Abstract
Background
Stapler-assisted hepatectomy has not been well established, as a routine procedure, although few reports exist in the literature. This analysis assesses the safety and outcome of the method based on peri-operative data.
Materials and Methods
From February 2005 to December 2006, endo GIA vascular staplers were used for parenchymal liver transection in 62 consecutive cases in our department. There were 18 (29%) patients with hepatocellular carcinoma (HCC), 31 (50%) with metastatic lesions and 13 (21%) with benign lesions [adenoma, focal nodular hyperplasia (FNH), simple cysts]. Twenty-one patients underwent major resections (33.9%) (i.e. removal of three segments or more) and 41 (66.1%) minor hepatic resections.
Results
Median blood loss was 260 ml. The median total operative time was 150 min and median transection time was 35 min. No patient required more than 2 days of intensive care unit (ICU) treatment. The median hospital stay was 8 days. Surgical complications included two (3%) cases of bile leak, two (3%) cases of pneumonia, two (3%) cases with wound infection and two (3%) cases with pleural effusion. The peri-operative mortality was zero. In a 30-month median follow-up, all patients with benign lesions were alive and free of disease. The 3-year disease-free survival for patients with HCC was 61% (57% for patients with colorectal metastases) and the 3-year survival 72% (68% for patients with colorectal metastases).
Conclusion
Stapler-assisted liver resection is feasible with a low incidence of surgical complications. It can be used as an alternative for parenchyma transection especially in demanding hepatectomies for elimination of the operating time and control of bleeding.
Keywords: stapler, liver resection, hepatectomy, endo GIA stapler
Introduction
Surgical technique in the era of hepatobiliary surgery affects the overall operative mortality, as it is directly connected to intra-operative bleeding.1,2 Various methods and instruments have been developed for safe and tissue-preserving liver parenchyma resection (Cavitron Ultrsonic Surgical Aspirator, Hydro Jet Cutter, radiofrequency assisted devices, etc).3–10 Vascular staplers are unique devises offering speed and safety during liver resection. In addition, its use in dividing hepatic veins and portal branches during hemihepatectomy minimizes blood loss.11–14
In this article, we provide prospective acquired data on liver resection with endo GIA vascular staplers in a single centre, regarding morbidity, blood loss, transection time, disease-free survival and long-term outcome. We also address the issue of negative tumour margin status when stapler technique is used for hepatectomy.
Materials and methods
From February 2002 to August 2008, a total of 257 patients underwent liver resection, in a tertiary referral Hepatobiliary and Pancreatic Unit, Agia Olga Hospital, Athens, Greece. From February 2005 to December 2006, 62 consecutive patients had liver resection using staplers for both extrahepatic vascular control and parenchyma transection. There were 21 major and 41 minor hepatectomies performed with the use of endo GIA vascular staplers for a variety of lesions (Table 1). Major hepatectomy was defined as resection of three or more liver segments. Patient characteristics and treatment applied are presented in Table 1. The potential for resection was assessed by ultrasound, computed tomography (CT) scan and sometimes magnetic resonance imaging (MRI) or MR angiography. Liver function was evaluated using Child–Pugh classification, liver biochemistry and model for end-stage liver disease (MELD) score in cirrhotics. All patients in this cohort were Child grade A and most of them had a MELD score ≤9 (Table 1). Ascites, encephalopathy, abnormal bilirubin level (>2 mg/dl) and portal hypertension were contraindications for liver resection. Portal hypertension was assessed by both platelet count as a surrogate marker and CT or Doppler imaging. Patients with a previous history of variceal bleeding were also excluded from this study. Resections were performed under low central vein pressure (CVP) anaesthesia (0–5 mmHg) and the supervision of two hepatobiliary and pancreas surgeons (S.D., Ch.D.). Each patient underwent an intra-operative ultrasound to define tumour location and relation to the major vascular structures. Intermittent portal triad clamping was not performed. During parenchyma transaction, clips, sutures and ligatures were used selectively in combination with an endo GIA vascular stapler. Transection time was defined as the duration between the beginning and the end of parenchyma transection. Total operating time was recorded from the beginning of parenchyma transection until completion of haemostasis. The amount of blood loss was estimated taking into account the suction volume after subtraction of rinse fluids. Indication for transfusion was a haemoglobin level <8 g/dl within 48 h after surgery. Drains were placed in all cases and remained until the 3rd post-operative day unless bile drainage or serosanguinous fluid above 500 cc was noted. Microscopic margins of resection were considered positive if there were tumour cells present at the tested margins. The presence of cirrhosis was evaluated by the Isaak fibrosis score15 and the degree of steatosis was graded as 0 (no steatosis or <10%) and 1 (steatosis >10%). Bile leak was defined as more than 50 ml of fluid drainage with bilirubin above 5 mg/dl.
Table 1.
Patient characteristics and operational data for stapler-assisted liver resection
| n = 62 | |
|---|---|
| Median (range) age (years) | 65 (59–76) |
| Gender ratio (male : female) | 41:21 |
| CRM metastasis | 28 |
| Post-chemotherapy steatosis (in the specimen) | 6 |
| Ovarian metastasis | 3 |
| Simple cyst | 9 |
| Adenoma | 2 |
| Focal nodular hyperplasia | 2 |
| HCC (diameter between 2cm and 5cm) | 18 |
| Non-cirhotics | 4 |
| Cirhotics (grade 2 and 3-by Isaak15) (Child A) | 14 |
| MELD≤9 | 12 |
| MELD>9 | 2 |
| Alcohol | 4 |
| Hepatitis B | 9 |
| Hepatitis C | 4 |
| Hepatitis D | 1 |
| Hemochromatosis | 1 |
| Type resection performed | |
| Right hepatectomy | 7 |
| Left hepatectomy | 14 |
| Segmental resection | 28 |
| Wedge resection | 13 |
| Total operation time (min) (median) | 150 (105 to 240) |
| Transection time (min) (median) | 35 (25 to 105) |
| Median hospital stay (days) (range) | 8 (6 to 13) |
| Median ICU stay (days) (range) | 0 (0 to 2) |
CRM, colorectal metastases; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; ICU, intensive care unit.
Surgical technique
Patients were placed in the supine position, prepared and draped in a sterile fashion. The abdomen was initially explored for extrahepatic disease through a roof-top incision (with or without extension in the midline to the xiphoid), a reversed L-shaped incision from the xiphoid to the tip of the 12th right rib, or a standard transverse abdominal incision; no thoracotomies were necessary. After some initial mobilization of the falciform triangular ligament, dissection was carried out to expose the hepatic veins and the porta hepatis. Short hepatic and caudate veins from the inferior vena cava (IVC) were clipped, ligated or divided with the endo GIA vascular stapler (Fig. 1) to fully mobilize the liver. If haemihepatectomy or extended haemihepatectomy was performed, the appropriate hepatic arterial branch was divided between ligation with sutures, followed by division of the portal vein branch with the vascular stapler or via suture. The appropriate hepatic vein(s) was then divided with the endo GIA 30-mm vascular stapler (Fig. 2). After the transectional line was marked, the liver capsule was divided with diathermy. For subsequent dissection of the hepatic parenchyma, liver tissue was fractured stepwise with a vascular clamp and subsequently divided with endo GIA 30-mm vascular staplers in a serial fashion without inflow occlusion (Fig. 3). If necessary, intra-operative ultrasound was used to guide this dissection. It is recommended that the thickness of parenchyma to be divided should not exceed 3 cm. After completed resection, the argon beam coagulation was applied to stop minor oozing. After haemostasis was secure, easy-flow drains were placed in the subphrenic and subhepatic space and the abdomen was closed. Using this method of liver resection, no Pringle's manoeuver or other vascular control was necessary.
Figure 1.
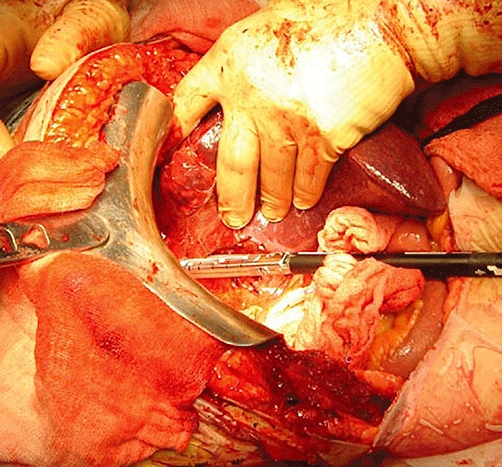
Dividing short hepatic veins with the stapler device
Figure 2.
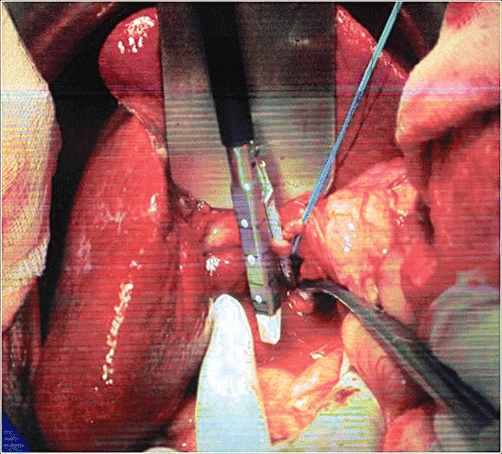
Dividing the vessels of the hepatic pedicle
Figure 3.
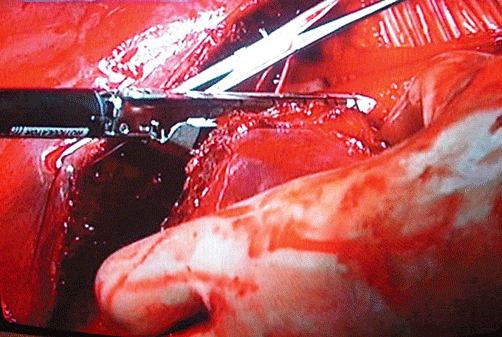
Use of the stapler for the parenchymal phase of liver resection
Results
No peri-operative death was documented. Median intra-operative blood loss was 260 ml (range: 150 to 750 ml), estimated by the aspirated fluid minus irrigation (Table 2). A blood transfusion was required in 10 patients; four of which were cirrhotics (Table 2). All of them had a hostile abdomen from previous operations including open cholecystectomy and choledoco-duodenostomy indicated by cholelithiasis and choledoholithiasis. Blood loss in these cases was mainly as a result of adhesiolysis, before transection of the liver. Two units of packed red blood cells (RBC) were given post-operatively (72 h after surgery) to maintain an Hb level above 8 mg/dl. Our team did not associate blood loss during transection and liver disease. Mean pre-operative haemoglobin was 12.5 mg/dl and post-operative was 11.8 mg/dl. The median transection time was 35 min (range 25 to 105 min) (Table 1). The median total operating time was 150 min (range 105 to 240 min) (Table 1). The operative time for parenchymal transection was primarily affected because of two independent factors: (i) the raw liver surface (major hepatectomies defined as more than three segments resected were prolonged with median transection time 45 min and range 35 to 105 min compared with minor liver resections in which median transaction time was 20 min) and (ii) the underlying liver disease. In cirrhotic patients, transection time was greater because of the fact that control of the haemorrhage from the cutting surface was necessary before we could proceed with the transection. In our study, steatosis because of chemotherapy is not associated with longer transection time. In fact we note that fatty infiltration makes liver tissue softer and more susceptible to the resection process.
Table 2.
Blood loss and transfusion requirements related to stapler-assisted liver resection
| Mean blood loss, ml | 500 (range 150 to 750) |
| Median blood loss, ml | 260 |
| Mean transfusion requirements, units | 0.32 (0 to 2) |
| Median transfusion requirement, units | 0 |
| Mean pre-operative haemoglobin, g/dl | 12.5 (range 10.5 to 15) |
| Mean discharge haemoglobin, g/dl | 11.8 (range 8.9 to 12.5) |
| Patients required FFP (for INR > 2.0) | 8 (12%) |
FFP, fresh frozen plasma; INR, International Normalized Ratio.
All patients had a post-operative increase of liver enzymes which normalized within 7 days. Serial monitoring of liver function in the first 7 post-operative days revealed peak serum aspartate aminotransferase on day 1 (median 230.5 u/l, range 99 to 456 u/l) and day 3 (median 133.04 u/l, range 26 to 173 u/l) with a rapid decline thereafter by day 7. Median peak post-operative serum bilirubin level was 1.18 mg/dl (range 0.41 to 1.78 mg/dl) on the first post-operative day.
Procedure-related complications included two cases of bile leak, which were managed conservatively and regressed after 5 to 10 days (Table 3). Pleural effusion, pneumonia and wound infection were also documented and managed conservatively (Table 3).
Table 3.
Morbidity after stapler-assisted liver resection
| Complication | No. of patients |
|---|---|
| Pleural effusion | 2 (3.2%) |
| Biliary fistula | 2 (3.2%) |
| Hyperbilirubinemia | 1 (1.6%) |
| Pneumonia | 2 (3.2%) |
| Wound infection | 2 (3.2%) |
Resected specimens were carefully examined regarding the tumour-free margin. The margin in most cases was larger than 10 mm and only in 15 cases ranged from 5 to 10 mm.
The length of hospital stay ranged from 4 to 10 days with a median value of 7 days. In a median follow-up of 30 months, all patients operated for benign lesions were alive without signs of recurrence. In seven patients operated for hepatocellular carcinoma, recurrent disease was evident between 18 and 32 months after surgery (3 year disease-free survival: 61%). Five of them died 20 to 32 months after the surgical intervention as a result of generalized disease (3-year survival: 72%) (Table 4).
Table 4.
Long-term results after stapler hepatectomy
| n | CRM metastases | Ovarian metastases | HCC | Simple cyst | Adenoma | FNH |
|---|---|---|---|---|---|---|
| 28 | 3 | 18 | 9 | 2 | 2 | |
| Median follow-up (months) | 30 | 27 | 32 | 40 | 25 | 22 |
| 1-, 3-year disease-free survival (%) | 69, 57 | 100, 66 | 79, 61 | 100, 100 | 100, 100 | 100, 100 |
| 1-, 3-year survival (%) | 74, 68 | 100, 100 | 83, 72 | 100, 100 | 100, 100 | 100, 100 |
CRM, colorectal metastases; HCC, hepatocellular carcinoma; FNH, focal nodular hyperplasia.
Patients operated for colorectal metastases reached a 3-year disease-free survival of 57% and a 3-year survival of 68%. Five of them were re-operated for recurrent disease (Table 4).
Regarding metastatic lesions from ovarian carcinoma, the 3-year disease-free survival was 66% and the 3-year survival was 100%, but as a result of the very small cohort (3 patients), solid results cannot be concluded (Table 4).
Discussion
The use of vascular staplers to divide hepatic veins and portal branches is considered an achievement that has aided in minimizing blood loss and thereby reduced the need for inflow occlusion. Recent publications reporting a number of techniques using stapling devices in liver surgery showed them to be extraordinarily useful in the safe ligation of inflow and outflow vessels.5,11,12,16–18 Recently, an ultrasound-directed application of vascular staplers, to selectively divide major intrahepatic blood vessels for inflow and outflow control during major liver resection, has been shown to achieve excellent results, reducing blood loss, warm ischaemia time and operative time.19 Furthermore, reports of laparoscopic left lateral segmentectomies performed with stapler and stapled wedge resections of the liver also showed favourable results.18,20 Staplers have been applied in living donor liver transplantation with promising results.21 Stapling devices can also be useful in patients with coagulopathy and in the treatment of complex liver abscesses.11,12,16,18
Our experience in 62 patients who underwent stapler hepatectomy shows that this technique for parenchymal dissection is applicable in a routine clinical setting based on both its feasibility and safety. In the present non-selective series, both mortality (0%) and total morbidity (14.5%) were as low as in a recently published large series of non-selected patients who underwent liver resection in other high-volume surgical centres.22–25
Control of operative blood loss is one of the immediate concerns when performing liver resection. Excessive blood loss is associated with increased peri-operative morbidity and, in cases of colorectal metastases, a shorter disease-free interval.1,2
In contrast to most series,22–24 we did not routinely apply the Pringle manoeuvre or other vascular control during resection. In our cohort, median blood loss was 260 ml during stapler hepatectomy. We consider this to be one of the advantages of stapler-assisted hepatectomy, as the vessels are sealed before they are transected. The same principle is in force for biliary structures as well (bile leak in our cohort: 3%).
Serious blood loss can theoretically occur when the stapler has sealed only half the diameter of the vessel or after misfire of the devise although we did not experience such a situation. Even in this or relevant cases of ongoing bleeding, serious blood loss does not usually occur because of the fact that stapler-assisted hepatic resection is completed promptly (median transection time in our cohort: 35 min) eliminating blood loss during the transaction phase. More to the point, in cases of difficult parenchymal transection with ongoing bleeding, the stapler device offers faster specimen removal giving the surgeon the opportunity to control the loss of blood from the raw liver surface. The obvious help that endo-GIA stapler provides, does not replace the specialized manipulations performed by a specialized HPB surgeon in a specialized centre.
Another potential danger from the use of staplers in the liver is tearing a major vein (e.g. right hepatic or vena cava), while placing the instrument. Usually after encircling of the hepatic vein, the articulated and rotating Endo-GIA vascular stapler is passed gently around the hepatic vein to staple and divide it. The thinner blade of the stapler is inserted in preference to the thicker blade because the space available is limited. As the thinner blade is not on the same axis as the instrument, difficulty may be encountered if the tip of the blade impinges on the liver tissue or on the major vein wall and tearing of the vein may occur. In order to avoid this complication, we use a right-angle clamp to grab the thinner blade and guide its insertion into the space between the liver parenchyma and major vein. This technique is also reported by other centres.26
During stapler-assisted hepatectomy, jamming and consequential failure to release or misfire with no staplers in the cartridge27 are other unfortunate events which may lead to serious, life-threatening haemorrhage. Such cases have been documented sporadically in liver surgery but are lower than 1% (L. H. Blumgart, personal communication). The hepatobiliary surgeon must be prepared to place a vascular clamp to control bleeding if such an incident occurs.
In our study, blood loss was minimal even after demanding operations with avoidance of inflow occlusion. Similar values were reported for blood loss during liver resection with portal triad clamping or extrahepatic control of the hepatic veins in other studies.25,28,29 More recent studies reported 750 ml as median intra-operative blood loss, and about 17% of their patients required transfusion using a variety of transection devices; however, 27% of these patients underwent intermittent inflow clamping during liver resection.24 A further decrease in intra-operative blood loss can be achieved with selective extrahepatic division of major blood vessels with staplers before dissection of the liver parenchyma.19,29 The Pringle manoeuvre itself may be associated with complications resulting from ischaemic injury of the remnant liver and from abdominal visceral venous stagnation30 especially in cases of chronic liver disease. Thus avoiding Pringle parenchyma can be easily transected by means of vascular staplers with minimal blood loss. In our series blood transfusions were required in 10 patients (16%). In all situations, two units of packed RBC were transfused in order for the haemoglobin to be kept above 8 mg/dl.
Biliary leakage and biliomas were present as major obstacles after liver resection. While the overall complication rate markedly decreased, the incidence of bile leakage still frequently occurs. In our series, a bile leak or bilioma was recorded in 3% of cases, significantly lower than in other reports.31 This low incidence of bile leak is in our opinion related to the sealing of all injured bile ducts some of which might have remained unsealed if the stapler was not used.
The median transection time in our series was 35 min and the median total operative time was 150 min (Table 1). For major hepatectomy a median time of 190 min was required whereas minor liver resection was as fast as 90 min. The innovative advantage in using staplers is that the procedure is fast, in contrast to others, such as radio-frequency (RF) or saline-enhanced linear radiofrequency devices.10,13
A negative tumour margin has been reported to be a serious factor determining disease-free and long-term survival in malignant lesions.32–35 More to the point with regard to hepatocellular carcinoma (HCC), it has been reported that for macroscopically solitary HCC, a resection margin aiming grossly at 2 cm, efficaciously and safely decreases the post-operative recurrence rate and improve survival outcomes when compared with a gross resection margin aiming at 1 cm, especially for HCC ≤2cm.32 In our cohort, patients with HCC had 72% 3-year survival, which is comparable with results reported from other centres when resection32,36,37 or even transplantation38 is performed. The largest report of resected patients comes from the Liver Cancer Study Group in Japan,37 which has reported 1-, 3-, 5-, and 10-year survival rates of 85%, 64%, 45%, and 21%, respectively, in 6785 cirrhotic patients treated using hepatic resection between 1988 and 1999.37 Survival rates as high as 60% at 5 years may be achieved in Child grade A patients with well-encapsulated tumours of ≤2cm in diameter. Although <10% of patients fit these selection criteria, such results, obtained in patients with good liver function who underwent anatomical resection, could be favourably compared with those of liver transplantation.38–40
Regarding colorectal metastatic lesions, a resection margin less than 5 mm was associated with a greater risk of recurrence on the surgical margin, with a lesser rate of overall and disease-free survival, compared with patients in which the resection margin was 1 cm or more.33–35 All patients though with microscopical clear margins from a tumour will benefit from hepatectomy.33–35 A surgical margin >1 cm can be hard to achieve at times. Surgeons may need to remove major vascular structures, such as the hepatic vein, with more liver tissue for an adequate margin. Sometimes the extent of margin has to be compromised in patients with poor liver function. Although there is a concern regarding tumour margin status after stapler hepatectomy, an inadequate margin (<0.5 cm) was not confirmed in our cohort. More precisely 49 patients had more than a 1-cm negative margin and 13 patients between 0.5 and 1 cm.
The average treatment costs in our series, bearing in mind that we used approximately four cartridges for each patient, was in agreement with those reported by others.14 Schemmer et al.14 in a recently performed evaluation of the intra-operative costs [including surgery, anaesthesioloy, blood products and the average number of endo-GIA vascular staplers (Tyco 030412, 60 cm; Tyco 030403, handset)], the median hospital stay and ICU stay added up to a total of EUR 11 382. Interestingly enough he reported that there was a cost-benefit when comparing stapler and conventional methods of more than EUR 2400 in favour of stapler hepatectomy.14
Regarding colorectal metastases, our long-term results were comparable with other centres when resection was performed to treat similar lesions, although using different technique.34,35,41 Nuzzo et al. reported 3-, 5- and 10-year survival rates to be 54.9%, 37.9%, and 22.9%, respectively.34 In his study, global and surgical margin recurrence rates increased as the tumour-free margin decreased (P = 0.01 and P < 0.001, respectively). Supplementary at univariate analysis, the width of surgical margin (P < 0.001), transfusion requirement, major hepatectomy, R1 resection, number of metastases, high pre-operative carcinoembryonic antigen (CEA) and increasing tumour size (P-value from 0.001 to 0.03) were associated with lesser rates of long-term survival. Rees et al.35 reported 5- and 10-year cancer-specific survival of 36% and 23%, respectively. In his study, multivariate analysis, revealed seven risk factors to be independent predictors of poor survival: number of hepatic metastases >3, node positive primary, poorly differentiated primary, extrahepatic disease, tumour diameter ≥5 cm, carcinoembyonic antigen level >60 ng/ml and positive resection margin. The first six of these criteria were used in a pre-operative scoring system and the last six in the post-operative setting. Patients with the worst post-operative prognostic criteria had an expected median cancer-specific survival of 0.7 years and a 5-year cancer-specific survival of 2%. Conversely, patients with the best prognostic post-operative criteria had an expected median cancer-specific survival of 7.4 years and a 5-year cancer-specific survival of 64%. When tested both predictive models fitted the data well with no significant differences between observed and predicted outcomes (P > 0.05).
Conclusion
Stapler hepatectomy is both an effective and safe surgical procedure, but controlled clinical trials are needed to further investigate and develop this liver resection technique. As treatment costs become more and more a focus in clinical medicine, novel methods need to be evaluated not only for patient's safety but also for their cost-effectiveness.
Conflicts of interest
None declared.
References
- 1.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 2.Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–505. doi: 10.1097/00000658-199210000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasberg SM, Drebin JA, Lineban D. Use of a bipolar vessel-sealing device for parenchymal transection during liver surgery. J Gastrointest Surg. 2002;6:569–574. doi: 10.1016/s1091-255x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 4.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habbib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–563. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Fong Y, Jarnagin WR, Blumgart LH. Recent advances in hepatic resection. Semin Surg Oncol. 2000;19:200–207. doi: 10.1002/1098-2388(200009)19:2<200::aid-ssu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, et al. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166–172. doi: 10.1007/s00268-003-7167-5. [DOI] [PubMed] [Google Scholar]
- 7.Sundaram CP, Rehman J, Venkatesh R, Lee D, Rageb MM, Kibel A, et al. Hemostatic laparoscopic partial nephrectomy by a water-cooled, high density, monopolar device without renal vascular control. Urology. 2003;61:906–909. doi: 10.1016/s0090-4295(02)02550-5. [DOI] [PubMed] [Google Scholar]
- 8.Tabuse K, Katsumi M, Kobayashi Y, Noguchi H, Egawa H, Aoyama O, et al. Microwave surgery: hepatectomy using a microwave tissue coagulator. World J Surg. 1985;9:136–143. doi: 10.1007/BF01656265. [DOI] [PubMed] [Google Scholar]
- 9.Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–823. doi: 10.1097/01.sla.0000189121.35617.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delis SG, Bakoyiannis A, Tassopoulos N, Athanasiou K, Madariaga J, Dervenis C. Radiofrequency-assisted liver resection. Surg Oncol. 2008;17:81–86. doi: 10.1016/j.suronc.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 11.McEntee GP, Nagorney DM. Use of vascular staplers in major hepatic resections. Br J Surg. 1991;78:40–41. doi: 10.1002/bjs.1800780114. [DOI] [PubMed] [Google Scholar]
- 12.Fong Y, Blumgart LH. Useful stapling techniques in liver surgery. J Am Coll Surg. 1997;185:93–100. doi: 10.1016/s1072-7515(01)00889-4. [DOI] [PubMed] [Google Scholar]
- 13.DeMatteo RP, Fong Y, Jarnagin WR, Blumgart LH. Recent advances in hepatic resection. Semin Surg Oncol. 2000;19:200–207. doi: 10.1002/1098-2388(200009)19:2<200::aid-ssu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Schemmer P, Friess H, Hinz U, Mehrabi A, Kraus TW, Z'graggen K, et al. Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg. 2006;30:419–430. doi: 10.1007/s00268-005-0192-9. [DOI] [PubMed] [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 16.Voyles CR, Vogel S. Hepatic resection using stapling devices to control the hepatic veins. Am J Surg. 1989;158:459–460. doi: 10.1016/0002-9610(89)90286-9. [DOI] [PubMed] [Google Scholar]
- 17.Wrightson WR, Edwards MJ, McMasters EM. The role of the ultrasonically activated shears and vascular cutting stapler in hepatic resection. Am Surg. 2000;66:1037–1040. [PubMed] [Google Scholar]
- 18.Jurim O, Colonna JO, II, Colquhoun SD, Shaked A, Busuttil RW. A stapling technique for hepatic resection. J Am Coll Surg. 1994;178:510–511. [PubMed] [Google Scholar]
- 19.Smith DL, Arens JF, Barnett CC, Izzo F, Curley SA. A prospective evaluation of ultrasound-directed transparenchymal vascular control with linear cutting staplers in major hepatic resections. Am J Surg. 2005;190:23–29. doi: 10.1016/j.amjsurg.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Lefor AT, Flowers JL. Laparoscopic wedge biopsy of the liver. J Am Coll Surg. 1994;178:307–308. [PubMed] [Google Scholar]
- 21.Eguchi S, Kawashita Y, Takatsuki M, Kanematsu T. Application of endovascular stapler in living-donor liver transplantation. Am J Surg. 2007;193:258–259. doi: 10.1016/j.amjsurg.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred fourty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 23.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection. Ann Surg. 2002;236:397–407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–1056. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 26.Wang WX, Fan ST. Use of the Endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003;26:193–196. doi: 10.1016/S1015-9584(09)60301-8. [DOI] [PubMed] [Google Scholar]
- 27.Kurian MS, Gagner M, Murakami Y, Andrei V, Jossart G, Schwartz M. Hand-assisted laparoscopic donor hepatectomy for living related transplantation in the porcine model. Surg Laparosc Endosc Percutan Tech. 2002;12:232–237. doi: 10.1097/00129689-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 29.Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155–161. doi: 10.1097/00000658-199608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YI, Kitano S. Segment VIII resection of the cirrhotic liver under continuous Pringle maneuver with in situ cooling followed by temporary portal decompression. Am J Surg. 1999;177:244–246. doi: 10.1016/s0002-9610(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484–489. doi: 10.1016/s1072-7515(02)01288-7. [DOI] [PubMed] [Google Scholar]
- 32.Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43. doi: 10.1097/01.sla.0000231758.07868.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143:384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 36.Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, et al. Prognosis of patients with intrahepatic recurrence after hepatic resection for hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2008 doi: 10.1016/j.ejso.2008.01.027. in press. [DOI] [PubMed] [Google Scholar]
- 37.Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21–29. doi: 10.1016/j.hepres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–1007. doi: 10.1245/s10434-007-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belghiti J, Cortes A, Abdalla EK, Regimbau JM, Prakesh K, Durand F, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885–892. doi: 10.1097/01.sla.0000098621.74851.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cha CH, Ruo L, Fong Y, Jarnagin WR, Shia J, Blumgart LH, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315–321. doi: 10.1097/01.sla.0000086548.84705.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, Shimada H, Ueda M, Matsuo K, Endo I, Togo S. Role of hepatectomy in treating multiple bilobar colorectal cancer metastases. Surgery. 2008;143:259–270. doi: 10.1016/j.surg.2007.08.015. [DOI] [PubMed] [Google Scholar]


