Abstract
Background and purpose:
Mucosal microcirculation is compromised during gastric damage induced by non-steroidal anti-inflammatory drugs, such as aspirin. Consequently, oxygen supply to epithelial cells is decreased. The trefoil factor (TFF) peptides are involved in mechanisms of defence and repair in the gastrointestinal tract but their regulation at sites of gastric injury is unknown.
Experimental approach:
Hypoxia and expression of TFF genes and peptides were measured in the damaged stomach of aspirin-treated rats. In a human gastric cell line (AGS cells), the effects of hypoxia and of hypoxia inducible factor (HIF)-1 (through transient transfection of HIF-1α siRNA or over-expression of HIF-1α) on TFF gene expression were evaluated.
Key results:
Hypoxyprobe immunostaining, up-regulation of TFF2 (1.9-fold) and TFF3 (1.8-fold) and a non-significant increase of TFF1 (1.5-fold) mRNA were observed in the damaged stomach of aspirin-treated rats, compared with control animals. Hypoxia (3% O2, 16 h) induced mRNA for TFF1 (5.8-fold), TTF2 (9.1-fold) and TFF3 (9.3-fold) in AGS cells, an effect mediated by HIF-1, as transient transfection of HIF-1α siRNA reduced the effects of hypoxia. Over-expression of HIF-1α by transfection in non-hypoxic epithelial cells produced a similar pattern of TFF induction to that observed with hypoxia and transactivated a TFF1 reporter construct.
Conclusions and implications:
Hypoxia inducible factor-1 mediated the induction of TFF gene expression by hypoxia in gastric epithelial cells. Low oxygen levels and up-regulation of TFF gene expression in the damaged stomach of aspirin-treated rats suggest that hypoxia induced expression of TFF genes at sites of gastric injury.
Keywords: epithelial cells, gastric damage, HIF-1, hypoxia, TFF genes, TFF peptides
Introduction
Gastric damage induced by non-steroidal anti-inflammatory drugs (NSAIDs) constitutes one of the most common adverse reactions associated with pharmacological treatment. It is generally believed that the ability of these agents to inhibit gastric prostaglandin generation (Vane, 1971) constitutes the main causal factor in the reduction of gastric mucosal blood flow (McCarthy, 1995), which consequently diminishes the supply of oxygen to the epithelial cells. In recent years, the discovery of a widespread system of cellular oxygen sensing that controls gene expression has led to the study of its role in many pathological circumstances. The hypoxia inducible factor-1 (HIF-1) has been identified as the master regulator of the transcriptional response to hypoxia (Semenza, 2001; 2003; Huang and Bunn, 2003). HIF-1, composed of two subunits (HIF-1α and HIF-1β), binds to a 5′-RCGTG-3′ hypoxia-response element (HRE) in the promoter regions of target genes. HIF-1 activity is regulated mainly through the post-translational control of protein stability by oxygen. In normoxia, HIF-1α is continuously degraded by specific enzymes which use oxygen as a major substrate (Bruick, 2003; Lando et al., 2003). When the oxygen level decreases, HIF-1α escapes hydroxylation and accumulates in the nucleus, where it binds to HIF-1β, forming an HIF-1 complex that becomes transcriptionally active.
The trefoil factor (TFF) family is a group of peptides synthesized and secreted by mucosal epithelia (Sands and Podolsky, 1996; Taupin and Podolsky, 2003) composed of three members: TFF1 (or pS2), TFF2 (or spasmolytic polypeptide, SP) and TFF3 (or intestinal TFF, ITF). In the gastrointestinal tract, TFF peptides are involved in mechanisms of defence and repair by interacting with mucins to form the mucus barrier and promote the process of restitution (Dignass et al., 1994; Playford et al., 1995; Kato et al., 1999; Hoffmann, 2005). The expression of these peptides in the gut occurs in a tissue- and cell-specific manner; TFF1 and TFF2 are constitutively expressed in the stomach, with TFF1 being restricted mostly to pit cells and TFF2 to mucous neck cells in gastric glands. In contrast, TFF3 is expressed mainly in goblet cells of the small and large intestine. Transcriptional regulation plays a major role in the expression of TFF genes and transcription factors such as GATA-6 mediate the constitutive expression of TFF1 and TFF2 (Al azzeh et al., 2000), while goblet cell-specific transcriptional elements are involved in TFF3 expression (Baus-Loncar and Giraud, 2005). Interestingly, when the integrity of the mucosa is threatened, this regionally specific expression disappears and induction of all three genes is observed in gastric tissue (Alison et al., 1995; Longman et al., 2000).
Hypoxia has been reported to increase TFF3 mRNA expression in intestinal epithelial cells, which is interpreted as a mechanism for maintenance of barrier function when oxygen levels are low (Furuta et al., 2001; Louis et al., 2006). In addition, a binding site for HIF-1 has been characterized in the human TFF3 gene promoter (Furuta et al., 2001). Analysis of the TFF1 and TFF2 genes shows presence of some HRE consensus sequences in their promoters. Considering the protective and healing effects of these TFF peptides on gastric ulceration (Playford et al., 1995; Babyatsky et al., 1996; McKenzie et al., 2000) we have analysed the effects of hypoxia and the role of HIF-1 on expression of TFF genes in gastric epithelial cells. Our results show for the first time, an HIF-1-dependent induction of TFF1 and TFF2 mRNA expression by hypoxia and that gastric cells also up-regulate TFF3 in response to low oxygen levels.
Methods
Animal model
All animal protocols complied with European Community guidelines for the use of experimental animals, and were approved by the ethics committee of the Faculty of Medicine, University of Valencia.
Male Sprague-Dawley rats (250–300 g) (Harlan Laboratories, Barcelona, Spain) were maintained on standard Purina laboratory chow and tap water ad libitum, and were housed at a controlled temperature (21 ± 1°C) and lighting regime of 0700–1900 h. On the day of the experiment, fasted (24 h) rats were treated with a vehicle (1% carboxymethylcellulose, p.o) or a gastro-toxic dose of aspirin (150 mg kg−1, p.o.). Six hours later, animals were killed, their stomachs were excised and two strips of the corpus were obtained; one was fixed in 4% formaldehyde solution for immunohistochemical studies. The second sample was snap frozen in liquid nitrogen and stored at −80°C for subsequent reverse transcription-polymerase chain reaction (RT-PCR) analysis.
Detection of tissue hypoxia
Tissue hypoxia was assessed in the gastric corpus using Hypoxyprobe-1 solution (Hypoxyprobe™-1 Plus, Chemicon Int. Temecula, CA, USA). Hypoxyprobe-1 is an exogenous nitroaromatic compound, which is metabolized in a stepwise reduction pathway by cellular nitroreductase enzymes that are able to use the nitroaromatic compounds as alternative electron acceptors in conditions of low physiological pO2. The consequent fragmentation of the imidazole ring leads to formation of chemical adducts with several macromolecular components of cells that can be detected by a specific antibody.
In our experiments, both vehicle and aspirin-treated rats were given Hypoxyprobe solution (60 mg kg−1 i.p.) (Morani et al., 2006) and 15 min later the animals were killed and tissues removed for analysis. Rats that were not injected with Hypoxyprobe-1 were used as negative controls.
Immunohistochemical studies
Hypoxyprobe-1, TFF1 and TFF3 were detected in representative 5 µm sections of paraffin-embedded tissues from vehicle or aspirin-treated rats. After antigen retrieval with 10 mmol L−1 citrate at 95°C, sections of tissues from rats given Hypoxyprobe-1 were sequentially incubated with a mouse FITC-conjugated IgG1 primary antibody against Hypoxyprobe-1 (Chemicon Int, 1:50) and a horseradish peroxidase (HRP)-conjugated secondary monoclonal antibody against FITC (Chemicon Int, 1:50). TFF1 and TFF3 detection were carried out in slides of aspirin or vehicle-treated rats incubated with a rabbit polyclonal antibody against rat TFF1 (kindly provided by Dr Giraud, 1:200) or with a rabbit polyclonal antibody against rat TFF3 (kindly provided by Dr Podolsky, 1:200) respectively. A goat anti-rabbit antibody conjugated with HRP (Vector Laboratories, Burlingame, CA, USA, 1:200) was used as secondary antibody. Finally, all tissues were incubated with DAB Enhanced Liquid substrate System for Immunohistochemistry (Sigma Chemical Co) and were counterstained with haematoxylin. The specificity of the immunostaining was confirmed in all cases by the absence of staining in analogous tissue sections when either the primary or the secondary antibodies were omitted. In addition, an immunizing peptide blocking experiment was performed to determine the specificity of the rat TFF3 and TFF1 antibodies. Western blot analysis of extracts (30 µg) from rat jejunum using anti-rat-TFF3 (1:10 000) or stomach with anti-rat-TFF1 (1:1000), revealed a major band of 7 and 12 kDa respectively. These signals were blocked with increasing amounts of TFF3 (1, 2 or 4 µg) and TFF1 (0.8, 1.6 3.2 µg) peptides respectively. Transfers were stripped and reprobed for actin as a loading control.
Cell culture and transfection
AGS cells, purchased from ATCC (Manassas, VA USA), were cultured in F12K medium (Invitrogen Life Technologies Carlsbad, CA, USA) supplemented with 10% FCS 100 U ml−1 penicillin and 100 µg ml−1 streptomycin, at 37°C in a humidified atmosphere (21% O2) with 5% CO2. Cells were exposed to a hypoxic atmosphere in which there was 3% O2 (with increased proportions of N2). Cells were routinely transfected at 60–80% confluence with Lipofectamine™ 2000 (Invitrogen). Forty-eight hours after transfection, cells were processed for Western blot or RT-PCR analysis as described below. In some experiments, AGS cells were incubated in normoxia with the nitric oxide (NO) donor DETA-NO (10, 50 or 100 µmol L−1, diethylenetriamine/NO, Alexis Co., Nottingham, UK) for 60 min, followed by 16 h incubation in hypoxia.
HIF-1α overexpression and transient silencing
HIF-1α was overexpressed in cells by using a plasmid that encoded a mutated HIF-1α (pcDNA4-HIF1α; kindly provided by Dr J. Mateo, CNIC, Madrid, Spain) or an empty vector (pcDNA4). Mutation of the proline residues at 402 and 564 and the asparagine residue at 803 allows HIF-1α to escape oxygen-dependent hydroxylation (personal communication from Dr Mateo). The resulting expressed protein is, therefore, stable in normoxia.
In order to silence endogenous HIF-1α we used RNA interference (RNAi) by employing a vector-driven system (pBS U6/Pol III) as previously described (Apostolova et al., 2006), using the HIF-1α-specific sequence 5′-GTCTCGAGATGCAGCCAGA-3′ (Erler et al., 2004), which we named siHIF-1α. Control transfections included an siRNA targeted to green fluorescent protein (GFP) (Apostolova et al., 2006) and called siGFP.
Luciferase reporter gene assay
The 5′-flanking region of human TFF1 (Genbank Accession NM_003225) was amplified by PCR from a sample of genomic DNA (Roche Diagnostics, Indianapolis, IN, USA) using the primers, 5′-GCCTCGAGTACAGGAGAGCAGGAGGCTGT-3′ and ATAAGCTTGCCTCCTCTCTGCTCCAAAGG-3′, which are engineered to contain a Xho I and an Hind III site respectively (underlined). The PCR product was gel-purified and cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA), using a T-A cloning strategy. Correct fragments were then cloned into the pGL3 luciferase reporter plasmid (Promega). Transient co-transfection assays of the luciferase reporter construct of the human TFF1 promoter and pcDNA4-HIF1α were carried out and luciferase analysis was performed as described (Apostolova et al., 2006). Finally luciferase activity was defined as the ratio between the constitutively active luciferase and the luciferase reporter plasmid.
Western blot analysis of HIF-1α and TFF peptides
Preparation of total protein extracts and membrane transfer was carried out as described (Mateo et al., 2003). Total protein concentration in extracts was determined with the Pierce BCA protein assay kit (Pierce Chemicals, Boulder, CO, USA), using BSA to generate a standard curve. Membranes were blocked with 5% non-fat dry milk in TBS-T (20 mmol L−1 Tris/HCl pH 7.2, 150 mmol L−1 NaCl and 0.1% Tween 20) and incubated overnight with a monoclonal antibody against HIF-1α (dilution 1:250 BD Biosciences, San Jose, CA, USA) or a polyclonal antibody against h-TFF1 (dilution 1:250 Santa Cruz Biotechnology), h-TFF2 (dilution 1:1000, Abnova) and h-TFF3 (dilution 1:200, Santa Cruz Biotechnology) or actin (dilution 1:4000; Sigma). Protein bands were detected by incubation with HRP-conjugated goat anti-mouse IgG (dilution 1:2500; DakoCytomation, Glostrup, Denmark) or goat anti-rabbit IgG (1:5000; Vector Laboratories), followed by treatment with supersignal west pico chemiluminescent substrate (Pierce) and revealed using LAS-3000 (Fujifilm).
Real-time quantitative RT-PCR
Total RNA from gastric samples or AGS cells was isolated with TriPure Isolation Reagent (Roche Diagnostics GmbH. Mannheim, Germany) and the RNeasy Mini kit (Qiagen, Valencia, CA, USA) respectively. The protocol was followed as described previously (Quintana et al., 2004). Specific primers for rat TFF1, TFF2 and TFF3 and human TFF1, TFF2, TFF3 and Glut-1 were designed according to reported sequences (Table 1) and cyclophilin A (rat and human, CyPA) was employed as a housekeeping gene. To quantify input amounts of templates, a standard curve was obtained with serial dilutions of total RNA of a positive control (Table 1) for each analysed gene also after RT-PCR. Specificity of PCR was confirmed by melting curve analysis and agarose gel electrophoresis. To normalize the results, interpolated values for each sample were divided by values for the corresponding housekeeping gene CyPA, and results are expressed as the fold induction of the TFF/CyPA ratio for each treatment versus the corresponding control group.
Table 1.
Primer sequences, reaction data and characteristics of specific PCR products for each gene analysed
| Target gene | Primer sequences (5′-3′) | Tann (°C) | PCR cycles | Size (bp) | Positive control |
|---|---|---|---|---|---|
| rCyc | CGTCTGCTTCGAGCTGTTTG (s) GTAAAATGCCCGCAAGTCAA (as) | 60 | 35 | 464 | Rat stomach |
| rTFF1 | TTGCCCAGAACCAGGAAG (s) GTGCCGAGTCTTGATGTAACC (as) | 60 | 30 | 227 | Rat stomach |
| rTFF2 | GTGCCCCTCTCTTGGTAGTG (s) GACGCTTGGTTTGGAAGT G (as) | 59 | 35 | 240 | Rat stomach |
| rTFF3 | ATGGAGACCAGAGCCTTCT (s) GGATGCTGGAGTCAAAACAG (as) | 59 | 40 | 193 | Rat intestine |
| hCyc | CGTCTCCTTTGAGCTGTTTG (s) GGTGATCTTCTTGCTGGTCT (as) | 58 | 35 | 415 | AGS cells |
| hGlut1 | ATGAAGGAAGAGAGTCGGCA (s) TGAAGAGTTCAGCCACGATG(as) | 57 | 45 | 547 | AGS cells in hypoxia |
| hTFF1 | GCAAATAAGGGCTGCTGTTTC (s) GAAGCGTGTCTGAGGTGTCC(as) | 61 | 40 | 209 | AGS cells in hypoxia |
| hTFF2 | CCCCCATAACAGGACGAAC (s) ATGAAGTTGGAGAAGCAGCAC (as) | 60 | 45 | 231 | AGS cells in hypoxia |
| hTFF3 | TCCCCAAGCAAACAATCC(s) TGAAACACCAAGGCACTCC(as) | 60 | 45 | 317 | AGS cells in hypoxia |
as, anti-sense; PCR, polymerase chain reaction; s, sense; Tann, annealing temperature.
Statistical analysis
Data are reported as the mean ± SEM. Comparisons between groups were made by means of an unpaired Student's t-test or one-way analysis of variance (anova) followed by a Newman-Keuls test where appropriate. Graphpad Prism version 3.03 (GraphPad, San Diego, CA, USA) was used to perform statistical analysis; P values < 0.05 were considered statistically significant.
All drug and molecular target nomenclature conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Gastric damage induced by aspirin is associated with hypoxia and increased TFF1, TFF2 and TFF3 mRNA expression in the gastric mucosa
Administration of a single dose of aspirin (150 mg kg−1, p.o.) to fasted rats caused haemorrhagic lesions in the corpus region of the stomach when analysed 6 h later. Histological analysis revealed mucosal erosions affecting around the third upper part of the mucosa (Fig. 1A). In normal rat gastric tissue immunohistochemical analysis of TFF1 expression revealed the surface mucous cells as the specific location of this peptide along the epithelium. After this single gastro-toxic dose of aspirin, expression of TFF1 was lost specifically at focal sites of injury where there had been loss of surface mucous cells (Fig. 1A). However, a broad band of TFF1 immunostaining was uniformly distributed along the gastric corpus of aspirin-treated rats (Fig. 1A). TFF3 was minimally expressed in the gastric corpus of control animals and it was localized at the surface mucous cells (Fig. 1A). A moderate immunostaining was observed in the third upper part of the mucosa of the gastric corpus of aspirin-treated rats (Fig. 1A).
Figure 1.
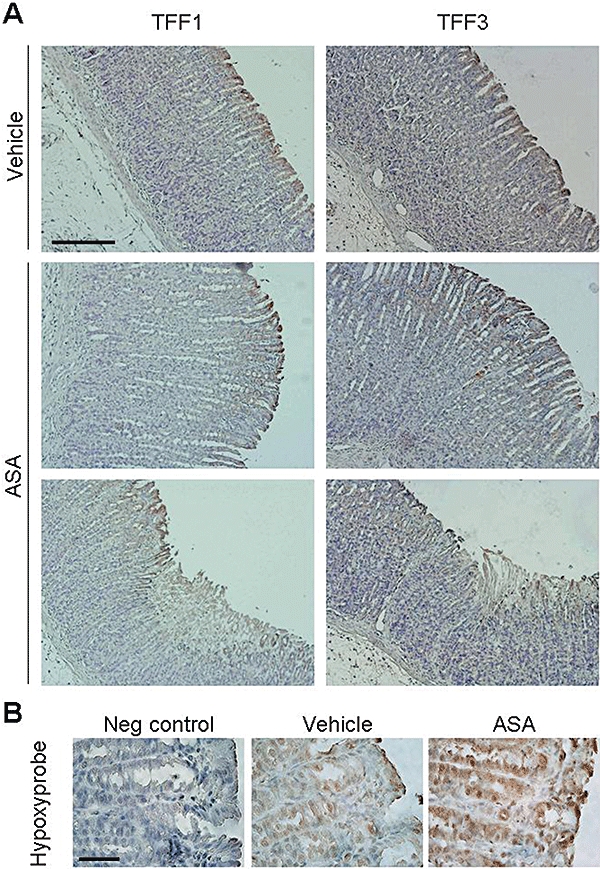
Aspirin-induced gastric damage in rats is associated with increased expression of TFF1 and TFF3 peptides and with hypoxia in the gastric corpus. Sections of gastric corpus of the stomach of vehicle- or aspirin-treated rats (6 h after 150 mg kg−1, p.o.) were excised, formalin-fixed, paraffin-embebbed and cut into 5 µm-sections. (A) Immunohistochemical detection of TFF1 and TFF3 in the gastric corpus of vehicle- or aspirin-treated rats (representative of n ≥ 3 assays) Scale bar: 200 µm. (B) Hypoxyprobe immunostaining in the superficial epithelium of the gastric corpus of vehicle or aspirin-treated rats (representative of n ≥ 3 assays; note the magnification is greater than in A). Scale bar: 50 µm. TFF, trefoil factor; ASA, aspirin.
A strong staining of hypoxyprobe was present along the gastric glands and pits of both non-damaged and damaged regions of the gastric corpus of aspirin-treated rats. A limited immunostaining was observed in the gastric mucosa of control animals (Fig. 1B), indicating that gastric damage induced by aspirin results in significant tissue hypoxia within the epithelium (Fig. 1B).
Real-time quantitative RT-PCR (qPCR) analysis of mRNA expression of TFF genes in a section of the control rat gastric corpus revealed significant differences among the three genes. TFF1 was abundantly expressed in the rat gastric corpus as its basal mRNA expression is significantly higher than that of CyPA. The expression levels of TFF2 mRNA were around half that of CyPA while TFF3 mRNA was much less expressed (Fig. 2A). Interestingly, a significant increase of TFF2 and TFF3 mRNA and a non-significant increase of TFF1 mRNA were observed in the stomach of aspirin-treated rats compared with control animals while the amount of CyPA was not significantly different among the experimental groups (Fig. 2B).
Figure 2.
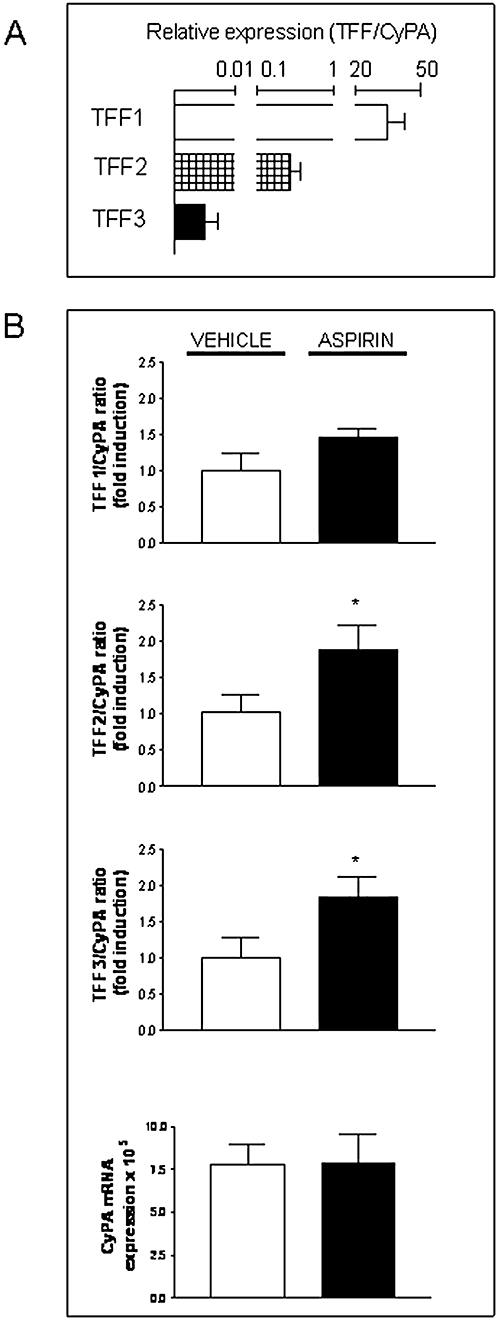
Aspirin-induced gastric damage in rats is associated with increased mRNA expression of TFF1, TFF2 and TFF3 in the gastric corpus. (A) Relative mRNA expression levels of TFF1, TFF2 and TFF3 versus the housekeeping gene CyPA in the gastric corpus of Sprague-Dawley control rats. (B) Ratio between mRNA expression levels of each TFF and CyPA in the homogenised stomach of aspirin-treated rats, expressed as fold induction versus the corresponding vehicle-treated group. CyPA mRNA expression in the gastric corpus of vehicle or aspirin-treated rats are also shown. Bars represent the mean ± SEM (n ≥ 3). Comparisons between groups were performed using the unpaired Student's t-test. Significant difference from the respective vehicle group is shown by *P < 0.05. TFF, trefoil factor.
Hypoxia induces HIF-1α stabilization and increases TFF genes and peptides expression in gastric epithelial cells (AGS cell line)
Constitutive expression of mRNA for TFF1, TFF2 and TFF3 was observed in AGS cells, and the amount of all three TFF mRNAs is lower than that of CyPA (Fig. 3A). As observed in the rat gastric mucosa, TFF1 mRNA was the most abundant while the expression levels of TFF3 mRNA was very low (Fig. 3A). In these cells, hypoxia (3% oxygen) induced HIF-1α stabilization in a time-dependent manner (Fig. 3B) and significantly increased TFF1, TFF2 and TFF3 mRNA, while it did not significantly modify CyPA mRNA levels (Fig. 3B). Western blot analysis of TFF peptides in AGS cells showed constitutive expression of TFF1 and TFF2 while TFF3 was almost undetectable. Hypoxia increased in all cases the amount of TFF peptides in whole cell extracts (Fig. 3C).
Figure 3.
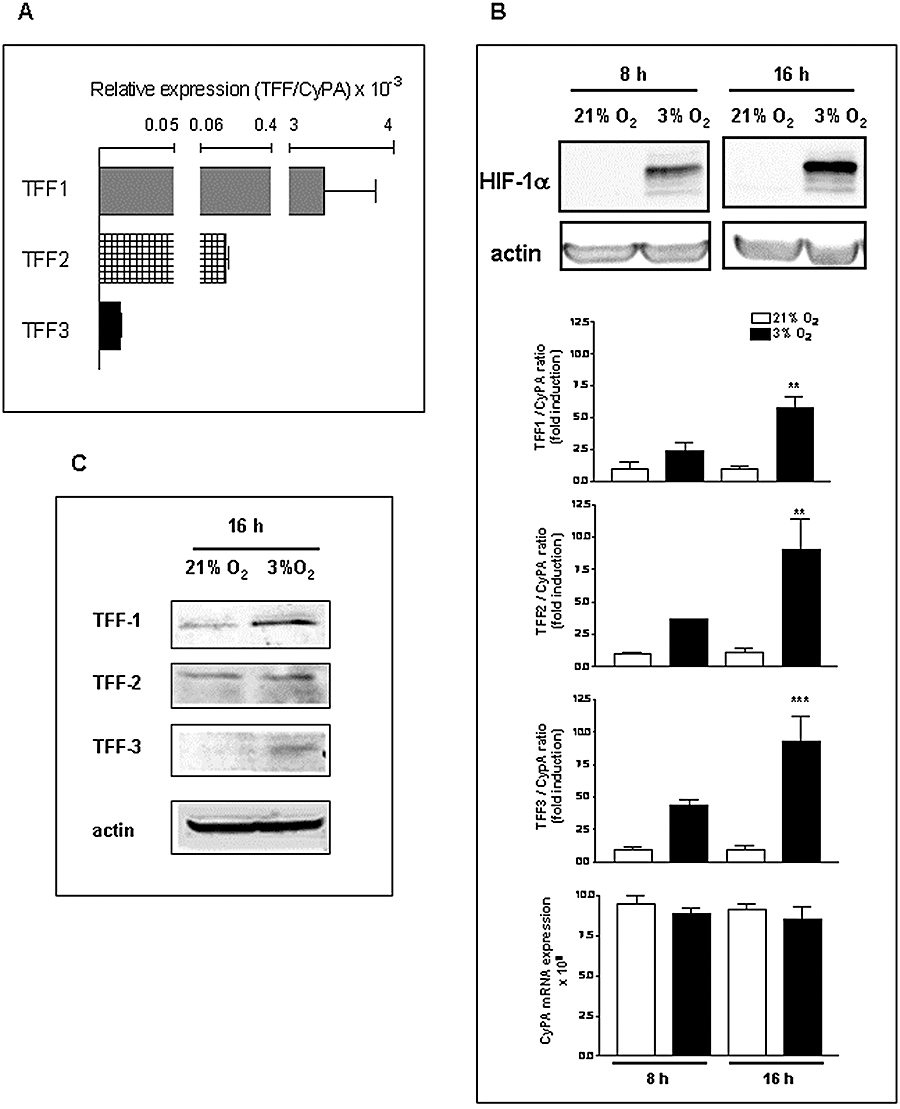
Hypoxia induces HIF-1α stabilization and increases TFF1, TFF2 and TFF3 gene expression in AGS cells (gastric epithelial cell line). (A) Relative mRNA expression levels of TFF1, TFF2 and TFF3 versus the housekeeping gene CyPA in AGS cells. (B) Cells were incubated in normoxia (21% O2) or hypoxia (3% O2) for 8 and 16 h and results show the levels of HIF-1α and actin in whole cell extracts (by Western blot) and, in the lower graphs, the ratio between mRNA expression levels of each TFF and CyPA, expressed as fold induction versus the corresponding normoxia-treated group and CyPA mRNA expression in the different experimental groups. Bars in the graphs represent the mean ± SEM (n ≥ 3). Comparisons between groups were performed using anova followed by Newman Keuls test. Significant difference from the respective normoxic group is shown by **P < 0.01 or ***P < 0.001. (C) Western blot detection of TFF1, TFF2, TFF3 and actin in cells incubated 16 h in normoxia or hypoxia. Blots are representative of results obtained in three separate experiments. HIF, hypoxia inducible factor; TFF, trefoil factor.
TFF1, TFF2 and TFF3 mRNA expression induced by hypoxia is HIF-1-dependent
In AGS cells, the NO donor compound, DETA-NO, induced a dose-dependent destabilization of hypoxia-induced HIF-1α (Fig. 4A) as previously demonstrated in other cell lines (Mateo et al., 2003). In parallel, DETA-NO (100 µmol L−1) induced a significant decrease in the hypoxia-induced expression of TFF1, TFF2 and TFF3 mRNA (Fig. 4B). In addition, transfection of siHIF-1 into AGS cells displayed a significantly lower level of steady-state HIF-1α protein after incubation in hypoxia, than cells transfected with a control siGFP which in turn exhibited a significant HIF-1α stabilization compared with the same cells under normoxia (Fig. 5). The reduction in HIF-1α protein observed in siHIF-1α cells was paralleled by a significant reduction in the hypoxic induction of TFF1, TFF2 and TFF3 mRNA (Fig. 5). Finally, no significant changes in CyPA mRNA levels were observed between siHIF-1 and siGFP cells (Fig. 5).
Figure 4.
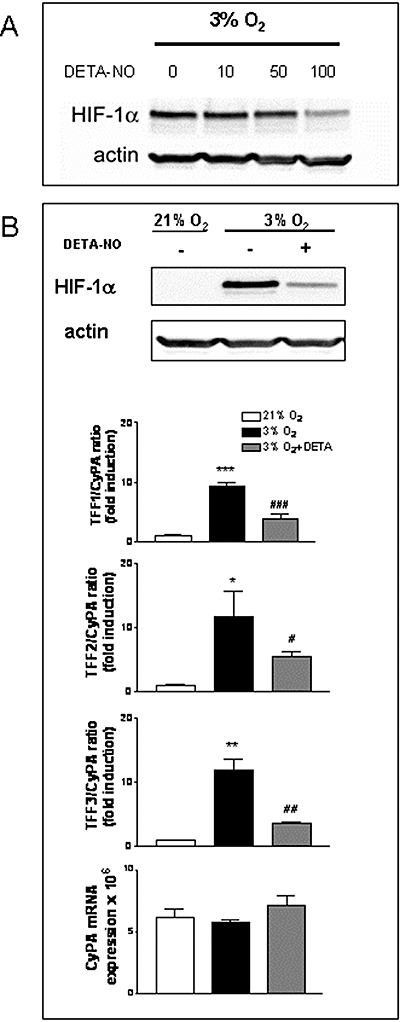
Inhibition of HIF-1α accumulation by nitric oxide decreases hypoxic induction of TFF1, TFF2 and TFF3 gene expression. (A) Western blot detection of HIF-1α and actin in whole cell extracts from AGS cells, pre-treated for 1 h with increasing doses of DETA-NO, before incubation in hypoxia for 16 h. (B) AGS cells were incubated for 16 h in normoxia, hypoxia, or hypoxia plus 100 µmol L−1 DETA-NO. Results show Western blots for HIF-1 and actin and, in the lower graphs, the ratio between mRNA expression levels of each TFF and CyPA, expressed as fold induction versus the corresponding normoxia-treated group and CyPA mRNA expression for each experimental group. Bars in the graphs represent the mean ± SEM (n ≥ 3). Comparisons between groups were performed using anova followed by Newman Keuls test. Significant difference from the respective control group in normoxia is shown by *P < 0.05, **P < 0.01 or ***P < 0.001 and from the respective group in hypoxia is shown by #P < 0.05, ##P < 0.01 or ###P < 0.001. Blots are representative of results obtained in three separate experiments. HIF, hypoxia inducible factor; NO, nitric oxide; TFF, trefoil factor.
Figure 5.
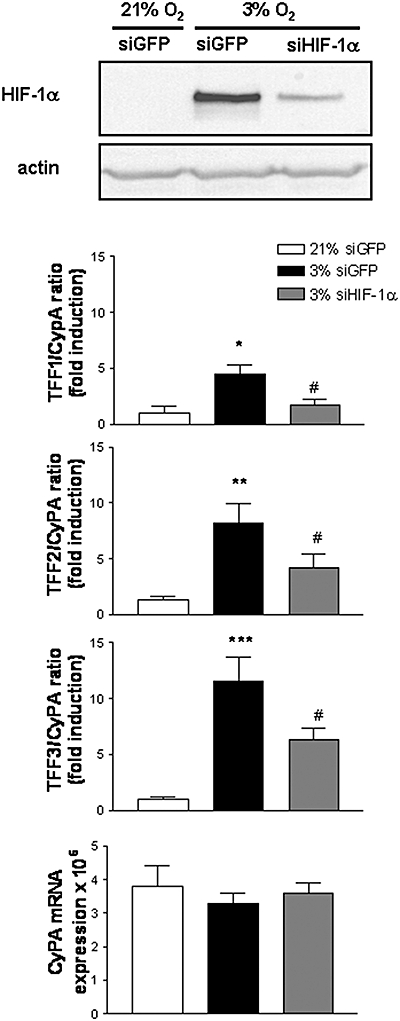
Hypoxia inducible factor (HIF)-1α suppression by siRNA decreases hypoxic induction of TFF1, TFF2 and TFF3 gene expression. AGS cells were transiently transfected with a plasmid expressing a siRNA against HIF-1α or green fluorescent protein (GFP) and incubated for 16 h in hypoxia or normoxia. (A) Western blot detection of HIF-1α and actin in whole cell extracts. Blots are representative of results obtained in three separate experiments. (B) Graphs show the ratio between mRNA expression levels of each TFF and CyPA, expressed as fold induction versus the corresponding normoxia-treated group and CyPA mRNA expression for each experimental group. Bars in the graphs represent the mean ± SEM (n ≥ 3). Comparisons between groups were performed using the anova followed by Newman Keuls test. Significant difference from the respective control group in normoxia is shown by *P < 0.05, **P < 0.01 or ***P < 0.001 and from the respective control group in hypoxia as #P < 0.05. TFF, trefoil factor.
Overexpression of HIF-1α up-regulates TFF1, TFF2 and TFF3 mRNA in normoxia
Transfection of AGS cells with an oxygen-stable HIF-1α plasmid (pcDNA-HIF1α) resulted in robust protein expression, as detected by Western blot analysis (Fig. 6) while no HIF-1α was observed in empty vector-transfected control cells (pcDNA4). This protein was transcriptionally active, as levels of Glut1 mRNA, a well-established HIF-1 responsive gene, were higher in these cells than in control cells (Fig. 6). A parallel significant increase in TFF1, TFF2 and TFF3 mRNA was observed in HIF-1α transfected cells while no significant changes were observed in CyPA mRNA levels.
Figure 6.
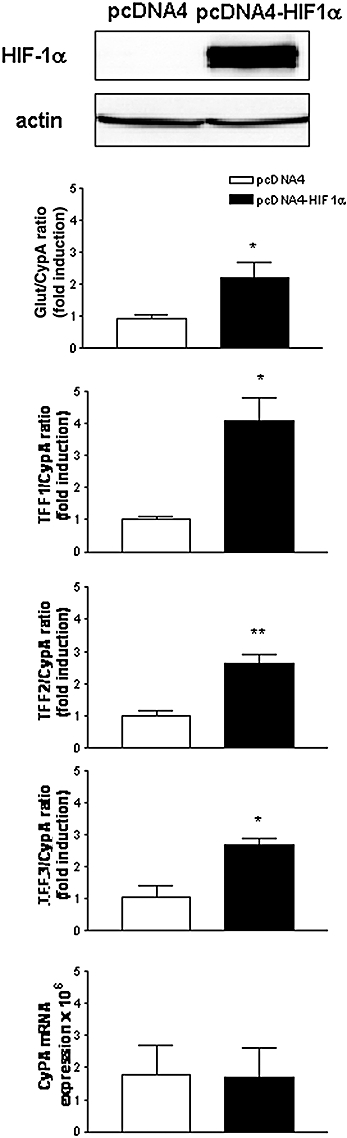
Hypoxia inducible factor (HIF)-1α overexpression increases TFF1, TFF2 and TFF3 gene expression in normoxia. AGS cells were transiently transfected with a plasmid expressing a mutant HIF-1α (P402A/P564A/N803A) or the empty vector for 48 h. (A) Western blot detection of HIF-1α and actin in whole cell extracts. Blots are representative of results obtained in three separate experiments. (B) Graphs show the ratio between mRNA expression levels of each TFF and CyPA, expressed as fold induction versus the corresponding control group and CyPA mRNA expression for each experimental group. Bars in the graphs represent the mean ± SEM (n ≥ 3). Comparisons between groups were performed using the unpaired Student's t-test. Significant difference from the respective control group is shown by *P < 0.05 or **P < 0.01. TFF, trefoil factor.
Functional response of TFF1 promoter region to HIF-1
Structural analysis of the TFF1 and TFF2 genes shows an HRE at −552/−562 and −283/−293, relative to the transcription start site in their respective promoters. We therefore assessed whether the human TFF1 promoter is responsive to HIF-1. As shown in Figure 7, cells that over-express HIF-1 in normoxia, transiently transfected with the full-length human TFF1 promoter, showed an approximately threefold increase in luciferase ratio over cells transfected with an empty vector P < 0.05.
Figure 7.
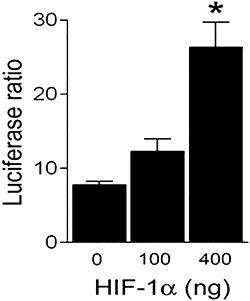
Hypoxia inducible factor (HIF)-1α over-expression activates the TFF1 proximal promoter in normoxia. Gastric epithelial cells were co-transfected with a luciferase reporter construct of the human TFF1 promoter and with different doses of the pcDNA4-HIF1α or pcDNA4. Graphs show the luciferase ratio between the constitutively active luciferase and the luciferase reporter plasmid. Bars in the graphs represent the mean ± SEM (n ≥ 3) and comparisons between groups were performed using anova followed by the Newman Keuls test. Significant difference from the control group is shown by *P < 0.05. TFF, trefoil factor.
Discussion
The present study demonstrates, for the first time, an HIF-1-dependent induction of TFF1 and TFF2 mRNA expression by hypoxia and extends to another location, gastric epithelial cells, the phenomenon of HIF-1-dependent regulation of TFF3. The presence of hypoxia and the up-regulation of TFF genes in the damaged stomach of aspirin-treated rats suggest that an endogenous mechanism is triggered by hypoxia to repair the damaged mucosa at sites of gastric injury.
Histological analysis of the gastric corpus of aspirin-treated rats show superficial erosions characterized by loss of surface and foveolar epithelial cells without affecting the muscularis mucosa. As shown in the present and other studies (Rio et al., 1988) most of the cells lost in the course of mucosal erosion are those in which constitutive TFF peptides are mainly expressed. Despite this observation a net increase in expression of mRNA from TFF genes was still detected in the gastric corpus of aspirin-treated rats which suggests that new cells and/or additional mechanisms are involved in the induction of TFF genes in the damaged stomach. Immunohistochemical analysis revealed a broad band of TFF1 expression in the corpus mucosa of aspirin-treated rats, compared with that in the control mucosa. Previous studies have reported up-regulation of this peptide in deeper cells of the gastric mucosa but it has usually been associated with severe gastric ulceration (Ulaganathan et al., 2001). Aspirin treatment also extends the cellular pattern of expression of TFF3, a peptide that is hardly expressed in the gastric corpus but is more abundant in the antrum and the duodenum (Taupin and Podolsky, 2003; Kouznetsova et al., 2004). Although our study has been restricted to the area containing the mucosal erosions induced by aspirin, i.e. the gastric corpus, it is possible that the increased expression of TFF3 in this area may be partly a consequence of its release into the gastric juice from more distal parts in the stomach, as the ability of TFF peptides to induce their expression in a paracrine manner has been described (Taupin et al., 1999; Baus-Loncar and Giraud, 2005; Hoffmann, 2005).
Several factors associated with aspirin-induced gastric damage may be related to TFF induction. Aspirin itself has been described as promoting TFF2 gene activation in gastric cell lines (Azarschab et al., 2001), while several cytokines, which could be released as part of the inflammatory process associated with damage, have been related to modulation of TFF genes (Dossinger et al., 2002; Blanchard et al., 2004). A previous study has reported TFF3 regulation by hypoxia in intestinal epithelial cells (Furuta et al., 2001). Interestingly, in the present study both TFF1 over-expression and induction of TFF3 in the damaged stomach of aspirin-treated rats took place in cells that were positively stained for hypoxia, results suggesting that low oxygen levels associated with cellular damage may induce the coordinated expression of the members of the TFF family in the rat gastric corpus.
The effect of hypoxia on TFF genes expression has been analysed in a gastric epithelial cell line, AGS. As expected from the gastric origin of these cells, basal relative expression of TFF genes was similar to that in the rat gastric corpus. Hypoxia induced in a time-dependent manner, transcriptional regulation of TFF1, TFF2 and TFF3, an effect that correlated with HIF-1α stabilization. This factor seems to be involved in the increased transcription of these genes by low oxygen levels as both pharmacological destabilization of HIF-1α with an NO donor and the more selective decrease of endogenous HIF-1α through gene silencing with siRNA, significantly reduced hypoxia-induced TFF gene expression. In contrast to hypoxia, no HIF-1α stabilization was observed in AGS cells in normoxia, suggesting that this transcription factor does not modulate the constitutive levels of TFF mRNA detected in these cells. Transcription factors such as GATA-6, present in AGS cells (Watson et al., 2002) have been reported to activate TFF1 and TFF2 promoters and regulate the constitutive expression of these genes (Al azzeh et al., 2000; Taupin and Podolsky, 2003).
In addition to HIF-1, a number of other transcription factors are also activated directly or indirectly by hypoxia (Cummins and Taylor, 2005). In an attempt to bypass the complex situation of hypoxia, the specific involvement of HIF-1 in the transcriptional regulation of TFF gene expression was assessed in cells transiently transfected with a plasmid that over-expresses HIF-1 in normoxia. Under these circumstances, an increased up-regulation of TFF1, TFF2 and TFF3 mRNA expression was detected, a result that establishes a direct link between HIF-1 and the TFF genes. It is interesting to point out that this up-regulation was less than that observed in hypoxia. Although differences in the experimental models used in both studies could explain the quantitative changes observed, the possibility that other transcription factors activated by hypoxia are also involved in the up-regulation of TFF genes cannot be ruled out. Finally, because of several HRE sequences (5′-RCGTG-3′; Semenza, 1999) in human TFF1 and TFF2 promoters and the results obtained in the present study showing the dose-dependent activation of a minimal TFF1 reporter construct by HIF-1 over-expression in gastric epithelial cells, we would strongly suggest that human TFF1 and TFF2 are target genes for HIF-1. Interestingly, a similar situation may exist in the gastric corpus of the rat, as the same HRE consensus sequences are present in the rat TFF1, TFF2 and TFF3 promoters (−105/−115, −294/−314 and 3/−13, respectively, relative to the transcription start site), which leads us to propose that HIF-1 may act as a transcription factor in the up-regulation of TFF genes observed in the damaged stomach of aspirin-treated rats. Further studies are needed to address this question.
In the present study, TFF genes were up-regulated in two situations: in the damaged stomach of aspirin-treated rats and in human gastric epithelial cells by hypoxia. Both conditions involved a transcriptional mechanism that required several hours to obtain active proteins. TFF peptides have been described as mediating different steps of tissue repair, particularly by modulating cell-cell contacts and cell migration, processes that start immediately after the damage occurs (Hoffmann, 2005). Considering the results of the present study, it seems unlikely that the mechanism described here initiates the rapid re-epithelization of the mucosa. We propose that the constitutively high levels of these peptides trigger the initiation of repair in vivo, while transcriptional regulation of TFF gene expression at sites of gastric damage could be involved in the subsequent stages of remodelling. Consistent with this hypothesis, TFF peptides have also been related to mechanisms associated with later stages of mucosal repair (Kinoshita et al., 2000; Bossenmeyer-Pourie et al., 2002).
In conclusion, our results demonstrate an HIF-1-dependent induction of TFF1, TFF2 and TFF3 gene expression during hypoxia in gastric epithelial cells. The presence of hypoxic cells and the increased expression of TFF genes in the damaged stomachs of aspirin-treated rats lead us to propose that mucosal hypoxia activates an endogenous mechanism, intended to repair gastric damage.
Acknowledgments
We thank Dr Podolsky and Dr Giraud for providing us with the TFF antibodies. KJM and SC acknowledge support from the ‘Ramon y Cajal’ programme of Spain. The study was supported by CIBER CD06/04/0071 (Ministerio de Sanidad), SAF2004-06211, SAF2007-064201 (Ministerio de Educación y Cultura), ACOMP06-237 and ACOMP07-297 (Generalitat Valenciana).
Glossary
Abbreviations:
- HIF-1
hypoxia inducible factor
- TFF
trefoil factor
Conflict of interest
None.
References
- Al azzeh ED, Fegert P, Blin N, Gott P. Transcription factor GATA-6 activates expression of gastroprotective trefoil genes TFF1 and TFF2. Biochim Biophys Acta. 2000;1490:324–332. doi: 10.1016/s0167-4781(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl.)(2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alison MR, Chinery R, Poulsom R, Ashwood P, Longcroft JM, Wright NA. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995;175:405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Cervera AM, Victor VM, Cadenas S, Sanjuan-Pla A, Alvarez-Barrientos A, et al. Loss of apoptosis-inducing factor leads to an increase in reactive oxygen species, and an impairment of respiration that can be reversed by antioxidants. Cell Death Differ. 2006;13:354–357. doi: 10.1038/sj.cdd.4401776. [DOI] [PubMed] [Google Scholar]
- Azarschab P, Al Azzeh E, Kornberger W, Gott P. Aspirin promotes TFF2 gene activation in human gastric cancer cell lines. FEBS Lett. 2001;488:206–210. doi: 10.1016/s0014-5793(00)02422-4. [DOI] [PubMed] [Google Scholar]
- Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- Baus-Loncar M, Giraud AS. Multiple regulatory pathways for trefoil factor (TFF) genes. Cell Mol Life Sci. 2005;62:2921–2931. doi: 10.1007/s00018-005-5480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, et al. IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol. 2004;172:3775–3783. doi: 10.4049/jimmunol.172.6.3775. [DOI] [PubMed] [Google Scholar]
- Bossenmeyer-Pourie C, Kannan R, Ribieras S, Wendling C, Stoll I, Thim L, et al. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J Cell Biol. 2002;157:761–770. doi: 10.1083/jcb200108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossinger V, Kayademir T, Blin N, Gott P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell Physiol Biochem. 2002;12:197–206. doi: 10.1159/000066279. [DOI] [PubMed] [Google Scholar]
- Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004;24:2875–2889. doi: 10.1128/MCB.24.7.2875-2889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–2938. doi: 10.1007/s00018-005-5481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- Kato K, Chen MC, Nguyen M, Lehmann FS, Podolsky DK, Soll AH. Effects of growth factors and trefoil peptides on migration and replication in primary oxyntic cultures. Am J Physiol. 1999;276:G1105–G1116. doi: 10.1152/ajpgi.1999.276.5.G1105. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Taupin DR, Itoh H, Podolsky DK. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: structure-function analysis of human intestinal trefoil factor. Mol Cell Biol. 2000;20:4680–4690. doi: 10.1128/mcb.20.13.4680-4690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova I, Peitz U, Vieth M, Meyer F, Vestergaard EM, Malfertheiner P, et al. A gradient of TFF3 (trefoil factor family 3) peptide synthesis within the normal human gastric mucosa. Cell Tissue Res. 2004;316:155–165. doi: 10.1007/s00441-004-0854-1. [DOI] [PubMed] [Google Scholar]
- Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781–790. doi: 10.1046/j.1432-1033.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–1627. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- McCarthy DM. Mechanisms of mucosal injury and healing: the role of non-steroidal anti-inflammatory drugs. Scand J Gastroenterol Suppl. 1995;208:24–29. doi: 10.3109/00365529509107758. [DOI] [PubMed] [Google Scholar]
- McKenzie C, Thim L, Parsons ME. Topical and intravenous administration of trefoil factors protect the gastric mucosa from ethanol-induced injury in the rat. Aliment Pharmacol Ther. 2000;14:1033–1040. doi: 10.1046/j.1365-2036.2000.00796.x. [DOI] [PubMed] [Google Scholar]
- Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, et al. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta−/−) mice. Proc Natl Acad Sci USA. 2006;103:7165–7169. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford RJ, Marchbank T, Chinery R, Evison R, Pignatelli M, Boulton RA, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Quintana E, Hernandez C, Alvarez-Barrientos A, Esplugues JV, Barrachina MD. Synthesis of nitric oxide in postganglionic myenteric neurons during endotoxemia: implications for gastric motor function in rats. FASEB J. 2004;18:531–533. doi: 10.1096/fj.03-0596fje. [DOI] [PubMed] [Google Scholar]
- Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, Chenard MP, et al. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Sands BE, Podolsky DK. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Perspectives on oxygen sensing. Cell. 1999;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- Taupin D, Wu DC, Jeon WK, Devaney K, Wang TC, Podolsky DK. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999;103:R31–R38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaganathan M, Familari M, Yeomans ND, Giraud AS, Cook GA. Spatio-temporal expression of trefoil peptide following severe gastric ulceration in the rat implicates it in late-stage repair processes. J Gastroenterol Hepatol. 2001;16:506–512. doi: 10.1046/j.1440-1746.2001.02469.x. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Watson F, Kiernan RS, Dimaline R. GATA proteins are potential negative regulators of HDC gene expression in the gastric epithelium. Biochim Biophys Acta. 2002;1576:198–202. doi: 10.1016/s0167-4781(02)00301-9. [DOI] [PubMed] [Google Scholar]


