Abstract
Background
Little is known concerning the impact of HIV status disclosure on quality of life, leaving clinicians and families to rely on research of children with other terminal illnesses.
Objectives
The purpose of this work was to examine the impact of HIV disclosure on pediatric quality of life and to describe the distribution of age at disclosure in a perinatally infected pediatric population.
Methods
A longitudinal analysis was conducted of perinatally HIV-infected youth ≥5 years of age enrolled in a prospective cohort study, Pediatric AIDS Clinical Trials Group 219C, with ≥1 study visit before and after HIV disclosure. Age-specific quality-of-life instruments were completed by primary caregivers at routine study visits. The distribution of age at disclosure was summarized. Six quality-of-life domains were assessed, including general health perception, symptom distress, psychological status, health care utilization, physical functioning, and social/role functioning. For each domain, mixed-effects models were fit to estimate the effect of disclosure on quality of life.
Results
A total of 395 children with 2423 study visits were analyzed (1317 predisclosure visits and 1106 postdisclosure visits). The median age at disclosure was estimated to be 11 years. Older age at disclosure was associated with earlier year of birth. Mean domain scores were not significantly different at the last undisclosed visit compared with the first disclosed visit, with the exception of general health perception. When all of the visits were considered, 5 of 6 mean domain scores were lower after disclosure, although the differences were not significant. In mixed-effects models, disclosure did not significantly impact quality of life for any domain.
Conclusions
Age at disclosure decreased significantly over time. There were no statistically significant differences between predisclosure and postdisclosure quality of life; therefore, disclosure should be encouraged at an appropriate time.
Keywords: quality of life, HIV, children and adolescents
What's Known on This Subject.
Current recommendations regarding diagnostic disclosure to children with HIV/AIDS are based on lessons from pediatric oncology. The limited number of disclosure-related QoL studies among HIV-infected pediatric populations have focused on psychological and emotional health, with inconsistent outcomes reported after disclosure.
What This Study Adds.
This is the first longitudinal study to examine the impact of disclosure of HIV status on health-related QoL using multivariate analytical methods and broad-based QoL outcomes.
Since the advent of HAART, children with HIV experience a less symptomatic early course and survive to older ages1–4, which increasingly raises questions about disclosure of diagnosis. Many families are reluctant to discuss the nature of the illness with their infected child or adolescent, yet delays in disclosure of HIV infection may potentially result in negative consequences. Lack of disclosure may impair treatment understanding and participation and increase psychological and behavioral problems.5–7 As children reach adolescence and begin risk-taking behaviors, knowledge about their disease becomes essential for both personal health maintenance and HIV prevention within the larger population.
Despite strong recommendations from the American Academy of Pediatrics that children and adolescents with HIV be told their diagnosis,8 published rates of disclosure among this population are widely varied, ranging from 18% to 77%.9–15 The rationale for HIV disclosure is based on the pediatric oncology model because of sparse empiric evidence from HIV-infected populations.6,16,17 Studies on childhood cancer report better long-term emotional health, improved self-esteem, and closer relationships with parents after disclosure.18–21 Yet, parents and guardians of children with HIV voice reasons that are not applicable to cancer for resisting disclosure, including the social stigma of HIV infection, guilt over perinatal transmission, and loss of another family member to AIDS.6,10,22 Research on the impact of HIV disclosure is limited and has focused primarily on psychological and emotional health outcomes, with varying results.23 Thus, it is important to better understand the effect of HIV disclosure on health-related quality of life (QoL) to develop more effective disclosure strategies in this population.
Health-related QoL measures are designed to be multidimensional and provide a standardized approach to assess patient and caregiver perspectives of health and functioning, areas that are important considerations in the clinical management of chronic progressive illnesses, such as HIV infection.24–26 QoL instruments also capture aspects of health commonly missed by standard clinical end points, such as survival and disease progression. Our study sought to describe the distribution of age at disclosure and to evaluate the impact of disclosure on health-related QoL by assessing within-patient changes in QoL for those with ≥1 predisclosure and ≥1 postdisclosure QoL measurement.
Methods
Study Population
This longitudinal analysis was based on data collected for the Pediatric AIDS Clinical Trials Group (PACTG) 219C prospective cohort study, which was designed to examine the long-term effects of HIV and antiretroviral therapies in children exposed in utero, postnatally, or both. HIV-infected or perinatally HIV-exposed children <21 years of age at entry were eligible for enrollment. Institutional review boards at participating sites in the United States and Puerto Rico approved the research protocol, and written informed consent was obtained from each child's parent/guardian. The study population used in this analysis was restricted to perinatally HIV-infected study participants ≥5 years of age, enrolled in PACTG 219C between September 15, 2000, and December 31, 2005. Patients were excluded if they did not have ≥1 predisclosure and ≥1 postdisclosure QoL measurement.
Data Collection
At study enrollment, a physical examination was performed, demographic information was recorded, and laboratory measurements were collected, including CD4+ cell percentage and HIV-1 RNA viral load. Children were assigned a Centers for Disease Control and Prevention (CDC) clinical classification.27,28 Clinical records were abstracted to obtain complete histories of antiretroviral therapy (ART) regimens and clinical diagnoses. Data on new diagnoses, laboratory immunologic and virologic measurements, ART use, and QoL were collected at routine study visits.
Disclosure status, age at disclosure, and the QoL outcomes were collected from the PACTG 219C parent/caregiver QoL form. Disclosure status (disclosed or not disclosed) was assessed by a question that asked whether the patient knows he/she is infected, and, if so, the age at disclosure. The actual date of disclosure was not collected; therefore, we used 2 approaches to estimate age at disclosure. The first approach was to estimate the age of the child at the midpoint between the last undisclosed visit date and the first disclosed visit date. The second approach was to use the first caregiver-reported age at disclosure, because the indicated ages were not always consistently reported over time.
QoL Instruments
The General Health Assessment for Children (GHAC) was used to gather QoL data. This modular tool, developed for use in PACTG 219, used previously validated instruments to evaluate 4 QoL domains: general health perception, symptom distress, psychological status, and physical functioning.24 In addition, the GHAC measured social/role functioning and health care utilization. The GHAC demonstrates both good reliability and validity.24 In this study, all of the previously validated QoL domains demonstrated good internal consistency with Cronbach's α scores ranging from .87 (general health perception) to .97 (physical functioning). QoL outcomes assessed with the GHAC have been studied in relation to negative life events,29 antiretroviral therapy,30,31 severity of illness,30 and pain32 in children and youth with HIV infection.
Outcome Measures
Each QoL domain score was constructed using multiple items.24 Domain scores were standardized on a 0 to 100 scale and scored independently of other domains.33 In rare cases, caregivers responded to the majority of questions within a QoL domain but failed to complete ≥1 item. In these instances, which occurred in <5% of domain scores overall, missing items were assigned the mean value of nonmissing items within that domain, provided that at least half of the items within the score were completed.34 Reverse coding of all symptom distress, physical functioning, and health care utilization items and some social/role functioning items was conducted to ensure that higher scores indicated better health.
Statistical Analyses
Frequencies and percentages were calculated for demographic and clinical variables at the first visit. Unadjusted mean domain scores were calculated at the first undisclosed visit, the last undisclosed visit, the first disclosed visit, and the last disclosed visit; within-subject changes were compared using the Wilcoxon signed-rank test. Descriptive statistics are presented for age at disclosure using both the first reported age and the estimated age at disclosure. The agreement between reported and estimated age at disclosure was assessed via a Spearman's correlation coefficient. Univariate linear regression was used to assess the association between age at disclosure and calendar time.
A multivariate mixed-effects model was fit within each separate domain to estimate the effect of disclosure on QoL while taking into account within-subject correlations between measurements over time and the influence of potential confounding variables. Covariates included demographic information (age, gender, race/ethnicity, primary caregiver, and primary caregiver education level), immunologic and virologic measures of health status (CD4%, viral load, and CDC class), ART regimen, number of hospitalizations since last visit, and negative life-event score. Negative life-event score, a caregiver-reported indicator of social/environmental context,31 was calculated as the unweighted sum of 18 possible life events (range: 0–18) that occurred in the previous 12 months.35 Life events represented areas of financial stability, family structure, illness or death of family/friends, and changes in environment (eg, new school or mother starting work).
For each QoL domain, 2 random-effects models were considered, and the appropriate covariance structure was selected based on minimizing the Akaike Information Criterion. In the first model, which included 4 random effects (intercept, disclosure status, time in 6-month intervals, and the interaction between disclosure status and time), it was assumed that there was an increasing or decreasing trend in QoL scores before disclosure, a change in QoL at the time of disclosure, and then a possibly different trend over time in QoL scores after disclosure. In the second model, which included 2 random effects (intercept and disclosure status), it was assumed that QoL scores were, on average, constant over time until disclosure, at which time there may have been an increase or decrease in QoL, but then average QoL scores were again assumed to remain at this new constant level over time after disclosure. After fitting the multivariate model for each domain, we calculated and plotted predicted QoL score means for the reference group by time from disclosure. The use of a repeated-measures model with 395 children would provide 80% power to detect a difference of 3 to 5 points in QoL scores between undisclosed and disclosed children. Statistical analyses were performed by using SAS 9.1 (SAS Institute, Inc, Cary, NC).
Results
Baseline Patient Characteristics
Of the 2563 perinatally HIV-infected children and adolescents enrolled into the PACTG 219C Study as of December 31, 2005, 2325 were ≥5 years of age at their first QoL assessment. Caregivers of 1424 children (61%) reported disclosure of HIV infection, and 1014 of these were already aware of their HIV status at study entry. Of the remaining 410 children, 15 were excluded for being <5 years of age at disclosure (according to reported age at disclosure), leaving 395 children with ≥1 undisclosed and ≥1 disclosed visit available for analysis.
The study sample reported QoL measurements at 2423 visits, including 1317 predisclosure visits and 1106 postdisclosure visits. The median number of visits per patient was 7 (range: 2–11 visits). The median time between the last undisclosed visit and the first disclosed visit was 280 days (interquartile range: 182–390 days). Table 1 summarizes the distribution of demographic and clinical variables at the first predisclosure visit. The study population was 50% male, 56% black, 31% Hispanic, and 13% white or other race/ethnicity. At the first visit, 19% of children were classified as CDC class C (AIDS), 71% had CD4% ≥25% (no immune suppression), 40% had viral load ≤400 copies per mL (nondetectable status), and 72% were using an ART regimen of HAART with a protease inhibitor (PI). At the first visit, the source of QoL information was a biological parent for 39% of children, another relative for 25%, and another primary caregiver for 37%. Only 15 subjects (4%) had a change in the caregiver who reported on QoL between their first and last visit; exclusion of these subjects did not impact the results of the analysis.
Table 1. Characteristics of 395 Perinatally HIV-Infected Children at the First Predisclosure Study Visit.
| Variable | Data |
|---|---|
| Age, y | |
| Mean ± SD | 9.1 ± 2.3 |
| CDC class, n (%) | |
| N/A | 127 (32) |
| B | 149 (38) |
| C | 75 (19) |
| Missing/unknown | 44 (11) |
| CD4% | |
| Median | 30.0 |
| <15%, n (%) | 29 (7) |
| 15%–24%, n (%) | 77 (19) |
| ≥25%, n (%) | 280 (71) |
| Missing/unknown, n (%) | 9 (2) |
| HIV RNA, copies per mL | |
| Median | 1564.5 |
| ≤400, n (%) | 159 (40) |
| 401–5000, n (%) | 91 (23) |
| 5001–50 000, n (%) | 96 (24) |
| >50 000, n (%) | 40 (10) |
| Missing/unknown, n (%) | 9 (2) |
| ART, n (%) | |
| HAART with PI | 284 (72) |
| Other therapy | 104 (26) |
| Not on ART | 5 (1) |
| Missing/unknown | 2 (1) |
| Primary caregiver, n (%) | |
| Biological parent | 153 (39) |
| Other relative | 97 (25) |
| Other/unknown | 145 (37) |
| Primary caregiver education, n (%) | |
| Grade 1–11 | 135 (34) |
| High school graduate or higher | 215 (54) |
| Missing | 45 (11) |
| Negative life-event score | |
| Median | 1.0 |
| 0, n (%) | 188 (48) |
| 1, n (%) | 66 (17) |
| ≥2, n (%) | 140 (35) |
| Missing, n (%) | 1 (0) |
Age at Disclosure
Among 395 subjects, 57% always reported the same age at disclosure at each visit, 35% had ≥1 inconsistent report of age at disclosure, and 8% failed to report age at disclosure. Among those who reported age at disclosure, the median age at disclosure was 11.0 years (interquartile range: 9.0–12.0 years; Fig 1). Discrepancies in reported age at disclosure within a single subject ranged from 1.0 to 12.0 years, with an average maximum difference in reports of 2.2 years. The median estimated age at disclosure at the midpoint between the last undisclosed and first disclosed visits was 11.0 years (interquartile range: 9–12 years). The first reported age at disclosure was highly correlated with estimated age at disclosure (Spearman's r = 0.90; P < .001). Older age at disclosure was associated with earlier year of birth (Pearson's r = −0.79; P < .001), suggesting that the age of disclosure has had a significant decline over calendar time (Fig 2).
Figure 1.
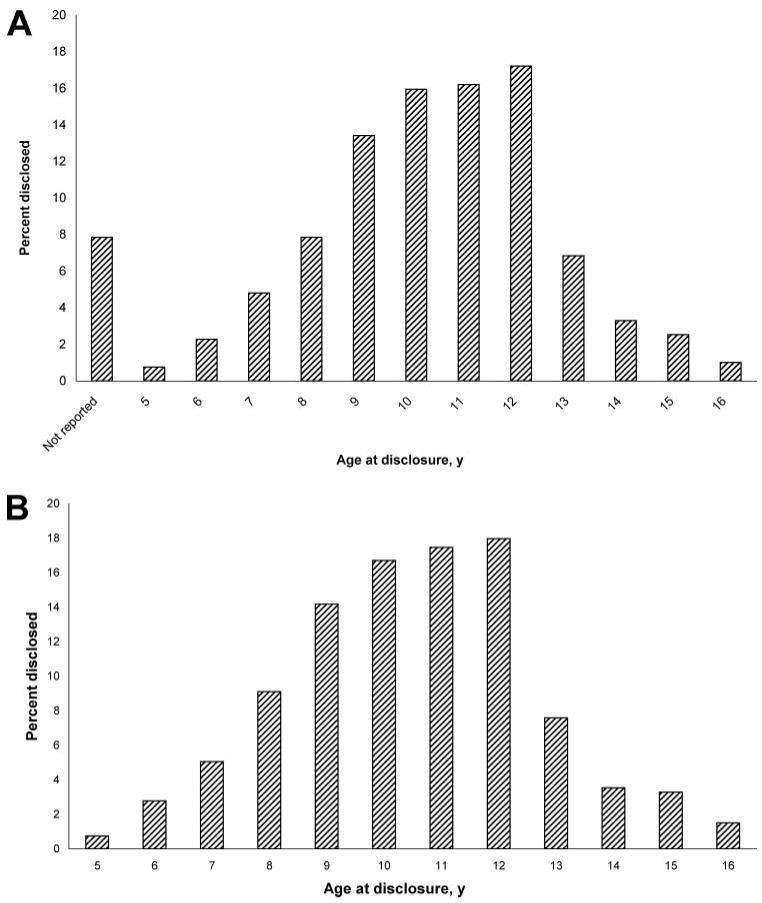
Distribution of age at disclosure. A, Reported age at disclosure. B, Reported and estimated ages at disclosure, where age at the midpoint between the last predisclosure visit and the first postdisclosure visit are presented for 31 subjects without reported age at disclosure.
Figure 2.

Mean age of disclosure reported by caregiver according to year of birth for 395 subjects with both predisclosure and postdisclosure visits (age at the midpoint between the last predisclosure visit and the first postdisclosure visit is presented for 31 subjects without reported age at disclosure).
Study Visit, Disclosure, and QoL
At the first undisclosed visit, mean domain scores ranged from 81.3 for psychological status to 94.0 for health care utilization (Table 2). The mean domain score for general health perception increased by 1.1 points between the last undisclosed and first disclosed visit (P = .04). However, there were no other significant changes in mean domain scores based on comparing the last undisclosed to first disclosed visit or comparing the first undisclosed to the last disclosed visit, with all of the changes within 0.7 points. When all of the visits were considered, 5 of 6 domain mean scores were lower after disclosure, although the differences were not significant.
Table 2. Unadjusted Mean Domain Scores According to Disclosure Status and Visit.
| QoL Domain | Predisclosure | Postdisclosure | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First Visit | Last Visit | All Visits | First Visit | Last Visit | All Visits | First vs Lasta | Last vs Firstb | Undisclosed vs Disclosedc | |
| General health perception | 84.4 | 83.9 | 84.5 | 85.0 | 84.3 | 84.4 | .96 | .04 | .70 |
| Symptom distress | 92.6 | 92.2 | 92.4 | 92.1 | 92.1 | 92.3 | .37 | >0.999 | .72 |
| Psychological status | 81.3 | 80.7 | 81.5 | 80.6 | 81.9 | 81.3 | .93 | .10 | .51 |
| Physical functioning | 82.9 | 82.0 | 82.6 | 82.6 | 82.9 | 82.0 | .70 | .44 | .89 |
| Social/role functioning | 83.3 | 83.5 | 83.2 | 82.9 | 83.7 | 83.4 | .83 | .60 | .81 |
| Health care utilization | 94.0 | 93.9 | 93.9 | 93.2 | 93.6 | 93.4 | .34 | .26 | .61 |
QoL scores for each domain range from 0 to 100, with higher scores indicating better QoL. P values are based on Wilcoxon signed-rank tests comparing mean QoL scores.
Data show first undisclosed visit versus last disclosed visit.
Data show last undisclosed visit versus first disclosed visit.
Data show the mean of all undisclosed versus the mean of all disclosed, within-subject comparison.
Disclosure was not significantly associated with QoL in crude or adjusted mixed-effects model analyses, indicating that QoL scores did not change because of disclosure of HIV infection status (Table 3). Figure 3 displays predicted scores for each of the 6 QoL domains over time before and after disclosure. There was a marginally significant decline in symptom distress scores (P = .07) over the study period. There were no significant changes over time in general health perception, psychological status, physical functioning, social/role functioning, or health care utilization domains. There was also no significant difference between time trends in QoL scores before and after disclosure of HIV status. For all of the domains, the more complex model structure allowing separate slopes for QoL changes before and after disclosure was preferable based on the Akaike Information Criterion, but there was no qualitative difference regarding the impact of disclosure on QoL for the simpler model structure.
Table 3. Unadjusted and Adjusted Effects of Disclosure on QoL Scores.
| Domain | Unadjusted for Covariatesa | Adjusted for Covariates: Full Modelb | ||||||
|---|---|---|---|---|---|---|---|---|
| Effect of Disclosure | Effect of Time by Disclosure | Effect of Disclosure | Effect of Time by Disclosure | |||||
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | |
| General health perception | 0.546 | .61 | −0.076 | .82 | 0.410 | .70 | −0.040 | .91 |
| Symptom distress | 0.588 | .30 | 0.154 | .45 | 0.588 | .31 | 0.170 | .41 |
| Psychological status | 0.164 | .87 | 0.488 | .20 | 0.005 | >0.999 | 0.448 | .23 |
| Physical functioning | 0.686 | .73 | −0.283 | .68 | 0.536 | .79 | −0.333 | .63 |
| Social/role functioning | 0.423 | .65 | 0.004 | .99 | 0.380 | .69 | 0.002 | >0.999 |
| Health care utilization | −0.261 | .63 | 0.079 | .63 | −0.275 | .61 | 0.075 | .64 |
Data are based on fitting a mixed–effects model for each domain. The effects of disclosure represent the difference in QoL scores between predisclosure and postdisclosure visits. Negative values indicate poorer QoL scores after disclosure.
All of the models have effects of disclosure adjusted for time since disclosure (6–month intervals) and the interaction between disclosure and time since disclosure.
Full model is adjusted for age (5–9, 10–11, 12–13, and ≥14 years), gender, race/ethnicity (white non-Hispanic/other, black non-Hispanic, or Hispanic), CD4% (<14%, 15%–24%, or >25%), viral load (≤400 or >400 copies per mL), CDC class (N/A/B or C), primary caregiver (biological parent, other relative, or other/unknown), primary caregiver education level (grade 1-11 or high school graduate or higher), antiretroviral regimen (HAART with PI, other therapy, or not on ART), number of hospitalizations since last visit (0,1, or ≥2), negative life-event score (0,1,or ≥2), time since disclosure, and the interaction between disclosure and time since disclosure.
Figure 3.

Predicted scores for 6 QoL domains for 395 subjects with both predisclosure and postdisclosure QoL assessments. All of the models are adjusted for age (5–9, 10–11, 12–13, and ≥14 years), gender, race/ethnicity (white non-Hispanic/other, black non-Hispanic, or Hispanic), CD4% (<14%, 15%–24%, or >25%), viral load (≤400 or >400 copies per mL), CDC class (N/A/B or C), primary caregiver (biological parent, other relative, or other/unknown), primary caregiver education level (grade 1–11 or high school graduate or higher), antiretroviral regimen (HAART with PI, other therapy, or not on ART), number of hospitalizations since last visit (0, 1, or ≥2), negative life-event score (0, 1, or ≥2), disclosure, time since disclosure, and the interaction between disclosure and time since disclosure. Time of disclosure is denoted by the line at month 0.
Although disclosure of HIV status seemed to have no impact on QoL scores, other factors were associated with QoL. Girls scored significantly higher than boys in social/role functioning QoL, and blacks reported higher average scores than whites, whereas Hispanics had lower average scores in all of the domains except psychological status. In 4 domains, subjects receiving no ART reported the highest average scores, and those receiving a regimen other than HAART with PIs had the lowest average scores. Subjects with more previous hospitalizations had significantly lower general health perception, and those with more negative life events had significantly lower symptom scores and physical functioning. Other covariates were not associated with any of the QoL domains but were retained in the model to control for confounding.
Discussion
The results of this investigation suggest that disclosure did not significantly affect QoL in a population of perinatally, HIV-infected children and adolescents. Although primary caregivers reported lower QoL scores after disclosure for all of the domains except social/role functioning, these differences were not significant, even after adjustment for demographic and clinical factors. Current recommendations regarding diagnostic disclosure to children with HIV/AIDS are based on lessons learned from pediatric oncology. However, the psychological benefits of disclosure apparent among youth with cancer may be offset by HIV-related stigma and discrimination toward the infected child and other family members.16 A more comprehensive understanding of the effect of HIV disclosure on QoL is important for developing appropriate strategies for disclosure to children and adolescents with HIV.
The limited number of disclosure-related QoL studies among HIV-infected pediatric populations have focused on psychological and emotional health, with inconsistent outcomes reported after disclosure. Anecdotal clinician reports describe beneficial effects of HIV disclosure to children with normal cognitive development, such as reduced anxieties and disruptive behaviors, increased family intimacy, and better long-term health and emotional well-being.2,16,17,36 In a study of 40 children informed of their HIV diagnosis in the context of a supportive environment, most youth (70%) had feelings of normalcy 6 months postdisclosure and positive psychosocial adjustment.37 Bachanas et al7 reported that children and adolescents in families where disclosure has not taken place were more likely to report internalizing behavior problems and psychological adjustment problems. Riekert et al38 found that children with HIV who knew of their diagnosis scored significantly lower on depression and anxiety measures than children who did not know their diagnosis. Other studies that examined the psychological impact of disclosure found little or no difference in psychological functioning between disclosed and nondisclosed children.5,23 Inconsistencies regarding the impact of disclosure on psychological and emotional QoL may be attributable to variation among QoL measures, limited sample size, or lack of multivariate methods. Furthermore, the majority of quantitative studies used cross-sectional designs, which only allow for the evaluation of the presence of associations. This is the first longitudinal study to date examining the impact of disclosure of HIV status on health-related QoL using multivariate analytical methods and broad-based QoL outcomes.
An interesting finding of this study was the significant decrease in age at disclosure over time. This may be the result of a decline in the social stigma surrounding HIV, as well as improved long-term survival because of advances in antiretroviral therapy, most notably, the introduction of HAART in 1996.3,4 More than two thirds of our population initiated HAART before their first study visit. As increasing numbers of HIV-infected children are surviving well into their adolescent and young adult years, knowledge about their own disease becomes essential for both personal health maintenance and HIV prevention within the larger population.2 The decline in age at disclosure offers an additional explanation for inconsistent psychological and emotional QoL outcomes in the disclosure literature.
This study is limited by missing information regarding the disclosure process. Caregivers reported disclosure as a yes/no variable; however, the process and context within which the disclosure occurred are unknown. Although disclosure has commonly been examined as a single, binary event, the process is increasingly being viewed as an ongoing progression from nondisclosure to disclosure.16,39 Another limitation is the possibility of less reliable caregiver reports of QoL for adolescents compared with younger children. Compared with self-reports, proxy reporting by parents and caregivers has been shown to be closely correlated for younger children but more variable for older children.40,41 Despite these limitations, our longitudinal data allowed us to examine the relationships between QoL and disclosure, time from disclosure, and their interaction. We were unable to demonstrate that disclosure significantly impacts QoL over time.
The PACTG 219C cohort is the largest pediatric HIV cohort in the world, representing 22% of the perinatally infected US population. These analyses had excellent power, and the results are generalizable to the US perinatally infected population. Multivariate mixed-effects models were used to estimate the effect of disclosure on QoL, which are particularly important for taking into account the within-subject correlations between measurements over time and the influence of potential confounding variables. To our knowledge, this is the most in-depth and comprehensive analysis of the impact of disclosure on health-related QoL.
Conclusions
This study suggests that HIV disclosure does not significantly change QoL scores and that diagnostic disclosure to children with HIV should not be delayed because of fear of a negative impact on QoL. Disclosure is occurring at younger ages, which may suggest a decline in the stigma and fear surrounding an HIV diagnosis. Additional work is needed to describe important factors that are related to optimal strategies in disclosing an HIV diagnosis. Such knowledge may offer critical guidance to pediatric providers in counseling caregivers of HIV-infected children and adolescents.
Acknowledgments
The study was funded by National Institute of Allergy and Infectious Diseases grant U01AI068632 and the National Institute of Child Health and Human Development grant NO1 HD33345. This work was also supported by the Statistical and Data Analysis Center of the PACTG at Harvard School of Public Health, under National Institute of Allergy and Infectious Diseases cooperative agreement 5U01AI41110, and 1U01AI068616 with the International Maternal Pediatric Adolescent AIDS Clinical Trials Group.
The following institutions and individuals participated in the PACTG Protocol 219C, in order of enrollment: Baylor Texas Children's Hospital: F. Minglana, M. E. Paul, and C. D. Jackson; University of Florida, Jacksonville: M. H. Rathore, A. Khayat, K. Champion, and S. Cusic; Chicago Children's Memorial Hospital: R. Yogev and E. Chadwick; University of Puerto Rico, University Children's Hospital AIDS Program: I. Febo-Rodriguez and S. Nieves; Bronx Lebanon Hospital Center: M. Purswani, S. Baksi, E. Stuard, and M. Dummit; San Juan Hospital: M. Acevedo, M. Gonzalez, L. Fabregas, and M. E. Texidor; University of Miami: G. B. Scott, C. D. Mitchell, L. Taybo, and S. Willumsen; University of Medicine and Dentistry of New Jersey: L. Bettica, J. Amour, B. Dashefsky, and J. Oleske; Charity Hospital of New Orleans and Earl K. Long Early Intervention Clinic: M. Silio, T. Alchediak, C. Boe, and M. Cowie; University of California San Diego Mother, Child, and Adolescent HIV Program: S. A. Spector, R. Viani, M. Caffery, and L. Proctor; Howard University: S. Rana, D. Darbari, J. C. Roa, and P. H.Yu; Jacobi Medical Center: M. Donovan, R. Serrano, M. Burey, and R. Auguste; St Christopher's Hospital for Children, Philadelphia: J. Chen and J. Foster; Baystate Medical Center Children's Hospital: B. W. Stechenberg, D. J. Fisher, A. M. Johnston, and M. Toye; Los Angeles County Medical Center/University of Southern California: J. Homans, M. Neely, L. S. Spencer, and A. Kovacs; Children's Hospital Boston: S. Burchett and N. Karthas; Children's Hospital of Michigan: E. Moore and C. Cromer; St Jude Children's Research Hospital, Memphis: P. M. Flynn, N. Patel, M. Donohoe, and S. Jones; New York University School of Medicine/Bellevue Hospital: W. Borkowsky, S. Chandwani, N. Deygoo, and S. Akleh; Children's Hospital at Downstate: E. Handelsman, H. J. Moallem, D. M. Swindell, and J. M. Kaye; Columbia Presbyterian Medical Center and Cornell University New York Presbyterian Hospital: A. Higgins, M. Foca, P. LaRussa, and A. Gershon; Children's Hospital of Philadelphia: R. M. Rutstein, C. A. Vincent, S. D. Douglas, and G. A. Koutsoubis; Children's Hospital of Oakland: A. Petru and T. Courville; University of California San Francisco, Moffitt Hospital: D. Wara and D. Trevithick; Children's Hospital, University of Colorado, Denver: E. McFarland and C. Salbenblatt; Johns Hopkins University Pediatrics: N. Hutton, B. Griffith, M. Joyner, and C. Kiefner; Children's Hospital and Regional Medical Center, Washington: M. Acker, R. Croteau, C. McLellan, and K. Mohan; Metropolitan Hospital Center: M. Bamji, I. Pathak, S. Manwani, and E. Patel; Children's National Medical Center: H. Spiegel and V. Amos; University of Massachusetts Medical School: K. Luzuriaga and A. Sharples; University of Alabama at Birmingham: R. Pass and M. Crain; University of Maryland Medical Center: J. Farley and K. Klipner; Schneider Children's Hospital: V. R. Bonagura, S. J. Schuval, C. Colter, and L. Campbell; Boston Medical Center: S. I. Pelton and A. M. Reagan; University of Illinois: K. C. Rich, K. Hayani, and M. Bicchinella; State University of New York Stony Brook: S. Nachman, D. Ferraro, and S. Madjar; North Broward Hospital District: A. Puga; Duke University: F. Wiley, K. Whitfield, O. Johnson, and R. Dizney; Harlem Hospital: S. Champion, M. Frere, M. DiGrado, and E. J. Abrams; Cook County Hospital: J. Martinez; University of South Alabama: M. Mancao; Connecticut Children's Medical Center: J. Salazar and G. Karas; University of North Carolina at Chapel Hill: T. Belho, B. Pitkin, and J. Eddleman; Ruiz Arnau University Hospital: W. Figueroa and E. Reyes; State University of New York Upstate Medical University: L. B. Weiner, K. A. Contello, W. A. Holz, and M. J. Famiglietti; Children's Medical Center of Dallas; University of Florida at Gainesville: R. Lawrence, J. Lew, C. Delany, and C. Duff; Children's Hospital at Albany Medical Center: A. D. Fernandez, P. A. Hughes, N. Wade, and M. E. Adams; Lincoln Medical and Mental Health Center and Phoenix Children's Hospital: J. P. Piatt, J. Foti, and L. Clarke-Steffen; Public Health Unit of Palm Beach County: J. Sleasman and C. Delaney; Medical College of Georgia: C. S. Mani; Yale University School of Medicine: W. A. Andiman, S. Romano, L. Hurst, and J. de Jesus; Vanderbilt University Medical Center: G. Wilson; University of Rochester Medical Center: G. A. Weinberg, F. Gigliotti, B. Murante, and S. Laverty; St Josephs Hospital and Medical Center, New Jersey: N. Hutchcon and A. Townley; Emory University Hospital: S. Nesheim and R. Dennis; University of South Florida: P. Emmanuel, J. Lujan-Zilberman, C. Graisberry, and S. Moore; Children's Hospital of the King's Daughters: R. G. Fisher, K. M. Cunnion, T. T. Rubio, and D. Sandifer; Medical University of South Carolina: G. M. Johnson; University of Mississippi Medical Center: H. Gay and S. Sadler; Harbor-University of California Los Angeles Medical Center: M. Keller, J. Hayes, A. Gagajena, and C. Mink; Mount Sinai Medical Center: D. Johnson; Children's Hospital of Los Angeles: J. Church, T. Dunaway, and C. Salata; Long Beach Memorial: A. Deveikis and L. Melton; Robert Wood Johnson Medical School: S. Gaur, P. Whitley-Williams, A. Malhotra, and L. Cerracchio; Sinai Children's Hospital: M. Dolan, J. D'Agostino, and R. Posada; Medical Center, Pediatric Columbus, Georgia: C. Mani and S. Cobb; Medical College of Virginia: S. R. Lavoie and T. Y. Smith; Cooper Hospital-University Medical Center: A. Feingold and S. Burrows-Clark; University of Cincinnati: J. Mrus and R. Beiting; Columbus Children's Hospital: M. Brady, J. Hunkler, and K. Koranyi; Sacred Heart Children's Medical Services of Florida: W. Albritton; St Luke's/Roosevelt Hospital Center: R. Warford and S. Arpadi; Incarnation Children's Center, New York: A. Gershon and P. Miller; Montefiore Medical-Albert Einstein College of Medicine: A. Rubinstein and G. Krienik; Children's Hospital of Los Angeles: A. Kovacs and E. Operskalski; San Francisco General Hospital: D. Wara, A. Kamrin, and S. Farrales; Cornell University New York Presbyterian: R. Johan-Liang and K. O'Keefe; St Louis Children's Hospital: K. A. McGann, L. Pickering, and G. A. Storch; North Shore University Hospital: S. Pahwa and L. Rodriquez; and Oregon Health and Science University: P. Lewis and R. Croteau.
We are grateful for the contributions of Joyce Kraimer, Barbara Heckman, Shirley Traite, and Nathan Tryon. We also thank the children and families for their participation in PACTG 219C and the individuals and institutions involved in the conduct of 219C.
Abbreviations
- HAART
highly active antiretroviral therapy
- QoL
quality of life
- PACTG
Pediatric AIDS Clinical Trials Group
- CDC
Centers for Disease Control and Prevention
- ART
antiretroviral therapy
- GHAC
General Health Assessment for Children
- PI
protease inhibitor
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
The National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development were involved in the design, data collection, and conduct of protocol 219C but were not involved in the present analysis, the interpretation of the data, the writing of the article, or the decision to submit for publication; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- 1.Berk DR, Falkovitz-Halpern MS, Hill DW, et al. Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. JAMA. 2005;293(18):2221–2231. doi: 10.1001/jama.293.18.2221. [DOI] [PubMed] [Google Scholar]
- 2.Wiener L, Lyon M. HIV disclosure: who knows? who needs to know?: clinical and ethical considerations. In: Lyon M, D'Angelo L, editors. Teenagers, HIV, and AIDS: Insights From Youths Living With the Virus. Westport, CT: Praeger Publishers; 2006. pp. 105–126. [Google Scholar]
- 3.Patel K, Hernan MA, Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46(4):507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 4.de Martino M, Tovo PA, Balducci M, et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection: Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA. 2000;284(2):190–197. doi: 10.1001/jama.284.2.190. [DOI] [PubMed] [Google Scholar]
- 5.Lester P, Chesney M, Cooke M, et al. When the time comes to talk about HIV: factors associated with diagnostic disclosure and emotional distress in HIV-infected children. J Acquir Immune Defic Syndr. 2002;31(3):309–317. doi: 10.1097/00126334-200211010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Instone SL. Perceptions of children with HIV infection when not told for so long: implications for diagnosis disclosure. J Pediatr Health Care. 2000;14(5):235–243. doi: 10.1067/mph.2000.107338. [DOI] [PubMed] [Google Scholar]
- 7.Bachanas PJ, Kullgren KA, Schwartz KS, et al. Predictors of psychological adjustment in school-age children infected with HIV. J Pediatr Psychol. 2001;26(6):343–352. doi: 10.1093/jpepsy/26.6.343. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Committee on Pediatrics AIDS. Disclosure of illness status to children and adolescents with HIV infection. Pediatrics. 1999;103(1):164–166. doi: 10.1542/peds.103.1.164. [DOI] [PubMed] [Google Scholar]
- 9.Lee CL, Johann-Liang R. Disclosure of the diagnosis of HIV/AIDS to children born of HIV-infected mothers. AIDS Patient Care STDS. 1999;13(1):41–45. doi: 10.1089/apc.1999.13.41. [DOI] [PubMed] [Google Scholar]
- 10.Lester P, Chesney M, Cooke M. Diagnostic disclosure to HIV-infected children: how parents decide when and what to tell. Clin Child Psychol Psychiatry. 2002;7:85–99. [Google Scholar]
- 11.Steele RG, Nelson TD, Cole BP. Psychosocial functioning of children with AIDS and HIV infection: review of the literature from a socioecological framework. J Dev Behav Pediatr. 2007;28(1):58–69. doi: 10.1097/DBP.0b013e31803084c6. [DOI] [PubMed] [Google Scholar]
- 12.Thorne C, Newell ML, Peckham CS. Disclosure of diagnosis and planning for the future in HIV-affected families in Europe. Child Care Health Dev. 2000;26(1):29–40. doi: 10.1046/j.1365-2214.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Thorne C, Newell ML, Botet FA, et al. Older children and adolescents surviving with vertically acquired HIV infection. J Acquir Immune Defic Syndr. 2002;29(4):396–401. doi: 10.1097/00126334-200204010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Brady MT, Clark C, Weedy C, Fowler M, Mofenson L, Oleske J. Disclosure of HIV diagnosis to children in ACTG clinical trials (ACTG 219) (Poster We.D. 131). Presented at the XI International Conference on AIDS; July 7–12, 1996; Vancouver, British Columbia. [Google Scholar]
- 15.Grubman S, Gross E, Lerner-Weiss N, et al. Older children and adolescents living with perinatally acquired human immunodeficiency virus infection. Pediatrics. 1995;95(5):657–663. [PubMed] [Google Scholar]
- 16.Lipson M. Disclosure of diagnosis to children with human immunodeficiency virus or acquired immunodeficiency syndrome. J Dev Behav Pediatr. 1994;15(3 suppl):S61–S65. [PubMed] [Google Scholar]
- 17.Mellins CA, Brackis-Cott E, Dolezal C, Richards A, Nichols SW, Abrams EJ. Patterns of HIV status disclosure to perinatally HIV-infected children and subsequent mental health outcomes. Clin Child Psychol Psychiatry. 2002;7(1):101–114. [Google Scholar]
- 18.Slavin LA, O'Malley JE, Koocher GP, Foster DJ. Communication of the cancer diagnosis to pediatric patients: impact on long-term adjustment. Am J Psychiatry. 1982;139(2):179–183. doi: 10.1176/ajp.139.2.179. [DOI] [PubMed] [Google Scholar]
- 19.Spinetta J, Maloney J. Death anxiety in the outpatient leukemic child. Pediatrics. 1975;56(6):1035–1037. [PubMed] [Google Scholar]
- 20.Van Dongen-Melman JE, Sanders-Woudstra JA. Psychosocial aspects of childhood cancer: a review of the literature. J Child Psychol Psychiatry. 1986;27(2):145–180. [PubMed] [Google Scholar]
- 21.Claflin CJ, Barbarin OA. Does “telling” less protect more?: relationships among age, information disclosure, and what children with cancer see and feel. J Pediatr Psychol. 1991;16(2):169–191. doi: 10.1093/jpepsy/16.2.169. [DOI] [PubMed] [Google Scholar]
- 22.Wiener LS, Battles HB, Heilman N, Sigelman CK, Pizzo PA. Factors associated with disclosure of diagnosis to children with HIV/AIDS. Pediatr AIDS HIV Infect. 1996;7(5):310–324. [PubMed] [Google Scholar]
- 23.Wiener L, Mellins CA, Marhefka S, Battles HB. Disclosure of an HIV diagnosis to children: history, current research, and future directions. J Dev Behav Pediatr. 2007;28(2):155–166. doi: 10.1097/01.DBP.0000267570.87564.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gortmaker S, Lenderking WR, Clark C, Lee S, Fowler MG, Oleske JM. Development and use of a pediatric quality of life questionnaire in AIDS clinical trials: reliability and validity of the General Health Assessment for Children (GHAC) In: Drotar D, editor. Assessing Pediatric Health-Related Quality of Life and Functional Status: Implications for Research, Practice and Policy. Mahwah, NJ: Lawrence Feldbaum, Associates; 1998. pp. 219–235. [Google Scholar]
- 25.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 26.Testa MA, Lenderking WR. Quality of life considerations in AIDS clinical trials. In: Finkelstein DM, Schoenfeld DA, editors. AIDS Clinical Trials. New York, NY: Wiley-Liss; 1992. pp. 213–241. [Google Scholar]
- 27.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR17):1–19. [PubMed] [Google Scholar]
- 28.1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Recomm Rep. 1994;43(RR12):1–10. [Google Scholar]
- 29.Howland LC, Storm DS, Gortmaker SL, Crawford SL, Ma Y, Oleske JM. Negative life events: risk to health-related quality of life in children and youth with HIV Infection. J Assoc Nurses AIDS Care. 2007;18(1):3–11. doi: 10.1016/j.jana.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Storm DS, Boland MG, Gortmaker SL, et al. Protease inhibitor combination therapy, severity of illness, and quality of life among children with perinatally acquired HIV-1 infection. Pediatrics. 2005;115(2) doi: 10.1542/peds.2004-1693. Available at: www.pediatrics.org/cgi/content/full/115/2/e173. [DOI] [PubMed]
- 31.Lee GM, Gortmaker SL, McIntosh K, Hughes MD, Oleske JM, Pediatric AIDS Clinical Trials Group Protocol 219C Team Quality of life for children and adolescents: impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117(2):273–283. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 32.Gaughan DM, Hughes MD, Seage GR, III, et al. The prevalence of pain in pediatric human immunodeficiency virus/acquired immunodeficiency syndrome as reported by participants in the Pediatric Late Outcomes Study (PACTG 219) Pediatrics. 2002;109(6):1144–1152. doi: 10.1542/peds.109.6.1144. [DOI] [PubMed] [Google Scholar]
- 33.Patrick DL, Erickson P. Health Status and Health Policy: Allocating Resources to Health Care. New York, NY: Oxford University Press; 1993. [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 35.Zimmerman M. Weighted versus unweighted life event scores: is there a difference? J Human Stress. 1983;9(4):30–35. doi: 10.1080/0097840X.1983.9935028. [DOI] [PubMed] [Google Scholar]
- 36.Walker G. In the Midst of Winter. New York, NY: Norton; 1991. [Google Scholar]
- 37.Blasini I, Chantry C, Cruz C, et al. Disclosure model for pediatric patients living with HIV in Puerto Rico: design, implementation, and evaluation. J Dev Behav Pediatr. 2004;25(3):181–189. doi: 10.1097/00004703-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Riekert KA, Wiener L, Battles HB. Prediction of psychological distress in school-age children with HIV. Child Health Care. 1999;28(3):201–220. [Google Scholar]
- 39.Ng WYK, Mellins CA, Ryan S. The mental health treatment of children and adolescents perinatally infected with HIV. Abrams E, editor. [February 1, 2008];Topic of the Month. 2004 2-1-0008. Available at: www.archive.org/web/20040419162219/hivfiles.org/topic.html.
- 40.Brunner HI, Klein-Gitelman MS, Miller MJ, et al. Health of children with chronic arthritis: relationship of different measures and the quality of parent proxy reporting. Arthritis Rheum. 2004;51(5):763–773. doi: 10.1002/art.20689. [DOI] [PubMed] [Google Scholar]
- 41.Chang PC, Yeh CH. Agreement between child self-report and parent proxy-report to evaluate quality of life in children with cancer. Psychooncology. 2005;14(2):125–134. doi: 10.1002/pon.828. [DOI] [PubMed] [Google Scholar]


