Abstract
Background
In addition to the biochemical components secreted in bile, aquaporin (AQP) water channels exist in hepatocyte membranes to form conduits for water movement between the sinusoid and the bile canaliculus. The aim of the current study was to analyse AQP 9 expression and localization in human hepatocellular carcinoma (HCC) and non-tumourigenic liver (NTL) tissue from patients undergoing hepatic resection.
Methods
Archived tissue from 17 patients was sectioned and analysis performed using an antibody raised against AQP 9. Slides were blind-scored to determine AQP 9 distribution within HCC and NTL tissue.
Results
Aquaporin 9 was predominantly expressed in the membranes of hepatocytes and demonstrated zonal distribution relative to hepatic sinusoid structure in normal liver. In HCC arising in the absence of cirrhosis AQP 9 remained membrane-localized with zonal distribution in the majority of NTL. By contrast, AQP 9 expression was significantly decreased in the HCC mass vs. pair-matched NTL. In HCC in the presence of cirrhosis, NTL was characterized by extensive AQP 9 staining in the membrane in the absence of zonal distribution and AQP 9 staining in NTL was significantly greater than that observed in the tumour mass.
Conclusions
These data demonstrate that human HCC is characterized by altered AQP 9 expression and AQP 9 localization in the NTL mass is dependent on underlying liver pathology. Given the central role of AQPs in normal liver function and the potential role of AQPs during transformation and progression, these data may prove valuable in future diagnostic and/or therapeutic strategies.
Keywords: aquaporin, normal liver, hepatocyte, hepatocellular carcinoma
Introduction
The synthesis, secretion and modification of bile by hepatocytes represent a primary physiological function of the liver. For bile synthesis to occur, hepatocytes must demonstrate cell polarity in which distinct basolateral and canalicular membranes exist.1 The biochemical components of bile are actively transported from the hepatocyte across the canalicular membrane into the bile canaliculus.2,3 In addition to the biochemical constituents, ≈95–98% of bile is water.2,4 Water is able to cross the plasma membrane of cells in one of two ways: directly through the lipid bilayer (a slow, unregulated process), or via proteinacious water channels termed aquaporins (AQPs).5,6 Within the hepatocyte at least three distinct AQP populations have been identified at the mRNA and protein level: AQPs 0, 8 and 9,1,7,8 whereas AQP 11 has been reported at the mRNA level.9 Under normal conditions AQPs 0, 8 and 9 are constitutively expressed, AQPs 0 and 8 are located (predominantly) intracellularly and AQP 9 is localized, for the most part, to the basolateral membrane.4,8,10,11 However, following appropriate stimulation, AQP 8 translocates to the canalicular membrane1,8,12 and, in conjunction with basolateral AQP 9, may act to form a conduit for rapid water movement from the hepatic sinusoid to the bile canaliculus during the process of bile synthesis and modification.7,8
Hepatocellular carcinoma (HCC) represents one of the most common gastrointestinal malignancies diagnosed worldwide and accounts for >80% of all primary liver tumours.13–15 Unlike in other common cancers, familial markers for HCC development are rare; HCC most commonly arises following exposure to one or more ‘environmental’ factors, the most common of which are viral hepatitis infection, aflatoxins (naturally occurring mycotoxins produced by species of Aspergillus) and/or chronic alcohol consumption.14,16,17 Despite the varied nature of these risk factors, underlying hepatic cirrhosis following exposure represents the most common precursor to subsequent HCC development.15,18 Unlike normal liver cells, the transformed cells that comprise HCC undergo cellular end-stage de-differentiation, are characterized by altered responsiveness to mitogenic and apoptotic stimuli and, to varying degrees, fail to perform the normal physiological functions of the parent hepatocytes, including bile synthesis and secretion.13,14,18
In a previous study carried out by our laboratory, we reported that AQP 8 and 9 expression is significantly decreased in a rat model of HCC, an event that correlates with altered responsiveness to exogenous and endogenous apoptotic stimuli.19 However, this model of HCC was generated by inoculating transformed hepatic epithelial cells (H4IIE) into the parenchyma of healthy animals, a technique that results in reproducible tumour formation in the absence of changes to the underlying integrity of the liver.19,20 By contrast, the underlying pathology from which HCC arises in humans is varied.13,14,18 The aims of the current study were to analyse AQP 9 expression and cellular localization in human HCC samples and adjacent non-tumourigenic liver (NTL) tissue from patients who had undergone HCC resection therapy.
Materials and methods
Tissue specimens
Paraffin-embedded surgical specimens from the pathological files of 17 patients undergoing hepatic resection at the Carolinas Medical Center were obtained, following institutional review board guidelines. In two cases normal liver, in the absence of hepatic tumours, was obtained from non-transplanted livers. Of the 15 samples with HCC, seven had underlying micro (six of seven) or macro (one of seven) hepatic cirrhosis and eight had HCC in the setting of ostensibly normal liver architecture. Representative tissue sections were used for immunohistochemical (IHC) study and the diagnosis for each lesion was confirmed. Clinicopathological data for HCC patients are shown in Table 1. The stage of disease was determined after the surgical resection of the tumour and the histological grade was determined according to the degree of tumour differentiation.
Table 1.
Group and individual patient pathology data
| Group summaries | No underlying cirrhosis | Underlying cirrhosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender distribution | 2 female; 5 male | 1 female; 7 male | |||||||||||||
| Age, years, mean ± SEM | 61.29 ± 3.87 (69.00 ± 9.00 F; 58.20 ± 3.87 M) | 58.88 ± 3.67 (46 F; 60.71 ± 3.67 M) | |||||||||||||
| Race Distributiona | 3 AA, 4 Ca | 1 AA, 7 Ca | |||||||||||||
| Individual patient parameters | |||||||||||||||
| Gender distribution | F | F | M | M | M | M | M | F | M | M | M | M | M | M | M |
| Tumour gradeb | WD | WD | PD | MD | MD | PD | MD | MD | MD | PD | PD | PD | MD | PD | MD |
| TNM stage | NK | IIIB | IIIB | I | IIIA | II | I | II | II | I | II | IIIA | II | NK | I |
| Focalityc | NK | SF | MF | SF | MF | MF | NK | MF | MF | SF | MF | MF | SF | SF | SF |
| Tumour size, cm | NK | ≤5 | 5–10 | >10 | >10 | ≤5 | ≤5 | 5–10 | ≤5 | 5–10 | ≤5 | 5–10 | ≤5 | ≤5 | ≤5 |
| Resection status (clean margin) | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Vascular invasiond | No | No | NK | No | Yes | NK | No | Yes | No | No | Yes | Yes | No | No | No |
| Lymph invasion | No | No | NK | No | NK | NK | No | Yes | Yes | No | No | Yes | No | No | No |
| Serum AFPe | NK | Norm | Incr | Incr | NK | Norm | Incr | Incr | Incr | NK | Incr | Incr | Incr | Norm | Incr |
| Associated disease risk factorsf | Cryp | Cryp | HBV | Et | HCV, HBV | Cryp | Cryp | Et, HCV | Et, HCV | Et, HCV, HBV | Cryp | DILI | HCh | HCV | HCV, Et |
| Cytoplasmic AQP 9 NTL | 1.1 | 0.5 | 1.1 | 0 | 0.6 | 0.2 | 1.0 | 1.4 | 0.9 | 0.3 | 0.3 | 1.4 | 0.3 | 0.6 | 1.0 |
| Membrane AQP 9 NTL | 1.9 | 3.8 | 3.5 | 2.8 | 1.0 | 3.4 | 3.7 | 3.6 | 3.9 | 3.4 | 3.4 | 2.3 | 3.8 | 4.0 | 1.6 |
| Cytoplasmic AQP 9 HCC | 0.1 | 0.3 | 0.70 | 1.4 | 0.10 | 0.5 | 1.0 | 0.9 | 0.5 | 0.3 | 0.3 | 1.0 | 0 | 0.8 | 0 |
| Membrane AQP 9 HCC | 1.8 | 0.9 | 0.60 | 2.0 | 1.2 | 0 | 0.6 | 0.8 | 0.1 | 1.0 | 1.0 | 1.3 | 0.8 | 0.7 | 0.6 |
AA, African-American; Ca, Caucasian
Tumour grade: WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated
Focality: NK, not known; SF, single focus; MF, multiple foci
NK, not known
Incr, increased; Norm, normal; NK, not known
Et, ethanol; HCV, hepatitis C viral infection; HBV, hepatitis B viral infection; Cryp, cryptogenic; DILI, drug-induced liver injury; HCh, haemachromatosis
SEM, standard error of the mean; F, female; M, male; AFP, alpha-fetoprotein; NTL, non-tumour liver; AQP, aquaporin; HCC, hepatocellular carcinoma
Immunohistochemical study
Formalin-fixed paraffin-embedded tissues were cut into 5-µm sections and dried onto Superfrost-plus glass slides (Fisher Scientific, Inc., Pittsburgh, PA, USA). Sections were then deparaffinized with standard xylene and hydrated through graded alcohols into water. Antigen retrieval was performed using citrate buffer and heating (10 min; 100 °C). Slides were placed into a 3% hydrogen peroxide blocking medium (20 min) and allowed to react with a custom rabbit IgG anti-human AQP 9 polyclonal antibody (1:500 dilution, 30 min) raised and previously characterized by our laboratory.19 Immunodetection was performed using an avidin-biotin complex system in accordance with the manufacturer's instructions (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Slides were counterstained with haematoxylin (Sigma-Aldrich Corp., St Louis, MO, USA) and dehydrated through graded alcohols and mounted with a cover-slip. Appropriate positive controls were used (normal liver, n = 2) and negative controls were performed by omitting antiserum from the primary incubation. Specimen slides were viewed randomly, without clinical data, by two of the authors (SP and AMS). Scoring of slides for zonal distribution and staining intensity was based upon a predetermined scale created using representative tissue sections (Fig. 1).
Figure 1.
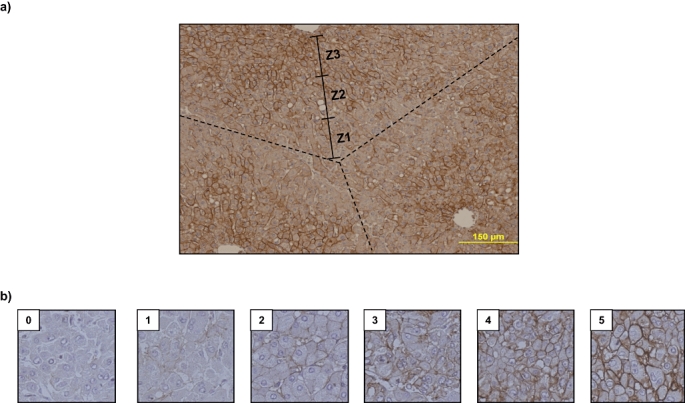
Standards used for determining zonal staining and degree of staining for aquaporin (AQP) 9. (a) Representative immunohistochemical (IHC) image of normal liver (NL) section following IHC staining using an anti-human AQP 9 antibody. Superimposed are the representative lobular structure (dashed lines) and relative zones within the lobule (solid lines) used as a standard for analysis of tissue. (b) Different levels of AQP 9 membrane staining were assigned numerical scores of 0–5 based on stain intensity. These standards were used for subsequent blind scoring of diseased tissue
Immunofluorescent histochemical analysis
Formalin-fixed paraffin-embedded tissues were cut into 5-µm sections, mounted on glass slides, deparaffinized, hydrated and subjected to antigen retrieval, blocking and reaction with the rabbit IgG anti-human AQP 9 polyclonal antibody (1:500 dilution, 30 min), as previously. Detection was performed using an Alexa488-conjugated goat anti-rabbit secondary antibody (Invitrogen Corp., Carlsbad, CA, USA), counterstained with DAPI (Invitrogen Corp.) and dehydrated through graded alcohols prior to cover-slip mounting with an anti-fade medium (Invitrogen Corp.). The sections were examined by laser scanning confocal microscopy (Olympus America, Inc., Melville, NY, USA), each channel recorded independently and superimposed images generated.
Statistical analysis
Scoring data from two independent analysts for five random fields (HCC and non-HCC tissue) were combined and averaged for each sample. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). A P-value of <0.05 was considered significant.
Results
Aquaporin 9 expression is predominantly membrane-localized and demonstrates zonal distribution in normal liver
Immunohistochemical analysis was performed on sections of normal human liver (NL) without tumour masses (n = 2). Usingthis approach, AQP 9 protein was readily detected and, at low magnification, it was apparent that AQP 9 was not evenly distributed throughout the liver and, instead, a clear lobular-zonal distribution was identified (Z3 >> Z2 > Z1; Fig. 2). At higher magnification AQP 9 was observed to be (predominantly) localized in the plasma membrane of hepatocytes and, to a significantly lower degree, within the cytoplasm (3.65 ± 0.60 vs. 0.82 ± 0.1, membrane vs. cytoplasm; values are means of five separate fields scored independently by two different investigators, n = 2 separate samples) (Fig. 2).
Figure 2.
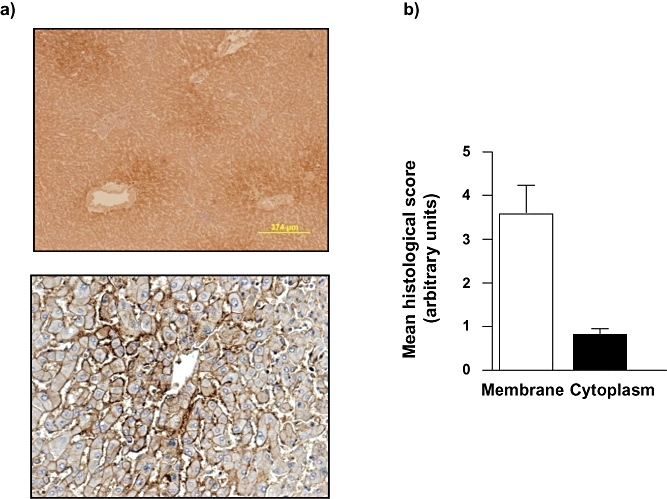
Normal liver in the absence of hepatocellular carcinoma (HCC) is characterized by zonal aquaporin (AQP) 9 distribution and is predominantly localized to the plasma membrane. (a) Representative immunohistochemical (IHC) images of normal liver (NL) section following IHC staining using an anti-human AQP 9 antibody. Note the zonal distribution (upper panel) and the membrane localization (lower panel). (b) Cumulative scoring analysis of membrane vs. cytoplasmic staining for AQP 9 in hepatocytes in NL. Values are means ± standard error of the mean of five separate fields scored independently by two different investigators; n = 2 separate samples. *P < 0.05
AQP 9 expression in HCC samples in the absence of underlying hepatic cirrhosis
In patients in whom HCC arose in the absence of underlying cirrhosis, the NTL tissue was not distinguishable from normal liver architecture observed in the absence of HCC tumours. At low magnification in NTL tissue, four of seven specimens demonstrated moderate to high zonal distribution of AQP 9 as detected by IHC and immunofluorescent histochemistry (IFHC) (Z3 >> Z2 > Z1; Fig. 3a, b), whereas in the remaining three specimens, zonal distribution was either low or not apparent. It is of note that two of the three specimens in which NTL zonal distribution was not apparent represented the largest HCC samples resected within this study group (mean tumour diameters: 130 mm and 150 mm).
Figure 3.
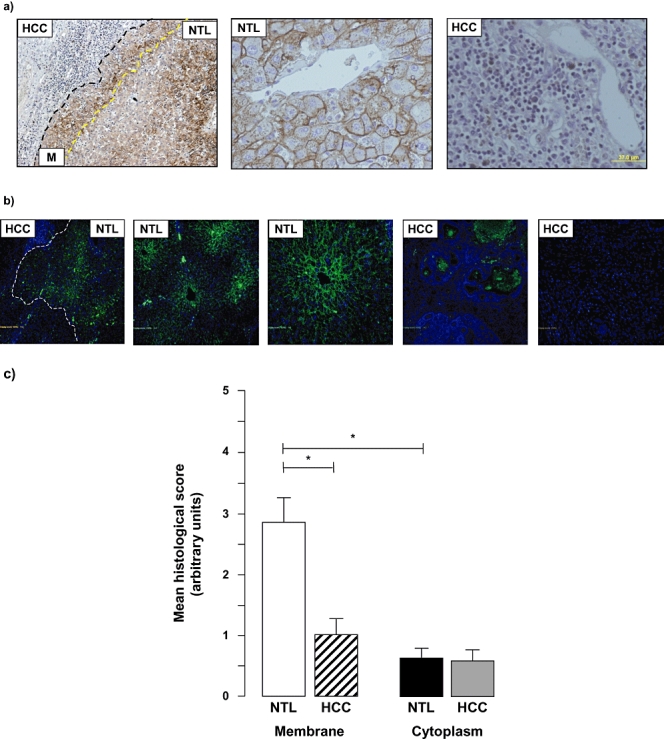
Hepatocellular carcinoma (HCC)in the absence of underlying cirrhosis is characterized by decreased aquaporin (AQP) 9 expression. (a) Representative immunohistochemical (IHC) images of HCC non-tumour liver (NTL) sections following IHC staining using an anti-human AQP 9 antibody. Note the continued zonal distribution (left and middle panels) in the NTL that occurred in four of seven samples analysed and the relative absence of AQP 9 staining in the tumour (left panel). (b) Representative immunofluorescent histochemical (IFHC) images of HCC and NTL sections following IFHC staining using an anti-human AQP 9 antibody and detection with a Fluro488 labelled secondary antibody (green). Sections were counterstained with DAPI (blue) for nuclear localization. The HCC/NTL margin is identified by a dashed line. (c) Cumulative scoring analysis of membrane vs. cytoplasmic staining for AQP 9 in NTL vs. HCC. Values are means ± standard error of the mean of five separate fields scored independently by two different investigators; n = 7 separate samples. *P < 0.05
At higher magnification, AQP 9 detection occurred to a significantly higher degree in the membrane than the cytoplasm of NTL in all samples analysed (2.87 ± 1.05 vs. 0.64 ± 0.44, membrane vs. cytoplasm; values are means of five separate fields scored independently by two different investigators, n = 7 separate samples; P < 0.05) (Fig. 3). Analysis of the HCC region of the specimens demonstrated significantly lower AQP 9 expression in the membrane of cells within the HCC mass vs. NTL (1.01 ± 0.71 vs. 2.87 ± 0.71; values are means of five separate fields scored independently by two different investigators, n = 7 separate samples; P < 0.05) (Fig. 3c). By contrast, whereas cytoplasmic staining was significantly lower than membrane in all instances, no significant differences in cytoplasmic AQP 9 staining were detected between HCC and NTL (Fig. 3c).
AQP 9 expression in HCC samples in the setting of underlying hepatic cirrhosis
In patients in whom HCC arose in the setting of underlying cirrhosis, the NTL mass was clearly distinguishable from normal liver architecture by the presence of scar tissue and fibrous septae that encompassed nodules of hepatocytes (Fig. 4a, b). At low magnification, following staining for AQP 9, the NTL mass was further distinguishable by the complete absence of zonal AQP 9 staining in the cirrhotic tissue (Fig. 4a, b). In all cases, a clear distinction could be made between the non-tumourigenic hepatic mass and the HCC tumour, with the HCC demonstrating significantly lower AQP 9 expression than the surrounding cirrhotic tissue (Fig. 4a, b) (3.40 ± 0.98 vs. 0.84 ± 0.41, NTL vs. HCC; values are means of five separate fields scored independently by two different investigators, n = 8 separate samples; P < 0.05; Fig. 4c). At higher magnification analysis, the NTL tissue demonstrated extensive membrane staining for AQP 9 and showed that the AQP 9 staining was significantly higher in the membrane than the cytoplasm (3.40 ± 0.46 vs. 0.98 ± 0.40, membrane vs. cytoplasm; values are means of five separate fields scored independently by two different investigators, n = 8 separate samples; P < 0.05; Fig. 4). In the HCC mass, a non-significant trend was observed for decreased cytoplasmic AQP 9 staining vs. cytoplasmic staining in the surrounding cirrhotic liver tissue (decreased in six of eight cases, approximately equal staining in one of eight cases and increased in one of eight cases in HCC vs. NTL). Of further note, the case in which AQP 9 staining was increased in HCC cytoplasm (vs. the NTL) represented the only sample in which the patient tested normal for serum α-fetoprotein and was the smallest tumour resected within this group (mean tumour diameter: 41 mm).
Figure 4.
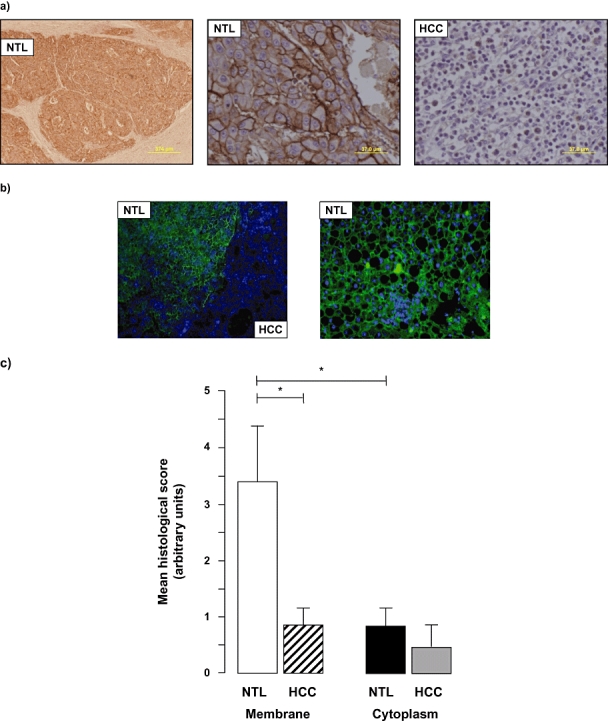
Hepatocellular carcinoma (HCC)in the presence of underlying cirrhosis is characterized by decreased aquaporin (AQP) 9 expression. (a) Representative immunohistochemical (IHC) images of HCC non-tumour liver (NTL) sections following IHC staining using an anti-human AQP 9 antibody. Note the formation of hepatocyte-containing nodules bordered by fibrous septae in the NTL and lack of zonal distribution of AQP 9 (left and middle panels) in the NTL that occurred in all eight of the samples analysed coupled with the relative absence of AQP 9 staining in the tumour (left panel). (b) Representative immunofluorescent histochemical (IFHC) images of HCC and NTL sections following IFHC staining using an anti-human AQP 9 antibody and detection with a Fluro488 labelled secondary antibody (green). Sections were counterstained with DAPI (blue) for nuclear localization. (c) Cumulative scoring analysis of membrane vs. cytoplasmic staining for AQP 9 in NTL vs. HCC. Values are means ± standard error of the mean of five separate fields scored independently by two different investigators; n = 8 separate samples. *P < 0.05
Analysis of AQP 8 expression
In addition to AQP 9, AQP 8 has been identified as another physiologically important AQP in hepatocytes. Studies using rodent models have identified that AQP 8 exists (predominantly in the cytoplasmic regions) in hepatocytes and, following appropriate stimuli, translocates to the canalicular membrane. Using commercially available antibodies, we next sought to determine the relative expression and localization of AQP 8 in HCC and NTL sections. Despite varying the IHC techniques, we were unable to reproducibly detect AQP 8 protein expression in either HCC or NTL, nor within sections prepared from the same sample (data not shown).
Discussion
Previous studies using rodent liver tissue identified AQP 0, 8 and 9 expression within hepatocytes at the mRNA and protein levels,1,12,21 and AQP 11 at the mRNA level only.9 In these model systems, the authors report uneven levels of expression of AQPs 0, 8 and 9, whereby AQP 8 is expressed to a greater degree than AQP 9, which is in turn expressed to a greater degree than AQP 0.1 Researchers using mouse and rat tissue reported AQPs 0 and 8 to be most strongly expressed in hepatocytes that surround the central vein, and AQP 9 to be uniformly distributed throughout the liver.1 In addition, AQPs 0 and 8 appear to be predominantly located intracellularly in these rodent models, whereas AQP 9 is most strongly detected in the basal membrane.1 By contrast with these findings, our analysis of normal human liver in the absence of HCC burden demonstrated clear zonal distribution of AQP 9 within the sinusoid, where staining for AQP 9 occurred in Z3 >> Z2 > Z1. However, as with rodent hepatic tissue, the cellular distribution of AQP 9 in normal human liver was localized to the membrane of hepatocytes in human liver samples and membrane expression was significantly greater than that detected in the cytoplasmic region.
Within the healthy liver the role of AQPs in normal and abnormal hepatic function during disease pathogenesis is a relatively new, although intense, area of research interest.4,8 To date considerable evidence exists suggesting a role for cyclic-AMP (cAMP) in regulating AQP 8 translocation from cytoplasmic compartments to the canalicular membrane during bile synthesis in response to enteric stimulation (glucagon).1,12,22,23 By contrast, relatively little is known about the regulation and function of AQP 9. The (apparently) constitutive expression of AQP 9 and (predominant) localization to the basolateral membrane in hepatocytes has led to speculation that, in conjunction with AQP 8 translocation to the canalicular membrane, these two AQPs act to form a conduit to allow the rapid passage of water between the hepatic sinusoid and the bile canaliculus.4,8 However, unlike AQP 8, AQP 9 (along with AQPs 3, 7 and 10) belongs to a family of AQPs termed aquaglyceroporins because of their ability to transport specific small, uncharged molecules through the pore structure of the protein, including glycerol and urea.24,25 Hence it is increasingly speculated that AQP 9 may play a central role during hepatocyte glycerol uptake, especially during ‘starvation states’ in which glycerol deriving from adipose lipolysis is a major substrate for hepatic gluconogenesis.8,26,27 Similarly, the constitutive basolateral expression and ability of AQP 9 to transport urea has also led to speculation that these channel structures are further involved in metabolic regulation.4,8
Given the potential range of roles that AQP 9 may play in normal hepatic physiology, it was unsurprising to us that AQP 9 expression in NTL in the absence of underlying cirrhosis (i.e. ostensibly normal hepatic architecture) resembled that observed in normal liver in the absence of tumour burden in four of the seven cases analysed (i.e. zonal distribution coupled with membrane localization). Of interest, it was noted that in two of seven cases in which zonal distribution no longer occurred (in the NTL of HCC-burdened patients in the absence of cirrhosis) these were the largest tumour masses resected in which, one might predict, the highest degree of compromise of normal hepatic function might be found. More striking were the differences in AQP expression in livers of patients with underlying cirrhosis compared with those without. No zonal distribution of AQP 9 was apparent in any of the samples analysed. Rather, extensive membrane-localized AQP 9 expression was detected throughout the NTL and was localized to the nodules of hepatocytes located between fibrogenic septae.
Given the clear differences in AQP location detected in the NTL of cirrhotic and non-cirrhotic patients, we next sought to analyse the expression of AQP 9 in HCC masses. These data revealed significantly lower AQP 9 expression in HCC compared with NTL of patients with or without underlying cirrhosis. Furthermore, no significant differences were observed between AQP 9 localized to the membrane compared with the cytoplasm in the tumour mass. Initially, these observations may seem logical: as well as no longer responding to normal mechanisms that regulate cell proliferation, end-stage transformed, tumourigenic HCC cells are also characterized by de-differentiation and failure, to varying degrees, to perform the functions of the parent cell population (hepatocytes).14,15 Hence, given the emerging role(s) of AQP 9 in normal hepatic physiology, the absence of AQP 9 in cancerous cells may simply reflect the failure of these cells to participate in normal hepatic functions or homeostasis. However, studies in colorectal cancer tissue report increased expression of AQPs 1 and 5, neither of which are detected in non-transformed cells, in the early stages of tumourigenesis and subsequently throughout the course of the disease.28 In this instance, the authors speculate that increased AQP expression may lead to transformed cells gaining an advantage during the increased rates of replication and enhanced metabolic demands of these cells.28 In previous research by our group, we reported that, in rodent models, HCC tumourigenic tissue and cells demonstrate decreased AQP 9 expression compared with NTL.19 In this instance we also demonstrated that decreased AQP protein expression correlates with increased resistance to apoptotic stimuli as a result of the inability of cells to undergo the highly conserved biological process termed the apoptotic volume decrease (AVD) that is a prerequisite for subsequent apoptotic progression.29–31
In light of these data it is tempting to speculate on a potentially significant role for AQP 9 during both the tumourigenic and progression stages of HCC. That is, during initial transformation the cell no longer responds to normal signalling mechanisms that regulate fundamental hepatocyte features and functions, including bile synthesis and secretion. In this instance, failure to produce defined polar membranes may lead to diminished AQP 9 expression. Subsequently, these cells may no longer be susceptible to apoptotic deletion as a result of the absence of membrane-localized AQP 9 and a failure to undergo the AVD,29,30 a feature that will confer inherent advantages for transformed cell survival during progression.
In conclusion, the current study demonstrates a significant difference in AQP 9 expression and localization in NTL depending upon the underlying pathology of the patient (i.e. cirrhotic vs. non-cirrhotic). However, regardless of underlying hepatic pathology, HCC demonstrates significantly decreased AQP 9 expression compared with NTL. At present the precise role of AQP 9 (and other AQP forms normally expressed in hepatocytes) during cell transformation and HCC progression remain to be fully elucidated. However, the data presented in this study indicate that increasing the sample size for further analysis and identifying suitable antibodies for use in IHC analysis of human specimens warrants further investigation.
Conflicts of interest
None declared.
References
- 1.Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J Biol Chem. 2002;277:22710–22717. doi: 10.1074/jbc.M202394200. [DOI] [PubMed] [Google Scholar]
- 2.Arrese M, Accatino L. From blood to bile: recent advances in hepatobiliary transport. Ann Hepatol. 2002;1:64–71. [PubMed] [Google Scholar]
- 3.Suchy FJ, Ananthanarayanan M. Bile salt excretory pump: biology and pathobiology. J Pediatr Gastroenterol Nutr. 2006;43(Suppl.)(1):S10–S16. doi: 10.1097/01.mpg.0000226385.71859.5f. [DOI] [PubMed] [Google Scholar]
- 4.Masyuk AI, LaRusso NF. Aquaporins in the hepatobiliary system. Hepatology. 2006;43(2)(1) Suppl.:S75–S81. doi: 10.1002/hep.20996. [DOI] [PubMed] [Google Scholar]
- 5.Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Kwon TH, Frokiaer J, Agre P. Regulation and dysregulation of aquaporins in water balance disorders. J Intern Med. 2007;261:53–64. doi: 10.1111/j.1365-2796.2006.01760.x. [DOI] [PubMed] [Google Scholar]
- 7.Marinelli RA, LaRusso NF. Aquaporin water channels in liver: their significance in bile formation. Hepatology. 1997;26:1081–1084. doi: 10.1002/hep.510260539. [DOI] [PubMed] [Google Scholar]
- 8.Portincasa P, Palasciano G, Svelto M, Calamita G. Aquaporins in the hepatobiliary tract. Which, where and what they do in health and disease. Eur J Clin Invest. 2008;38:1–10. doi: 10.1111/j.1365-2362.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- 9.Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gradilone SA, Tietz PS, Splinter PL, Marinelli RA, LaRusso NF. Expression and subcellular localization of aquaporin water channels in the polarized hepatocyte cell line, WIF-B. BMC Physiol. 2005;5:13. doi: 10.1186/1472-6793-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinelli RA, Gradilone SA, Carreras FI, Calamita G, Lehmann GL. Liver aquaporins: significance in canalicular and ductal bile formation. Ann Hepatol. 2004;3:130–136. [PubMed] [Google Scholar]
- 12.Garcia F, Kierbel A, Larocca MC, Gradilone SA, Splinter P, LaRusso NF, et al. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem. 2001;276:12147–12152. doi: 10.1074/jbc.M009403200. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald GA. Pathogenesis of hepatocellular carcinoma. Clin Liver Dis. 2001;5:69–85. doi: 10.1016/s1089-3261(05)70154-9. [DOI] [PubMed] [Google Scholar]
- 14.McKillop IH, Moran DM, Jin X, Koniaris LG. Molecular pathogenesis of hepatocellular carcinoma. J Surg Res. 2006;136:125–135. doi: 10.1016/j.jss.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K. Hepatocellular carcinoma – history, current status and perspectives. Dig Liver Dis. 2002;34:613–616. doi: 10.1016/s1590-8658(02)80200-6. [DOI] [PubMed] [Google Scholar]
- 16.Colombo M, Sangiovanni A. Aetiology, natural history and treatment of hepatocellular carcinoma. Antiviral Res. 2003;60:145–150. doi: 10.1016/j.antiviral.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 17.McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol. 2005;35:195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Rocken C, Carl-McGrath S. Pathology and pathogenesis of hepatocellular carcinoma. Dig Dis. 2001;19:269–278. doi: 10.1159/000050693. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski EM, Mattocks MA, Sokolov E, Koniaris LG, Hughes FM, Jr, Fausto N, et al. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007;250:36–46. doi: 10.1016/j.canlet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran DM, Koniaris LG, Jablonski EM, Cahill PA, Halberstadt CR, McKillop IH. Microencapsulation of engineered cells to deliver sustained high circulating levels of interleukin-6 to study hepatocellular carcinoma progression. Cell Transplant. 2006;15:785–798. doi: 10.3727/000000006783981477. [DOI] [PubMed] [Google Scholar]
- 21.Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, et al. Immunolocalization of AQP 9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun. 2000;276:1118–1128. doi: 10.1006/bbrc.2000.3505. [DOI] [PubMed] [Google Scholar]
- 22.Gradilone SA, Garcia F, Huebert RC, Tietz PS, Larocca MC, Kierbel A, et al. Glucagon induces the plasma membrane insertion of functional aquaporin-8 water channels in isolated rat hepatocytes. Hepatology. 2003;37:1435–1441. doi: 10.1053/jhep.2003.50241. [DOI] [PubMed] [Google Scholar]
- 23.Marinelli RA, Tietz PS, Caride AJ, Huang BQ, LaRusso NF. Water transporting properties of hepatocyte basolateral and canalicular plasma membrane domains. J Biol Chem. 2003;278:43157–43162. doi: 10.1074/jbc.M305899200. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquaglyceroporins AQP 7 and AQP 9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, et al. Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- 26.Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP 9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci U S A. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, et al. Co-ordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51:2915–2921. doi: 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 28.Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- 29.Bortner CD, Cidlowski JA. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002;9:1307–1310. doi: 10.1038/sj.cdd.4401126. [DOI] [PubMed] [Google Scholar]
- 30.Bortner CD, Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 2004;448:313–318. doi: 10.1007/s00424-004-1266-5. [DOI] [PubMed] [Google Scholar]
- 31.Jablonski EM, Webb AN, McConnell NA, Riley MC, Hughes FM., Jr Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease. Am J Physiol Cell Physiol. 2004;286:C975–C985. doi: 10.1152/ajpcell.00180.2003. [DOI] [PubMed] [Google Scholar]


