The multi-factorial etiology of atherosclerosis includes inflammatory and immune processes that involve numerous cell types including monocytic and lymphocytic leukocytes, the endothelium, smooth muscle cells and fibroblasts. It is likely that each of these cell types, as a contributor to the atherosclerotic process, possesses a gene expression profile unique to its role in disease pathogenesis, though perhaps also dependent on the disease stage as well as on genetic and environmental factors. Modulation of gene expression patterns by various transcription factors will therefore dictate how a particular cell type contributes to the atherosclerotic process. Kruppel-like factors (KLFs) are a subclass of zinc-finger transcription factors originally implicated in cell growth and differentiation. KLF2 and KLF4 in particular regulate the expression of certain genes relevant to atherosclerosis in a shear-dependent manner in endothelial cells and monocytes1. It is therefore important to understand the mechanisms and consequences of KLF activation in these cell types in vitro and in vivo. Such studies have the potential to uncover previously unknown transcriptional links between shear-dependent monocytic and endothelial processes that may underlie the pathogenesis of atherosclerosis.
Studies by Atkins, et al2 in this volume address this issue head on using mice with a hemizygous deficiency of KLF2 [KLF2(+/−)] and on the ApoE(−/−) background to examine the role of KLF2 in atherosclerosis. Aortic lesion extent was increased 30–35% in KLF2(+/−) mice compared to control littermates. The increase in atherosclerosis in the KLF2(+/−) mice was associated with no alterations in aortic expression of the pro-inflammatory genes endothelial nitric oxide synthase (eNOS), thrombomodulin, or vascular cell adhesion molecule-1 (VCAM-1). Lesion macrophage content was not significantly increased in KLF2(+/−) mice, but both lipid uptake and expression of a lipid chaperone protein (aP2/FABP4) were increased in macrophages from KLF2(+/−) mice and were concomitantly reduced in a macrophage cell line over-expressing KLF2. These observations suggest that KLF2 expression is an important modulator of foam cell formation and thereby atherogenesis via regulation of aP2/FABP4 expression.
Atkins et al.2 show for the first time that an approximate 50% reduction of KLF2 results in an increase in the extent of atherosclerosis in vivo. These experiments imply that global expression of KLF2 exerts an atheroprotective effect in vivo, but they do not determine in which cells types gene expression is being regulated by KLF2 to protect against atheroma formation. Interestingly, Das et al.3 recently modulated KLF2 expression to show that KLF2 negatively regulates the expression of proinflammatory genes and processes in monocytes. They also showed that monocytes from patients with severe atherosclerosis and elevated levels of markers for inflammation have an approximate 30% reduction in KLF2 expression. The in vivo data from the KLF2 hemizygous mice therefore correspond closely to KLF2 expression changes in circulating cells from humans with atherosclerosis, highlighting the importance of monocyte/macrophage KLF2 expression levels in the regulation of atherogenesis.
Due to the global nature of the KLF2 deletion, the atheroprotective effects of KLF2 identified in the present studies cannot be attributed to a particular cell type. The data of Das et al.3 discussed above implicate KLF2 regulation of gene expression within the monocytic lineage as a likely mediator, at least in part, of the phenotype. However, KLF2 has been implicated in differentiation of additional cell types that participate in atherogenesis (Figure 1). KLF2 functions as a key molecular switch in endothelial cells, regulating expression of numerous genes that modulate the inflammatory, thrombotic, angiogenic, and vasoactive properties of endothelial cells1. KLF2 expression also serves as a regulator of T-cell development, and directs their trafficking and recirculation via modulating their chemokine receptor expression patterns4, 5. Finally, KLF2 is a negative regulator of adipogenesis and modulator of lipid metabolism as a result of its ability to inhibit PPARγ expression in adipocytes6. Each of these cell types participates in the pathogenesis of atherosclerosis, either directly or indirectly, and therefore may contribute to the observed phenotype. The atheroprone phenotypes in mice with a global reduction of KLF2 expression are quite possibly a compound effect of consequences in multiple cell types. Thus, cell-type specific deletions of KLF2 will be required to determine the relative contribution to the phenotype of KLF2-mediated processes in each cell type.
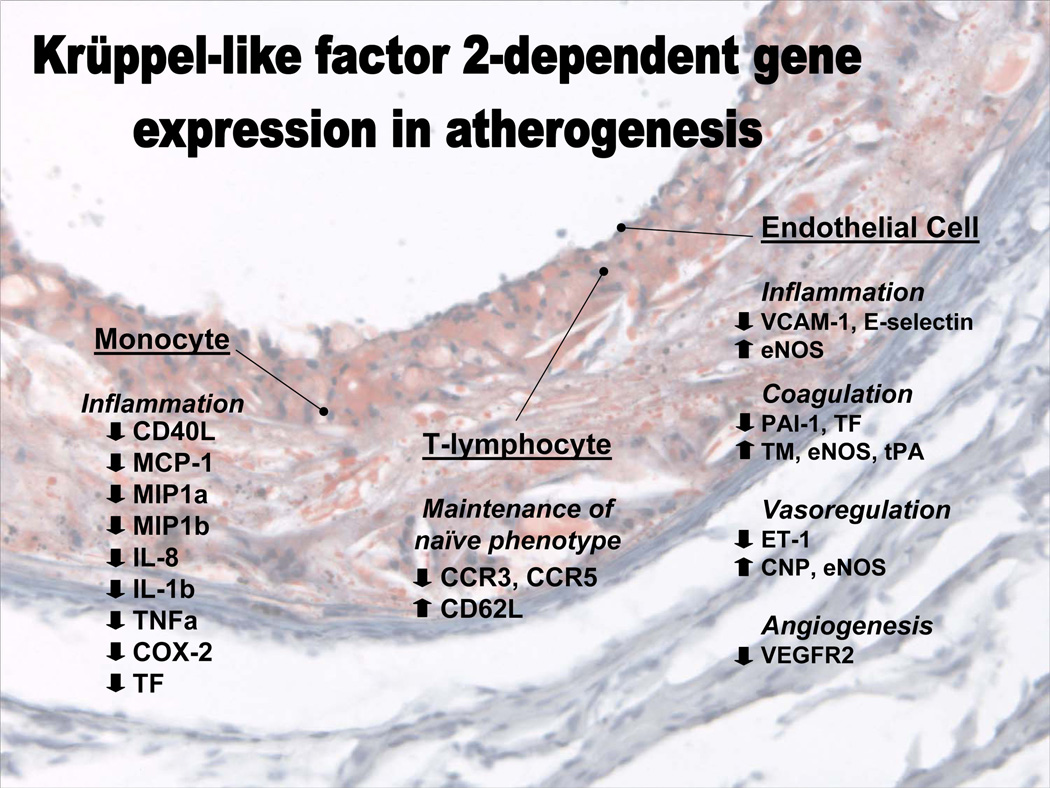
Figure 1.
Schematic diagram showing for certain cells involved in atherogenesis the known genes whose expression is regulated by Kruppel-like factor 2. Text is superimposed on a frozen section micrograph of a low density lipoprotein receptor-deficient mouse aorta stained with oil red O and hematoxylin. CD 40L, CD40 ligand; MCP-1, monocyte chemotactic protein-1; MIP, macrophage inflammatory protein; IL, interleukin; TNFα, tumor necrosis factor α, COX-2, cyclooxygenase 2; TF, tissue factor; CCR, C-C type chemokine receptor; CD62L, L-selectin; VCAM-1, vascular cell adhesion molecule-1; eNOS, endothelial nitric oxide synthase; PAI-1, plasminogen activator inhibitor-1; TM, thrombomodulin; tPA, tissue plasminogen activator; ET-1, endothelin-1; CNP, C naturetic peptide; VEGFR2, vascular endothelial growth factor 2.
The present studies also document the important observation that the Kruppel-like factors may exhibit some amount of redundancy. KLF4 expression was increased by 40% in the KLF2(+/−) mice. Such compensatory changes may serve to maintain homeostasis, at least in endothelial cells where both KLF2 and 4 are both known to regulate eNOS and VCAM-1 expression in a similar manner7. The possibility of redundancy is supported in part by phylogenetic studies showing that in man, mouse8, xenopus9, and zebrafish10, KLF2 and KLF4 are closely related members of the larger KLF family, suggesting the possibility of a gene duplication event. Functional redundancy of these two family members is also suggested based on the observation that both increase eNOS expression and decrease VCAM-1 expression in endothelial cells7. However, it is not clear whether this overlap in gene expression can be generalized to other KLF2-regulated genes such as E-selectin, tissue factor, endothelin-1, vascular endothelial growth factor receptor 2, or others1. It is also not clear whether functional similarities between KLF2 and KLF4 are present in other cell types such as monocytic cells, or T-lymphocytes. It is possible that further investigation will reveal that the functional relationship between KLF2 and KLF4 is similar to that between PPARα, PPARβ/δ, and PPARγ. Initial studies demonstrated that these transcription factors can each regulate a common set of genes. However, more detailed studies have demonstrated differences in their gene regulatory actions based on cell-specific activity modulation by numerous coactivator and corepressor molecules11. The extent and functional consequences of the proposed redundancy of the KLFs will require additional studies of the actions of KLF2 and especially KLF4 in endothelial cells as well as other cell types. Such studies will likely require the use of compound loss-of-function mutations for both KLFs as well as tissue-specific loss-of-function mutations for one or both KLFs.
Given the remarkable observations of Atkins and colleagues2, it is intriguing to muse about the possibility that pharmacologic manipulation of KLF2 expression may be an effective therapeutic strategy for the treatment of atherosclerosis and/or other cardiovascular disease processes. In fact, there is evidence suggesting that a current and common therapeutic treatment for atherosclerosis works in part by modulating KLF2 expression. Several groups have shown that HMGCoA reductase inhibitors (commonly referred to as statins) increase KLF2 expression in monocytic cells12 and in endothelial cells13. To the extent that the atheroprotective effects of statins exceed what is expected due to their lipid-lowering effects in humans, the additional protective effects of statins independent of lipid effects may arise due to increased KLF2 expression. Future experiments to determine whether the beneficial effects of statins are mediated in part by KLF2 will be extremely interesting and important to our understanding of the mechanisms of atherosclerotic disease and to the application of this knowledge to treat the most common cause of death and disability in our society.
Acknowledgments
Sources of Funding:
Work in the authors’ laboratories is supported by an American Heart Association Fellow to Faculty Transition Award 0275023N and NIH grant HL090823 (J.H.) and NIH grants GM61728, HL65619, AG02482 and HL61656 (C.P.) C.P. is an Established Investigator of the American Heart Association and a Burroughs Wellcome Fund Clinical Scientist in Translational Research.
Footnotes
See also Atkins et al., Hemizygous Deficiency of Kruppel-like Factor 2 Augments Experimental Atherosclerosis, this issue
Disclosures: None.
References
- 1.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100(12):1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 2.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103 doi: 10.1161/CIRCRESAHA.108.184663. XXX – XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das H, Kumar A, Lin Z, Patino WD, Hwang PM, Feinberg MW, Majumder PK, Jain MK. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(17):6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nature immunology. 2008;9(3):292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 5.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442(7100):299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. The Journal of biological chemistry. 2003;278(4):2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 7.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. The Journal of biological chemistry. 2007;282(18):13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 8.Bieker JJ. Kruppel-like factors: three fingers in many pies. The Journal of biological chemistry. 2001;276(37):34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 9.Lavallee G, Andelfinger G, Nadeau M, Lefebvre C, Nemer G, Horb ME, Nemer M. The Kruppel-like transcription factor KLF13 is a novel regulator of heart development. The EMBO journal. 2006;25(21):5201–5213. doi: 10.1038/sj.emboj.7601379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oates AC, Pratt SJ, Vail B, Yan Y, Ho RK, Johnson SL, Postlethwait JH, Zon LI. The zebrafish klf gene family. Blood. 2001;98(6):1792–1801. doi: 10.1182/blood.v98.6.1792. [DOI] [PubMed] [Google Scholar]
- 11.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Progress in lipid research. 2006;45(2):120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Tuomisto TT, Lumivuori H, Kansanen E, Hakkinen SK, Turunen MP, van Thienen JV, Horrevoets AJ, Levonen AL, Yla-Herttuala S. Simvastatin has an anti-inflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc Res. 2008;78(1):175–184. doi: 10.1093/cvr/cvn007. [DOI] [PubMed] [Google Scholar]
- 13.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112(5):720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]