Abstract
Background
Milan and University of California San Francisco (UCSF) Criteria have been used for selection of patients with hepatocellular carcinoma (HCC) for liver transplantation (LTx). The aims of this study were to analyse the results of LTx for HCC in Australia and New Zealand with emphasis on the effects of discordance between pre-LTx radiological and post-LTx pathological staging.
Methods
A total of 186 LTx for HCC carried out between July 1985 and August 2003 were included. Patients were categorized according to the Milan and UCSF Criteria.
Results
The median follow-up was 6.55 years (range 2.96–20.93 years). Pre-LTx factors associated with better survival include tumour size ≤5 cm, number of tumours ≤3, staging within Milan and UCSF Criteria and more recent transplantation (1996–2003). In all, 14 patients had a pre-LTx stage outside the Milan but within the UCSF Criteria. One- and 5-year patient survival rates were, respectively, 88% and 74% within the Milan Criteria, and 87% and 73% within the UCSF Criteria. Vascular invasion, capsular invasion, lymph node invasion and pathological stage outside UCSF Criteria were associated with poor outcome. Of patients within the Milan and UCSF Criteria pre-LTx, 24% and 18%, respectively, were outside the same criteria post-LTx. These patients had poorer survival rates.
Conclusions
The use of the UCSF Criteria in this cohort increased the number of patients eligible for LTx without compromising 5-year survival rates. Patients whose explant tumours were outside the Milan or UCSF Criteria had poorer outcomes compared with those whose explants remained within these criteria.
Keywords: liver cancer, hepatectomy, Milan Criteria, UCSF Criteria
Introduction
Liver transplantation (LTx) is the treatment of choice for patients with hepatocellular carcinoma (HCC) on a background of liver cirrhosis. In most centres, only patients whose HCC meets the Milan Criteria are considered for LTx.1 These criteria, based on tumour size and number, are surrogate markers for favourable tumour biology. However, LTx patients outside these criteria can still expect a modest 5-year survival (25–45%),2 indicating that size and number are not reliable determinants of tumour biology. Nevertheless, in the absence of more specific criteria to assess tumour biology preoperatively, tumour size and number remain the most important selection criteria for HCC patients undergoing LTx. They are, in fact, surrogate markers for vascular invasion, which indicates a poor prognosis.3
Inevitably there is a delay between placing the patient on the waiting list and subsequent LTx, during which tumour progression may occur. Periodic radiological imaging is performed tokeep track of tumour status4 and those who progress beyond specific criteria, such as the Milan or University of California San Francisco (UCSF) Criteria,5 are removed from waiting lists because of their predicted poorer post-transplant outcome.
The aims of this study were two-fold. Firstly, we aimed to determine the outcome of all patients who underwent LTx for HCC in Australia and New Zealand over an 18-year period and to identify the impact of pre- and post-LTx factors, including the application of the Milan and UCSF Criteria. Secondly, we wanted to examine the effect of discordance between pre-LTx radiological staging (using the Milan and UCSF Criteria) and post-LTx pathological staging on LTx outcome.
Materials and methods
A retrospective analysis was performed of all patients who underwent LTx for HCC in Australia and New Zealand between 1985 and 2003. All LTx patients for whom HCC was confirmed in the pathology of the explanted liver were included. All transplants were performed using deceased organs. Data examined included details of patient demographics, disease aetiology, preoperative imaging results and pre-transplant tumour treatment, as well as postoperative outcomes, including histopathological findings, patient survival, tumour recurrence and further treatments.
Pre-transplant variables analysed included age, sex, serum α-fetoprotein (α-FP) levels and radiological tumour size and number. Any pre-LTx treatment of the tumour by any modality was also noted. These factors were analysed with respect to patient survival and recurrence-free survival. The Milan and UCSF Criteria as previously published1,5 were applied to this cohort of patients using both pre-LTx (radiological) and post-LTx (pathological) classification.
The effect of era of LTx on patient survival was evaluated. We divided the cohort into three eras. The early era (1985–1990), when LTx was first established in Australia, was marked by a lack of objective selection criteria for transplanting patients with known HCC and by suboptimal radiological imaging. During the middle era (1990–1995) selection criteria were more restrictive, whereas the latter era (1996–2003) followed the introduction of the Milan Criteria.
The pathological features of the explanted liver including tumour size and number, differentiation of tumour, presence of vascular or capsular invasion and involvement of lymph nodes were analysed. The effects of additional treatment post-LTx on survival were also evaluated. In addition, we sought to identify the prognostic indicators for survival after LTx in this cohort of patients.
Finally, we examined the effect of discordance between radiological stage and pathological stage in patients who satisfied the Milan or UCSF Criteria at listing. Any discordance in either number of tumours and/or tumour size resulting in staging outside the two respective criteria sets was considered a ‘stage migration’ for analysis purposes. Survival outcomes in this stage migration group were compared with outcomes for patients whose explanted tumours remained within preoperative Milan and UCSF Criteria, respectively.
Data analysis
Univariate analysis was carried out using the log rank test with patient and graft survival difference as the outcome measures. Continuous variables such as age and tumour size were converted into categorical variables according to previously published ranges.1,5 Determinants that were significant on univariate analysis were then subjected to multivariate analysis with Cox multi-regression analysis. All statistical analyses were carried out using spss Version 11.0.1 (SPSS, Inc., Chicago, IL, USA).
Results
In the period between July 1985 and Aug 2003, a total of 186 patients with HCC underwent LTx within the six liver transplant units in Australia and New Zealand. The presence of tumour was confirmed from the histology of the explanted tumour. The majority of the patients were males (83%). Their mean age was 52.8 years (range 12–73 years). Overall patient survival rates following LTx were 83.7% at 1 year, 75.8% at 3 years and 67.1% at 5 years (Fig. 1).
Figure 1.
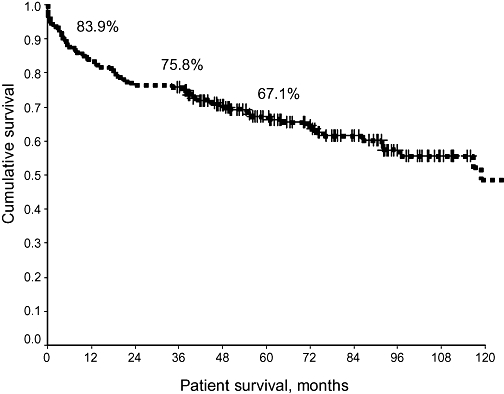
Overall patient survival following liver transplantation in hepatocellular carcinoma (1985–2003)
Aetiologies of the primary liver disease and cirrhosis are shown in Table 1. Viral hepatitis was the major cause of the primary liver disease in 70% of cases.
Table 1.
Aetiology of liver disease
| n | % | |
|---|---|---|
| HBV | 61 | 33.0 |
| HCV | 52 | 28.0 |
| HBV + HCV | 16 | 9.0 |
| Alcohol | 16 | 9.0 |
| Cryptogenic | 11 | 6.0 |
| Haemochromatosis | 6 | 3.2 |
| Primary biliary cirrhosis | 5 | 2.7 |
| Primary sclerosing cholangitis | 2 | 1.1 |
| Unknown | 1 | 0.6 |
HBV, hepatitis B virus; HCV, hepatitis C virus
A total of 146 (78.5%) patients were known to have HCC preoperatively based on a combination of radiological imaging (computed tomography [CT] scans), biopsy or serum α-FP assay. In 38 patients, the tumour was diagnosed incidentally from the histology of the explanted liver. Pre-transplant CT scans allowed us to stage tumours in 143 patients using the Milan and UCSF Criteria for 112 (71.1%) and 126 (88.1%) patients, respectively. Those staged within the Milan or UCSF Criteria had better survival than patients outside the criteria (Fig. 2a, b) (P < 0.02).
Figure 2.
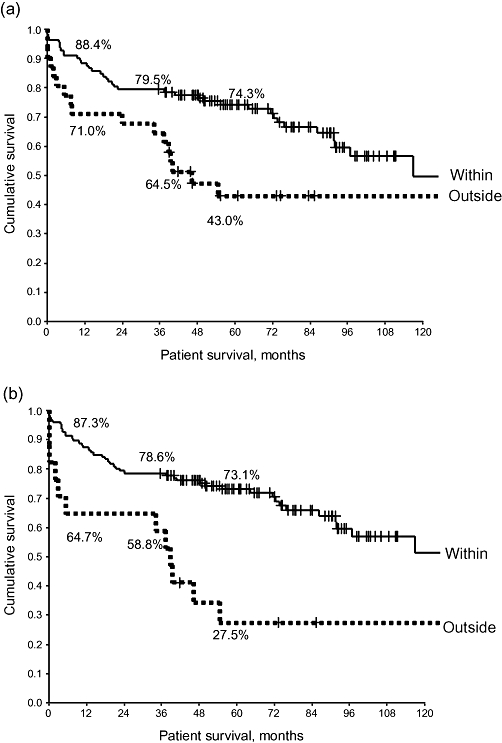
Survival curves for patients staged using the (a) Milan and (b) University of California San Francisco Criteria. There is a significant decrease in patient survival in patients staged outside the Milan Criteria on pre-liver transplant imaging (log rank P < 0.02)
Pre-transplant variables
The effects of various pre-transplant variables on patient survival are shown in Table 2. Both HCC staged within either the Milan or UCSF Criteria (P < 0.02) and LTx performed in the latest era (1996–2003) were associated with significantly better patient survival. A number of tumours ≤3 was associated with better outcome. Age and sex did not influence outcome. In 56 patients, HCC was treated pre-LTx. The majority (n = 29) of these patients underwent chemoembolization. The various modalities of pre-LTx treatment performed are shown in Table 3. Patients who had or required pre-transplant treatment had similar outcomes.
Table 2.
Overall survival of hepatocellular carcinoma patients after liver transplantation based on preoperative variables
| Parameters | n |
Survival, % |
P-value | ||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
| Gender | |||||
| Male | 155 | 82.6 | 74.2 | 61.9 | 0.3072 |
| Female | 31 | 90.0 | 83.3 | 64.0 | |
| Age, years | |||||
| ≤40 | 19 | 83.3 | 83.3 | 59.8 | 0.6749 |
| 41–50 | 56 | 91.1 | 78.6 | 76.8 | |
| 51–60 | 79 | 73.8 | 68.8 | 66.1 | |
| >60 | 32 | 96.9 | 84.4 | 59.5 | |
| α-fetoprotein, KIU/l | |||||
| <50 | 119 | 86.6 | 81.5 | 71.2 | 0.2072 |
| 50–200 | 33 | 84.9 | 72.7 | 65.2 | |
| >200 | 30 | 73.3 | 56.7 | 53.1 | |
| Number of tumours | |||||
| >3 | 11 | 72.7 | 72.7 | 32.7 | 0.0412 |
| ≤3 | 142 | 83.8 | 73.9 | 67.6 | |
| Tumour size | |||||
| >5 cm | 11 | 54.6 | 36.4 | 18.2 | 0.0002 |
| ≤5 cm | 131 | 87.0 | 79.4 | 72.4 | |
| Milan Criteria | |||||
| Within | 112 | 88.4 | 79.5 | 74.3 | 0.0164 |
| Outside | 31 | 71.0 | 64.5 | 43.0 | |
| UCSF Criteria (radiological) | |||||
| Within | 126 | 87.3 | 78.6 | 73.1 | 0.0009 |
| Outside | 17 | 64.7 | 58.8 | 27.5 | |
| Pre-transplant treatment of HCC | |||||
| Yes | 56 | 83.2 | 65.2 | 60.9 | 0.9736 |
| No | 121 | 87.5 | 78.6 | 67.9 | |
| Unknown | 9 | – | – | – | |
| Era of transplant | |||||
| 1985–1990 | 16 | 62.5 | 50.0 | 37.4 | 0.0500 |
| 1991–1995 | 13 | 92.3 | 92.3 | 76.9 | |
| 1996–2003 | 157 | 85.4 | 77.1 | 69.3 | |
| Diagnosis of HCC | |||||
| Pre-LTx | 146 | 82.2 | 72.6 | 64.9 | 0.1336 |
| Incidental | 38 | 89.5 | 86.8 | 74.3 | |
UCSF, University of California San Francisco; HCC, hepatocellular carcinoma; LTx, liver transplantation
Table 3.
Treatment of hepatocellular carcinoma prior to liver transplantation
| n | |
|---|---|
| None | 117 |
| Missing data | 5 |
| Treated | 56 |
| Chemoembolization | 29 |
| Chemotherapy | 3 |
| Alcohol injection | 4 |
| Tamoxifen | 1 |
| Embolization | 9 |
| Multimodality | 6 |
| Resection | 3 |
| Radiofrequency ablation | 1 |
In the evaluation of patient survival with respect to LTx era, the 16 patients who underwent LTx during 1985–1990 had the poorest outcome (Fig. 3). During the middle era (1991–1995), only 13 patients were transplanted as a result of more cautious patient selection, but the best outcomes were achieved. The most recent era, after the introduction of the Milan Criteria, saw the largest number of HCC patients transplanted (n = 157) and witnessed survival outcomes comparable with those of the middle era.
Figure 3.
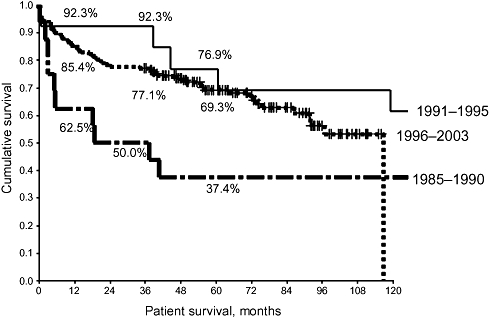
Patient survival rates following liver transplantation (LTx) in hepatocellular carcinoma (HCC). In the first period (1985–1999), some large and multiple HCC were transplanted with poor results (P < 0.05). As a result, the second period (1991–1995) saw relatively few transplants for this indication. The publication of the Milan Criteria resulted in the highest number of LTx for HCC in the last era (1996–2003)
Using Cox regression multivariate analysis, the significant factors associated with poorer patient survival outcome on multivariate analysis included a tumour number >3 and tumour size >5 cm. Being within the Milan or UCSF Criteria on pre-LTx radiology were independent predictors of a good outcome on multivariate analysis.
Tumour characteristics
Explanted tumour sizes >5 cm or >6.5 cm were associated with poorer patient survival following LTx. Vascular micro- or macroscopic invasion, capsular invasion and the presence of positive lymph nodes were associated with significantly poorer post-LTx outcomes. Surprisingly, the degree of tumour differentiation did not have a significant effect on outcome. Tumours that were outside UCSF pathological criteria were associated with poorer longterm outcomes (Fig. 4). Table 4 shows tumour characteristics.
Figure 4.
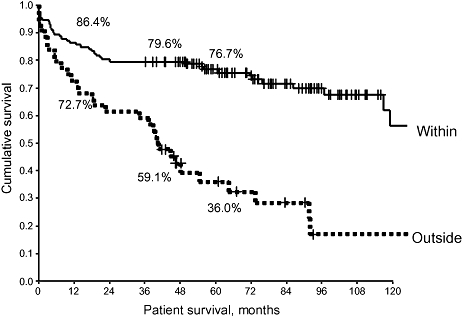
The pathological staging of the tumour using University of California San Francisco (UCSF) Criteria was evaluated for patient survival. When the explanted liver met UCSF Criteria, patient survival was significantly improved (P < 0.0005). A similar trend was noted for recurrence-free survival
Table 4.
Overall survival of hepatocellular carcinoma patients after liver transplantation based on tumour characteristics
| Parameters | n |
Survival, % |
P-value | ||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
| Largest tumour diameter | |||||
| <5.0 cm | 152 | 87.0 | 81.2 | 74.4 | <0.0001 |
| ≥5.0 cm | 24 | 54.2 | 33.3 | 20.1 | |
| <6.5 cm | 161 | 85.7 | 78.3 | 71.8 | 0.0001 |
| ≥6.5 cm | 15 | 53.3 | 40.0 | 13.3 | |
| Tumour number | |||||
| >3 | 32 | 80.7 | 74.2 | 45.1 | 0.0026 |
| ≤3 | 144 | 85.4 | 76.4 | 73.0 | |
| Milan Criteria (explant) | |||||
| Within | 117 | 87.2 | 80.3 | 77.1 | 0.0002 |
| Outside | 59 | 74.6 | 62.7 | 46.1 | |
| UCSF Criteria | |||||
| Within | 132 | 86.4 | 79.6 | 76.7 | <0.0001 |
| Outside | 44 | 72.7 | 59.1 | 36.0 | |
| Capsular invasion | |||||
| Absent | 157 | 84.7 | 79.6 | 71.7 | 0.0039 |
| Present | 19 | 79.0 | 42.1 | 30.1 | |
| Vascular invasion | |||||
| None | 142 | 86.8 | 81.9 | 74.1 | <0.0001 |
| Micro | 14 | 85.7 | 64.3 | 64.3 | |
| Macro | 20 | 55.0 | 30.0 | 13.3 | |
| Lymph nodes involvement | |||||
| No | 170 | 84.3 | 76.7 | 68.3 | 0.0034 |
| Yes | 6 | 50.0 | 16.7 | 16.7 | |
| Degree of differentiation | |||||
| Well | 38 | 89.5 | 81.6 | 75.3 | 0.3854 |
| Moderate | 67 | 85.1 | 74.6 | 64.8 | |
| Poor | 21 | 81.0 | 71.4 | 71.4 | |
UCSF, University of California San Francisco
Using Cox regression multivariate analysis, vascular invasion (macro- and microscopic), tumour number (>3), tumour size (>5 cm, >6.5 cm), and tumours exceeding the Milan and UCSF Criteria on explant pathology were independent predictors of poorer survival.
Recurrence-free survival
Factors that significantly influenced HCC recurrence-free survival post-transplantation were identical to those affecting patient survival, as shown in Table 5.
Table 5.
Recurrence-free survival of hepatocellular carcinoma patients after liver transplantation based on tumour characteristics
| Parameters | n |
Survival, % |
P-value | ||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
| Gender | |||||
| Male | 155 | 81.9 | 72.9 | 65.9 | 0.7488 |
| Female | 31 | 83.3 | 70.0 | 58.7 | |
| Age, years | |||||
| ≤40 | 19 | 88.9 | 72.2 | 60.1 | 0.8313 |
| 41–50 | 56 | 85.7 | 75.0 | 72.7 | |
| 51–60 | 79 | 76.3 | 70.0 | 63.7 | |
| >60 | 32 | 87.5 | 75.0 | 57.9 | |
| α-fetoprotein, KIU/l | |||||
| <50 | 119 | 85.7 | 77.3 | 70.3 | 0.1141 |
| 50–200 | 33 | 81.8 | 72.7 | 68.5 | |
| >200 | 32 | 70.0 | 56.7 | 46.4 | |
| Milan Criteria | |||||
| Within | 112 | 84.8 | 77.7 | 71.5 | 0.0202 |
| Outside | 31 | 77.4 | 58.1 | 42.3 | |
| UCSF Criteria (pre-LTx) | |||||
| Within | 126 | 84.1 | 77.0 | 70.6 | 0.0041 |
| Outside | 17 | 76.5 | 47.1 | 27.5 | |
| Pre-transplant treatment of HCC | |||||
| Yes | 56 | 85.7 | 76.8 | 68.1 | 0.8648 |
| No | 121 | 80.2 | 71.1 | 64.5 | |
| Era of transplant | |||||
| 1985–1990 | 16 | 56.3 | 31.3 | 25.0 | 0.0014 |
| 1991–1995 | 13 | 92.3 | 76.9 | 76.9 | |
| 1996–2003 | 157 | 84.1 | 75.8 | 67.9 | |
| Tumour number | |||||
| >3 | 32 | 74.2 | 67.7 | 45.4 | 0.0038 |
| ≤3 | 144 | 84.7 | 75.0 | 70.8 | |
| Largest tumour diameter | |||||
| <5.0 cm | 154 | 86.4 | 79.2 | 71.6 | <0.0001 |
| ≥5.0 cm | 22 | 58.3 | 29.2 | 25.0 | |
| <6.5 cm | 161 | 84.5 | 77.0 | 69.7 | 0.0019 |
| ≥6.5 cm | 15 | 60.0 | 26.7 | 20.0 | |
| Milan Criteria (explant) | |||||
| Within | 117 | 87.2 | 79.5 | 74.3 | 0.001 |
| Outside | 59 | 72.9 | 59.3 | 48.4 | |
| UCSF Criteria (explant) | |||||
| Within | 132 | 86.4 | 79.6 | 75.0 | <0.0001 |
| Outside | 44 | 70.5 | 52.3 | 36.3 | |
| Capsular invasion | |||||
| Absent | 157 | 85.4 | 76.4 | 69.7 | 0.0053 |
| Present | 19 | 57.9 | 42.1 | 30.1 | |
| Vascular invasion | |||||
| None | 144 | 85.4 | 78.5 | 70.3 | <0.0001 |
| Micro | 14 | 85.7 | 64.3 | 64.3 | |
| Macro | 20 | 55.0 | 30.0 | 25.0 | |
| Lymph nodes involvement | |||||
| No | 172 | 83.7 | 73.8 | 66.4 | 0.011 |
| Yes | 6 | 33.3 | 16.7 | 16.7 | |
| Degree of differentiation | |||||
| Well | 38 | 92.1 | 76.3 | 73.2 | 0.3591 |
| Moderate | 67 | 80.6 | 71.6 | 63.1 | |
| Poor | 21 | 76.2 | 76.2 | 70.3 | |
UCSF, University of California San Francisco; LTx, liver transplantation; HCC, hepatocellular carcinoma
On follow-up, 157 (84%) patients remained tumour-free. Recurrence of tumour occurred in 29 (16%) patients, two of whom survived beyond 5 years but not beyond 7 years.
Discordance between radiological and pathological stage and effect on outcome
Complete imaging information for accurate preoperative tumour staging according to the Milan and UCSF Criteria, as well as explant tumour pathology, was available for 137 patients.
Of the 108 patients in whom HCC fell within the Milan Criteria on pre-LTx staging, 26 (24%) were outwith the Milan Criteria on explant pathology. Of these 26 patients, 10 had more than three tumours, five had tumours >5 cm and tumours in 11 patients exceeded both size and number criteria. This group of patients had poorer survival than even those who were outwith the Milan Criteria prior to LTx (Fig. 5). Tumour characteristics fell within the UCSF Criteria on pre-LTx staging for 125 patients, 22 (18%) of whom migrated outside UCSF Criteria on explant pathology. This group had poorer survival compared with those whose explanted liver characteristics remained within the UCSF Criteria (Fig. 6).
Figure 5.
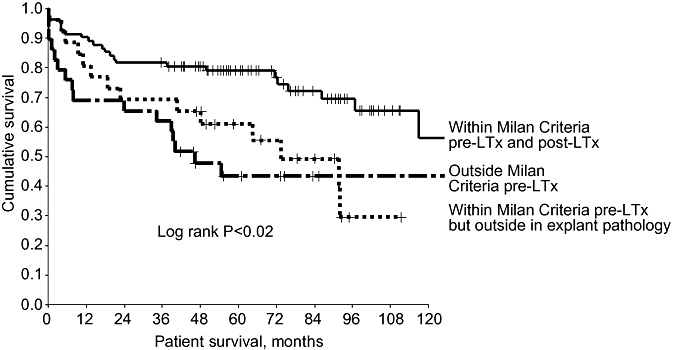
Patients in whom hepatocellular carcinoma (HCC) fell within the Milan Criteria pre- and post-liver transplantation (LTx) had significantly better survival rates than patients with HCC outside the Milan Criteria in explanted liver. This discordance between pre- and post-LTx characteristics probably reflects tumour progression. Patients with HCC outside the Milan Criteria on pre-LTx imaging had worse outcomes
Figure 6.
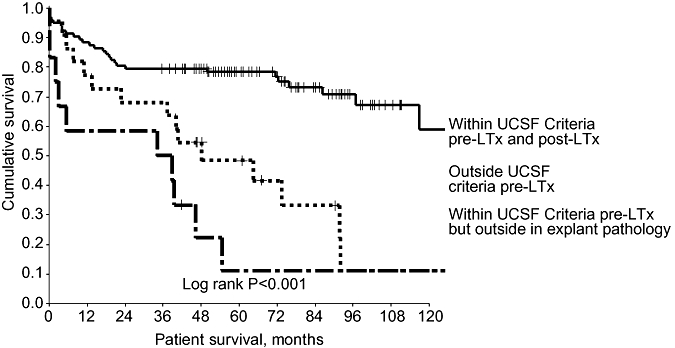
When the original pathological staging University of California San Francisco (UCSF) Criteria are applied to pre-LTx imaging, the survival trend mirrors that in Fig. 5. Patients in whom tumour progressed beyond the pre-LTx UCSF Criteria did more badly than those whose explanted liver remained within the criteria
Discussion
Recently, commentators have suggested that despite the usefulness of both the Milan and UCSF Criteria for selecting patients with HCC for LTx, staging criteria perhaps need to move beyond tumour size and number.6,7 These parameters are surrogate markers of tumour biology, particularly of vascular invasion.3 We now know that some patients whose tumour characteristics fall within these criteria do poorly after LTx and others, whose HCC exceed the criteria, do well.2 In the absence of more specific readily obtainable markers to assist us in differentiating good from aggressive tumour biology, the Milan and UCSF Criteria (applied pre-transplant) remain the current standards.
This series is unique in that all liver transplant units in Australia and New Zealand participated in the study. The series spans a period of almost two decades. During this period, overall 1- and 5-year patient survival rates following LTx for all indications improved from 70% and 59% (1985–1989) to 91% and 83% (2000–2004), respectively.8 This result is in line with similar improvements demonstrated in other registries. This cohort of HCC patients had a median follow-up of >6 years. The number of LTx procedures as well as the results very much reflect the prevailing management of HCC in patients with cirrhosis during three time periods. The post-Milan Criteria era (1996–2003) was associated with the largest number of transplants for HCC. Overall 5-year patient survival rates were 67.1%, and 74.3% and 73.1%, respectively, for cases falling within the Milan and UCSF Criteria (Fig. 2a, b), which compare favourably with rates in other published series (Table 6).
Table 6.
Patient survival after liver transplantation in hepatocellular carcinoma within the Milan Criteria
| References | Year | n | 1 year | 3 years | 4 years | 5 years |
|---|---|---|---|---|---|---|
| Romani et al.15 | 1994 | 27 | 82 | 71 | – | – |
| Mazzaferro et al.1 | 1996 | 48 | – | – | 75 | – |
| Figueras et al.16 | 1997 | 38 | 82 | 75 | – | 63 |
| Llovet et al.17 | 1998 | 58 | 84 | 74 | – | 74 |
| Bechstein et al.18 | 1998 | 52 | 88 | – | – | 71 |
| Yao et al.5 | 2001 | 46 | 91 | – | – | 72 |
| Moya et al.19 | 2002 | 104 | 74 | 66 | – | 63 |
| Baccarani et al.20 | 2006 | 50 | 83 | 77 | – | 72 |
| Decaens et al.9 | 2006 | 279 | – | – | – | 60 |
| Current series | 2007 | 112 | 87 | 80 | – | 74 |
The independent pathological variables that significantly influenced outcome include tumour size, tumour number and vascular invasion. These findings are consistent with previously published data.1 It is of interest that the degree of differentiation was not significant in the current series.
One of the aims of this study was to validate the prognostic significance of the Milan and UCSF Criteria in this cohort of patients. The results of applying both sets of criteria, based on pre-LTx imaging, to this cohort of patients produced almost identical patient survival rates, similar to the original publications from Milan and UCSF, respectively.1,5
According to our data, expanding the Milan Criteria to the UCSF Criteria would increase the number of patients eligible for LTx by 9.8% and would decrease post-transplant patient survival by only 1.2%. Thus, the application of UCSF Criteria in this cohort confirms the findings of other series which have demonstrated that expanding the Milan Criteria to the UCSF Criteria would not significantly compromise post-transplant survival. A recent French series found that patient survival was inferior for patients falling outside the Milan but within the UCSF Criteria.8 This may reflect the inclusion of an earlier cohort of LTx cases (1985–1990), in which outcomes were poorer overall.4 Previous retrospective series5,9,10 have revealed that only about 5% oflisted and subsequently transplanted patients exceeded the Milan but adhered to UCSF Criteria, compared with the 9.8% in our series.
With respect to disconcordance between pre-LTx staging and explant pathological staging, it was not surprising that 24% and 18% of patients who were within Milan and UCSF Criteria, respectively, exceeded the criteria on explant pathology. These groups had significantly worse outcomes. The discordance in each case was caused by inaccurate radiological staging or true tumour progression between imaging and transplantation. Factors affecting the latter include waiting time and tumour biology. Other studies comparing pre-LTx staging with explant pathology found an accuracy of around 60%.11 These findings have implications for the frequency of restaging of HCC patients on waiting lists. Given the relative shortage of donor livers and the need to best utilize this scarce resource, more vigilant and frequent restaging may result in delisting some of these patients and hence improve the utility of donor organs. Two previous studies looking at the progression of HCC beyond the Milan Criteria in the explanted liver revealed a much higher proportion (43%) of radiologically under-staged (by Milan Criteria) explant HCC.4,12 Both studies demonstrated similar decreased survival outcomes. The authors suggested that increasing CT imaging surveillance to intervals shorter than 3 months might be beneficial.4
Two recent studies have demonstrated that down-staging of HCC with various modalities such as transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) may select a group of patients outside the usual criteria who can be considered for LTx with acceptable results. Yao et al. demonstrated that, in a group of patients with large tumour, down-staging with RFA and TACE can produce results as good as those for patients who fall within the UCSF Criteria.13 However, as yet no survival benefit has been demonstrated with TACE. The more recent study by a German group demonstrated that the response of tumour to TACE is a good prognostic indicator for subsequent transplant outcome.14 In patients with large HCC that respond to TACE, the survival outcome was acceptable. However, in patients who progressed while under treatment with TACE, the outcome was poor despite the smaller tumour size in some cases. It would appear from these two studies that a positive response to down-staging perhaps reflects good tumour biology.
Conclusions
This unique Australasian study validated the use of the Milan and UCSF Criteria for selection of patients with HCC for LTx. Expanding the Milan Criteria to meet the UCSF Criteria did not decrease 5-year survival significantly. Overall, 24% and 18% of patients who satisfied the Milan and UCSF Criteria, respectively, on pre-LTx imaging exceeded these limits in explant pathology, suggesting either tumour progression or an under-estimation of disease on imaging. This group of patients had poorer survival outcomes.
Acknowledgments
The authors acknowledge the support of all the liver transplant unit co-ordinators, especially Libby John and Nicole Williams of the South Australia Liver Transplant Unit, in the collection of data. This study received no funding from any industry source.
Conflicts of interest
None declared.
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003;9:693–696. doi: 10.1053/jlts.2003.50086. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumour size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 4.Shah SA, Tan JC, McGilvray ID, Cattral MS, Cleary SP, Levy GA, et al. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation. 2006;81:1633–1639. doi: 10.1097/01.tp.0000226069.66819.7e. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumour size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY. Selection criteria for liver transplantation in patients with hepatocellular carcinoma: beyond tumour size and number? Liver Transpl. 2006;12:1189–1191. doi: 10.1002/lt.20853. [DOI] [PubMed] [Google Scholar]
- 7.Freeman RB., Jr. Transplantation for hepatocellular carcinoma: the Milan Criteria and beyond. Liver Transpl. 2006;12(Suppl)(2):S8–S13. doi: 10.1002/lt.20936. [DOI] [PubMed] [Google Scholar]
- 8.Lynch S, Balderson G, Cormark D. Australia & New Zealand Registry, 19th Report. [Accessed 20 August 2008]. http://www.anzltr.org/thisYear/report.pdf.
- 9.Decaens T, Roudot-Thoraval F, Hadni-Bresson S, Meyer C, Gugenheim J, Durand F, et al. Impact of UCSF Criteria according to pre- and post-OLT tumour features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl. 2006;12:1761–1769. doi: 10.1002/lt.20884. [DOI] [PubMed] [Google Scholar]
- 10.Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, et al. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10:911–918. doi: 10.1002/lt.20140. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M. Liver transplantation: the preferred treatment for early hepatocellular carcinoma in the setting of cirrhosis? Ann Surg Oncol. 2006;14:548–552. doi: 10.1245/s10434-006-9157-y. [DOI] [PubMed] [Google Scholar]
- 12.Sotiropoulos GC, Malago M, Molmenti E, Paul A, Nadalin S, Brokalaki E, et al. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumour classification before transplantation realistic? Transplantation. 2005;79:483–487. doi: 10.1097/01.tp.0000152801.82734.74. [DOI] [PubMed] [Google Scholar]
- 13.Yao FY, Hirose R, LaBerge JM, Davern TJ, III, Bass NM, Kerlan RK, Jr, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 14.Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 15.Romani F, Belli LS, Rondinara GF, DeCarlis L, Rimoldi P, Riolo F, et al. The role of transplantation in small hepatocellular carcinoma complicating cirrhosis of the liver. J Am Coll Surg. 1994;178:379–384. [PubMed] [Google Scholar]
- 16.Figueras J, Jaurrieta E, Valls C, Benasco C, Rafecas A, Xiol X, et al. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: a comparative study. Hepatology. 1997;25:1485–1489. doi: 10.1002/hep.510250629. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bruix J, Fuster J, Castells A, Garcia-Valdecasas JC, Grande L, et al. Liver transplantation for small hepatocellular carcinoma: the tumour-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–1577. doi: 10.1002/hep.510270616. [DOI] [PubMed] [Google Scholar]
- 18.Bechstein WO, Guckelberger O, Kling N, Rayes N, Tullius SG, Lobeck H, et al. Recurrence-free survival after liver transplantation for small hepatocellular carcinoma. Transpl Int. 1998;11(Suppl)(1):S189–S192. doi: 10.1007/s001470050458. [DOI] [PubMed] [Google Scholar]
- 19.Moya A, Berenguer M, Aguilera V, Juan FS, Nicolas D, Pastor M, et al. Hepatocellular carcinoma: can it be considered a controversial indication for liver transplantation in centres with high rates of hepatitis C? Liver Transpl. 2002;8:1020–1027. doi: 10.1053/jlts.2002.35664. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani U, Adani GL, Avellini C, Lorenzin D, Curro G, Beltrami A, et al. Comparison of clinical and pathological staging and longterm results of liver transplantation for hepatocellular carcinoma in a single transplant centre. Transplant Proc. 2006;38:1111–1113. doi: 10.1016/j.transproceed.2006.02.015. [DOI] [PubMed] [Google Scholar]


