Abstract
Background and purpose:
Atrial inotropic responses to 5-HT mediated through 5-HT4 receptors fade, presumably through phosphodiesterase (PDE) activity. We investigated the influence of a selective inhibitor of PDE3 (cilostamide) or of PDE4 (rolipram) on the fade of 5-HT responses in atrial muscle.
Experimental approach:
5-HT responses were compared, ex vivo, on sinoatrial beating rate of newborn piglets, porcine atrial and ventricular force, and human atrial force. cAMP levels were assessed in piglet atrium.
Key results:
5-HT-evoked sinoatrial tachycardia did not fade and was not potentiated by cilostamide (300 nmol·L−1) or rolipram (1 µmol·L−1). Inotropic responses to 5-HT faded in atria from piglets, adolescent pigs and humans. Cilostamide reduced atrial fade of 5-HT responses in adolescent pigs and humans but not in newborn piglets. Cilostamide disclosed 5-HT ventricular responses in newborn, but not adolescent pigs. Rolipram reduced fade of atrial 5-HT responses in newborn and adolescent pigs but not in humans. Concurrent cilostamide + rolipram abolished fade of 5-HT responses in porcine left atria and facilitated ventricular 5-HT responses, but did not reduce residual fade in human atrium in the presence of cilostamide. 5-HT-evoked increases in cAMP faded; fade was abolished by concurrent cilostamide + rolipram.
Conclusions and implications:
PDE3-induced control of porcine 5-HT responses differed in atrium and ventricle and changed with age. PDE3 and PDE4 jointly prevented fade of inotropic and cAMP responses to 5-HT in porcine atrium. Unlike porcine atria, only PDE3 induced fade of 5-HT responses in human atria.
Keywords: Phosphodiesterases-3 and -4, porcine and human atrium, sinoatrial node, ventricle, 5-HT4 receptors, cAMP
Introduction
Human (Kaumann et al., 1990; Sanders and Kaumann, 1992; Brattelid et al., 2004) and porcine (Kaumann, 1990; Parker et al., 1995) hearts express functional 5-HT4receptors that mediate cardiostimulation including arrhythmias (Kaumann, 1994; Kaumann and Sanders, 1994; Rahme et al., 1999; Pau et al., 2003; Leftheriotis et al., 2005) through cAMP-dependent pathways (see Kaumann and Levy, 2006a). Cyclic AMP is hydrolysed by phosphodiesterases (PDEs) and recent evidence has disclosed that the role of these enzymes is so important that they virtually prevent the functional manifestation of effects of 5-HT through human and porcine ventricular 5-HT4receptors. Only when PDE activity is inhibited with the non-selective PDE inhibitor, isobutyl-methylxanthine (IBMX), can positive inotropic and lusitropic effects of 5-HT, as well as stimulation of cAMP-dependent protein kinase (PKA) and even arrhythmias mediated by ventricular 5-HT4 receptors, become apparent (Brattelid et al., 2004), but which PDE isoenzymes are responsible is unknown.
The inotropic responses to 5-HT and 5-HT4 receptor partial agonists tend to fade in human (Kaumann et al., 1991; Sanders and Kaumann, 1992) and porcine (Parker et al., 1995) atria. De Maeyer et al.(2006) recently reported that IBMX prevents the fade of 5-HT responses in porcine atria but which PDE isoenzymes are mainly involved is still an open question (Kaumann and Levy, 2006b).
At least four PDE isoenzymes, PDEs 1–4, are expressed in the human heart and PDEs 2–4 have also been found in porcine heart (Zimmermann et al., 1994). PDE3 and PDE4 are the most important isoenzymes responsible for the hydrolysis of inotropically relevant cAMP, generated through receptor activation, but there are important species differences. The inotropic effects of noradrenaline, mediated through β1-adrenoceptors, are controlled mainly by PDE4 in murine (Galindo-Tovar and Kaumann, 2008) and rat (Katano and Endoh, 1992; Vargas et al., 2006) hearts, but controlled by PDE3 in human heart (Christ et al., 2006; Kaumann et al., 2007).
As the PDE isoenzymes that blunt 5-HT4-receptor-mediated cardiostimulation were unknown (Kaumann and Levy, 2006b), we sought to investigate the role of PDE3 and PDE4 in three cardiac regions of the heart of newborn piglets, a model for human cardiac 5-HT4receptors. Cilostamide and rolipram were used to selectively inhibit PDE3 and PDE4 respectively (Vargas et al., 2006; Galindo-Tovar and Kaumann, 2008). We investigated the effects of the PDE inhibitors on 5-HT-evoked sinoatrial tachycardia, as well as left atrial inotropic and cAMP responses to 5-HT in isolated atria from newborn piglets. To investigate how relevant data from newborn piglets were to older pigs and humans, we also assessed the effects of the PDE inhibitors on the fade of 5-HT responses in atrial trabeculae from adolescent pigs and adult humans. Finally, we investigated whether selective inhibition of PDE3 and/or PDE4 could uncover effects of 5-HT on ventricular trabeculae of newborn and adolescent pigs.
Methods
The drug/molecular target nomenclature conforms to the BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Patients
This study was approved by the Ethical Committees of the University of Murcia and the University Hospital. The patients provided written informed consent. Right atrial appendages were obtained from 10 patients (mean age 59 ± 4 years, 8 men, 2 women) with stable sinus rhythm and not in heart failure (ejection fraction 68 ± 5%), undergoing cardiac surgery for aortic valve replacement (7) and coronary artery bypass (2) or both (1) at the University Hospital. Two coronary patients and two aortic valve patients were chronically treated with the β-blockers atenolol and bisoprolol respectively. Two aortic valve patients took salbutamol. Other chronically administered drugs included an angiotensin-converting enzyme inhibitor (1), H+pump antagonists (6), aspirin (5), lipid lowering drugs (simvastatin/atorvastatin, 7), diuretics (7), hypoglycemic agents (3), calcium antagonist (1), benzodiazepines (3) and nitrates (4).
Human right atrial trabeculae
After excision, the atrial appendages were immediately placed into oxygenated, modified cold Tyrode's solution and transported to the laboratory in less than 5 min. Up to eight atrial trabeculae were dissected in oxygenated, modified Tyrode's solution at room temperature containing (mmol·L−1): NaCl 136.9, KCl 5.0, CaCl2 1.8, MgCl2 1.5, NaHCO3 11.9, NaH2PO4 0.4, EDTA 0.04, ascorbic acid 0.2, pyruvate 5 and glucose 5.0. The solution was maintained at pH 7.4 by bubbling a mixture of 5% CO2 and 95% O2. The atrial trabeculae were mounted in pairs, attached to Swema 4-45 strain gauge transducers in an apparatus containing the above solution at 37°C, paced at 1 Hz, and stretched as described previously (Kaumann et al., 1990). Force was recorded on a 12-channel Watanabe polygraph.
Isolated tissues from pigs
All animal care and procedures complied with the guidelines of the European Communities Council Directive of 23 March 1998 (1999/575/CE) and were approved by the Ethical Committee of the University of Murcia. Newborn piglets (1–2 days, either sex, 0.9 ± 0.05 kg) and adolescent pigs (12–14 weeks old, either sex, 22 ± 2 kg) were obtained from the animal farm of the School of Veterinary Sciences, University of Murcia. Newborn piglets and pigs were anaesthetized with sodium pentobarbital, 100 mg·kg−1 i.p. and 50 mg·kg−1 i.v. respectively. The hearts were removed and dissected in oxygenated, modified Tyrode's solution, as detailed above, at room temperature. Spontaneously beating right atria (Kaumann, 1990), left atrial strips (Parker et al., 1995) and right ventricular trabeculae (width <1 mm, Brattelid et al., 2004) from newborn piglets, as well as left atrial trabeculae (width <1 mm) (De Maeyer et al., 2006) and ventricular trabeculae from adolescent pigs were rapidly dissected and mounted to contract in modified Tyrode's solution at 37°C, often in pairs. Two to four atrial strips (newborn piglets) or trabeculae (adolescent pigs) were obtained from a single left atrium. Left atrial preparations and right ventricular trabeculae were paced at 1 Hz and stretched as described earlier (Parker et al., 1995; Brattelid et al., 2004). Contractile force of left atrial trabeculae from adolescent pigs and human right atrium was recorded on a 12-channel Watanabe polygraph. Force and time to peak force of left atrial strips from newborn piglets were recorded through PowerLab amplifiers on a Chart for Windows, Version 5.5.6 recording programme (ADInstruments, Castle Hill, NSW, Australia).
Protocols
All experiments were carried out in the presence of (-)-propranolol (200 nmol·L−1) to avoid indirect effects due to 5-HT-evoked release of noradrenaline and interaction with β-adrenoceptors.
Cumulative concentration–effect curves for 5-HT were carried out on spontaneously beating right atria and paced left atrial strips of newborn piglets in the absence and presence of the PDE3 inhibitor cilostamide (300 nmol·L−1) or PDE4 inhibitor rolipram (1 µmol·L−1) (Vargas et al., 2006; Galindo-Tovar and Kaumann, 2008), followed by the administration of a saturating concentration of (-)-isoprenaline (200 µmol·L−1). For inotropic studies, the experiments were terminated by elevating the CaCl2concentration to 9 mmol·L−1.
Measurement of cyclic AMP
In order to investigate whether contractile responses correlated with cyclic AMP levels in the same left atrial strip, basal force and agonist-induced force were measured and the tissues frozen in liquid nitrogen immediately 2 or 20 min after the addition of a maximum inotropically effective concentration of 5-HT (10 µmol·L−1). The effects of a maximum inotropically effective concentration of (-)-isoprenaline (200 µmol·L−1), incubated for 2 min, were studied for comparison. Two to four strips from one left atrium of a newborn piglet were set up into separate organ baths and exposed to two to four different experimental conditions (for a representative experiment, see Figure 1).
Figure 1.
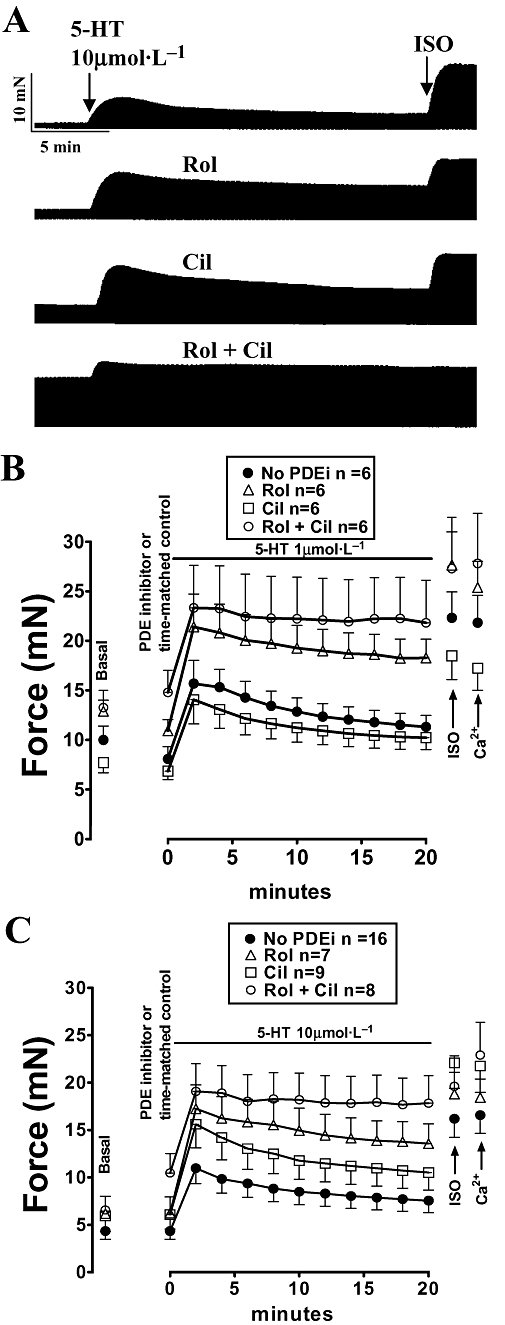
Rolipram (Rol) reduces fade and concurrent rolipram + cilostamide (Rol + Cil) abolished fade of the inotropic response to 5-HT on left atria from newborn piglets. (A) Represtative experiment showing the response to 10 µmol·L−1 5-HT in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide on four strips obtained from the same atrium. After the 20th min of 5-HT administration, (-)-isoprenaline (200 µmol·L−1, ISO) was added. (B) Data of experiments with fade of the response to 1 µmol·L−1 5-HT. Experiments were carried out on two strips from each atrium. (C) Data of experiments with fade of the response to 10 µmol·L−1 5-HT. Experiments were carried out on two to four strips from each atrium. n refers to number of strips. The experiments were terminated by raising the Ca2+ concentration to 9 mmol·L−1 (Ca2+). PDEi, PDE inhibitor.
Levels of cyclic AMP were measured by radioimmunoassay [125I]TME-S-cAMP (Diagnostic Pasteur, France), according to the manufacture's instructions. Incubation time with the PDE inhibitors was 30 min, instead of 15 min used by other authors (Katano and Endoh 1992; Verde et al., 1999). After freezing, the tissue was weighed and homogenized in cold perchloric acid (0.3 mol·L−1; 1:30, weight : volume) with a Polytron homogenizer (setting 8 for 15 s) and centrifuged (10 000× g, 4°C, 15 min). The supernatants were treated with potassium hydroxide until pH 6.7 was reached. The sensitivity of the assay was 2 pmol·mL−1. The pellet was re-disolved in KOH 2 mol·L−1for protein determination. Intra- and inter-assay coefficients of variation were 7.7% and 8.2% respectively. The antibody cross-reacted 100% with 3′, 5′-cyclic AMP and less than 0.3% with other nucleotides. Cyclic AMP concentrations were expressed as pmol·mg−1 of tissue.
Statistics
The data are expressed as mean ± SEM of n = number of right atria, left atrial strips or trabeculae and ventricular trabeculae. −LogEC50M values of 5-HT were estimated from fitting a Hill function with variable slopes to concentration–effect curves from individual experiments. Significance of differences between means was assessed with either paired and unpaired Student's t-test using GraphPad 4 Software Inc. (San Diego, CA) and/or anova (Tukey's multiple comparison test). P < 0.05 was considered significant.
Drugs
5-Hydroxytryptamine HCl, (-)-propranolol, rolipram and cilostamide were purchased from Sigma Chemicals (Madrid, Spain). Rolipram and cilostamide were dissolved in dimethylsulphoxide and Tyrode's solution (20% dimethylsulphoxide in Tyrode's solution). Drugs were added to the organ bath so that the concentration of dimethylsulphoxide was less than 0.1%, which by itself did not modify contractile force.
Results
Cilostamide and rolipram increased sinoatrial rate but did not potentiate the effects of 5-HT in newborn piglets
Rolipram (1 µmol·L−1) significantly (P < 0.05, n = 15) increased beating rate by 13 ± 3% (Figure 2A–C). Cilostamide (300 nmol·L−1) tended to increase sinoatrial beating rate by 10 ± 4% of the effect of (-)-isoprenaline (200 µmol·L−1) (Figure 2A–C), but the effect did not reach statistical significance (P = 0.09, n = 17, paired Student's t-test). Concurrent rolipram + cilostamide increased sinoatrial rate by 39 ± 12% (P < 0.05, n = 7) of the effect of (-)-isoprenaline (Figure 2A–C).
Figure 2.
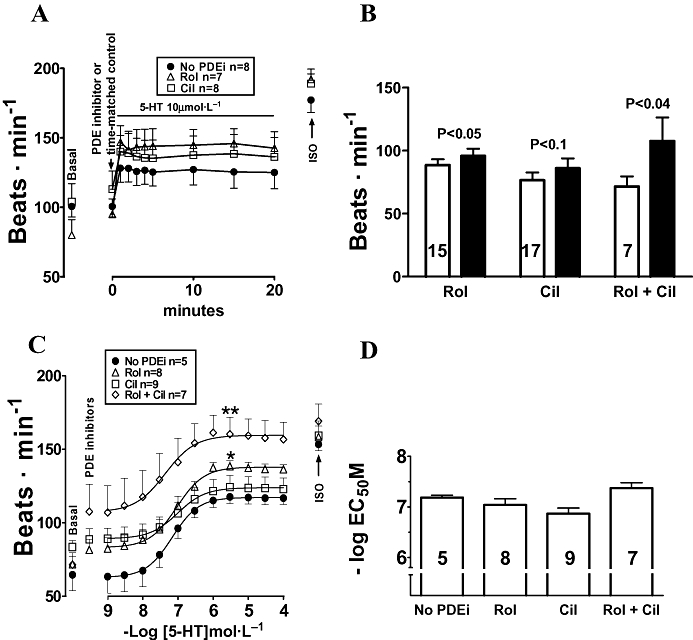
(A) 5-HT induced stable sinoatrial tachycardia on right atria from newborn piglets. (B) Rolipram (Rol) and concurrent rolipram + cilostamide (Rol + Cil) cause sinoatrial tachycardia. Open and black columns represent sinoatrial rate in the absence and presence of PDE inhibitors (PDEi). Data were from A, B or a pool of both. (C) The effects of 5-HT were not potentiated by rolipram, cilostamide or concurrent rolipram + cilostamide. (D) −LogEC50M data from the experiments in (C). *P < 0.05 Emax of 5-HT with rolipram, **P < 0.01 Emax of 5-HT with concurrent rolipram + cilostamide, compared with the Emax of 5-HT in the absence of PDE inhibitors. Numbers in open columns refer to number of right atria.
5-HT (10 µmol·L−1) elicited tachycardia that was maximal approximately at 1 min and remained stable until the 20th min of observation in the absence and presence of rolipram and cilostamide (Figure 2A).
5-HT caused concentration-dependent increases of sinoatrial rate in the absence and presence of cilostamide, rolipram and concurrent rolipram + cilostamide (Figure 2C). The PDE inhibitors, given separately or in combination, did not significantly change the chronotropic potency of 5-HT (Figure 2D). Rolipram and concurrent rolipram + cilostamide, but not cilostamide, significantly increased the Emax of 5-HT (Figure 2C).
Rolipram reduced fade and concurrent rolipram + cilostamide abolished fade of inotropic responses to 5-HT in left atria of newborn piglets
Rolipram (1 µmol·L−1) and cilostamide (300 nmol·L−1) did not significantly increase contractile force but concurrent rolipram + cilostamide caused variable increases in left atrial force with an average of 30 ± 6% of (-)-isoprenaline (P < 0.001, n = 23, pooled data of Figs 1 and 3). The responses to 1 and 10 µmol·L−15-HT were maximal by approximately the second min and faded to 39 ± 7 and 45 ± 2% by the 20th min of administration respectively (Figure 1). Cilostamide did not significantly affect the residual responses at the 20th min to 1 µmol·L−1 (54 ± 7%) and 10 µmol·L−1 (42 ± 6%) 5-HT, but rolipram significantly enhanced the residual responses to 77 ± 7% (P < 0.01) and 67 ± 3% (P < 0.001) respectively (Figure 1). Concurrent rolipram + cilostamide nearly abolished fade of the responses to both 1 µmol·L−1 and 10 µmol·L−1 5-HT at the 20th min (91 ± 6 and 87 ± 8%, respectively, of the maximum response) (Figure 1).
Figure 3.
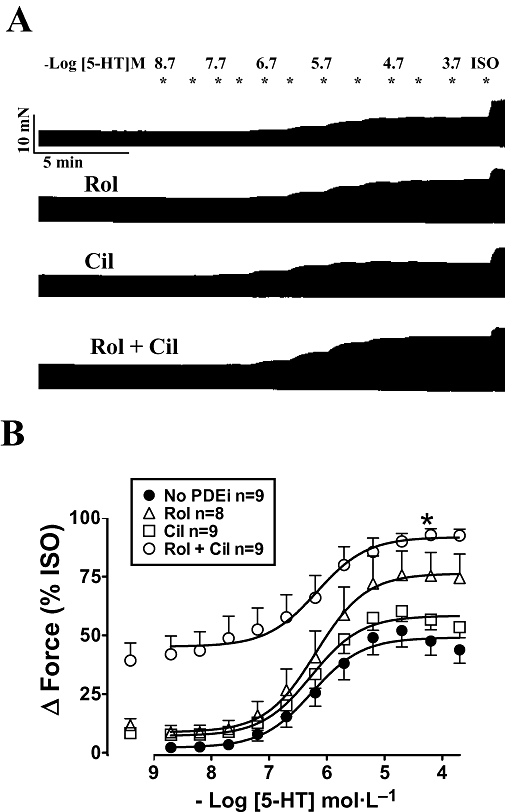
Rolipram and cilostamide, administered separately or in combination, did not potentiate the effects of 5-HT on left atria from newborn piglets. (A) Representative experiment of cumulative concentration–effect curves of 5-HT in the absence and presence of rolipram (Rol), cilostamide (Cil) and concurrent rolipram + cilostamide (Rol + Cil) on four strips from the same left atrium. The experiment was terminated with 200 µmol·L−1 (-)-isoprenaline (ISO). (B) Data from n strips of nine piglets. Basal force was 7.0 ± 0.8 mN and force in the presence of (-)-isoprenaline was 14.2 ± 1.5 mN (n = 35 atrial strips).
Cilostamide and rolipram failed to potentiate the inotropic effects 5-HT in left atria from newborn piglets
Cumulative concentration–effect curves to 5-HT were not significantly shifted to the left by rolipram, cilostamide and concurrent rolipram + cilostamide. The potency of 5-HT was not significantly changed (Figure 3B). −LogEC50 values for 5-HT were 6.04 ± 0.21 (n = 9), 6.04 ± 0.22 (n = 8), 6.20 ± 0.14 (n = 9) and 6.44 ± 0.24 (n = 9) in the absence of PDE inhibitors and presence of rolipram, cilostamide and concurrent rolipram + cilostamide respectively. Concurrent rolipram + cilostamide increased the Emax of 5-HT with respect to the effect of (-)-isoprenaline (Figure 3B).
Concurrent rolipram + cilostamide reduced fade of both the inotropic response and cAMP response to 5-HT in left atria from newborn piglets
Two minutes after administration, 5-HT (10 µmol·L−1) increased contractile force in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide by 3.9 ± 0.6, 9.2 ± 1.0, 9.8 ± 1.2 and 8.5 ± 1.3 mN respectively (Figure 4). The increase in force by the second min of 5-HT administration was significantly greater (anova) in the presence of rolipram (P < 0.05), cilostamide (P < 0.01) and concurrent rolipram + cilostamide (P < 0.05) compared with the absence of PDE inhibitors (Figure 4). Twenty minutes after administration, the increases in force by 5-HT were reduced to (mN) −0.6 ± 0.3, 4.5 ± 0.8 (P < 0.01), 3.5 ± 0.8 (P < 0.05) and 4.7 ± 0.9 (P < 0.01) in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide respectively. The inotropic responses to 5-HT at the 20th min in the separate presence of rolipram and cilostamide, but not in the presence of concurrent rolipram + cilostamide, were significantly smaller (P < 0.05, Figure 4) than the corresponding response to 5-HT at the second min of administration.
Figure 4.
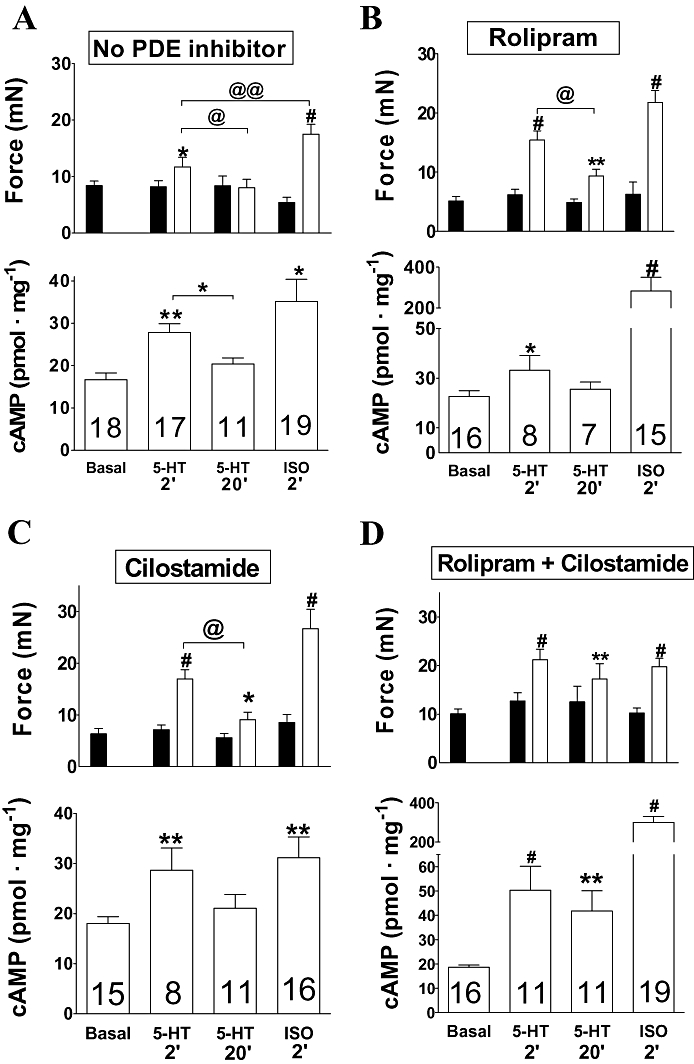
Fade of the inotropic and cAMP responses to 5-HT in left atria from newborn piglets in the absence (A) and presence of rolipram (1 µmol·L−1, B) and cilostamide (300 nmol·L−1, C), but lack of fade with concurrent rolipram + cilostamide (D). Top and bottom columns of each panel represent contractile force data and cAMP data from the same tissues. Black columns: contractile force in the absence of 5-HT. Top open columns of each panel represent the inotropic effects of 5-HT (10 µmol·L−1) at the second min and 20th min, and of (-)-isoprenaline (200 µmol·L−1, ISO). Numbers in each open column refers to number of atrial strips from at least five piglets. *P < 0.05, **P < 0.01, #P < 0.001 compared with the absence of 5-HT or (-)-isoprenaline (paired Student's t-test). @P < 0.05 between the response to 5-HT at the second min and 20th min of administration (unpaired Student's test). @@P < 0.02 between the response to 5-HT at the second min and the response to (-)-isoprenaline. Please note the expansion of the cAMP ordinate for (-)-isoprenaline (200 µmol·L−1, ISO) in (B) and (D).
Rolipram, cilostamide and concurrent rolipram + cilostamide did not significantly increase left atrial cAMP levels (anova) (Figure 4). At the second min of administration, 5-HT (10 µmol·L−1) significantly increased the cAMP levels in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide by 66%, 46%, 59% and 173% of basal cAMP levels respectively (Figure 4). The cAMP levels in the presence of both 5-HT (second min) and concurrent rolipram + cilostamide were significantly higher (P < 0.05) than in the presence of 5-HT alone (Figure 4A,D). At the 20th min of administration, 5-HT (10 µmol·L−1) increased the cAMP levels in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide by 22%, 13%, 17% and 124% of basal cAMP levels respectively (Figure 4). Only the cAMP level in the presence of both 5-HT (20th min) and concurrent rolipram + cilostamide was significantly higher (P < 0.01) than in the presence of 5-HT alone (Figure 4A,D).
Rolipram, but not cilostamide, markedly increased the left atrial cAMP signal produced by (-)-isoprenaline in newborn piglets
(-)-Isoprenaline, incubated for 2 min, increased the cAMP level approximately twofold in the absence or presence of cilostamide. The effects of (-)-isoprenaline on cAMP levels in the absence and presence of cilostamide were not significantly different to the corresponding effects of 5-HT (10 µmol·L−1) at the second min of administration (Figure 4A,C). In contrast, with rolipram, the cAMP levels in the presence of (-)-isoprenaline were considerably higher (P < 0.02) than in the presence of 5-HT. With concurrent rolipram + cilostamide, the cAMP levels in the presence of (-)-isoprenaline were also markedly higher (P < 0.001) than in the presence of 5-HT (Figure 4B,D), but virtually the same as with rolipram alone. The (-)-isoprenaline-evoked increases of contractile force were not significantly different in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide (Figure 4A–D).
5-HT accelerated the onset of relaxation in left atria of newborn piglets
Both 5-HT (10 µmol·L−1) and (-)-isoprenaline (200 µmol·L−1) shortened the time to peak force by the second min of administration, but the effect of 5-HT was significantly smaller (Figure 5A). Rolipram, cilostamide and concurrent rolipram + cilostamide also shortened time to peak force (Figure 5B–D). In the presence of rolipram, cilostamide and concurrent rolipram + cilostamide 5-HT caused shortenings of time to peak force (Figure 5B–D), which were not significantly different from the effects of (-)-isoprenaline under these conditions (Figure 5B–D). The shortening of the time to peak force caused by 5-HT persisted by the 20th min in the absence and presence of the PDE inhibitors (Figure 5A–D).
Figure 5.
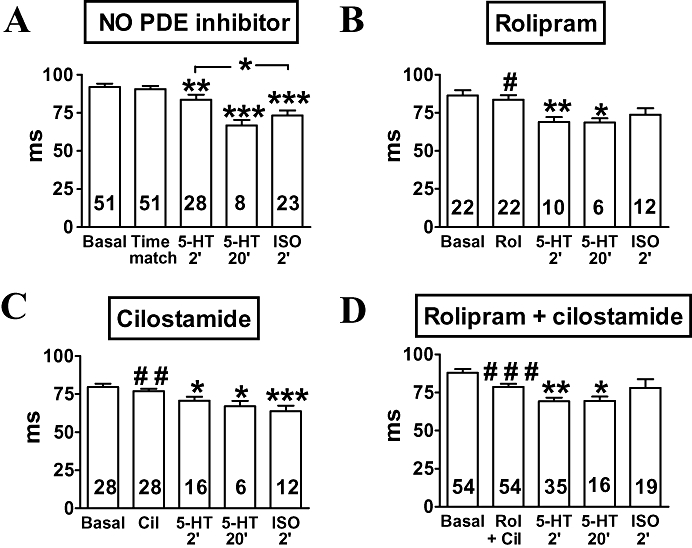
Faster onset of atrial relaxation by 5-HT (10 µmol·L−1) (A) in newborn piglets. Effects of rolipram (1 µmol·L−1, Rol) (B), cilostamide (300 nmol·L−1, Cil) (C) and concurrent rolipram + cilostamide (Rol + Cil) (D), incubated for 30 min, and comparison with (-)-isoprenaline (200 µmol·L−1, ISO). Data shown are means (± SEM) of time to peak force measurements. *P < 0.05, **P < 0.01, ***P < 0.001 with respect to time-matched control or after 30 min incubation with indicated phosphodiesterase inhibitors. #P < 0.05, ##P < 0.01, ###P < 0.001 with respect to basal.
Cilostamide but not rolipram disclosed 5-HT responses in ventricular trabeculae from newborn piglets
5-HT did not enhance contractile force of ventricular trabeculae from newborn piglets unless all PDE activity was inhibited with IBMX (Brattelid et al., 2004). Cilostamide and concurrent rolipram + cilostamide, but not rolipram alone, disclosed significant inotropic responses to 5-HT (Figure 6A). However, the response to 5-HT in the presence of concurrent rolipram + cilostamide was not significantly greater than in the presence of cilostamide alone (P = 0.2).
Figure 6.
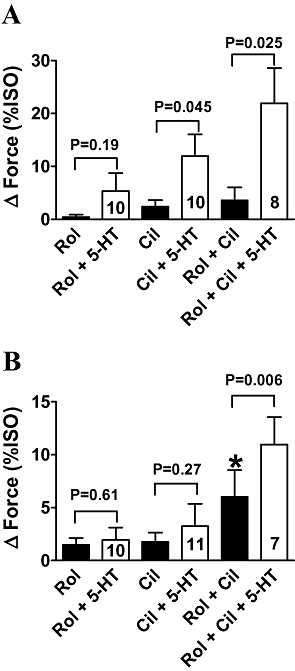
Cilostamide (300 nmol·L−1, Cil) disclosed ventricular 5-HT responses in newborn piglets (A) but not in adolescent pigs (B). 5-HT enhances ventricular force in the presence of concurrent rolipram (1 µmol·L−1, Rol) + cilostamide in both newborn piglets (A) and adolescent pigs (B). Basal force and force in the presence of (-)-isoprenaline (200 µmol·L−1, ISO) were 2.7 ± 0.5 and 10.0 ± 1.7 mN, respectively, in newborn (28 trabeculae from 20 piglets), and 2.5 ± 0.5 and 10.1 ± 1.1 mN in adolescents (28 trabeculae from 10 pigs). *P < 0.05 with respect to absence of rolipram. Open and closed columns summarize data in the presence and absence of 5-HT (10 µmol·L−1).
Concurrent rolipram + cilostamide, but not rolipram or cilostamide alone, disclosed 5-HT responses in ventricular trabeculae from adolescent pigs
5-HT does not increase contractions of ventricular trabeculae from newborn and adult pigs unless PDEs are inhibited with IBMX (Brattelid et al., 2004). Rolipram and cilostamide separately did not change force, but concurrent rolipram + cilostamide significantly increased force (Figure 6B). In the separate presence of either rolipram or cilostamide, 5-HT failed to increase force (Figure 6B). In contrast, concurrent rolipram + cilostamide allowed 5-HT to significantly increase force (Figure 6B).
Cilostamide reduced fade, and concurrent rolipram + cilostamide abolished fade of the response to 5-HT in atria from adolescent pigs
The data are shown in Figure 7. Rolipram alone did not significantly increase contractile force. Cilostamide tended to increase force but the effect was not significant (P = 0.11, Student's paired t-test). Concurrent rolipram + cilostamide increased force by 31 ± 12% of the effect of 200 µmol·L−1(-)-isoprenaline (P < 0.01). The positive inotropic response to 5-HT faded and disappeared between the 20th min and 30th min of administration. In the separate presence of rolipram and cilostamide, 20.2 ± 8.8% (P = 0.19) and 30.6 ± 9.7% (P < 0.04) of the initial response were observed, respectively, by the 30th min of administration. Concurrent rolipram + cilostamide completely prevented fade of the 5-HT response (P < 0.001).
Figure 7.
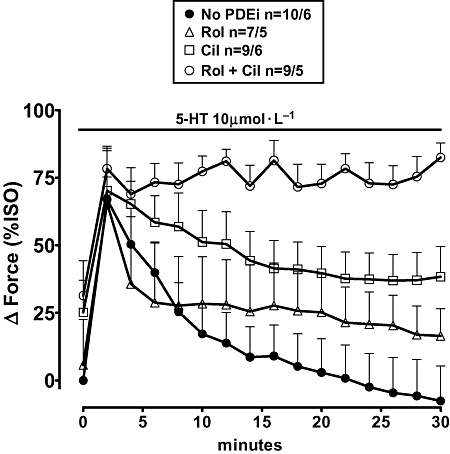
Fade of the inotropic response to 5-HT (10 µmol·L−1) in the absence and presence of phosphodiesterase inhibitors (PDEi) on left atrial trabecule of adolescent pigs. Marginal partial reduction of fade by rolipram (Rol), partial reduction by cilostamide (Cil) and prevention of fade by concurrent rolipram + cilostamide (Rol + Cil). Basal force and force in the presence of (-)-isoprenaline was 2.5 ± 0.3 and 5.4 ± 0.7 respectively (35 trabeculae from 9 pigs).
Cilostamide but not rolipram partially prevented fade of the inotropic response to 5-HT in human atrial trabeculae
Rolipram and cilostamide, administrated separately or together, did not significantly change atrial force. Force (mN) was 3.4 ± 0.5 (n = 33 trabeculae), 4.4 ± 1.0 (n = 10), 4.3 ± 0.9 (n = 10) and 2.7 ± 0.9 (n = 5) in the absence and presence of rolipram, cilostamide and concurrent rolipram + cilostamide respectively. The inotropic response to 5-HT (1 µmol·L−1) was approximately maximal at the third min and faded to 15 ± 6% and 14 ± 5% by the 60th min of administration in the absence and presence of rolipram respectively (Figure 8). Cilostamide but not rolipram partially reduced fade of the 5-HT response. Concurrent rolipram + cilostamide did not reduce fade more than cilostamide. In the presence of cilostamide and concurrent rolipram + cilostamide, the response to 5-HT faded at the 60th min to 54 ± 5% and 61 ± 4% of the maximum response (P < 0.001), compared with fade in the absence and presence of rolipram.
Figure 8.
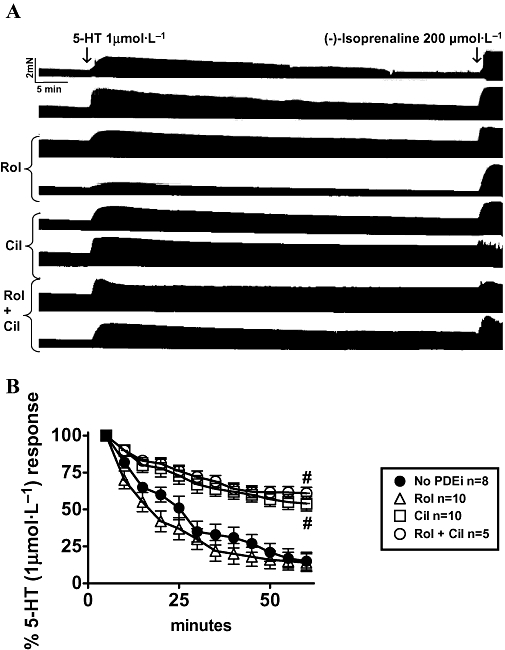
Cilostamide, but not rolipram, partially prevents fade of the inotropic response to 5-HT in human atrial trabeculae. (A) Representative experiment on eight trabeculae from a 73-year-old man with aortic insufficiency without heart failure (ejection fraction 63%). Pairs of trabeculae were set up into the same organ bath, in the absence and presence of rolipram (1 µmol·L−1, Rol), cilostamide (300 nmol·L−1, Cil) and concurrent rolipram + cilostamide. After 1 h incubation with 5-HT (1 µmol·L−1), (-)-isoprenaline (ISO) was administered. (B) Data from the fade of the response to 5-HT in the absence and presence of indicated PDE inhibitors. Basal contractile force was 3.4 ± 0.5 mN, and force in the presence of (-)-isoprenaline 7.2 ± 0.8 mN (n = 33 trabeculae). Number of trabeculae (n) from 10 patients.
Discussion
Our main findings were: (i) PDE3 and PDE4 reduced heart rate but neither isoenzyme affected the stable tachycardia elicited by 5-HT, mediated through sinoatrial 5-HT4 receptors of newborn piglets; (ii) the fade of the porcine atrial inotropic responses and cAMP responses to 5-HT was caused by both PDE3 and PDE4; (iii) the control of atrial 5-HT responses by PDEs switched from a predominant role of PDE4 in newborn piglet to a predominant role of PDE3 in adolescent pigs; (iv) in porcine ventricle, PDE3 prevented 5-HT responses in newborn but, PDE3 and PDE4, acting in concert, prevented 5-HT responses in adolescents; and (v) fade of human atrial responses to 5-HT was due to the action of PDE3, but not that of PDE4.
PDE3 and PDE4 reduced sinoatrial beating rate but did not modulate 5-HT4 receptor-mediated tachycardia
High cAMP levels and activity of cAMP-dependent PKA of sinoatrial cells are obligatory for the maintenance of basal heart beat (Vinogradova et al., 2006). Partial inhibition of PKA (Maltsev et al., 2006; Vinogradova et al., 2006) or reducing sinoatrial cAMP levels through PDE-catalysed hydrolysis (Vinogradova et al., 2008) slows sinoatrial beating rate. The PDE isoenzymes that control sinoatrial rate depend on species, PDE3 in the rabbit (Vinogradova et al., 2008), PDE4 in the rat (Christ et al., 2008) and both PDE3 and PDE4 in the mouse (Galindo-Tovar and Kaumann, 2008). The sinoatrial tachycardia produced by concurrent rolipram + cilostamide in newborn piglets was threefold greater than the tachycardia produced by each PDE inhibitor alone (Figure 2B). Therefore, both PDE3 and PDE4, acting in concert, reduced sinoatrial beating rate in newborn piglets.
In agreement with a similar observation by De Maeyer et al.(2006), we found that 5-HT-evoked tachycardia did not fade. Sinoatrial tachycardia elicited by 5-HT in newborn piglets is mediated through 5-HT4 receptors (Kaumann, 1990) and, as these receptors are coupled to Gs proteins, they are assumed to mediate a rise of cAMP levels in the neighbourhood of both the receptors and ion channels critically involved in increasing pacemaker firing rate. Atrial channel currents that increase upon 5-HT4 receptor stimulation include the L-type Ca2+ channel with an obligatory participation of PKA (Ouadid et al., 1992) and the pacemaker current If, activated by hyperpolarization (Pino et al., 1998). The stable tachycardia induced by 5-HT is probably due to the absence of PDE activity in the sinoatrial cAMP pool involved in the increase of pacemaker rate through 5-HT4 receptors. The lack of potentiation of the chronotropic effects by rolipram, cilostamide and concurrent rolipram + cilostamide (Figure 2) supports the hypothesis that the cAMP pool that governs 5-HT4 receptor-mediated sinoatrial tachycardia is protected from PDE3 and PDE4 and represents a compartment that is distinct from the cAMP compartment in which both PDE3 and PDE4 reduce basal sinoatrial beating. The increases in Emax of 5-HT in the presence of rolipram and concurrent rolipram + cilostamide (Figure 2C) could merely be due to additivity of the tachycardia caused by 5-HT and the PDE inhibitors.
The distinct compartmentalization of cAMP between basal sinoatrial rate, controlled by PDEs, and 5-HT4 receptor-mediated tachycardia, unaffected by PDEs in newborn piglets, resembles similar situations for β1-adrenoceptor-evoked tachycardia. Basal sinoatrial beating rate is reduced by both PDE3 and PDE4 in the mouse (Galindo-Tovar and Kaumann, 2008) and by PDE4 in the rat (Christ et al., 2008), but in contrast, (-)-noradrenaline-evoked tachycardia, mediated through β1-adrenoceptors, is minimally affected by PDE3 and PDE4 activity in these species.
The fade of the inotropic and cAMP responses to 5-HT in left atria from newborn piglets was caused by PDE3 and PDE4 acting in concert
The positive inotropic response to 5-HT (10 µmol·L−1) by the second min of exposure was accompanied by an increase in the atrial cAMP level (Figure 4A). The fade of the inotropic response to 5-HT by the 20th min was mirrored by the fade of the cAMP response (Figure 4A) and concurrent rolipram and cilostamide prevented both fades (Figure 4D). This evidence indicates that PDE3 and PDE4 jointly reduced cAMP generated through 5-HT4 receptor activation. However, are the measured atrial cAMP signals inotropically relevant?
In two situations there were discrepancies between the cAMP level and inotropic response to 5-HT (10 µmol·L−1). First, cilostamide induced 5-HT to produce a significantly greater inotropic response by the second min of administration, compared with the absence of cilostamide, but the 5-HT-evoked cAMP signal was not increased. Furthermore, by the 20th min, when the cAMP response to 5-HT had completely faded, there was still a residual inotropic response in the presence of cilostamide (Figure 4C). In the second situation, the inotropic response to 5-HT by the second min, but not the cAMP response, was increased by rolipram, compared with the absence of rolipram (Figure 4A,B). Moreover, although rolipram reduced the inotropic fade of the 5-HT response by the 20th min, the corresponding cAMP signal disappeared (Figure 4B).
These discrepancies between inotropic responses and cAMP levels are inconsistent with the assumption that the measured cAMP levels reflect cAMP surges in inotropically relevant compartments. Clearly, hypothetical increases of cAMP that activate PKA in small compartments, such as the 5-HT4 receptor/L-type Ca2+ channel domain or a domain in the vicinity of the membrane of the sarcoplasmic reticulum (SR), have escaped detection. PDE3 and PDE4 are mainly associated with the SR and sarcolemma respectively (Lugnier et al., 1993, Lugnier, 2006). PKA-catalysed phosphorylation of phospholamban (PLB) dis-inhibits the SR Ca2+-ATPase, which then pumps Ca2+faster back into the SR, lowering sarcoplasmic Ca2+ with consequent faster relaxation of contractile proteins (MacLennan and Kranias, 2003). The reduction of the time to peak force observed with 5-HT on left atria from newborn piglets (Figure 5) is consistent with a faster onset of relaxation due to PKA-catalysed PLB phosphorylation and phosphorylation of other proteins involved in the temporal control of relaxation, such as troponin-I (Garvey et al., 1988). Hypothetical cAMP changes in left atria of newborn piglets,observed during 5-HT4 receptor activation in the absence and presence of PDE inhibitors are represented in Figure 9.
Figure 9.
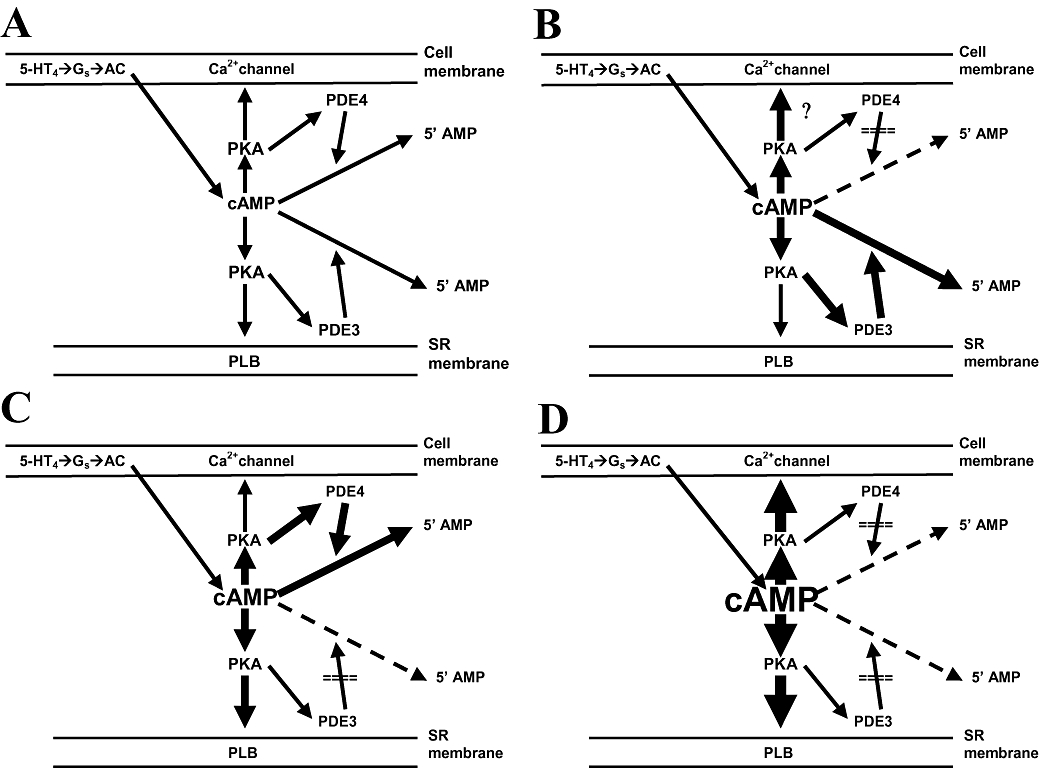
Schematic illustration of the decreases by phosphodiesterases PDE3 and PDE4 of hypothetical cAMP surges caused by 5-HT4 receptor (5-HT4) stimulation, subsequent coupling to Gs protein and activation of adenylyl cyclase (AC) in left atrial cells of newborn pigs. (A) PDE3 is assumed to hydrolyse cAMP mainly in the neighbourhood of the sarcoplasmic reticulum (SR), thereby partially reducing activation of protein kinase (PKA) and PKA-catalysed phosphorylation of phospholamban (PLB). PDE4 is assumed to hydrolyse cAMP mainly in the neighbourhood of the L-type Ca2+ channel, thereby partially preventing activation of PKA and PKA-dependent phosphorylation of the Ca2+ channel. (B) Inhibition of PDE4 would increase cAMP- and PKA-dependent phosphorylation of the Ca2+ channels, thereby initially enhancing Ca2+-induced Ca2+ release and transiently increase contractility. The increased cAMP would also cause PKA-catalysed phosphorylation and activation of PDE3, thereby reducing cAMP at the PLB domain and blunting the inotropic response to 5-HT. (C) Inhibition of PDE3 would increase cAMP and PKA-dependent phosphorylation of PLB, thereby augmenting contractility and accelerating the onset of relaxation. The increased cAMP would also cause PKA-catalysed phosphorylation and activation of PDE4, thereby reducing cAMP at the Ca2+ channel domain, decreasing Ca2+-induced Ca2+ release and contractility. (D) Inhibition of both PDE3 and PDE4 maintains stable cAMP concentrations in inotropically relevant compartments, thereby allowing sustained inotropic responses to 5-HT.
Inhibition of PDE4 with rolipram would increase cAMP and enhance PKA-dependent phosphorylation of the Ca2+channels (Ouadid et al., 1992), thereby initially stimulating Ca2+-induced Ca2+ release (Fabiato, 1985) and transiently increase contractility (Figure 9B) as observed experimentally with 5-HT (second min) in Figure 4B. The increased cAMP could also cause PKA-catalysed phosphorylation and activation of PDE3 (Gettys et al., 1987), thereby reducing cAMP at the PLB domain and blunting the inotropic response to 5-HT.
After inhibition of PDE3 with cilostamide, the contractile response to 10 µmol·L−15-HT was increased (Figure 4C) at the second min of administration and the onset of relaxation, compared with (-)-isoprenaline, further shortened (Figure 5C), consistent with enhanced PLB phosphorylation (Figure 9C). However, the inotropic response to 5-HT faded by the 20th min even in the presence of cilostamide (Figs 1 and 4c). Furthermore, the inotropic potency of 5-HT in the presence of cilostamide, estimated from cumulative concentration–effect curves to 5-HT, was not enhanced compared with the absence of cilostamide (Figure 3). The persistence of the fade of the inotropic response to 5-HT, despite the inhibition of PDE3 and the early enhanced inotropic response to 10 µmol·L−1 5-HT, suggests a time-dependent increase of cAMP hydrolysis. We propose that the persistent fade is due to accumulation of cAMP in a PDE3-inhibited compartment, followed by leak into compartments where PKA can be activated to phosphorylate and stimulate PDE4 (MacKenzie et al., 2002), thereby again reducing inotropically relevant cAMP. The activation of PDE4 and reduction of inotropically relevant cAMP take time, and are assumed to occur at several increasing 5-HT concentrations during the cumulative concentration–effect curve (Figure 3). The net effect of PDE4 activation in the presence of cilostamide is a lack of potentiation of the effects of 5-HT observed in Figure 3.
The fade of the inotropic cAMP responses to 5-HT was prevented by concurrent rolipram + cilostamide, consistent with the assumption that the combined activities of PDE3 and PDE4 converge to reduce cAMP in cell compartments that contribute to the increase in contractile force through activation of 5-HT4receptors (Figure 9D). cAMP may have overflowed from inotropically relevant compartments and flooded the sarcoplasm, consistent with the enhanced cAMP signals measured under concurrent rolipram + cilostamide (Figure 4D), compared with the absence of PDE inhibitors or separate presence of rolipram and cilostamide (Figure 4A–C).
In the absence of PDE inhibitors, 5-HT (2 min) accelerated the onset of relaxation, although less than (-)-isoprenaline (Figure 5A), consistent with PKA-dependent phosphorylation of PLB. This acceleration of the onset of relaxation by 5-HT and (-)-isoprenaline were, however, not significantly different in the presence of cilostamide (Figure 5C), rolipram (Figure 5B) or concurrent rolipram + cilostamide (Figure 5D). Interestingly, unlike the inotropic response to 5-HT that faded by the 20th min in the absence and presence of PDE inhibitors, the faster onset of relaxation persisted (Figure 5A–D), probably reflecting slow reversal kinetics due to dephosphorylation of some proteins involved in this lusitropic effect (e.g. troponin-I) (England, 1976; Perry, 1979; Garvey et al., 1988).
The (-)-isoprenaline-evoked cAMP signal was greatly reduced by PDE4 but not by PDE3 in left atria of newborn piglets
The twofold (-)-isoprenaline-evoked increase in cAMP was not affected by cilostamide, but was approximately 10-fold greater in the presence of rolipram or concurrent rolipram + cilostamide (Figure 4). Therefore, unlike the 5-HT4receptor-mediated increase of cAMP that is hydrolysed jointly by PDE3 and PDE4, the β-adrenoceptor-mediated increase of cAMP appears to be hydrolysed exclusively by PDE4. The β-adrenoceptor subtype that mediates the effects of (-)-isoprenaline is still unknown, but probably is mainly the β1-adrenoceptor.
The positive inotropic responses to (-)-isoprenaline were not significantly different in the absence and presence of the PDE inhibitors, presumably because at the high concentration used (200 µmol·L−1), the contractile system was saturated at each condition.
Ontogenic regional changes of the function of porcine PDE3 and PDE4
Early reports failed to find evidence for 5-HT-evoked increases of force in porcine (Schoemaker et al., 1992) and human ventricles (Jahnel et al., 1992; Schoemaker et al., 1993). However, mRNA for the 5-HT4receptor splice variants 5-HT4(a) and 5-HT4(b) receptors was detected in human ventricle by Bach et al.(2001). More recently, Brattelid et al.(2004) provided evidence for functional ventricular 5-HT4 receptors in newborn piglets, adult pigs and humans. They found that 5-HT increased ventricular force in the presence of the non-selective PDE inhibitor IBMX, suggesting that PDEs protect the ventricular myocardium against 5-HT4 receptor-mediated stimulation, but the PDE isoenzymes involved were not identified. Here, we found that in newborn piglets, but not adolescent pigs, cilostamide discloses increases of ventricular contractile force with 5-HT. Concurrent rolipram + cilostamide caused 5-HT to produce significant increases of ventricular force in adolescent pigs, suggesting that the preferential control by PDE3, found in newborn pigs, was lost in the adolescent hearts and that PDE3 and PDE4, acting in concert, suppressed the responses to 5-HT.
Curiously, in left atria there was also a change of the role of PDE3 and PDE4 with age, but somewhat opposite to the change observed in porcine ventricle. Rolipram partially prevented the fade of the inotropic response to 5-HT in atria from newborn, but not in atria from adolescent pigs. Conversely, cilostamide did not prevent fade of the inotropic response to 5-HT in newborn but reduced fade in adolescents. The mechanism of these age-dependent changes in the relative role of PDE3 and PDE4 in reducing inotropically relevant cAMP pools is unknown. Cardiac PDE activities have been reported to be markedly decreased in the left ventricle of 150-day-old pigs compared with newborn piglets (Mersmann et al., 1977). However, we found that the fade of the atrial inotropic 5-HT response was greater in adolescent pigs (≈100 days of age) than in newborn piglets, actually suggesting greater activities of both PDE3 and PDE4 in the former than in the latter.
Concurrent rolipram + cilostamide prevented the fade of both the inotropic and cAMP responses to 5-HT in newborn piglets, as well as inotropic fade in adolescent pigs. These results suggest that fade after 5-HT4 receptor stimulation is mostly produced by the activities of PDE3 and PDE4, but not by other PDE isoenzymes. Activation of 5-HT4 receptors produces rapid desensitization in several systems, as observed with the adenylyl cyclase responses to 5-HT in neurones of murine colliculi (Ansanay et al., 1995), rat oesophagus (Ronde et al., 1995) and recombinant receptors (Barthet et al., 2005; Ponimaskin et al., 2005). However, when both PDE3 and PDE4 were inhibited, responses to 1 and 10 µmol·L−15-HT were sustained during 20–30 min agonist exposure, inconsistent with 5-HT4 receptor desensitization.
PDE3, but not PDE4, induced fade of the inotropic response to 5-HT in human atrium
The inotropic response to 5-HT partially faded in human atrial trabeculae. Cilostamide but not rolipram reduced the fade, consistent with the hypothesis that PDE3 activity, but not PDE4, hydrolyses inotropically relevant cAMP, thereby contributing to tachyphylaxis. Concurrent rolipram + cilostamide did not reduce fade more than cilostamide alone, but a residual fade persisted under these two conditions (Figure 8). The residual fade could be produced by additional PDEs. However, the non-selective PDE inhibitor IBMX (10 µmol·L−1) does not potentiate more than cilostamide the positive inotropic effects of 5-HT on human atrial trabeculae (A. Kaumann, unpubl. experiments), consistent with an involvement of PDE3 but not of additional PDEs. By exclusion, the small cilostamide-resistant fade of the human atrial 5-HT responses could be due to 5-HT4 receptor desensitization.
PDE3, but not PDE4, is also responsible for the fade of inotropic responses to (-)-noradrenaline and (-)-CGP12177, mediated through β1-adrenoceptors in human atrium (Kaumann et al., 2007). Furthermore, the positive inotropic responses to (-)-adrenaline, mediated through human atrial β2-adrenoceptors, are only potentiated by cilostamide but not by rolipram (Christ et al., 2006). Thus, unlike effects mediated through porcine 5-HT4 receptors, which are blunted by both PDE3 and PDE4, and porcine β-adrenoceptors (β1?) controlled by PDE4, effects mediated through human Gs protein-coupled receptors appear to be controlled only by PDE3. This conclusion is reinforced by the observation that in human atria, concurrent rolipram + cilostamide does not reduce more than cilostamide alone, the fade of the inotropic responses to 5-HT through 5-HT4 receptors (this work) or to (-)-noradrenaline and (-)-CGP12177 through β1-adrenoceptors (Kaumann et al., 2007).
5-HT4 receptors of porcine cardiac tissues have been used as a model for human cardiac 5-HT4 receptors (Kaumann, 1990; Kaumann and Levy, 2006b). However, there are some important differences of 5-HT4receptor function in the two species. The density of 5-HT4 receptors is 10-fold lower in porcine atrium (Kaumann et al., 1995) than human atrium (Kaumann et al., 1996) and there are fundamental differences between the 5-HT4 receptor splice variants between the two species (De Maeyer et al., 2008). Our present work, showing that both PDE3 and PDE4 control porcine cardiac 5-HT responses but only PDE3 controls human atrial 5-HT responses, adds another functional difference between porcine and human 5-HT4 receptors. Therefore, the use of porcine cardiac 5-HT4 receptors as a model of human cardiac 5-HT4 receptors has to be treated with caution.
Conclusions
5-HT caused sustained sinoatrial tachycardia. PDE3 and PDE4 reduced sinoatrial beating rate but did not potentiate the 5-HT-evoked sinoatrial tachycardia, suggesting that these PDEs reduce the cAMP responsible for basal heart rate but do not have access to a cAMP compartment in the vicinity of the 5-HT4 receptors. PDE3 and PDE4 jointly prevent the fade of the inotropic and cAMP responses to 5-HT in left atria of both newborn and adolescent pigs, making unlikely any involvement of additional PDEs. However, while left atrial PDE4 was more active in the newborn, PDE3 was more active in adolescents. For still unknown reasons, there is a selective suppression of the ventricular 5-HT responses by PDE3 in newborn pigs that changes into a joint control by both PDE3 and PDE4 in older pigs. The inotropic response to 5-HT in human atrium partially fades but, unlike porcine myocardium, fade was caused only by PDE3 and not by PDE4.
Acknowledgments
This work was supported by Grant PI052338 Fondo Investigación Sanitaria of the Ministerio de Sanidad y Consumo, and the Séneca Foundation of Spain.
Glossary
Abbreviations:
- IBMX
isobutyl-methylxanthine
- PDE3
phosphodiesterase-3
- PDE4
phosphodiesterase-4
Conflicts of interest
The authors state no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L. cAMP-dependent, long-lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci USA. 1995;92:6635–6639. doi: 10.1073/pnas.92.14.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach T, Syversveen T, Kvingedal AM, Krobert KA, Brattelid T, Kaumann AJ, et al. 5-HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:146–160. doi: 10.1007/s002100000299. [DOI] [PubMed] [Google Scholar]
- Barthet G, Gaven F, Framery B, Shinjo K, Nakamura T, Claeysen S, Bockaert J, et al. Uncoupling and endocytosis of 5-hydroxytryptamine4 receptors. Dinstinct molecular events with different GRK2 requirements. J Biol Chem. 2005;280:27924–27934. doi: 10.1074/jbc.M502272200. [DOI] [PubMed] [Google Scholar]
- Brattelid T, Qvigstad E, Lynham JA, Molenaar P, Aass H, Geiran O, et al. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:157–166. doi: 10.1007/s00210-004-0963-0. [DOI] [PubMed] [Google Scholar]
- Christ T, Engel A, Ravens U, Kaumann AJ. Cilostamide potentiates more the positive inotropic effects of (-)-adrenaline through β2-adrenoceptors than the effects of (-)-noradrenaline through β1-adrenoceptors in human atrial myocardium. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:249–253. doi: 10.1007/s00210-006-0119-5. [DOI] [PubMed] [Google Scholar]
- Christ T, Galindo-Tovar A, Thoms M, Ravens U, Kaumann AJ. Inotropy and L-type Ca2+ current, activated by β1- and β2-adrenoceptors, are differently controlled by phosphodiesterases 3 and 4 in rat heart. Br J Pharmacol. 2008;156:62–83. doi: 10.1111/j.1476-5381.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer JH, Straetmann R, Schuurkes JAJ, Lefebvre RA. Porcine left atrial and sinoatrial 5-HT4 receptor induced responses: fading of the response and influence of development. Br J Pharmacol. 2006;147:140–157. doi: 10.1038/sj.bjp.0706497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer JH, Aerssens J, Verhasselt P, Lefebvre RA. Alternative splicing and exon duplication generates ten unique 5-HT4 receptor splice variants including a functional homofusion variant. Physiol Genomics. 2008;34:22–33. doi: 10.1152/physiolgenomics.00038.2008. [DOI] [PubMed] [Google Scholar]
- England PJ. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfusion of rat heart. Biochem J. 1976;160:295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Stimulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Tovar A, Kaumann AJ. Phosphodiesterase-4 blunts inotropism and arrhythmias but not sinoatrial tachycardia of (-)-adrenaline mediated through mouse cardiac β1-adrenoceptors. Br J Pharmacol. 2008;153:710–720. doi: 10.1038/sj.bjp.0707631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C protein, troponin I and phospholamban in isolated rabbit heart. Biochem J. 1988;249:709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettys TW, Blackmore PF, Redmon JB, Beebe SJ, Corbin JD. Short-term feedback regulation of cAMP by accelerated degradation in rat tissues. J Biol Chem. 1987;262:333–339. [PubMed] [Google Scholar]
- Jahnel U, Rupp J, Ertl R, Nawrath H. Positive inotropic responses to 5-HT in human atrial but not in ventricular heart muscle. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:482–485. doi: 10.1007/BF00169000. [DOI] [PubMed] [Google Scholar]
- Katano Y, Endoh M. Effects of a quinolinone derivative Y-20487 on the isoproterenol-induced positive inotropic action and cyclic AMP accumulation in rat ventricular myocardium: comparison with rolipram, Ro20-1724, milrinone, and isobutylmethylxanthine. J Cardiovasc Pharmacol. 1992;20:715–722. [PubMed] [Google Scholar]
- Kaumann AJ. Piglet sinoatrial 5-HT receptors resemble human atrial 5-HT4-like receptors. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:619–622. doi: 10.1007/BF00169055. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ. Do human atrial 5-HT4 receptors mediate arrhythmias? Trends Pharmacol Sci. 1994;15:451–455. doi: 10.1016/0165-6147(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006a;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Levy FO. Fading of 5-HT4 receptor-mediated inotropic responses to 5-hydroxytryptamine is caused by phosphodiesterase activity in porcine atrium. Br J Pharmacol. 2006b;147:128–130. doi: 10.1038/sj.bjp.0706501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Sanders L. 5-Hydroxytryptamine causes rate-dependent arrhythmias through 5-HT4 receptors in human atrium: facilitation by chronic β-adrenoceptor blockade. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:331–337. doi: 10.1007/BF00170877. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Sanders L, Brown AM, Murray KJ, Brown MJ. A 5-hydroxytryptamine receptor in human right atrium. Br J Pharmacol. 1990;100:879–885. doi: 10.1111/j.1476-5381.1990.tb14108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Sanders L, Brown AM, Murray KJ, Brown MJ. A 5-HT4-like receptor in human atrium. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:150–159. doi: 10.1007/BF00167212. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Lynham JA, Brown AM. Labelling with [125I]-SB207710 of a small 5-HT4 receptor population in piglet right atrium: functional relevance. Br J Pharmacol. 1995;115:933–936. doi: 10.1111/j.1476-5381.1995.tb15900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Lynham JA, Brown AM. Comparison of the densities of 5-HT4 receptors, β1- and β2-adrenoceptors in human atrium: functional implications. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:592–595. doi: 10.1007/BF00169181. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Semmler AL, Molenaar P. The effects of both noradrenaline and CGP12177, mediated through human β1-adrenoceptors, are reduced by PDE3 in human atrium but PDE4 in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:123–131. doi: 10.1007/s00210-007-0140-3. [DOI] [PubMed] [Google Scholar]
- Leftheriotis DI, Theodorakis GN, Poulis D, Flevari PG, Livanis EG, Iliodromitis EK, et al. The effects of 5-HT4 receptor blockade and stimulation during six hours of atrial fibrillation. Europace. 2005;7:560–568. doi: 10.1016/j.eupc.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Muller B, Le Bec A, Beaudry C, Rousseau E. Characterization of indolidan- and rolipram-sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J Pharmacol Exp Ther. 1993;265:1142–1151. [PubMed] [Google Scholar]
- MacKenzie SJ, Baillie GS, MacPhee I, MacKenzie C, Seamons R, McSorley T, et al. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- Mersmann HJ, Phinney G, Brown LJ, Steffen DG. Ontogeny of adenylate cyclase and phosphodiesterase activities in swine tissues. Biol Neonate. 1977;32:266–274. doi: 10.1159/000241028. [DOI] [PubMed] [Google Scholar]
- Ouadid H, Seguin J, Dumuis A, Bockaert J, Nargeot J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol Pharmacol. 1992;41:346–351. [PubMed] [Google Scholar]
- Parker SG, Taylor EM, Hamburger SA, Vimal M, Kaumann AJ. Blockade of human and porcine myocardial 5-HT4 receptors by SB203186. Naunyn Schmiedebergs Arch Pharmacol. 1995;335:28–35. doi: 10.1007/BF00168912. [DOI] [PubMed] [Google Scholar]
- Pau D, Workman AJ, Kane AK, Rankin AC. Electrophysiological effects of 5-hydroxytryptamine on isolated human atrial myocytes, and the influence of chronic β-adrenoceptor blockade. Br J Pharmacol. 2003;140:1434–1441. doi: 10.1038/sj.bjp.0705553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SV. The regulation of contractile activity in muscle. Biochem Soc Trans. 1979;7:593–617. doi: 10.1042/bst0070593. [DOI] [PubMed] [Google Scholar]
- Pino R, Cerbai E, Alajamo F, Porciatti F, Calamai G, Mugelli A. Effect of 5-hydroxytryptamine (5-HT) on the pacemaker current, if, in human atrial myocytes. Cardiovasc Res. 1998;40:516–522. doi: 10.1016/s0008-6363(98)00198-9. [DOI] [PubMed] [Google Scholar]
- Ponimaskin E, Dumuis A, Gaven F, Barthet G, Heine M, Glebov K, et al. Palmitoylation of the 5-hydroxytryptamine4a receptor regulates receptor phosphorylation, desensitization, and beta-arrestin-mediated endocytosis. Mol Pharmacol. 2005;67:1434–1443. doi: 10.1124/mol.104.008748. [DOI] [PubMed] [Google Scholar]
- Rahme MM, Cotter B, Leistad E, Wadhwa MK, Mohabir R, Ford AP, et al. Electrophysiological and antiarrhythmic effects of the atrial selective 5-HT4 receptor antagonist RS-100302 in experimental atrial flutter and fibrillation. Circulation. 1999;100:2010–2017. doi: 10.1161/01.cir.100.19.2010. [DOI] [PubMed] [Google Scholar]
- Ronde P, Ansanay H, Dumuis A, Miller R, Bockaert J. Homolognous desentization of 5-hydroxytrryptamine4 receptors in rat esophagus: functional and second messenger studies. J Pharmacol Exp Ther. 1995;272:977–983. [PubMed] [Google Scholar]
- Sanders J, Kaumann AJ. A 5-HT4-like receptor in human left atrium. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:382–386. doi: 10.1007/BF00176614. [DOI] [PubMed] [Google Scholar]
- Schoemaker RG, Du XY, Bax WA, Saxena PR. 5-Hydroxytryptamine increases contractile force in porcine right atrium but not in left ventricle. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:486–489. doi: 10.1007/BF00169001. [DOI] [PubMed] [Google Scholar]
- Schoemaker RG, Du XY, Bax WA, Bos E, Saxena PR. 5-Hydroxytryptamine stimulates human isolated atrium but not ventricle. Eur J Pharmacol. 1993;230:103–105. doi: 10.1016/0014-2999(93)90417-g. [DOI] [PubMed] [Google Scholar]
- Vargas ML, Hernandez J, Kaumann AJ. Phosphodiesterase PDE3 blunts the positive inotropic and cyclic AMP enhancing effects of CGP12177 but not of noradrenaline in rat ventricle. Br J Pharmacol. 2006;147:158–163. doi: 10.1038/sj.bjp.0706498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde I, Vandecasteele G, Lezoualc'h F, Fischmeister R. Characterization of the cyclic nuleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102:761–769. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- Zimmermann W, Scholz H, Schumacher C, Wenzlaff H, Haverich A. Effects of saterinone and its enantiomers R(+)-saterinone and S(−)-saterinone on the phosphodiesterase isoenzymes from ventricular tissue of failing human hearts and porcine hearts. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:611–618. doi: 10.1007/BF01258467. [DOI] [PubMed] [Google Scholar]


