpoint kinase 1 (Chk1)- and epidermal growth factor (EGFR)-molecularly targeted agents with gemcitabine-radiation therapy.
Gemcitabine biochemistry and radiosensitization
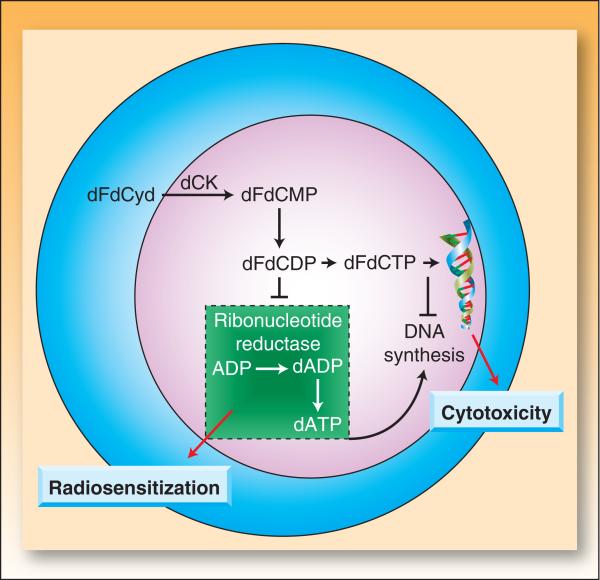
The antitumor activity of gemcitabine depends on a series of sequential phosphorylations. In the first rate limiting step, deoxycytidine kinase converts gemcitabine to the monophosphorylated metabolite, dFdCMP. (This has motivated the study of fixed-dose-rate infusion (10 mg/m2/min), which increases intracellular metabolites compared to bolus treatment (8, 9), but in the majority of trials does not significantly improve survival (10)). Subsequent phosphorylations lead to the accumulation of gemcitabine di- and triphosphate (dFdCDP and dFdCTP) which are both active metabolites (Figure 1). While dFdCTP can interfere with DNA synthesis by competing with endogenous dCTP for misincorporation into replicating DNA, dFdCDP is a potent inhibitor of ribonucleotide reductase, reducing the synthesis of deoxynucleoside triphosphates, primarily dATP (in solid tumor cells).1
Figure 1. Gemcitabine mechanisms of action.
Following cellular incorporation, gemcitabine (dFdCyd) undergoes a series of sequential phosphorylations mediated by deoxycytidine kinase. dFdCDP is a direct inhibitor of ribonucleotide reductase which results in inhibition of deoxyribonucleotide triphosphate synthesis, specifically deoxyadenosine triphosphate (dATP). Depletion of dATP pools is crucial for radiosensitization (19). dFdCTP is incorporated into DNA during synthesis and contributes to cytotoxicity (88-90).
The inhibition of ribonucleotide reductase by dFdCDP and subsequent depletion of dATP pools caused by gemcitabine suggested that it would be a good radiation sensitizer (Box 1) (12, 13). Early preclinical studies showed that, as anticipated, gemcitabine radiosensitized both solid tumor cell lines (12, 14-16) and mouse sarcoma (17). Subsequent studies showed that cells transduced with the active subunit of ribonucleotide reductase become relatively resistant to gemcitabine-mediated radiosensitization (18). Furthermore, radiosensitization does not correlate with intracellular concentrations of dFdCTP (19), suggesting that dATP pool depletion and not incorporation of dFdCMP into DNA underlies radiosensitization. Although gemcitabine-induced dATP pool depletion is necessary, it alone is not sufficient for radiosensitization. The ability of gemcitabine to cause redistribution of cells into S phase is also required for radiosensitization (20). Although high concentrations of gemcitabine cause near complete dATP pool depletion within just a few hours, cells irradiated at this time are minimally radiosensitized. Maximum sensitization requires both dATP pool depletion and sufficient time to permit redistribution of cells into early S-phase (15, 21). Sensitization is maximized in vivo by a fixed-dose-rate exposure to gemcitabine, compared to a bolus administration (22), presumably due to the production of more intracellular metabolites, as alluded to above.
Cellular effects of radiation and gemcitabine
DNA-directed effects
Based on the inhibition of dNTP synthesis by gemcitabine, it seemed likely that gemcitabine would have an effect on the repair of radiation-induced DNA damage, which may contribute in part to its radiosensitizing activity. When initial work showed that gemcitabine had no effect on the induction or repair of bulk DNA damage (23-25), individual repair pathways were explored. These studies found that DNA damage induced by ionizing radiation is primarily repaired by the non-homologous end joining (NHEJ) pathway and, to a lesser extent, through base-excision repair and homologous recombination repair (HRR) (26). While the NHEJ pathway is not required for gemcitabine-mediated radiosensitization (23) other studies suggest that HRR may be required. Whereas the radiation sensitivity of cells deficient in HRR is relatively unaffected by gemcitabine, cells that are HRR-competent, but unable to carry out base excision repair, are radiosensitized (27). The finding that ionizing radiation-induced Rad51 foci formation, a marker for HRR activity, is inhibited by gemcitabine pretreatment provides further evidence that gemcitabine inhibits this repair pathway in irradiated cells (27).
Another DNA repair pathway that may affect gemcitabine-mediated radiosensitization is the mismatch repair pathway (MMR). MMR-deficient cells display enhanced radiosensitization after a long exposure (24 hrs) to an IC50 concentration of gemcitabine (19, 28). These data suggest that MMR may antagonize the radiosensitizing effects of relatively low-dose gemcitabine, perhaps by facilitating the repair of gemcitabine-induced errors in DNA caused by nucleotide pool imbalance.
Roles of apoptosis and p53 expression
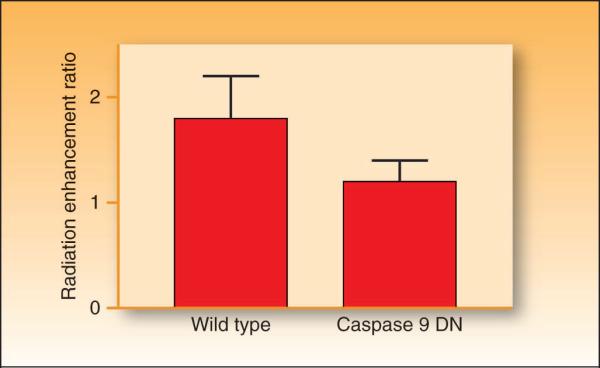
One potential consequence of the increase in residual DNA damage after radiation in gemcitabine-treated cells is an increase in radiation-induced apoptosis. Initial studies suggested that the extent of apoptosis produced by the combination of gemcitabine and radiation correlated with radiosensitization, and that the inhibition of apoptosis substantially reduced sensitization (16). To test this hypothesis directly, we performed studies using MCF-7 cells overexpressing a dominant negative form of caspase-9. We found that caspase-9 dominant negative overexpression blocks apoptosis and inhibits gemcitabine-mediated radiosensitization (Figure 2). (However, overexpression of the pro-apoptotic protein bcl-xS in fibroblasts, did not affect gemcitabine radiosensitization (29)). P53 does not seem to have a direct effect on gemcitabine-radiosensitization (30-32). Taken together these findings suggest that although apoptosis plays a role in radiosensitization by gemcitabine, this role depends on many factors, including the cell type and status of apoptotic regulators.
Figure 2. The effect of caspase 9 on gemcitabine-mediated radiosensitization.
Wild type or caspase 9 dominant negative (DN) MCF-7 cells were exposed to equicytotoxic concentrations of gemcitabine for 24 hours prior to treatment with 0 − 8 Gy radiation. Survival was determined by a clonogenic survival assay as previously described (91). Data are expressed as the radiation enhancement ratio which was calculated as the ratio of the mean inactivation dose for drug treated cells to non-drug treated cells (ER = 1). Data are from the mean of 3 independent experiments ± standard error.
Cell cycle checkpoints
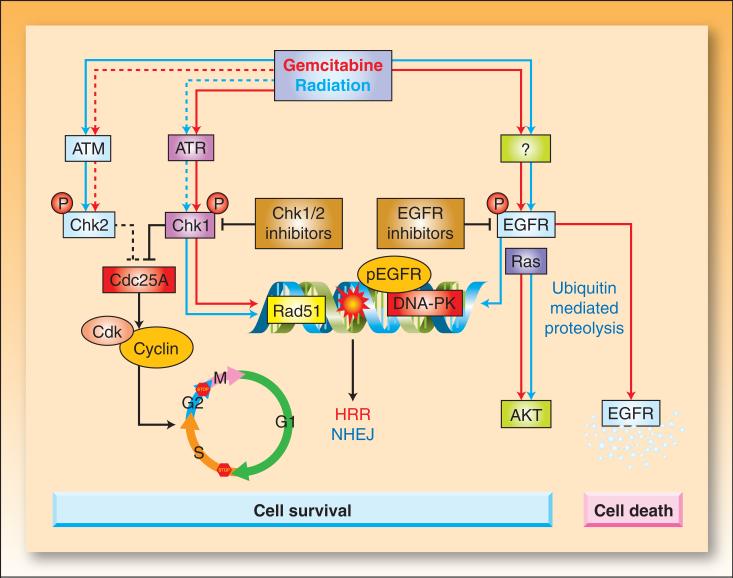
Because both gemcitabine-mediated cytotoxicity and radiosensitization depend on S-phase redistribution, efforts have been directed at understanding the mechanisms of gemcitabine-induced S-phase arrest. In response to DNA damage, ATM and ATR activate Chk1 and Chk2 kinases which result in Cdc25 phosphatase inhibition and cell cycle arrest (Figure 3). In general, gemcitabine treatment results in the accumulation of the phosphorylated forms of Chk1 and Chk2 and degradation of Cdc25A (33) (34-36). These observations led to the hypothesis that Chk1 and/or Chk2 activation were required for gemcitabine-induced early S-phase arrest. Initial studies found, however, that although Chk1 activity was required for gemcitabine-induced Cdc25A degradation, neither Chk1 nor Chk2 inhibition affected the gemcitabine-induced accumulation of cells in early S-phase (33, 35, 37). Instead, Chk1 inhibition abrogated the G2/M checkpoint, and permitted gemcitabine-treated cells with arrested DNA synthesis to enter mitosis with either a 4N DNA content (normal mitosis) or a sub-4N DNA content (premature mitosis). Thus, it appears that gemcitabine-induced Chk1 activation functions in part to coordinate cell cycle progression with DNA synthesis, preventing cells with stalled replication from prematurely entering mitosis (33).
Figure 3. The effects of gemcitabine and radiation on cell cycle checkpoints and EGFR signaling.
Radiation-induced double strand breaks or gemcitabine-induced replication stress trigger the activation of ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR) kinases, respectively (92). Active ATM/ATR phosphorylate and activate Chk1 and Chk2 (93-95) which phosphorylate Cdc25 phosphatases, leading to their inactivation through degradation (Cdc25A) or cytoplasmic sequestration (Cdc25C) (93, 96). In the absence of Cdc25 phosphatase activity, cyclin dependent kinases (Cdk1 and Cdk2) remain bound by inhibitory phosphorylations, resulting in arrest of the cell cycle at G1/S, intra-S, or G2/M. Treatment of cells with gemcitabine prior to radiation results in radiosensitization that can be attributed to a number of events (Box 1), including dATP depletion and S-phase arrest. Inhibition of Chk1 sensitizes cells to gemcitabine and radiation by a number of potential mechanisms including abrogation of cell cycle arrest, premature mitotic entry, and inhibition of Rad 51 focus formation resulting in impaired homologous recombination repair (HRR).
EGFR is phosphorylated in response to radiation or gemcitabine by an unknown mechanism(s) (97). Radiation triggers translocation of EGFR into the nucleus (58, 60). This process coincides with transport of Ku70/80 and protein phosphatase 1 into the nucleus, resulting in increases in DNA-PK, repair of DNA-strand breaks (NHEJ; nonhomologous endjoining), and cell survival. Activation of EGFR in response to gemcitabine can also result in activation of the survival signal AKT (46). Activating Ras mutations can result in activation of Ras-dependent pathways, such as PI3K/AKT, even in the presence of EGFR inhibitors. EGFR inhibitors prevent gemcitabine and/or radiation-mediated EGFR signaling and are thought to impair cell survival signals and DNA repair. EGFR inhibition blocks nuclear transport of EGFR and DNA-PK activity (60, 98). In some instances, phosphorylation of EGFR by gemcitabine promotes ubiquitination of the receptor leading to degradation along a proteosome/lysosome pathway (63). EGFR degradation results in down-regulation of the survival signal pAKT, leading to apoptosis. Blocking EGFR degradation at various steps of this pathway reduces gemcitabine-mediated cytotoxicity. Whether an EGFR-activating insult leads to cell survival or cell death may ultimately be determined by the severity and duration of the stress. The colored arrows indicate the effects mediated by gemcitabine (red) versus radiation (blue). Dotted lines indicate less pronounced effects.
The finding that gemcitabine activates Chk1 and Chk2 led to studies assessing the effects of checkpoint inhibition on gemcitabine-induced cytotoxicity. Inhibition of Chk1 by either siRNA-mediated Chk1 depletion (34, 37) or by small molecule Chk1 inhibitors (35, 36) enhanced gemcitabine cytotoxicity. Likewise, inhibition of other members of the Chk1 signaling pathway, such as Rad9, ATR, and ATM, enhanced gemcitabine cytotoxicity (34). Although, enhancement of gemcitabine cytotoxicity is accompanied by inhibition of Cdc25A degradation and induction of premature mitotic entry in some instances, we have found examples where these markers do not correlate with sensitization. Instead, our recent data demonstrate a stronger correlation between sensitization to gemcitabine by Chk1 inhibition and depletion of Rad51 protein, inhibition of Rad51 focus formation, and increased γ-H2AX (36). These findings suggest that sensitization to gemcitabine by Chk1 inhibition is mediated by inhibition of the DNA damage response.
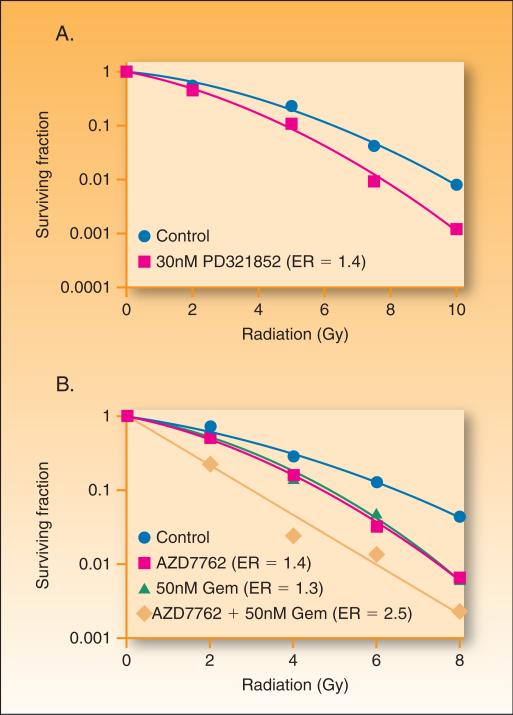
Chk1 may also play a role in radiosensitization by gemcitabine. Chk1 inhibitors such as PD-321852 (Pfizer) and AZD7762 (AstraZeneca; (38)) increase radiation sensitivity in a variety of model systems (39-42). Based on the ability of Chk1 inhibitors to sensitize to gemcitabine or radiation, we have initiated studies to examine whether Chk1 inhibition might enhance gemcitabine-mediated radiosensitization. PD-321852 (43) enhanced radiation sensitivity (Figure 4A) as well as gemcitabine-cytotoxicity (36) in pancreas tumor cell lines. Likewise, AZD7762 enhanced radiation sensitivity and further enhanced gemcitabine-mediated radiosensitization (Figure 4B). Chk1 inhibitors have now entered clinical trials (for a review see (44)).
Figure 4. The effects of Chk1 inhibition on radiation and chemoradiation sensitivity.
MiaPaca-2 cells were treated with 30 nM PD-321852 for 24 hrs pre- and post-ionizing radiation (0 −10 Gy) (A) or for 2 hours with gemcitabine (50nM) and then with AZD7762 (100nM) for 1 hour pre- and 24 hours post-irradiaiton (B). Cells were then plated at cloning densities and grown for 10 days to determine the surviving fraction, which represents the fraction of cells surviving radiation treatment relative to un-irradiated controls. Cell survival curves were then fitted using the linear quadratic equation, and the mean inactivation dose was calculated according to the method of Fertil et al. (99). The radiation enhancement ratio was calculated by dividing the mean inactivation dose under control conditions by the mean inactivation dose of Chk1 inhibitor-treated cells.
EGFR signaling
EGFR is a transmembrane receptor tyrosine kinase that is activated in response to binding of ligands such as EGF, transforming growth factor-α, or amphiregulin (for a review see 42). Ligand binding results in receptor dimerization and activation of a number of downstream pathways (STAT, AKT, ERK, PKC) which promote survival, angiogenesis, cell cycle progression, and transformation. A recent Phase III clinical trial in metastatic pancreatic cancer demonstrated a statistically significant but clinically modest improvement in overall survival for patients treated with gemcitabine plus erlotinib versus gemcitabine alone (6.2 vs. 5.9 months) (45). There are several mechanisms (discussed below) through which EGFR inhibitors might interact with gemcitabine and/or radiation including EGF receptor activity, cell cycle, and DNA repair.
In addition to nucleotide pool depletion, S-phase arrest, and cell cycle checkpoint activation, gemcitabine stimulates phosphorylation of EGFR both in head and neck as well as in pancreas cancer cells (46, 47). EGFR is also phosphorylated in response to a variety of other cytotoxic agents (48-51) and it is hypothesized that this phosphorylation may promote survival through stimulation of stress/survival response pathways as illustrated in Figure 3. This model provides an obvious rationale for the addition of EGFR inhibitors, such as the small molecule tyrosine kinase inhibitor, erlotinib or the anti-EGFR antibody, cetuximab to gemcitabine therapy. Initial studies in head and neck cancer xenografts demonstrated that gefitinib, which blocked gemcitabine-mediated EGFR phosphorylation, enhanced gemcitabine-mediated tumor growth delay (46). In other studies, both cetuximab and erlotinib were found to enhance pancreas tumor growth delay when combined with gemcitabine and radiation (7, 47).
The ability of EGFR inhibitors to sensitize to gemcitabine is sequence dependent. In head and neck cancer cells as well as xenografts, the combination of gemcitabine followed by gefitinib is superior to the reverse sequence (46). This observation has been supported in pancreatic cancer cells as well where treatment with gemcitabine prior to gefitinib produced additive to synergistic effects but antagonistic effects in response to the reverse sequence (52, 53). This schedule dependent cell killing may be attributable to the cell cycle effects of EGFR inhibitors since EGFR inhibitors upregulate the cyclin dependent kinase inhibitors, p27 (54, 55) and p21 (56) and thus produce G1 cell cycle arrest.
EGFR also plays a role in DNA repair. Ionizing radiation and chemotherapeutic agents produce a variety of types of DNA damage including single- and double-strand DNA breaks, DNA adducts, and DNA crosslinks. EGFR can physically interact with DNA-dependent protein kinase (DNA-PK) (57). In response to radiation, EGFR translocates to the nucleus which is associated with increased DNA-PK activity (58, 59). Inhibition of EGFR activation by cetuximab blocks nuclear EGFR import, DNA-PK activity, and radiation-induced DNA damage repair, and induces radiosensitization (60) (for a review see (61). Together, these results suggest that EGFR inhibitors could potentiate the efficacy of gemcitabine-radiation through inhibition of DNA repair.
In addition to kinase activity, EGFR may have important structural functions to inhibit cell death (62). In head and neck cancer, treatment with gemcitabine results in degradation of EGFR (63). EGFR degradation in response to gemcitabine is accompanied by inhibition of downstream EGFR signaling molecules such as AKT and ERK as well as cell death. In preclinical studies, EGFR degradation in response to gemcitabine correlated with response. In contrast, gemcitabine does not cause EGFR degradation in pancreatic cancer models (47). These differences in EGFR degradation may at least in part account for the greater sensitivity to gemcitabine in head and neck cancer versus pancreatic cancer models.
The finding that EGFR inhibitors produce much greater effects in head and neck versus pancreatic cancer tumor models (47, 64) illustrates the importance of the cellular context of EGFR activation or inhibition. One plausible explanation for the relative insensitivity of pancreatic cancers to EGFR inhibitors is the presence of mutant Ras in more than 85% of pancreatic cancers (65). Mutant Ras confers resistance to EGFR inhibition (66, 67). While Ras mutation confers resistance to EGFR inhibitor monotherapy and combination EGFR inhibitor-chemotherapy, some preclinical models have demonstrated radiosensitization by EGFR inhibitors in Ras mutant cell types, which could be explained by inhibition of EGFR/H-Ras (7, 68-70). While the role of Ras mutation status in patients treated with radiation and EGFR inhibitor therapies has not yet been determined, the consensus of the existing clinical data is that Ras mutation confers resistance to both EGFR inhibitor monotherapy as well as combination EGFR inhibitor-chemotherapy. Recent clinical studies in colorectal cancer and non-small-cell lung cancer demonstrated a lack of efficacy of EGFR inhibitors (as monotherapy and in combination with chemotherapy) against tumors with Ras mutations (71-73) Since Ras mutation is present in the majority of pancreatic cancers and EGFR inhibitors have produced limited benefit, retrospective studies to determine the influence of Ras mutation on EGFR inhibitor sensitivity should be conducted. Together, these studies indicate that the influence of EGFR inhibition on survival is influenced by the presence of other activated pathways, such as Ras.
Combining molecularly targeted agents with gemcitabine-radiotherapy in the clinic
Gemcitabine and radiation have been used in combination to treat a variety of solid tumors types including lung, head and neck, cervix, bladder, and breast (for a review see (74, 75). Based on its two distinct mechanisms of action (incorporation into DNA and ribonucleotide reductase inhibition), gemcitabine has been used clinically both as a chemotherapeutic agent and as a radiation sensitizer, effects separable by concentration. For example, early clinical trials in pancreatic cancer investigated low dose gemcitabine concurrent with standard radiation (50.4 Gy in 1.8 Gy fractions) in patients with locally advanced pancreatic cancer (76) and determined the maximum tolerated dose of gemcitabine to be approximately 40 mg/m2 given twice a week. In latter trials patients treated with 350−500 mg/m2 gemcitabine weekly and 30−33 Gy in 3 Gy fractions (77) experienced unacceptable toxicities (fatigue, anorexia, vomiting, etc.). It has been speculated that the relatively large standard radiation fields including clinically uninvolved regional lymph nodes increased the toxicity of the combination therapy. Our study used a standard chemotherapeutic dose of gemcitabine (1000mg/m2), which should maximize systemic control, with dose-escalated 3D conformal radiotherapy administered to the gross disease only, with exclusion of clinically uninvolved regional lymph nodes (78). We found this treatment was tolerable and produced a favorable objective response rate (10/33 patients) and median survival (11.6 months). The great majority of the recurrences were systemic, suggesting that the most important need was better systemic therapy. Subsequent preclinical and clinical trials have been carried out adding cisplatin or oxaliplatin to gemcitabine-radiation (4, 5, 79, 80).
Unfortunately, neither cisplatin-gemcitabine nor oxaliplatin-gemcitabine significantly prolong survival compared to gemcitabine alone in the treatment of metastatic disease (81, 82), suggesting that these combinations will only modestly improve the treatment of locally advanced, non-metastatic disease. Likewise, adding capecitabine to gemcitabine marginally improved the survival of patients with metastatic disease in one study (median 6 months to 7.4 months (83)) but not in another (84).
Therefore, we have turned to integrating targeted agents with gemcitabine-radiation with the goal of improving systemic disease control while maintaining or improving local radiosensitization. This has led us to combine EGFR or Chk1 inhibitors with gemcitabine-radiation. Because both preclinical and clinical studies have demonstrated that erlotinib plus gemcitabine is superior to gemcitabine alone, we have initiated studies combining EGFR inhibitors with gemcitabine and radiation. While clinical trials combining Chk1 inhibitors with gemcitabine are underway, a variety of preclinical models have demonstrated enhanced gemcitabine-mediated radiosensitization as well as cytotoxicity in response to Chk1 inhibitors (Figure 4) (36). These studies have prompted our ongoing investigation of Chk1 inhibitors in combination with gemcitabine-radiation.
Looking into the future
One of our current goals in gemcitabine-radiation therapy for pancreatic cancer is to integrate a third agent to gemcitabine-radiation therapy that improves systemic disease control (cytotoxicity) without reducing local tumor control (radiosensitization). In the previous decade we have successfully added other standard chemotherapeutic agents (i.e. cisplatin, oxaliplatin) to gemcitabine-radiation therapy. However, trials combining agents such as oxaliplatin, cisplatin, irinotecan, and 5-fluorouracil have not significantly improved survival (although capecitabine may). Likewise, targeted therapies such as marimastat (matrix metalloproteinase inhibitor), and tipifarnib (farnesyltransferase inhibitor) with gemcitabine have not produced significant survival improvements over gemcitabine alone (85). Thus, the finding that the addition of erlotinib to gemcitabine produced a significant (yet modest) improvement in survival (0.3 months) compared to gemcitabine alone is of interest (45).
How can laboratory studies help us improve on these results? One obvious strategy is better patient selection. For example, it is conceivable that the efficacy of the combination of gemcitabine with EGFR inhibitors could be improved upon by identifying populations of patients most sensitive to EGFR inhibition, such as those who lack Ras activation (71-73) or who develop a rash in response to EGFR inhibitor therapy (86). Another approach to improve the clinical efficacy of molecularly targeted agents in combination with gemcitabine or gemcitabine-radiation is through preclinical determination of the optimal sequence of gemcitabine, radiation, and EGFR inhibitor. For instance, in the aforementioned clinical trial, EGFR inhibitor was given concurrently with gemcitabine and produced a modest survival advantage. It seems possible that survival might have been improved if the most effective preclinical schedule (gemcitabine prior to EGFR inhibitor) had been used. Other targets, such as Chk1, need to be explored in combination with gemcitabine-radiation therapy. The utilization of better preclinical models such as tumor xenografts derived from primary human tumors will be crucial in order to translate results directly to the clinic. In addition, the effects of therapy combinations on tumor stem cells versus gross tumor (87) may provide insight into potential therapeutic efficacy. This decade will focus on preclinical studies in the best available model systems, combining molecularly targeted therapies with gemcitabine-radiation with the goal of producing better patient responses.
BOX 1. Mechanisms of radiosensitization by gemcitabine.
Footnotes
Conflict of Interest Statement: No conflicts of interest exist.
Statement of translational relevance
Gemcitabine with radiation is a standard of care in the treatment of locally advanced pancreatic cancer. However, even with the best available care patient median survival is in the range of one year. Thus we have focused our efforts on improving therapy for pancreatic cancer by using multi-modality therapies including gemcitabine, radiation, and cisplatin or oxaliplatin. However, the use of multiple cytotoxic agents produces additional toxicity. Thus it seems likely that the future of pancreatic cancer therapy will integrate molecularly targeted therapies with standard chemoradiation regimens. We describe here two promising molecular targets: EGFR and Chk1. Our hope is that this review will stimulate rationally designed clinical trials combining molecularly targeted agents with gemcitabine-radiation with the ultimate goal of improving survival.
In solid tumor cells treated with gemcitabine, dATP pools are depleted. This is in contrast to leukemia cells, which in response to gemcitabine display a pronounced depletion of dCTP pools 11.
Heinemann V, Xu YZ, Chubb S, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2',2'-difluorodeoxycytidine. Molecular pharmacology 1990;38: 567−72..
References
- 1.Safran H, Dipetrillo T, Iannitti D, et al. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a Phase I trial. International journal of radiation oncology, biology, physics. 2002;54:137–41. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 2.Blackstock AW, Melin SA, Butler JM, et al. Irinotecan/gemcitabine followed by twice-weekly gemcitabine/radiation in locally advanced pancreatic cancer. Oncology (Williston Park) 2002;16:25–8. [PubMed] [Google Scholar]
- 3.Kachnic LA, Shaw JE, Manning MA, Lauve AD, Neifeld JP. Gemcitabine following radiotherapy with concurrent 5-fluorouracil for nonmetastatic adenocarcinoma of the pancreas. International journal of cancer. 2001;96:132–9. doi: 10.1002/ijc.1008. [DOI] [PubMed] [Google Scholar]
- 4.Symon Z, Davis M, McGinn CJ, Zalupski MM, Lawrence TS. Concurrent chemoradiotherapy with gemcitabine and cisplatin for pancreatic cancer: from the laboratory to the clinic. International journal of radiation oncology, biology, physics. 2002;53:140–5. doi: 10.1016/s0360-3016(01)02790-0. [DOI] [PubMed] [Google Scholar]
- 5.Morgan MA, Meirovitz A, Davis MA, Kollar LE, Hassan MC, Lawrence TS. Radiotherapy combined with gemcitabine and oxaliplatin in pancreatic cancer cells. Translational Oncology. 2008;1:36–43. doi: 10.1593/tlo.07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianco C, Giovannetti E, Ciardiello F, et al. Synergistic antitumor activity of ZD6474, an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, with gemcitabine and ionizing radiation against pancreatic cancer. Clin Cancer Res. 2006;12:7099–107. doi: 10.1158/1078-0432.CCR-06-0833. [DOI] [PubMed] [Google Scholar]
- 7.Buchsbaum DJ, Bonner JA, Grizzle WE, et al. Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. International journal of radiation oncology, biology, physics. 2002;54:1180–93. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- 8.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–8. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 9.Grimison P, Galettis P, Manners S, et al. Randomized crossover study evaluating the effect of gemcitabine infusion dose rate: evidence of auto-induction of gemcitabine accumulation. J Clin Oncol. 2007;25:5704–9. doi: 10.1200/JCO.2007.10.7078. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi V. Questions about gemcitabine dose rate: answered or unanswered? J Clin Oncol. 2007;25:5691–4. doi: 10.1200/JCO.2007.13.6879. [DOI] [PubMed] [Google Scholar]
- 11.Heinemann V, Xu YZ, Chubb S, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2',2'-difluorodeoxycytidine. Molecular pharmacology. 1990;38:567–72. [PubMed] [Google Scholar]
- 12.Shewach DS, Hahn TM, Chang E, Hertel LW, Lawrence TS. Metabolism of 2',2'-difluoro-2'-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res. 1994;54:3218–23. [PubMed] [Google Scholar]
- 13.Shewach DS, Lawrence TS. Radiosensitization of human tumor cells by gemcitabine in vitro. Semin Oncol. 1995;22:68–71. [PubMed] [Google Scholar]
- 14.Rockwell S, Grindey GB. Effect of 2',2'-difluorodeoxycytidine on the viability and radiosensitivity of EMT6 cells in vitro. Oncol Res. 1992;4:151–5. [PubMed] [Google Scholar]
- 15.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2',2'-difluoro-2'-deoxycytidine. International journal of radiation oncology, biology, physics. 1996;34:867–72. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence TS, Davis MA, Hough A, Rehemtulla A. The role of apoptosis in 2',2'-difluoro-2'-deoxycytidine (gemcitabine)-mediated radiosensitization. Clin Cancer Res. 2001;7:314–9. [PubMed] [Google Scholar]
- 17.Milas L, Fujii T, Hunter N, et al. Enhancement of tumor radioresponse in vivo by gemcitabine. Cancer Res. 1999;59:107–14. [PubMed] [Google Scholar]
- 18.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Seminars in radiation oncology. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 19.Robinson BW, Im MM, Ljungman M, Praz F, Shewach DS. Enhanced radiosensitization with gemcitabine in mismatch repair-deficient HCT116 cells. Cancer Res. 2003;63:6935–41. [PubMed] [Google Scholar]
- 20.Pauwels B, Korst AE, Pattyn GG, et al. Cell cycle effect of gemcitabine and its role in the radiosensitizing mechanism in vitro. International journal of radiation oncology, biology, physics. 2003;57:1075–83. doi: 10.1016/s0360-3016(03)01443-3. [DOI] [PubMed] [Google Scholar]
- 21.Ostruszka LJ, Shewach DS. The role of cell cycle progression in radiosensitization by 2',2'-difluoro-2'-deoxycytidine. Cancer Res. 2000;60:6080–8. [PubMed] [Google Scholar]
- 22.Morgan MA, El Shaikh M, Abu-Isa E, Davis MA, Lawrence TS. Radiosensitization by gemcitabine fixed-dose-rate infusion versus bolus injection in a pancreatic cancer model. Translational Oncology. 2008;1:44–9. doi: 10.1593/tlo.07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Putten JWG, Groen HJM, Smid K, Peters GJ, Kampinga HH. Endjoining deficiency and radiosensitization induced by gemcitabine. Cancer Res. 2001;61:1585–91. [PubMed] [Google Scholar]
- 24.Gregoire V, Beauduin M, Bruniaux M, De Coster B, Octave Prignot M, Scalliet P. Radiosensitization of mouse sarcoma cells by fludarabine (F-ara-A) or gemcitabine (dFdC), two nucleoside analogues, is not mediated by an increased induction or a repair inhibition of DNA double-strand breaks as measured by pulsed-field gel electrophoresis. Int J Radiat Biol. 1998;73:511–20. doi: 10.1080/095530098142059. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol. 1997;24:S7-24–S7-8. [PubMed] [Google Scholar]
- 26.Jeggo P, Lobrich M. Radiation-induced DNA damage responses. Radiation protection dosimetry. 2006;122:124–7. doi: 10.1093/rpd/ncl495. [DOI] [PubMed] [Google Scholar]
- 27.Wachters FM, van Putten JW, Maring JG, Zdzienicka MZ, Groen HJ, Kampinga HH. Selective targeting of homologous DNA recombination repair by gemcitabine. International journal of radiation oncology, biology, physics. 2003;57:553–62. doi: 10.1016/s0360-3016(03)00503-0. [DOI] [PubMed] [Google Scholar]
- 28.van Bree C, Rodermond HM, de Vos J, Haveman J, Franken NA. Mismatch repair proficiency is not required for radioenhancement by gemcitabine. International journal of radiation oncology, biology, physics. 2005;62:1504–9. doi: 10.1016/j.ijrobp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Morgan MA, Poynter JN, Maybaum J, Lawrence TS. The role of Bcl-X(S) in radiation sensitivity. Radiation research. 2004;161:535–9. doi: 10.1667/rr3169. [DOI] [PubMed] [Google Scholar]
- 30.Pauwels B, Korst AE, Andriessen V, et al. Unraveling the mechanism of radiosensitization by gemcitabine: the role of TP53. Radiation research. 2005;164:642–50. doi: 10.1667/rr3445.1. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, Hough AM, Lawrence TS. The role of p53 in gemcitabine-mediated cytotoxicity and radiosensitization. Cancer Chemother Pharmacol. 2000;45:369–74. doi: 10.1007/s002800051004. [DOI] [PubMed] [Google Scholar]
- 32.Robinson BW, Shewach DS. Radiosensitization by gemcitabine in p53 wild-type and mutant MCF-7 breast carcinoma cell lines. Clin Cancer Res. 2001;7:2581–9. [PubMed] [Google Scholar]
- 33.Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65:6835–42. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 34.Karnitz LM, Flatten KS, Wagner JM, et al. Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Molecular pharmacology. 2005;68:1636–44. doi: 10.1124/mol.105.012716. [DOI] [PubMed] [Google Scholar]
- 35.Matthews DJ, Yakes FM, Chen J, et al. Pharmacological Abrogation of S-Phase Checkpoint Enhances the Anti-Tumor Activity of Gemcitabine In Vivo. Cell Cycle. 2007:6. doi: 10.4161/cc.6.1.3699. [DOI] [PubMed] [Google Scholar]
- 36.Parsels LA, Morgan MA, Tanska DM, et al. Gemcitabine sensitization by Chk1 inhibition correlates with inhibition of a Rad51 DNA damage response. Manuscript in preparation. [DOI] [PMC free article] [PubMed]
- 37.Morgan MA, Parsels LA, Parsels JD, Lawrence TS, Maybaum J. The relationship of premature mitosis to cytotoxicity in response to checkpoint abrogation and antimetabolite treatment. Cell Cycle. 2006;5:1983–8. doi: 10.4161/cc.5.17.3184. [DOI] [PubMed] [Google Scholar]
- 38.Zabludoff SD, Deng C, Grondine MR, et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA targeted therapies. Molecular cancer therapeutics. 2008 doi: 10.1158/1535-7163.MCT-08-0492. In press. [DOI] [PubMed] [Google Scholar]
- 39.Carrassa L, Broggini M, Erba E, Damia G. Chk1, but not Chk2, is involved in the cellular response to DNA damaging agents: differential activity in cells expressing or not p53. Cell Cycle. 2004;3:1177–81. [PubMed] [Google Scholar]
- 40.Playle LC, Hicks DJ, Qualtrough D, Paraskeva C. Abrogation of the radiation-induced G2 checkpoint by the staurosporine derivative UCN-01 is associated with radiosensitisation in a subset of colorectal tumour cell lines. British journal of cancer. 2002;87:352–8. doi: 10.1038/sj.bjc.6600492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syljuasen RG, Sorensen CS, Nylandsted J, Lukas C, Lukas J, Bartek J. Inhibition of Chk1 by CEP-3891 accelerates mitotic nuclear fragmentation in response to ionizing Radiation. Cancer Res. 2004;64:9035–40. doi: 10.1158/0008-5472.CAN-04-2434. [DOI] [PubMed] [Google Scholar]
- 42.Koniaras K, Cuddihy AR, Christopoulos H, Hogg A, O'Connell MJ. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene. 2001;20:7453–63. doi: 10.1038/sj.onc.1204942. [DOI] [PubMed] [Google Scholar]
- 43.Smaill JB, Lee HH, Palmer BD, et al. Synthesis and structure-activity relationships of soluble 8-substituted 4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-diones as inhibitors of the Wee1 and Chk1 checkpoint kinases. Bioorganic & medicinal chemistry letters. 2008;18:929–33. doi: 10.1016/j.bmcl.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 44.Janetka JW, Ashwell S, Zabludoff S, Lyne P. Inhibitors of checkpoint kinases: from discovery to the clinic. Current opinion in drug discovery & development. 2007;10:473–86. [PubMed] [Google Scholar]
- 45.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 46.Chun PY, Feng FY, Scheurer AM, Davis MA, Lawrence TS, Nyati MK. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66:981–8. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 47.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of EGFR inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-4072. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzariti A, Xu JM, Porcelli L, Paradiso A. The schedule-dependent enhanced cytotoxic activity of 7-ethyl-10-hydroxy-camptothecin (SN-38) in combination with Gefitinib (Iressa, ZD1839). Biochemical pharmacology. 2004;68:135–44. doi: 10.1016/j.bcp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Benhar M, Engelberg D, Levitzki A. Cisplatin-induced activation of the EGF receptor. Oncogene. 2002;21:8723–31. doi: 10.1038/sj.onc.1205980. [DOI] [PubMed] [Google Scholar]
- 50.Van Schaeybroeck S, Karaiskou-McCaul A, Kelly D, et al. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin Cancer Res. 2005;11:7480–9. doi: 10.1158/1078-0432.CCR-05-0328. [DOI] [PubMed] [Google Scholar]
- 51.Sumitomo M, Asano T, Asakuma J, Asano T, Horiguchi A, Hayakawa M. ZD1839 modulates paclitaxel response in renal cancer by blocking paclitaxel-induced activation of the epidermal growth factor receptor-extracellular signal-regulated kinase pathway. Clin Cancer Res. 2004;10:794–801. doi: 10.1158/1078-0432.ccr-0948-03. [DOI] [PubMed] [Google Scholar]
- 52.Morelli MP, Cascone T, Troiani T, et al. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(Suppl 4):iv61–8. doi: 10.1093/annonc/mdi910. [DOI] [PubMed] [Google Scholar]
- 53.Rosetti M, Tesei A, Ulivi P, et al. Modulation of drug cytotoxicity by Iressa (ZD1839) in pancreatic cancer cell lines. Cancer biology & therapy. 2005;4:1089–95. doi: 10.4161/cbt.4.10.1995. [DOI] [PubMed] [Google Scholar]
- 54.Ling YH, Li T, Yuan Z, Haigentz M, Jr., Weber TK, Perez-Soler R. Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27KIP1 up-regulation and nuclear translocation in association with cell growth inhibition and G1/S phase arrest in human non-small-cell lung cancer cell lines. Molecular pharmacology. 2007;72:248–58. doi: 10.1124/mol.107.034827. [DOI] [PubMed] [Google Scholar]
- 55.Busse D, Doughty RS, Ramsey TT, et al. Reversible G(1) arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27(KIP1) independent of MAPK activity. The Journal of biological chemistry. 2000;275:6987–95. doi: 10.1074/jbc.275.10.6987. [DOI] [PubMed] [Google Scholar]
- 56.Di Gennaro E, Barbarino M, Bruzzese F, et al. Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 (‘Iressa’), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cells. Journal of cellular physiology. 2003;195:139–50. doi: 10.1002/jcp.10239. [DOI] [PubMed] [Google Scholar]
- 57.Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. The Journal of biological chemistry. 1998;273:1568–73. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 58.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. The Journal of biological chemistry. 2005;280:31182–9. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 59.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. The radioprotector Bowman-Birk proteinase inhibitor stimulates DNA repair via epidermal growth factor receptor phosphorylation and nuclear transport. Radiother Oncol. 2008;86:375–82. doi: 10.1016/j.radonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005;76:157–61. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13:6555–60. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- 62.Weihua Z, Tsan R, Huang WC, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–93. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng FY, Varambally S, Tomlins SA, et al. Role of epidermal growth factor receptor degradation in gemcitabine-mediated cytotoxicity. Oncogene. 2007;26:3431–9. doi: 10.1038/sj.onc.1210129. [DOI] [PubMed] [Google Scholar]
- 64.Feng FY, Lopez CA, Normolle DP, et al. Effect of epidermal growth factor receptor inhibitor class in the treatment of head and neck cancer with concurrent radiochemotherapy in vivo. Clin Cancer Res. 2007;13:2512–8. doi: 10.1158/1078-0432.CCR-06-2582. [DOI] [PubMed] [Google Scholar]
- 65.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 66.Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. International journal of cancer. 2006;118:209–14. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- 67.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 68.Toulany M, Baumann M, Rodemann HP. Stimulated PI3K-AKT signaling mediated through ligand or radiation-induced EGFR depends indirectly, but not directly, on constitutive K-Ras activity. Mol Cancer Res. 2007;5:863–72. doi: 10.1158/1541-7786.MCR-06-0297. [DOI] [PubMed] [Google Scholar]
- 69.Cengel KA, Voong KR, Chandrasekaran S, et al. Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells. Neoplasia. 2007;9:341–8. doi: 10.1593/neo.06823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toulany M, Dittmann K, Kruger M, Baumann M, Rodemann HP. Radioresistance of K-Ras mutated human tumor cells is mediated through EGFR-dependent activation of PI3K-AKT pathway. Radiother Oncol. 2005;76:143–50. doi: 10.1016/j.radonc.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 71.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 72.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. British journal of cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 74.Spalding AC, Lawrence TS. New and emerging radiosensitizers and radioprotectors. Cancer investigation. 2006;24:444–56. doi: 10.1080/07357900600705706. [DOI] [PubMed] [Google Scholar]
- 75.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25:4043–50. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 76.Blackstock AW, Bernard SA, Richards F, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–12. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 77.Wolff RA, Evans DB, Gravel DM, et al. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res. 2001;7:2246–53. [PubMed] [Google Scholar]
- 78.McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–8. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 79.Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22:238–43. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 80.Desai SP, Ben-Josef E, Normolle DP, et al. Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol. 2007;25:4587–92. doi: 10.1200/JCO.2007.12.0592. [DOI] [PubMed] [Google Scholar]
- 81.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–16. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–52. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 83.Cunningham D, Chau I, Stocken D, et al. Phase III randomised comparison of gemcitabine (GEM) versus gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. Eur J Cancer. 2005;3(suppl 2005) [Google Scholar]
- 84.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–7. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 85.Van Cutsem E, Verslype C, Grusenmeyer PA. Lessons learned in the management of advanced pancreatic cancer. J Clin Oncol. 2007;25:1949–52. doi: 10.1200/JCO.2006.09.4664. [DOI] [PubMed] [Google Scholar]
- 86.Perez-Soler R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clinical lung cancer. 2006;8(Suppl 1):S7–14. doi: 10.3816/clc.2006.s.008. [DOI] [PubMed] [Google Scholar]
- 87.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 88.Huang P, Plunkett W. Fludarabine- and gemcitabine-induced apoptosis: incorporation of analogs into DNA is a critical event. Cancer Chemother Pharmacol. 1995;36:181–8. doi: 10.1007/BF00685844. [DOI] [PubMed] [Google Scholar]
- 89.Ruiz van Haperen VW, Veerman G, Vermorken JB, Peters GJ. 2',2'-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochemical pharmacology. 1993;46:762–6. doi: 10.1016/0006-2952(93)90566-f. [DOI] [PubMed] [Google Scholar]
- 90.Ostruszka LJ, Shewach DS. The role of DNA synthesis inhibition in the cytotoxicity of 2',2'-difluoro-2'-deoxycytidine. Cancer Chemother Pharmacol. 2003;52:325–32. doi: 10.1007/s00280-003-0661-5. [DOI] [PubMed] [Google Scholar]
- 91.Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. International journal of radiation oncology, biology, physics. 1988;15:953–8. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 92.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 93.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–7. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 94.Gatei M, Sloper K, Sorensen C, et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. The Journal of biological chemistry. 2003;278:14806–11. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- 95.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mailand N, Falck J, Lukas C, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–9. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 97.Sturla LM, Amorino G, Alexander MS, Mikkelsen RB, Valerie K, Schmidt-Ullrichr RK. Requirement of Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth factor receptor by ionizing radiation and modulation by SHP2. The Journal of biological chemistry. 2005;280:14597–604. doi: 10.1074/jbc.M413287200. [DOI] [PubMed] [Google Scholar]
- 98.Friedmann BJ, Caplin M, Savic B, et al. Interaction of the epidermal growth factor receptor and the DNA-dependent protein kinase pathway following gefitinib treatment. Molecular cancer therapeutics. 2006;5:209–18. doi: 10.1158/1535-7163.MCT-05-0239. [DOI] [PubMed] [Google Scholar]
- 99.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiation research. 1984;99:73–84. [PubMed] [Google Scholar]