Abstract
Background:
Aberrant arterial anatomy is a common finding during foregut surgery. Anomalies to the right hepatic lobe are especially relevant during pancreaticoduodenectomy (PD) and their recognition serves to protect the blood supply to the liver and bile ducts. We report our experience with aberrant right hepatic arterial anatomy (ARHAA) found during PD.
Methods:
All patients who underwent PD between February 2003 and June 2007 were retrospectively reviewed and those with ARHAA were identified. Preoperative imaging studies were assessed by one radiologist, graded according to the presence of ARHAA and compared with the original interpretations.
Results:
We found ARHAA in 31 of 191 patients (16.2%). Operative management included dissection and preservation in 24, transection and reconstruction in four, and transection and primary anastomosis in three patients. Reconstruction of ARHAA was carried out through interposition grafts in two patients and implantation into the gastroduodenal stump in two patients. No cases of arterial thrombosis, liver infarction, abscess formation or biliary fistula were demonstrated in the immediate postoperative period. Review of preoperative imaging interpretations found that only nine of 23 reports indicated the presence of ARHAA; however, the retrospective review of the images found that ARHAA was readily apparent in 24 patients.
Discussion:
Recognition of aberrant vasculature to the liver before PD is important. Preoperative imaging studies will often be adequate to identify these anomalies, but interpreting radiologists may not be aware of its clinical significance. Surgeons performing PD must be adept at managing ARHAA safely.
Keywords: Pancreaticoduodenectomy, replaced right hepatic artery, right hepatic artery, Whipple
Introduction
Variants in visceral arterial anatomy are commonly encountered, and many patients have one or more variations of the coeliac axis or superior mesenteric artery (SMA).1 Congenital deviations are thought to occur because of the persistence of vitelline arteries during embryologic development.2 These deviations can cause difficulties during surgery of the upper intestinal tract, pancreas, gallbladder and biliary tract, or during percutaneous transarterial procedures, and all surgeons should be aware of these variations. Arterial anomalies near the pancreas and liver are an especially important consideration during pancreaticoduodenectomy (PD), with aberrant right hepatic arterial anatomy (ARHAA) being the most common and relevant anomaly. Variations in arterial blood supply in this region occur in up to 15% of donor livers3 and in 17% of preoperative visceral angiograms.4 The most common anomalies, according to the Hiatt classification,3 involve a replaced or accessory right hepatic artery without (type III, 10.6%) or with (type IV, 2.3%) an accessory or replaced left hepatic artery. The common hepatic artery may originate from the SMA (type V) orthe aorta (type VI), but, together, they account for only 1.7% of deviations. Normal hepatic arterial anatomy is seen in 75.7% of all patients. Failure to preoperatively recognize anomalies risks accidental transection and vascular compromise of the liver and biliary ducts, resulting in liver ischaemia or bilioenteric breakdown.
Preoperative planning evaluations for PD commonly include a high-quality, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) examination. Either method can provide exceptionally detailed images of arterial anatomy if the examination is performed with close attention to technique.5 However, both the surgeon and the radiologist must be aware of the possibility of ARHAA. We report our experience with these arterial anomalies encountered during PD at a single centre during a period of 52 months.
Materials and methods
This study was conducted in accordance with the Helsinki Declaration. After the study had been approved by the Mayo Clinic Institutional Review Board (registration 06002114; 3 October 2006), all patients undergoing PD between 25 February 2003 and 4 June 2007 were identified. The subset of patients with ARHAA was identified from operative reports and their electronic medical records were retrospectively analysed. Additional review of all preoperative imaging studies for the 191 patients who underwent PD was performed to identify any references to ARHAA in the reports. A database was created to manage data abstracted from the records, including patient characteristics, findings from radiologic examinations, operative reports, pathology reports and postoperative course. The postoperative course was examined specifically for signs of hepatic arterial compromise and all imaging studies and liver function tests performed during this period were investigated for abnormalities.
Preoperative imaging studies were retrospectively reviewed by one radiologist (MDB), who was experienced in hepatobiliary imaging and was masked to the operative findings. These retrospective observations were recorded and compared with intraoperative findings and preoperative interpretations. The appearance of ARHAA on the preoperative images was evaluated for discernability.
Results
Of the 191 patients who underwent PD at our institution during the study period, 31 (16.2%) had ARHAA. Preoperative and intraoperative images of ARHAA are shown in Figs 1–3. Patients included 16 women and 15 men, with a mean age of 66.1 years. The type of PD procedure performed included standard PD (n = 17), pylorus-preserving PD (n = 12) and total pancreatectomy (n = 2). Table 1 shows indications for PD and operative management of patients with ARHAA. Details about the right hepatic artery anomalies and their overall prevalence rates are shown in Table 2. Three patients underwent arterial transection with primary anastomosis and four patients underwent arterial transection with reconstruction. Reconstruction was performed with a polytetrafluoroethylene jump graft to the gastroduodenal artery (GDA) in one patient, an interposition graft with the gonadal vein in one and a primary anastomosis to the GDA stump in two. Reasons for ARHAA transection included tumour involvement (n = 3) and thrombosis (n = 2); two transections were accidental.
Figure 1.
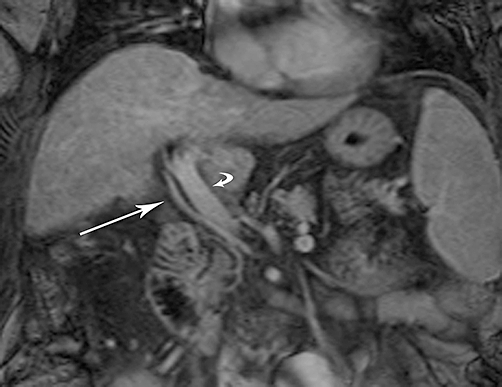
Coronal enhanced T1-weighted magnetic resonance image shows aberrant right hepatic arterial anatomy. As the replaced right hepatic artery (straight arrow) courses to the liver hilum, it is seen to be caudal and lateral to the portal vein (curved arrow), reflecting a variant origin from the superior mesenteric artery
Figure 3.
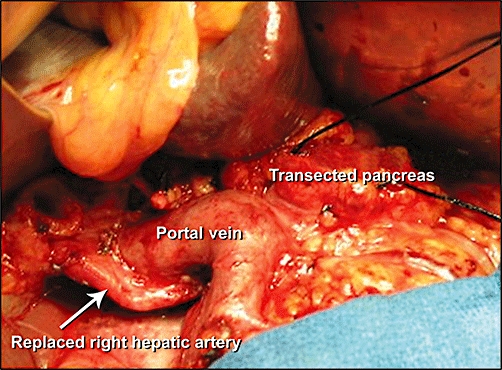
Intraoperative photograph showing a replaced right hepatic artery posterior to the portal vein
Table 1.
Indications for pancreaticoduodenectomy and management of aberrant right hepatic arterial anatomy
| Variable | Patients, n |
|---|---|
| Indication | |
| Pancreatic adenocarcinoma | 15 |
| Duodenal adenoma | 4 |
| Intraductal papillary mucinous neoplasm | 3 |
| Other malignant tumour | 6 |
| Chronic pancreatitis | 3 |
| Management | |
| Dissection and preservation | 24 |
| Transection and reconstruction | 4 |
| Transection and primary anastomosis | 3 |
Table 2.
Defects in right hepatic arteries
| Anatomic defect | Patients, n | Overall prevalence, %a |
|---|---|---|
| Replaced right hepatic artery from SMA | 23 | 12.0 |
| Accessory right hepatic artery from SMA | 5 | 2.6 |
| Accessory right hepatic artery from gastroduodenal artery | 1 | 0.5 |
| Replaced common hepatic artery from SMA | 1 | 0.5 |
| Replaced common hepatic artery from aorta | 1 | 0.5 |
The total number of patients undergoing pancreaticoduodenectomy was 191
SMA, superior mesenteric artery
Figure 2.
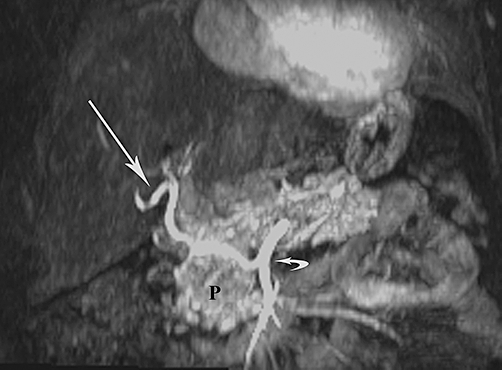
Maximum-intensity projection image from a coronal magnetic resonance angiographic acquisition shows aberrant right hepatic arterial anatomy. The replaced right hepatic artery is indicated by the straight arrow; the superior mesenteric artery is indicated by the curved arrow, and the pancreas is indicated by the P
No cases of hepatic arterial thrombosis, liver failure, infarction, abscess formation or biliary fistula were documented in the immediate postoperative period. However, a pseudoaneurysm developed in one patient, who had accidental ARHAA transection with subsequent primary anastomosis, and presented with an upper gastrointestinal bleeding episode 1 month after PD (Fig. 4). Gelfoam-and-coil embolization of the pseudoaneurysm was performed and postprocedural angiography of the left hepatic artery showed reconstitution of the right hepatic arterial system (Fig. 5). The patient's symptoms resolved without liver infarction or recurrent bleeding.
Figure 4.
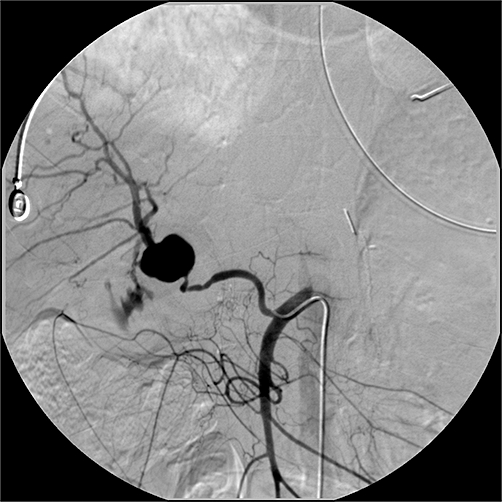
Selective visceral angiogram of the superior mesenteric artery showing the replaced right hepatic artery with pseudoaneurysm
Figure 5.
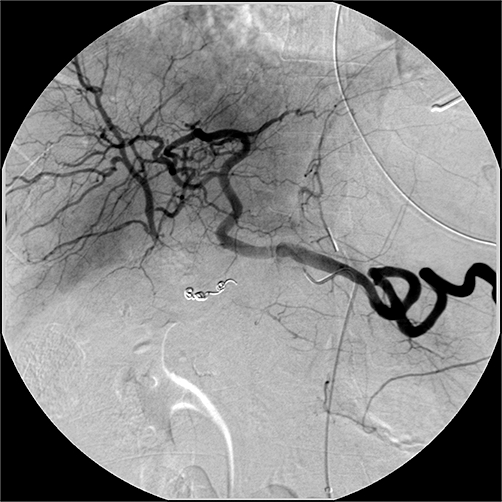
Selective visceral angiogram of the coeliac axis after embolization of the pseudoaneurysm. The right hepatic arterial system is reconstituted through intrahepatic flow from the left hepatic artery
A review of available preoperative CT and MRI interpretations for 26 patients showed that only nine indicated ARHAA. Retrospective review, however, accurately identified ARHAA in 24 of 25 preoperative imaging studies (one additional examination was available by report only). Additional review of preoperative imaging reports for the remaining 160 patients who underwent PD during the study period did not show any ARHAA observed during imaging only (i.e. in all patients with ARHAA findings on imaging studies, ARHAA was also observed intraoperatively).
Discussion
Surgical resection remains the cornerstone treatment for patients with pancreatic cancer6 and other diseases of the distal biliary tree, pancreatic head or duodenum. This case series showed that PD can be safely performed in patients with ARHAA and that variants of arterial anatomy are to be expected in nearly one in five patients undergoing pancreatic resections. Ideally, the aberrant vessels should be preserved, as they were in 77% of our patients. Recognition and appropriate management of ARHAA is critical during PD because complications of injury include hepatic ischaemia and bilioenteric anastomosis breakdown.7,8 Hepatic parenchymal ischaemia may be minimized by compensatory portal venous blood flow and intrahepatic collateral flow. However, biliary fistula is likely to occur if arterial blood flow is not maintained to the remaining bile duct at the bilioenteric anastomosis. Most of the arterial supply to the common bile duct is derived from the retroduodenal artery, which is a branch of the GDA. The proximal biliary tree is the susceptible watershed area; it is supplied by the right hepatic artery, which, in combination with the retroduodenal artery, produces an arterial plexus at the 3-o'clock and 9-o'clock positions of the duct.9 Because the GDA is routinely sacrificed during PD, the remaining bile duct becomes completely reliant on the right hepatic artery for its blood supply.
The most common type of ARHAA vessel is one that originates from the SMA, runs lateral to the portal vein in the hepatoduodenal ligament, and courses under the head of the pancreas and under the common bile duct toward the liver, as was observed in 28 of 31 patients with ARHAA. The other three patients had deviations in the originating vessel (ARHAA from the GDA [n = 1] and ARHAA from the aorta [n = 1]) or deviations in the course of the ARHAA vessel (replaced right hepatic artery on the ventral side of the pancreas [n = 1]). Many variations of this norm have been identified during PD,7,8,10–12 and the surgeon must be prepared to handle these variations. Although advance planning of ARHAA management is ideal, extemporaneous decisions may be required intraoperatively; such decisions are affected by tumour involvement and the specific anatomic variation. The four options for management of ARHAA vessels include: (i) sacrifice; (ii) preoperative embolization; (iii) dissection and preservation, and (iv) transection and reconstruction.8,11–15 Sacrifice is ideal for an accessory right hepatic artery of small calibre that impedes resection and is unlikely to have clinically significant consequences if it is sacrificed.11 Preoperative embolization is used to facilitate adequate collateralization to the right lobe and bile ducts before PD.14 This may be ideal for a larger aberrant artery that is sufficiently involved with the tumour to mandate resection for an adequate oncologic outcome. Dissection and preservation are ideal for all types of ARHAA, but may not always be possible. Transection and reconstruction may be accomplished by primary anastomosis or through implantation at a suitable arterial site. In cases of accidental transection or a small resection of the artery, a primary anastomosis can generally be achieved. If a large segment of artery is resected, re-implantation (direct implantation to the GDA stump) is best. However, interposition grafting is sometimes necessary and may be accomplished by using a gonadal vein or prosthetic graft, as was performed in two of our patients.
Preoperative recognition of ARHAA can permit modification of the PD technique, depending on the relationship of the tumour to the artery as it traverses the uncinate process of the pancreas. Noie et al.16 advocated a ventral approach to dissecting ARHAA instead of the traditional dorsal approach after a Kocher manoeuvre. The authors asserted that this approach facilitates a complete en bloc resection of the hepatoduodenal and para-aortic lymph nodes with the main lesion, theoretically improving the oncologic outcome. Varty et al.17 similarly described a modified technique for PD in patients with ARHAA. Their group performed an early retropancreatic dissection to expose the SMA (a right-to-left retropancreatic dissection), allowing the surgeon to directly visualize the aberrant artery and dissect it safely. The approach used in this study involved a similar dissection. A wide Kocherization of the duodenum, Cattel mobilization of the right colon and retropancreatic dissection were performed, encompassing the retrocholedochal lymph nodes en bloc with the head of the pancreas. If it is not identified preoperatively, ARHAA is generally discovered when the coeliac lymph node is removed and the hepatic artery is exposed. If the right hepatic arterial supply is diminutive or in an atypical location, the entire right hepatic arterial anatomy should be determined. However, caution must be taken during the Kocher manoeuvre and during retropancreatic dissections in patients with ARHAA because excessive retraction on the dorsum of the pancreatic head may cause thrombosis of the aberrant vessels. This occurred in two patients in this series, both of whom required reconstructive procedures.
Although high-quality images of the liver and pancreas are obtained in nearly every patient before PD, imaging methods are usually limited to MRI or CT. Routine visceral angiography before PD has been advocated by some.7,12,18 However, advances in cross-sectional imaging technology over the past decade have meant that angiography has largely fallen into disuse for preoperative evaluations. A properly performed CT or MRI will delineate the arterial anatomy and directly show the tumour with adjacent structures. Contrast-enhanced CT and MRI of the pancreas are generally performed to optimize the pancreatic parenchymal enhancement, but they can easily obscure small, aberrant arteries. Our institution has altered its imaging protocol to maximize enhancement during the arterial phase, which increases resolution of the arterial system.
Experienced surgeons have reported difficulty in palpating the tumour–vessel relationship when tumours are large or associated with pancreatitis.8,13 Well-performed preoperative imaging may clearly depict aberrant vessel anatomy, but the variant anatomy must also be discerned and described before surgical intervention. Therefore, the radiologist must be aware of ARHAA and its crucial importance during surgery, and communication between the surgeon and interpreting radiologist is essential. Only nine of the 26 preoperative imaging studies mentioned ARHAA in the original interpretations, but the retrospective review accurately identified ARHAA in 24 of 25 available examinations. Radiologists in our institution now routinely describe the hepatic arterial anatomy of patients with pancreatic malignancy.
Oncologic outcomes for those with aberrant vasculature and tumour involvement, as was the case in three of our patients, remain unknown. Although SMA involvement is a known contraindication for PD, tumour involvement of the aberrant artery is likely to have the same poor prognosis as a tumour with SMA invasion. This subject deserves further study in a larger patient cohort.
In conclusion, high-quality preoperative imaging and interpretation are essential when planning PD. Preoperative imaging can identify most ARHAA, but the interpreting radiologist must be alert to the clinical significance of these variants and the surgeon must be aware of ARHAA to avoid vessel damage during dissection. Although ARHAA may increase the level of difficulty of PD, our experience suggests that ARHAA can be managed safely without incurring an increase in perioperative PD-related morbidity.
Conflicts of interest
None declared.
References
- 1.Lin PH, Chaikof EL. Embryology, anatomy, and surgical exposure of the great abdominal vessels. Surg Clin North Am. 2000;80:417–433. xiv. doi: 10.1016/s0039-6109(05)70413-8. [DOI] [PubMed] [Google Scholar]
- 2.Reuter SR, Redman HC. Gastrointestinal Angiography. 2nd. Philadelphia, PA: Saunders; 1977. pp. 31–33. edn, pp. [Google Scholar]
- 3.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective coeliac and superior mesenteric angiographies. Surg Radiol Anat. 2004;26:239–244. doi: 10.1007/s00276-004-0229-z. [DOI] [PubMed] [Google Scholar]
- 5.Sahani D, Mehta A, Blake M, Prasad S, Harris G, Saini S. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367–1380. doi: 10.1148/rg.245035224. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 7.Traverso LW, Freeny PC. Pancreaticoduodenectomy: the importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg. 1989;55:421–426. [PubMed] [Google Scholar]
- 8.Woods MS, Traverso LW. Sparing a replaced common hepatic artery during pancreaticoduodenectomy. Am Surg. 1993;59:719–721. [PubMed] [Google Scholar]
- 9.Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa H, Shimada K, Iwata R, Moriyama N. A replaced common hepatic artery running through the pancreatic parenchyma. Surgery. 2000;127:711–712. doi: 10.1067/msy.2000.104485. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Kubota K, Rokkaku K, Nemoto T, Sakuma A. Disposal of replaced common hepatic artery coursing within the pancreas during pancreatoduodenectomy: report of a case. Surg Today. 2005;35:984–987. doi: 10.1007/s00595-005-3040-5. [DOI] [PubMed] [Google Scholar]
- 12.Rong GH, Sindelar WF. Aberrant peripancreatic arterial anatomy: considerations in performing pancreatectomy for malignant neoplasms. Am Surg. 1987;53:726–729. [PubMed] [Google Scholar]
- 13.Bold RJ, Charnsangavej C, Cleary KR, Jennings M, Madray A, Leach SD, et al. Major vascular resection as part of pancreaticoduodenectomy for cancer: radiologic, intraoperative, and pathologic analysis. J Gastrointest Surg. 1999;3:233–243. doi: 10.1016/s1091-255x(99)80065-1. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto N, Kodama Y, Endo H, Shimizu T, Miyasaka K, Tanaka E, et al. Embolization of the replaced common hepatic artery before surgery for pancreatic head cancer: report of a case. Surg Today. 2004;34:619–622. doi: 10.1007/s00595-004-2785-6. [DOI] [PubMed] [Google Scholar]
- 15.Nakano H, Kikuchi K, Seta S, Katayama M, Horikoshi K, Yamamura T, et al. A patient undergoing pancreaticoduodenectomy in whom involved common hepatic artery anomalously arising from the superior mesenteric artery was removed and reconstructed. Hepatogastroenterology. 2005;52:1883–1885. [PubMed] [Google Scholar]
- 16.Noie T, Harihara Y, Inoue K, Kubota K, Takayama T, Makuuchi M. Ventral dissection of replaced right hepatic artery during pancreatoduodenectomy. Hepatogastroenterology. 2001;48:999–1000. [PubMed] [Google Scholar]
- 17.Varty PP, Yamamoto H, Farges O, Belghiti J, Sauvanet A. Early retropancreatic dissection during pancreaticoduodenectomy. Am J Surg. 2005;189:488–491. doi: 10.1016/j.amjsurg.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Volpe CM, Peterson S, Hoover EL, Doerr RJ. Justification for visceral angiography prior to pancreaticoduodenectomy. Am Surg. 1998;64:758–761. [PubMed] [Google Scholar]


