Abstract
Background and purpose:
W/Wv and wild-type murine bladders were studied to determine whether the W/Wv phenotype, which causes a reduction in, but not abolition of, tyrosine kinase activity, is a useful tool to study the function of bladder interstitial cells of Cajal (ICC).
Experimental approach:
Immunohistochemistry, tension recordings and microelectrode recordings of membrane potential were performed on wild-type and mutant bladders.
Key results:
Wild-type and W/Wv detrusors contained c-Kit- and vimentin-immunopositive cells in comparable quantities, distribution and morphology. Electrical field stimulation evoked tetrodotoxin-sensitive contractions in wild-type and W/Wv detrusor strips. Atropine reduced wild-type responses by 50% whereas a 25% reduction occurred in W/Wv strips. The atropine-insensitive component was blocked by pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid in both tissue types. Wild-type and W/Wv detrusors had similar resting membrane potentials of −48 mV. Spontaneous electrical activity in both tissue types comprised action potentials and unitary potentials. Action potentials were nifedipine-sensitive whereas unitary potentials were not. Excitatory junction potentials were evoked by single pulses in both tissues. These were reduced by atropine in wild-type tissues but not in W/Wv preparations. The atropine-insensitive component was abolished by pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid in both preparations.
Conclusions and implications:
Bladders from W/Wv mice contain c-Kit- and vimentin-immunopositive ICC. There are similarities in the electrical and contractile properties of W/Wv and wild-type detrusors. However, significant differences were found in the pharmacology of the responses to neurogenic stimulation with an apparent up-regulation of the purinergic component. These findings indicate that the W/Wv strain may not be the best model to study ICC function in the bladder.
Keywords: Interstitial cells, nerve-mediated contraction, immunohistochemistry, intracellular recordings
Introduction
The functional role of interstitial cells of Cajal (ICC) in the physiology of the gastrointestinal tract that was suggested by numerous in vitro investigations of tissues and isolated cells, has been established by the use of mice that have mutations leading to deficiencies in their populations of ICC. Such animal models have been crucial in revealing the pacemaking and neurotransmission roles of several populations of ICC subtypes in different gastrointestinal tissues (Sanders et al., 1999; 2006; Sanders and Ward, 2007). Commercially available animal models that have been employed by researchers interested in ICC have essentially included animals with defects in their Kit signalling pathways either at the Kit receptor (W mutants) or by affecting the Kit receptor ligand, stem cell factor (Steel mutants). The murine W locus, located on chromosome 5, has been identified as being allelic with the c-Kit gene (Chabot et al., 1988; Geissler et al., 1988; Bernstein et al., 1990; Reith et al., 1990) and mice with mutations at the W locus typically have severe macrocytic anaemia, loss of hair pigmentation, defects in haematopoietic stem cells (Russell, 1979) and gastrointestinal motility disorders (Maeda et al., 1992). Homozygote W mutants (W/W) die before birth due to acute anaemia; however, heterozygotes with less severe anaemia from the W/Wv strain are used widely in research. Wv is a point mutation at amino acid 660 in Kit that causes a reduction in but not abolition of tyrosine kinase activity. W/Wv mice survive to adulthood although they have significant c-Kit-related defects, in particular the loss of some populations of ICC in the gut. Interestingly, other populations of gut ICC have apparently normal development (Burns et al., 1996).
The fact that W/Wv mice lack defined populations of ICC has allowed them to be an excellent model for studying the physiological function of these cells in specific gastrointestinal tissues.
The finding that ICC were absent in the myenteric plexus region of the small intestine (ICC-MY) in W/Wv animals was significant given that those tissues were electrically quiescent and normal slow wave (pacemaking) activity was absent (Ward et al., 1994; Huizinga et al., 1995), establishing a key pacemaker role for ICC-MY. Intramuscular ICC (ICC-IM), which are found in the smooth muscle layers in the gut, were found to be missing from the stomach and sphincteric regions of W/Wv mice. The significant defects in inhibitory and excitatory neurotransmission in these W/Wv tissues were explained by the loss of ICC-IM and not by changes in properties of neurons or smooth muscle cells (Burns et al., 1996; Ward et al., 1998; 2000; Beckett et al., 2002).
Knowledge of the function of ICC in the urinary bladder is at a comparatively early stage. While there is an emerging body of evidence on the physiological properties of bladder ICC from the detrusor and lamina-propria regions, both from studies of isolated cells and in situ preparations, the precise physiological roles of ICC in normal bladder function have not yet been elucidated (McCloskey and Gurney, 2002; Sui et al., 2002; Sui et al., 2004; Wu et al., 2004; Hashitani et al., 2004; McCloskey 2005; 2006; Brading and McCloskey, 2005; Johnston et al., 2008). The aim of the present investigation was to study urinary bladders from wild-type and W/Wv mice: in particular, to examine their populations of ICC using specific labelling antibodies and confocal microscopy, to compare their electrical and contractile responses to neurogenic stimulation, to determine the pharmacological identity of the innervation responsible for evoking the responses, to compare their contractile responses to exogenously applied agonists and to investigate their spontaneous electrical activity.
Methods
Preparation of urinary bladders
All animal procedures were in accordance with United Kingdom Animal Scientific Procedures Act, Schedule 1 (1986) and as approved by local university animal welfare and ethics committee or as approved by Care of Animals in the University of Nevada, Reno. Wild-type and mutant animals were available in both the University of Strathclyde and the University of Nevada laboratories. Comparisons between wild-type and mutant tissues in all of the experimental protocols were carried out in the same laboratory.
Urinary bladders were removed from C57BL or W/Wv mice (Jackson laboratories), which had been killed by cervical dislocation. Bladders were opened longitudinally and pinned to the Sylgard silicone elastomer (Dow Corning Corp., Midland, MI, USA) base of Petri dissecting dish. The mucosa was removed by peeling, to leave the underlying detrusor.
Contractile responses to electrical field stimulation
For contractile studies, strips of detrusor (5 mm × 1 mm × 1 mm) were mounted in organ baths with one end tied by thread to a fixed hook and the other to a tension transducer. The initial tension was set to 500 mg. Tissues were constantly superfused with oxygenated Krebs' solution at 35°C. Electrical field stimulation (EFS) was delivered by a Grass stimulator, via two platinum electrodes positioned close to the tissue. The mechanical signals were digitized and recorded to a personal computer running Acknowledge software (BIOPAC Systems) with a computerized data acquisition and analysis system (MP100; BIOPAC Systems, Inc, Goleta, CA).
Immunohistochemistry
Whole-mount or flat-sheet preparations of detrusor were fixed in acetone (for anti-c-Kit) or 4% paraformaldehyde (for anti-vimentin) and washed in PBS for immunohistochemical labelling. After blocking with 1% BSA to prevent nonspecific binding, the tissues were incubated in primary antibody (anti-c-Kit 1:200, Santa Cruz; anti-vimentin 1:100, Sigma, anti-vesicular acetylcholine transferase 1:200, Santa Cruz) containing 0.05% Triton X-100 for at least 24 h. Excess antibody was removed by washing with PBS and tissues were then incubated in fluorescent secondary antibody (Alexa Fluor 488 anti-rabbit immunoglobulin G or Alexa Fluor 594 anti-goat immunoglobulin G, 1:200; Molecular Probes, Eugene, OR). After washing in PBS, tissues were mounted with cover slips and examined with epi-fluorescence microscopy. Slides were imaged using a confocal microscope (Bio-Rad Radiance) equipped with an argon ion laser and a green HeNe laser to excite the fluorophores at 488 or 543 nm respectively, and emission filters to enable collection to a photo-multiplier tube at 520 nm (green) or above 570 nm (red). Double-labelled specimens were imaged sequentially to minimize any bleed-through of fluorophores. Digital images were acquired using Bio-Rad Lasersharp software and reconstructed using Metamorph software (Universal Imaging Inc.) and Adobe Photoshop.
Controls
Omission of the primary antibody showed no significant fluorescence of the secondary antibody. There was minimal auto-fluorescence in tissues that had not been incubated in either primary or secondary antibodies; smooth muscle bundles were only just visible over background levels. Positive controls were carried out on preparations of guinea-pig bladder.
Intracellular recordings
Preparations of detrusor (6 mm × 4 mm) were made as described above and pinned to the base of a Sylgard recording chamber with the serosal surface facing downward. Detrusor cells were impaled with glass microelectrodes that were filled with KCl (3 mol·L−1) and had resistances of 80–120 MΩ. Transmembrane potentials were recorded with a standard electrometer (Intra 767; World Precision Instruments, Sarasota, FL, USA). Data were recorded to a personal computer running AxoScope 8.0 data acquisition software (Axon Instruments, Union City, CA, USA) and results were analysed using Clampfit analysis software (Axon Instruments). In all experiments, parallel platinum electrodes were placed on either side of the muscle strips and neural responses were elicited by square wave pulses of EFS (single pulse; 0.1–0.75 ms pulse duration, 15 V) using a Grass S48 stimulator (Quincy, MA, USA).
Data analysis
Data were analysed using Microsoft Excel, Prism (Graphpad) and Origin (Microcal, Northampton, MA, USA) software and are expressed as mean ± standard error of the mean. Statistical comparisons were made using the Student's paired or unpaired t-test with P < 0.05 considered to be significant. Drug/molecular target nomenclature conform to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Drugs and solutions
Pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), acetylcholine, atropine, tetrodotoxin (TTX), carbachol and nifedipine were all obtained from Sigma Chemical Company (St. Louis, MO). Drugs were dissolved in distilled H2O as stock solutions and added to the organ bath to achieve the stated concentrations. Nifedipine was made up as a stock solution in ethanol that was then diluted in Krebs' solution to its final concentration. Krebs' solution contained the following (mmol·L−1); 120.4 NaCl, 5.9 KCl, 15.5 NaHCO3, 11.5 glucose, 1.2 MgCl2, 1.2 NaH2PO4 and 2.5 CaCl2; pH 7.3–7.4 at 36°C when bubbled with 97% O2/3% CO2.
Results
Bladders from both wild-type and W/Wv animals were emptied of urine by making a longitudinal incision through the detrusor, blotted dry on absorbent tissue and weighed before being placed immediately into saline solution. The mean mass of bladders from W/Wv mice was significantly higher than bladders from age-matched wild-type animals (mass of wild type 0.022 ± 0.002 g compared with W/Wv 0.03 ± 0.003 g, P = 0.042; n = 8 for each group). Macroscopic examination of the bladders did not indicate marked differences in appearance between the two groups.
Immunohistochemistry
Preparations of bladder detrusor from C57BL wild-type and W/Wv mutant mice were labelled with an antibody to the c-Kit receptor (an established marker of ICC) and imaged with a confocal microscope. Wild-type detrusor preparations had c-Kit-positive cells with the typical morphology of ICC reported for guinea-pig bladder (McCloskey and Gurney, 2002; Davidson and McCloskey, 2005) and were spindle-shaped with several lateral branches (Figure 1a,b). They were observed throughout the thickness of the detrusor in all regions of the bladder, located on the boundary of the smooth muscle bundles that were just visible due to their auto-fluorescence, running in parallel with the bundle orientation. Bladder preparations from W/Wv mice also had c-Kit-positive ICC-like cells (Figure 1d,e). These were of similar morphology and distribution to those observed in detrusors from wild-type mice. We did not observe marked differences in the numbers or distribution of c-Kit-positive cells between the wild-type (n = 8) and the mutant mouse bladders (n = 8). These results reveal that the W/Wv mice detrusor, like some regions of the gastrointestinal tract, contains apparently normal ICC. Double-labelling with anti-c-Kit and an antibody to vesicular acetylcholinetransferase showed that c-Kit-positive cells (red) were located close to cholinergic nerves (green) in both wild-type and W/Wv bladders (Figure 1c,f).
Figure 1.
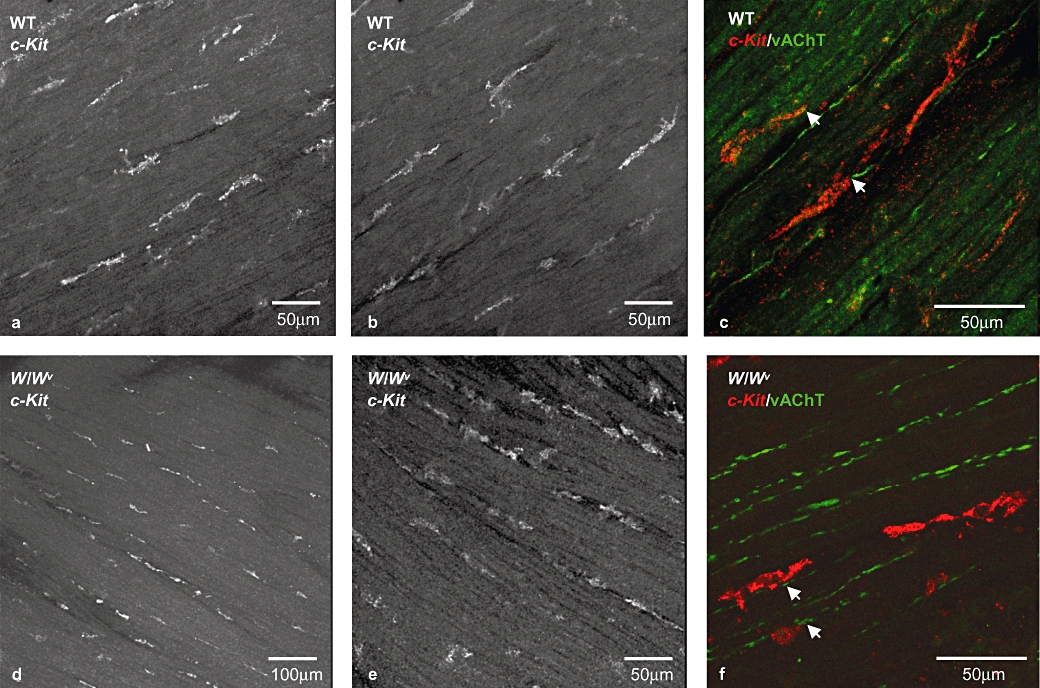
c-Kit-positive cells in wild-type and W/Wv bladder. c-Kit-positive cells in whole-mount preparations of wild-type (a,b) and W/Wv (d,e) mouse detrusor. Immunoreactive cells were elongated with several small lateral branches and were orientated parallel to the detrusor muscle fibres. Double-labelling with an antibody to vesicular acetylcholine transferase (vAChT, green) and anti-c-Kit (red) revealed close proximity between interstitial cells of Cajal and cholinergic neurons in both tissue types (c,f). All images are projections of a stack of optical sections captured with a confocal microscope.
Vimentin antibodies are often used to label ICC as vimentin (intermediate) filaments are found in ICC but are absent from smooth muscle cells. This approach is less specific than the use of c-Kit antibodies as vimentin filaments are found in other cell phenotypes including fibroblasts; however, it is an important method to confirm the presence of ICC in tissue preparations. In the present study, both wild-type and W/Wv detrusors contained vimentin-positive cells that had a variety of morphologies: some were elongated and were located on the boundary of the smooth muscle bundles; others were stellate with several branches emanating from a central nuclear region; others had a more rounded shape (Figure 2). The vimentin-positive cell population is likely to contain both ICC and fibroblasts. Double-labelling experiments with anti-c-Kit and anti-vimentin were not carried out as each antibody requires a specific fixative (detailed in Methods). Clear differences between the pattern of vimentin immunolabelling between wild-type and W/Wv bladders were not detected.
Figure 2.
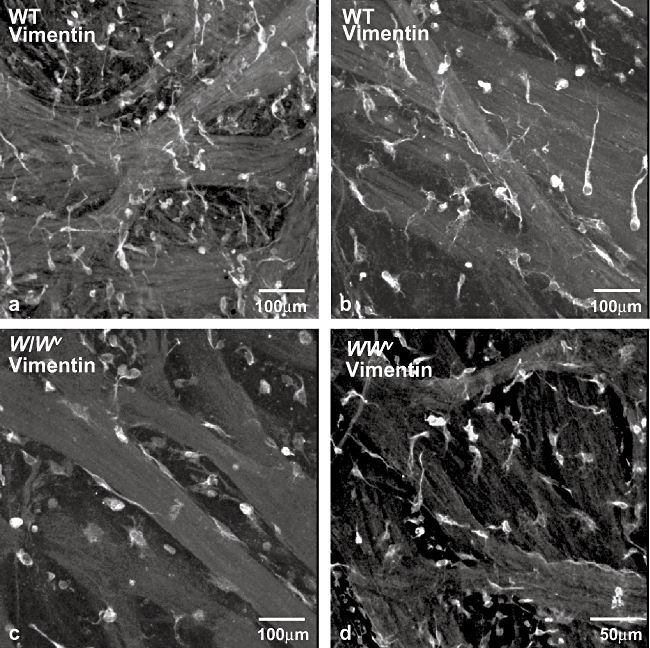
Vimentin-positive cells in wild-type and W/Wv bladder. Vimentin-positive cells in whole-mount preparations of wild-type (a,b) and W/Wv (c,d) mouse detrusor. Vimentin (intermediate) filaments are found in interstitial cells of Cajal (ICC) and fibroblasts but not in smooth muscle cells and vimentin antibodies are often used to label ICC. Vimentin-positive cells had several morphologies including rounded, stellate or elongated cell bodies with branched processes and are likely to include both ICC and fibroblasts. All images are projections of a stack of optical sections captured with a confocal microscope.
Contractile response to EFS
Strips of bladder from wild-type and W/Wv mice were mounted in organ baths and allowed to equilibrate for up to 1 h. Spontaneous contractile activity typically did not develop during this period whether strips were mounted to an initial tension of 100, 200 or 500 mg, consistent with the findings of others (Petkov et al., 2001). Preparations were initially stimulated at 8 Hz for 10 s (100 V, pulse width 0.3 ms) and responded with a contraction that was maintained for the duration of the stimulation. The stimulation was repeated after 5 min to ensure reproducibility of responses. Figure 3 shows contractile responses of wild-type and W/Wv bladder strips to stimulations of 1, 2, 4, 8 and 16 Hz. These were abolished in the presence of TTX (0.5 µmol·L−1), indicating that the stimulation parameters were directly stimulating nerves. Overall, there was no significant difference in the amplitude of EFS-induced contractions produced by strips from W/Wv bladders (n = 16 strips from six animals) compared with those from wild type (n = 12 strips from four animals) at all the frequencies tested [amplitude evoked by 16 Hz stimulation; 738 ± 138 mg (wild type), compared with 612 ± 138 mg (W/Wv); P = 0.53].
Figure 3.
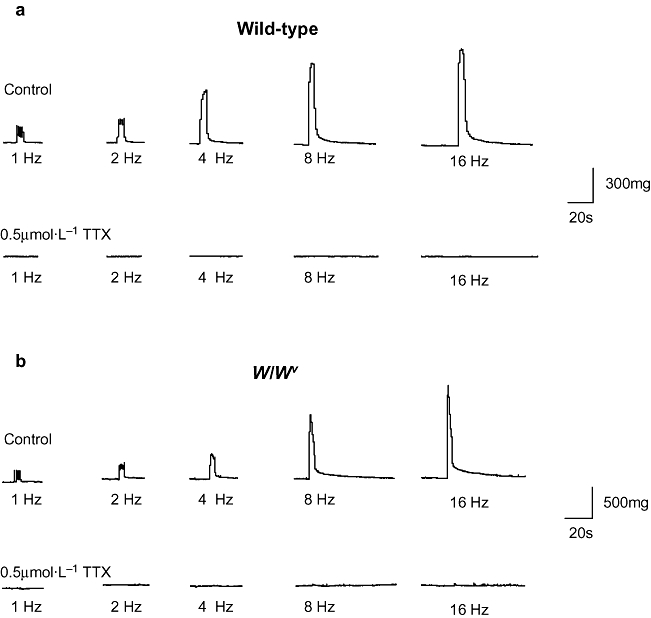
Contractile responses to electrical field stimulation. Detrusor strips from wild-type (a) and W/Wv (b) animals contracted in response to electrical field stimulation applied for 10 s at frequencies of 1, 2, 4, 8 and 16 Hz; pulse width durations of 0.3 ms. All contractions were abolished in the presence of tetrodotoxin (TTX) (0.5 µmol·L−1).
Cholinergic and purinergic mediated contractile responses to EFS
Tissues were perfused with the muscarinic antagonist atropine (1 µmol·L−1) after responses to the range of stimulations had been obtained in the absence of drugs. In strips from wild-type bladders, atropine significantly reduced the contractions at all frequencies (except 1 Hz). Subsequent addition of the purinergic receptor antagonist, PPADS (30 µmol·L−1), abolished the atropine-resistant contractions. A typical experiment is shown in Figure 4a and data for n = 9 strips from three animals are summarized in Figure 4c. Atropine also caused a significant reduction in the EFS contractions in W/Wv animals at all frequencies and the residual component was abolished by the subsequent addition of PPADS (n = 9 strips from three animals, Figure 4b). While both wild-type and W/Wv bladder strips had both a cholinergic and a purinergic component of neurogenic contractions, it is interesting to note that the relative proportions were different. The amplitude of the contraction to 16 Hz in wild-type detrusor strips was 52% cholinergic and 48% purinergic (control 571 ± 185 mg reduced to 272 ± 97 mg in atropine and further reduced to 24 ± 8 mg by PPADS), whereas responses in W/Wv detrusor strips were comprised of 27% cholinergic and 73% purinergic components (control 1054 ± 115 mg, atropine 774 ± 115 mg and PPADS 76 ± 15 mg).
Figure 4.
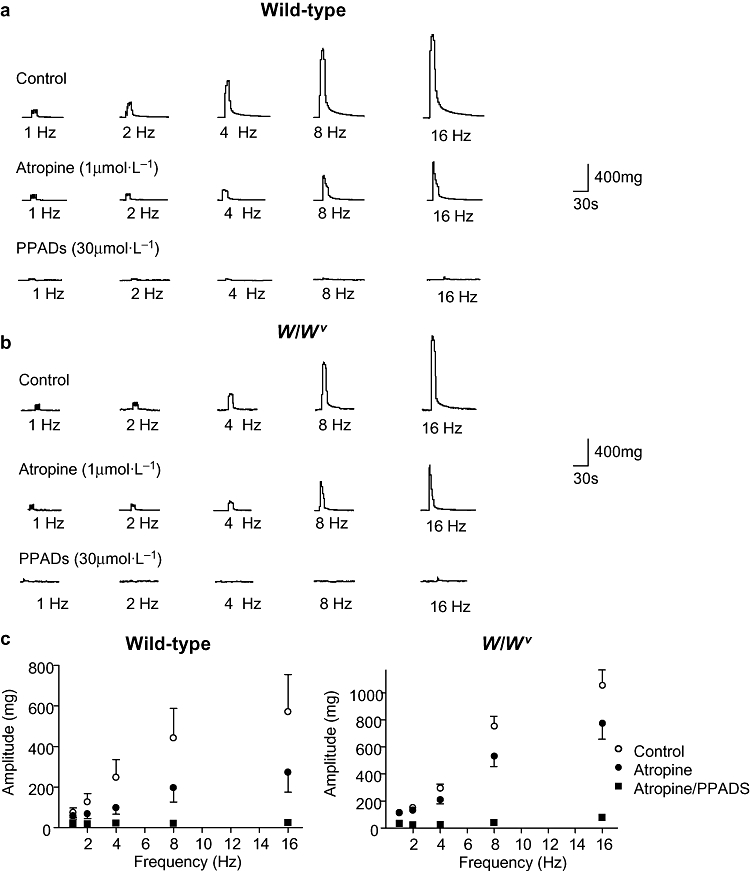
Cholinergic and purinergic components of neurogenic-evoked contractions. Detrusor strips from wild-type (a) and W/Wv (b) animals contracted in response to electrical field stimulation as before. In both tissue types, atropine (1 µmol·L−1) reduced the amplitude of the contractions with the remaining responses inhibited by pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) (30 µmol·L−1). Summary graphs (c) reveal that atropine blocked approximately 50% of the contraction in wild-type strips, whereas in W/Wv strips, atropine reduced the contractions by only about 25%. Contraction amplitude at 16 Hz in wild-type strips was 52% cholinergic and 48% purinergic (control 571 ± 185 mg reduced to 272 ± 97 mg in atropine and further reduced to 24 ± 8 mg by PPADS), whereas responses in W/Wv strips were comprised of 27% cholinergic and 73% purinergic components (control 1054 ± 115 mg, atropine 774 ± 115 mg and PPADS 76 ± 15 mg).
Addition of the muscarinic agonist, carbachol, to wild-type and W/Wv detrusor strips evoked contractions in a concentration-dependent fashion. Typical traces are shown in Figure 5. Concentrations of carbachol (0.5, 1, 5, 10 µmol·L−1) were applied to the tissue for 2 min and then washed out for at least 20 min before the next concentration was added. Experiments showed that this time was sufficient to allow reproducible responses. The area under the curve for the first 5 min of the carbachol-induced contraction at each concentration was measured and normalized to that of the maximum concentration. Mean data were plotted against concentration and fitted with a Hill equation so that the EC50 could be calculated (E/Emax = Emin + (Emax − Emin)[xn/(kn + xn)] where E/Emax was the relative response to carbachol, kn was the EC50 and n was the Hill coefficient). Wild-type strips had an EC50 of 1.2 ± 0.05 µmol·L−1 whereas W/Wv strips had an EC50 of 2.6 ± 0.5 µmol·L−1, indicating that the W/Wv tissues were perhaps less sensitive to carbachol. The shape of the carbachol-induced contractions did not differ between wild-type and W/Wv strips.
Figure 5.
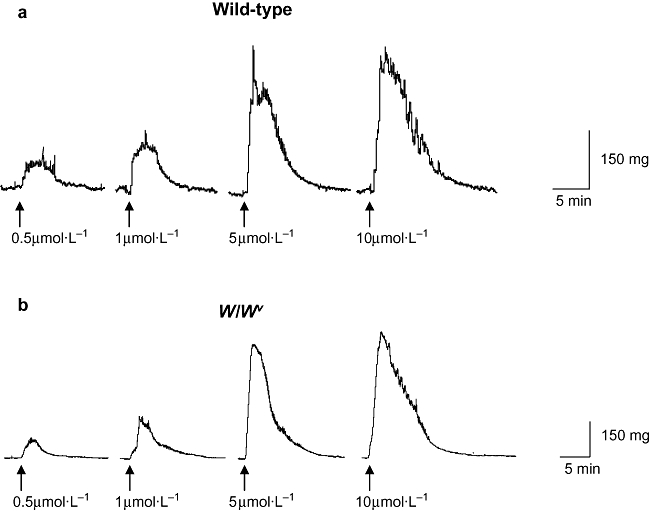
Exogenously applied carbachol. Carbachol was applied to detrusor strips from wild-type (a) and W/Wv (b) bladders as indicated on the traces. Agonist was applied for 2 min and tissues were subsequently washed for at least 20 min before the next concentration was added.
Intracellular recordings
Wild-type detrusor preparations had mean resting membrane potentials of −47.5 ± 1.1 mV (n = 18 impalements of tissues from six animals) and those from W/Wv detrusors had mean resting membrane potentials of −47.7 ± 1.0 mV (n = 12 impalements of tissues from three animals). There was no significant difference between the two groups (P = 0.8966). Spontaneous electrical activity was recorded in both wild-type and W/Wv preparations. This activity typically comprised spontaneous unitary potentials and action potentials. Both wild-type and W/Wv tissues exhibited similar electrical activity and typical recordings are illustrated in Figure 6. Action potentials were fired at a frequency of 3.3 ± 1.6 min−1 (range: 0–14 min−1, n = 11 from four animals) in wild-type tissues and 11.3 ± 3.4 min−1 in W/Wv (range: 2–26 min−1, n = 8 from two animals). This apparent difference in frequency was not tested statistically as tissues from only two mutant animals were available. The mean amplitude of action potentials in wild-type and W/Wv tissues were 30 ± 3.9 and 36.0 ± 4.04 mV respectively. Unitary potentials were fired at a frequency (and amplitude) of 7.7 ± 1.2 min−1 (6.3 ± 1.0 mV) in wild-type (n = 11 from six animals) and 10.9 ± 2.5 min−1 (6.2 ± 0.5 mV) in W/Wv tissues. Action potentials were inhibited by 1 µmol·L−1 nifedipine. However, unitary potentials remained and continued to fire regularly (9.4 ± 2.6 min−1 in wild type and 7.0 ± 2.2 min−1 in W/Wv (n = 5 and n = 4 respectively, P > 0.05 in both wild type and W/Wv). Addition of atropine (1 µmol·L−1) did not affect the frequency or amplitude of unitary potentials nor the resting membrane potential, but addition of PPADS (100 µmol·L−1) caused a small depolarization (in the region of 5 mV) and abolished unitary potentials in both wild-type (n = 2) and W/Wv (n = 3) tissues.
Figure 6.
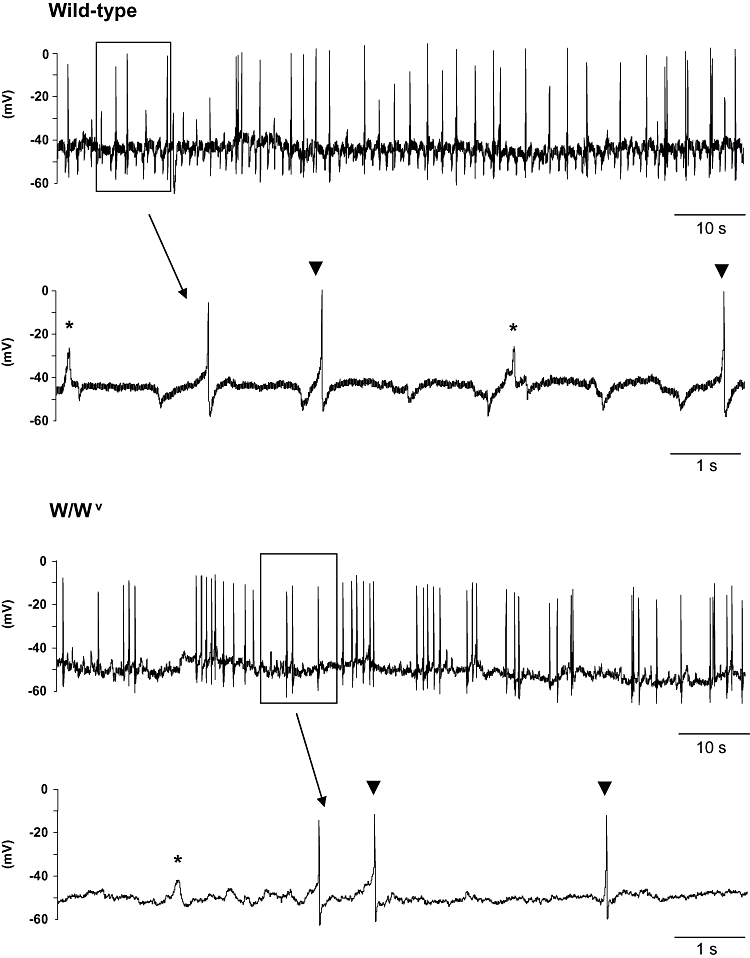
Intracellular recordings of spontaneous electrical activity. Wild-type and W/Wv detrusor preparations exhibited electrical activity comprising spontaneous unitary potentials (denoted by asterisks) and larger events resembling action potentials (indicated by arrowheads). Nifedipine (1 µmol·L−1) inhibited action potentials in both tissue types; however, unitary potentials were unaffected (trace not shown).
Preparations were stimulated with single pulses having a range of durations (0.1, 0.2, 0.3, 0.4, 0.5, 0.75 ms), which typically evoked excitatory junction potentials (EJPs). In the absence of drugs, similar responses were elicited in both wild-type and W/Wv tissues (Figure 7). Application of atropine (1 µmol·L−1) caused a small reduction in the amplitude of the EJPs in wild-type preparations but did not inhibit those from W/Wv detrusors (see expanded traces). In contrast, PPADS (100 µmol·L−1) abolished the atropine-resistant component of EJPs in all tissues.
Figure 7.
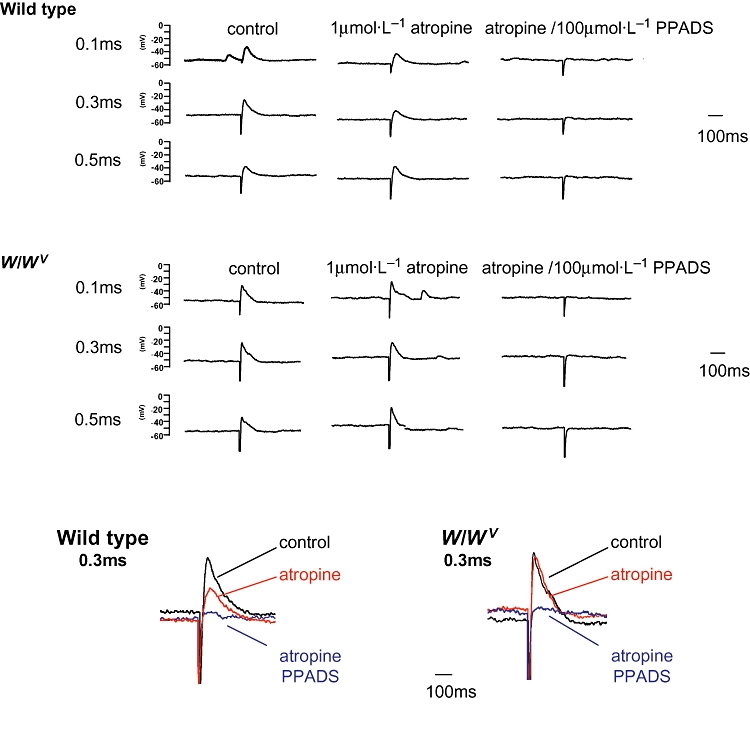
Neurogenic-evoked electrical responses. Wild-type and W/Wv detrusor preparations were stimulated with single pulses having durations of 0.1, 0.3 and 0.5 ms, which typically evoked excitatory junction potentials (EJPs). In the absence of drugs, similar responses were elicited in both wild-type and W/Wv tissues. Application of atropine (1 µmol·L−1) caused a small reduction in the amplitude of the EJPs in wild-type preparations but did not inhibit those from W/Wv detrusors (see expanded traces of the responses to a pulse of 0.3 ms). In contrast, PPADS (100 µmol·L−1) abolished the atropine-resistant component of EJPs in all tissues.
Discussion
The study of ICC in the urinary bladder has been aided by the use of the specific marker anti-c-Kit that has enabled the cells to be identified in fixed tissues, in preparations ‘in situ’ and in enzymically dispersed cells. While progress has been encouraging on investigations of the morphological characteristics of ICC, their structural interactions with nerves and smooth muscle cells, their Ca2+-signalling response to cholinergic stimulation and their electrophysiological properties, the study of mice having mutations in Kit-signalling pathways could potentially provide the opportunity of studying bladders devoid of ICC and therefore help determine the functional role of ICC in the bladder. In the present study, the bladders of W/Wv mice were investigated and compared with wild-type bladders to ascertain whether these mutants are a useful tool in the assessment of the role of ICC in the urinary bladder.
Identification of ICC in W/Wv bladder
Our immunohistochemistry experiments demonstrated that c-Kit-positive ICC and vimentin-positive ICC-like cells were present in both wild-type and W/Wv murine bladders. ICC in the guinea-pig bladder are immunopositive for both c-Kit and vimentin antibodies (Davidson and McCloskey, 2005), comparable with the ICC in the gastrointestinal tract. Vimentin (intermediate) filaments are found in ICC and fibroblasts but not smooth muscle cells and vimentin-labelling is therefore a useful tool in the identification of ICC in smooth muscle tissues. The vimentin-positive cells in the present study will include both ICC and fibroblasts and therefore represents a larger, heterogeneous population compared with the c-Kit-positive cells. The c-Kit-positive ICC had the typical morphology and arrangement of ICC in the guinea-pig bladder; moreover, there was no evidence of differences in quantities or arrangement of ICC in wild-type or W/Wv bladders. This finding was rather disappointing as it indicates that the W/Wv strain is not the model of choice for study of the role of ICC in the bladder. However, it is consistent with findings in gastrointestinal tissues where several classes of ICC in the gut are apparently unaffected by W/Wv mutations. ICC-MY are present in the W/Wv stomach comparable with those in wild-type animals (Ordög et al., 2002;Hirst et al., 2002; Sanders et al., 2006 for review) and it has been reported that while ICC are reduced in the colons of W/Wv mice, no class of ICC are entirely lost in this region (Sanders and Ward, 2007).
There are other strains of mice with W mutations that might be potentially useful in the study of bladder ICC. W/W is a homozygotic lethal mutation arising from a c-KitW allele that encodes a shortened protein lacking the transmembrane portion, leading to non-expression of the Kit receptor on the cell membrane. The majority of W/W animals die in utero or before postnatal day 10 due to anaemia, significantly limiting their ability to be used in ICC-related research. Waskow et al. (2004) generated viable transgenic erythropoietin-overexpressing W/W mice, termed WEPO, which are potentially useful to analyse the role of c-Kit-positive ICC in mature animals. Although these animals do not have surface expression of c-Kit, transgenic expression of erythropoietin appears to overcome the lethality caused by the W/W mutation and the typical macrocytic anaemia is reduced to at a level that enables survival.
General properties of W/Wv smooth muscle
The electrical and mechanical properties of W/Wv detrusor smooth muscle in our study did not appear to be different from wild-type smooth muscle. There was no significant difference between resting membrane potentials, patterns of spontaneous electrical activity or the maximal amplitude of contraction in response to nerve stimulation in the two strains of animals. This is first consistent with the c-Kit immunohistochemistry, which indicated that ICC were present in both tissues and second indicates that W/Wv bladder smooth muscles are not affected by Kit mutations. Studies of W/Wv gastrointestinal smooth muscles in which ICC-IM were absent showed that nerve processes were present as in the controls; there was no reduction in transmitter release; there was no loss of response to exogenous transmitter (showing that integrity of smooth muscle contraction had not been compromised) and that there was no loss of nerve cell bodies or fibres (Ward et al., 1994; 2000; Burns et al., 1996;Beckett et al., 2002).
Up-regulation of purinergic responses
In spite of the presence of ICC in W/Wv detrusors and the apparent normality of the smooth muscle, the contractile response to electrical nerve stimulation had a different pharmacology from that recorded in strips from wild-type bladders. While both wild-type and W/Wv strips responded to nerve stimulation with TTX-sensitive contractions of comparable amplitude, the proportion of cholinergic and purinergic components were notably different. Overall, wild-type bladder strips exhibited an approximate 50: 50 ratio of cholinergic versus purinergic components of contractions, whereas this was about 25: 75 in W/Wv strips. Our control data are consistent with published results for EFS-induced contractions in mouse bladder in which atropine causes a 50% reduction (Werner et al., 2007). The reason for the difference in W/Wv strips is unclear. However, it is reminiscent of several pathological bladder conditions in which purinergic-evoked contractions either emerge for the first time or, alternatively, are up-regulated.
Normal detrusors of many species of mammal including mouse, rat, guinea pig (Brading and Williams 1990; Kennedy et al., 2007) and rabbit (Chancellor et al., 1992) have both cholinergic and purinergic components of the contractile response to neurogenic stimulation (for review see Burnstock 2001). In human detrusor strips, EFS-mediated contractions are normally exclusively atropine-sensitive (see Fry et al., 2005 for review); however, in pathological conditions, an atropine-insensitive component emerges. Strips of detrusor from patients suffering from interstitial cystitis had an atropine-resistant portion of EFS-induced contraction, which was abolished after desensitization with α,β-methylene ATP, in contrast to control detrusor strips where an atropine-insensitive component did not exist (Palea et al., 1993). A purinergic component of EFS-induced contractions has also been reported for idiopathic unstable female human detrusor strips, which comprised 50% of the total response. This finding was explained by an up-regulation of P2X2 receptors in the idiopathic unstable samples and interestingly, other P2X receptors decreased (O'Reilly et al., 2002). A study of human detrusor strips from stable, unstable and obstructed bladders demonstrated that unstable and obstructed strips had atropine-resistant contractions that were sensitive to TTX or α,β-methylene ATP (Bayliss et al., 1999). The emergence of a purinergic component was apparently not due to differences in smooth muscle contractility as the response to direct muscle stimulation was similar in all three patient groups. Unstable bladders arising from a neuropathic instability conversely did not have atropine-resistant contractions.
In a rabbit model of detrusor instability secondary to bladder outlet obstruction, the purinergic component increased and cholinergic component decreased in the early hypertrophic stage (Calvert et al., 2001). However, in a model of partial bladder outlet obstruction in the same species, up-regulation of the P2X component of bladder contraction did not occur 3 weeks after the obstruction, leading the authors to suggest that it perhaps occurred later in bladder hypertrophy/pathology. They also noted no difference in P2X receptor subtypes expressed on detrusor smooth muscle between control and partially obstructed groups (Banks et al., 2006).
It is possible that the W/Wv bladder has undergone changes in the expression of cell types other than ICC, related to the W/Wv c-Kit mutation. For example, it has been reported that mast cells are absent in W/Wv bladder (Saban et al., 2001). D'Andrea et al. (2005) demonstrated spatial relationships between mast cells and nerves in the mucosa and detrusor regions of murine bladder. There is considerable evidence that purinergic mechanisms play key roles in inflammatory responses of many tissues (Kolachala et al., 2007) and it is likely that purinergic neurons contact mast cells in the bladder. It seems feasible that in the mast cell-deficient W/Wv detrusor, there may therefore be a richer innervation of smooth muscle cells leading to a larger neurogenic-evoked purinergic component. Alternatively, our findings might be explained by the W/Wv mutation causing an up-regulation of purinergic nerve fibre expression or indeed an increase in purinoceptor expression. An up-regulation of purinoceptor expression in W/Wv gastric fundus has been shown by Sergeant et al. (2002) who used cDNA microarrays to demonstrate that P2Y receptors were up-regulated in W/Wv muscles lacking ICC-IM.
In summary, the present study has shown that c-Kit-positive ICC and vimentin-positive ICC-like cells are present in the W/Wv bladder. There was little difference in spontaneous electrical activity or contractile properties between wild-type and W/Wv detrusor strips. The neurogenic contractile response had a greater proportion from purinergic nerves than cholinergic nerves in W/Wv tissues compared with wild type. Other strains of mutant mice may offer an advantage over W/Wv in the study of ICC in the urinary bladder.
Acknowledgments
We thank Dr. EAH Beckett (University of Nevada, Reno) for helpful advice on the intracellular recordings and Dr. C Lawrence (University of Strathclyde, Glasgow, UK) for donation of some W/Wv tissues. KDMcC was financially supported by the Biotechnology and Biological Sciences Research Council, the Wellcome Trust (Grant 074591/Z/04/Z) and Queen's University Belfast International Exchange Scheme. SMW received support from NIH DK57236 and DK41315 and KMS received support from DK41315 and DK40569.
Glossary
Abbreviations:
- ICC
interstitial cells of Cajal
- EFS
electrical field stimulation
- EJPs
excitatory junction potentials
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks FC, Knight GE, Calvert RC, Morgan RJ, Burnstock G. Alterations in purinergic and cholinergic components of contractile responses of isolated detrusor contraction in a rat model of partial bladder outlet obstruction. BJU Int. 2006;97:372–378. doi: 10.1111/j.1464-410X.2006.06010.x. [DOI] [PubMed] [Google Scholar]
- Bayliss M, Wu C, Newgreen D, Mundy AR, Fry CH. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J Urol. 1999;162:1833–1839. [PubMed] [Google Scholar]
- Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A, Chabot B, Dubreuil P, Reith A, Nocka K, Majumder S, et al. The mouse W/c-kit locus. Ciba Found Symp. 1990;148:158–166. 166–172. discussion. [PubMed] [Google Scholar]
- Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urinary tract – an assessment of current knowledge. Nat Clin Pract Urol. 2005;2(11):546–554. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- Brading AF, Williams JH. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha,beta-methylene ATP. Br J Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in the lower urinary tract. In: Abbracchio MP, Williams M, editors. Handbook of Experimental Pharmacology; Purinergic and Pyrimidinergic Signalling. Berlin: Springer; 2001. pp. 423–515. [Google Scholar]
- Calvert RC, Thompson CS, Khan MA, Mikhailidis DP, Morgan RJ, Burnstock G. Alterations in cholinergic and purinergic signaling in a model of the obstructed bladder. J Urol. 2001;166:1530–1533. [PubMed] [Google Scholar]
- Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Kaplan SA, Blaivas JG. The cholinergic and purinergic components of detrusor contractility in a whole rabbit bladder model. J Urol. 1992;148:906–909. doi: 10.1016/s0022-5347(17)36775-7. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Saban MR, Gerard NP, Wershil BK, Saban R. Lack of neurokinin-1 receptor expression affects tissue mast cell numbers but not their spatial relationship with nerves. Am J Physiol Regul Integr Comp Physiol. 2005;288:R491–500. doi: 10.1152/ajpregu.00452.2004. [DOI] [PubMed] [Google Scholar]
- Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–1390. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- Fry CH, Brading AF, Hussain M, Lewis SA, Takeda M, Tuttle JB, et al. In: Incontinence. Abrams P, Cardozo L, Khoury S, Wein A, editors. Plymouth: Health Publications; 2005. pp. 313–362. [Google Scholar]
- Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Beckett EA, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002;540:1003–1012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Johnston L, Carson C, Lyons AD, Davidson RA, McCloskey KD. Cholinergic induced Ca2+-signalling in Interstitial Cells of Cajal from the guinea-pig bladder. Am J Physiol Renal Physiol. 2008;294:F645–55. doi: 10.1152/ajprenal.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Tasker PN, Gallacher G, Westfall TD. Identification of atropine- and P2X1 receptor antagonist-resistant, neurogenic contractions of the urinary bladder. J Neurosci. 2007;27:845–851. doi: 10.1523/JNEUROSCI.3115-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic mechanisms in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2007;294:G401–410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- McCloskey KD. Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol. 2005;173:296–301. doi: 10.1097/01.ju.0000141581.00922.f4. [DOI] [PubMed] [Google Scholar]
- McCloskey KD. Calcium currents in interstitial cells from the guinea-pig bladder. BJU Int. 2006;97(6):1338–1343. doi: 10.1111/j.1464-410X.2006.06156.x. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit-positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- Ordög T, Baldo M, Danko R, Sanders KM. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/W mutant mice. Gastroenterology. 2002;123:2028–2040. doi: 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- O'Reilly BA, Kosaka AH, Knight GF, Chang TK, Ford AP, Rymer JM, et al. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990;3:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Saban R, Saban MR, Nguyen NB, Hammond TG, Wershil BK. Mast cell regulation of inflammation and gene expression during antigen-induced bladder inflammation in mice. Physiol Genomics. 2001;7:35–43. doi: 10.1152/physiolgenomics.00044.2001. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;5:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Large RJ, Beckett EA, McGeough CM, Ward SM, Horowitz B. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics. 2002;11:1–9. doi: 10.1152/physiolgenomics.00052.2002. [DOI] [PubMed] [Google Scholar]
- Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002;90:118–129. doi: 10.1046/j.1464-410x.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol. 2004;171:938–943. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C, Terszowski G, Costa C, Gassmann M, Rodewald HR. Rescue of lethal c-KitW/W mice by erythropoietin. Blood. 2004;104(6):1688–1695. doi: 10.1182/blood-2004-04-1247. [DOI] [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol. 2007;292:R616–R624. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol. 2004;559:231–243. doi: 10.1113/jphysiol.2004.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]


