Abstract
Background:
Neoadjuvant chemotherapy (NC+) and portal vein embolization (PVE) enables curative resection in more patients with colorectal-liver metastases (CRLM). However, after NC+, structural alterations have been reported with the risk of post-operative hepatic failure. We undertook to determine if NC+ toxicity limits future remnant liver (FRL) hypertrophy after PVE.
Methods:
PVE was performed in 20 patients, 13 (65%) of whom previously received a mean FOLFIRI (5-fluorouracil + leucovorin + irinotecan) regimen (NC+) of 6.6 cycles. The seven remaining patients served as the control group without NC (NC−).
Results:
CRLM were bilateral in 69% (NC+) and 57% (NC−), and synchronous in 84% (NC+) and 14% (NC−). The FRL hypertrophy rate was 54.1% (NC+) and 43.7% (NC−) (P= 0.3). CRLM were unresectable in four of our 20 patients, i.e. group NC+: one insufficient FRL hypertrophy and one severe steatosis; and group NC−: two tumoral progressions. In both groups, the operative parameters were comparable except for pedicular clamping: 8 (NC+) and 36 min (NC−), respectively (P < 0.05). Also, the surgical outcome rate and hospital stay were comparable. No significant pathological difference was observed between the two groups. No mortality occurred in either group.
Conclusion:
In view of our limited experience, we conclude that hypertrophy of the non-embolized liver (FRL) is not altered after FOLFIRI-based NC.
Keywords: portal vein embolization, liver hypertrophy, chemotherapy, colorectal-liver metastases, hepatectomy
Introduction
Liver resection is the sole curative approach to colorectal-liver metastases (CRLM) with a 5-year survival rate of about 50%, especially when it is combined with chemotherapy. However, only 10–15% of patients could be eligible for curative resection.1,2 The limiting factors are diffuse metastases and future remnant liver (FRL) volume that may be reduced by CRLM distribution.3 Additionally, patients can undergo curative resection with portal vein embolization (PVE) and systemic neoadjuvant chemotherapy (NC).3–8 FRL increases after PVE and can be amended to resection without post-hepatectomy dysfunction.3,7,9 NC reduces tumor size, controls micrometastatic disease and makes chemotherapy a predictive factor of successful liver resection.8,10 After chemotherapy, liver toxicity is well documented, with structural studies identifying hepatic alterations such as veno-occlusive lesions as well as steatosis and a possible worse post-operative course.11,12 NC and PVE have been used complementarily, and usually NC is interrupted for 1 month before PVE and consequently until surgery to avoid hepatic regeneration defects13 with, however, the risk of tumoral progression.14
To assess the impact of chemotherapy on hypertrophy of the FRL after right portal vein embolization, we retrospectively studied a group of 20 patients that underwent PVE to allow CRLM resection. We compared FRL hypertrophy in 13 patients receiving systemic NC (group NC+) and seven control patients without NC (group NC−). FRL hypertrophy and the tumoralresponse with resulting conversion to resectability and post-operative course were the main target points.
Patients and methods
From January 2004 to March 2006, 20 CRLM patients were admitted for PVE preceding planned liver resection. Primary tumors were found in the right (n= 4), transverse (n= 1) or left (n= 8) colon and rectum (n= 7). Mean age was 60 ± 2 years, and the mean of metastases was three (range 1–8) which were bilobular in 13/20 (65%) and synchronous to primary in 12/20 (60%). There were no extra-hepatic metastases. CRLM were judged to be primarily unresectable because of their number and/or distribution (two lobes). The NC− group underwent PVE for FRL <30% in three patients, and for FRL <40% in four patients with severe fatty liver or when a bilateral liver resection was planed. In the NC+ group, all patients underwent PVE because FRL was <40%.
Right PVE was performed by the contralateral transhepatic left portal vein puncture under light neuroleptanalgesia and ultrasound guidance. After portography, the right anterior and posterior portal branches were embolized with cyanoacrylate (Histoacryle; Braun Lab, Hamburg, Germany) and lipiodol (Lipiodol Ultrafluid; Guerbert Lab, Paris, France) in 18 patients and with an Amplazer vascular plug (AGA Medical Corporation, Plymouth, MN, USA) in two patients. PVE was extended to segment IV in four patients (1 NC− and 3 NC+) when extended right hepatectomy was planned. Surgery was scheduled at least 3 weeks after embolization. One patient submitted to a two-stage procedure: a left metastasectomy prior to right PVE. The post-PVE course was uneventful except for a hepatic hematoma that was reabsorbed spontaneously in one patient.
FRL was measured by a volumetric helicoidal CT scan before and 3–4 weeks after embolization. Liver volume was quantified by landmarks: the middle hepatic vein, gallbladder bed and umbilical portion of the left portal vein. FRL was computed as a percentage of total liver volume. FRL hypertrophy was assessed as follows: (FRL after PVE – FRL before PVE)*100/FRL before PVE. Parameters were recorded prospectively, including pre-operative phlebotomy, as described previously,15 red blood cell and fresh frozen plasma transfusion. During hospital stay, liver function tests were conducted, including aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and glutamyltransferase.
Post-surgical complications were defined as a deep venous thrombosis confirmed by Doppler ultrasound, pulmonary infection treated with antibiotics, wound infection, biliary leak, hepatic failure when prothrombin time dropped below 50% and a serum bilirubin level which peaked over 50 µmol/l on the fifth postoperative day or thereafter, ascites when abdominal drainage exceeded 500 ml/day and a pleural effusion which required drainage. Duration of hospital stay was recorded. Mortality was measured until 6 post-operative months. The histological parameters assessed systematically in 14 patients were: macrovacuolar steatosis, classified as the percentage of affected hepatocytes, and vascular lesions (veno-occlusive), categorized by the degree of severity. Continuous data were reported as means ± SEM. Fisher's exact t-test and the Mann–Whitney test were used to compare groups NC+ and NC−. The data were analyzed using Sigma software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered significant at P < 0.05.
Results
The only obstacle to curative resection was insufficient FRL. Before PVE, 13 patients (65%) received 6.6 cycles (range 2–18) of the FOLFIRI-based regimen (5-fluorouracil + leucovorin + irinotecan) (NC+) and seven control patients had no chemotherapy (NC−). NC was mostly given for synchronous metastases (11/13). FRL increased after PVE, from 29.5 ± 2.6% to 43 ± 2% (P < 0.001) and from 31.1 ± 2% to 43.9 ± 2% (P < 0.001) in the NC+ and NC− groups (Fig. 1), respectively. The FRL hypertrophy rate was 54.1 ± 7.9% (NC+) and 43.3 ± 6.8% (NC−), respectively (P= 0.38). FRL reached a threshold of resectability in all except one NC+ patient in whom it rose from 14.8% to 23.5%. Three others were unresectable because of significant steatosis discovered intra-operatively in one NC+ patient and tumoral progression after PVE in two NC− patients with bilobular metastases. Globally, 11/13 NC+ and 5/7 NC− patients underwent hepatectomy (Table 1). NC+ and NC− patients underwent surgery 58 (range 23–127) and 46 (range 22–66) days after PVE, respectively (NS). In NC+ patients, PVE was performed 14 days (range 1–37) after the end of chemotherapy, and liver resection, after 72 days (range 35–140). The operative parameters were comparable in both groups except for shorter pedicle clamping duration of 8 ± 3 vs. 36 ± 12 min (P < 0.05) (Table 1). Post-surgical complication rates were comparable: 5/11 (36%) in NC+ and 2/5 (40%) in NC− patients (NS). We observed biliary leakage in one patient from each group (NS), pleural effusion requiring drainage in one NC− patient (NS), and wound infection in one NC− and four NC+ patients (NS). Median hospital stay for NC+ and NC− patients was 7 and 8 days (P= 0.12), respectively, without mortality in both groups until 6 months. Pathology showed macrovacuolar steatosis ≤30% in two patients from each group (NS) and 30–60% in one NC+ patient who only received three cycles of the FOLFIRI regimen; in spite of a FRL hypertrophy rate of 39%, liver resection was cancelled. Also, liver specimens were slightly cholestatic in two NC− patients and moderately cholestatic in three NC+ and one NC− patients (NS) (Table 2).
Figure 1.
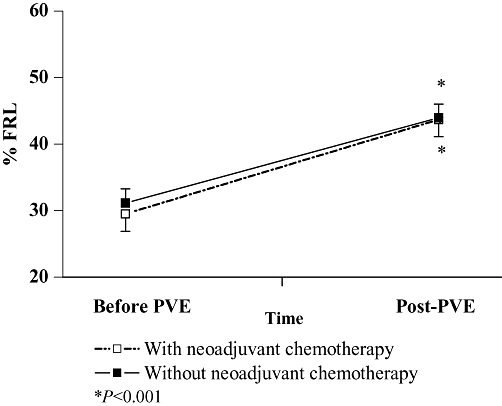
Future remnant liver (FRL) with or without chemotherapy after portal vein embolization (PVE)
Table 1.
Results of portal vein embolization (PVE) in chemotherapy and control groups
| Group NC+n= 13 | Group NC−n= 7 | ||
|---|---|---|---|
| PVE complication | 1 liver hematoma | 0 | NS |
| FRL hypertrophy rate | 54.1 ± 7.9% | 43.7 ± 6.8% | P= 0.3 |
| FRL at resectability threshold | 12/13 | 7/7 | |
| Failure of CRLM resection | 2/13 (13.4%) Steatosis, low FRLa | 2/7 (28.6%) Tumor progression | P= 0.3 |
| Pedicle clamping duration | 8 ± 3 min | 36 ± 12 min | P < 0.05 |
| Intra-operative bleeding | 1031.8 ± 191 ml | 1140 ± 290 ml | P= 0.7 |
| Red blood cell transfusion | 1 unit | 1 unit | NS |
| Phlebotomy15 | 540 ± 84 ml | 630 ± 131 ml | P= 0.5 |
| Segments retrieved | 4.4 (range 4–6) | 4 (range 3–5) | P= 0.2 |
FRL, future remnant liver; CRLM, colorectal-liver metastases
FRL before PVE was 14.78%
Table 2.
Histological parameters in chemotherapy and control groups
| NC+ group n= 9 | NC− group n= 5 | ||
|---|---|---|---|
| Steatosis | |||
| ≤30% | 2/9 | 2/5 | NS |
| >30% | 1/9a | 0 | |
| Sinusoidal alterations | |||
| Mild | 1 | 1 | NS |
| Moderate | 0 | 0 | |
| Cholestasis | |||
| Mild | 0 | 2/5 | NS |
| Moderate | 3/9 | 1/5 |
Patient with steatohepatitis in whom liver resection was cancelled
Discussion
In a large retrospective French study of 1439 CRLM patients, 76% were initially unresectable; after chemotherapy with a FOLFOX-based (5-fluorouracil + leucovorin + oxaliplation) regimen, 12.5% became resectable with 5-year survival and disease-free survival rates of 33% and 22%, respectively, and a median follow-up of 48.7 months.4 However, NC use increased the lower limit of FRL after resection from 30% to 40% and induced structural alterations.12,16 After NC, Rubbia-Brandt et al. identified sinusoidal dilatation in liver specimens with possible parenchyme hemorrhage related to rupture of the sinusoidal barrier in 51% persinusoidal and 48% veno-occlusive fibrosis.12 Oxaliplatin was a major risk factor, with 78% of patients developing sinusoidal congestion. No structural alterations were identified in patients without chemotherapy. Similar findings were reported by Karoui et al. with increased morbidity under chemotherapy but no mortality.17 After oxaliplatin-related chemotherapy, vascular lesions appeared to be the most significant structural alterations.1,12,18
Post-chemotherapy steatosis remains controversial and its prevalence is fluctuant in the literature, depending on the chemotherapy regimen, the duration and interval between the end of NC and the time of surgery.19 In a cohort study, Kooby et al. discerned that patients with marked steatosis more likely received NC than the controls (P < 0.01).20 Also, marked steatosis was sometimes associated with an increased risk of complications after hepatic resection but not mortality.19,21,22 Parikh et al. reported mild steatosis (<25%) in 15 and severe steatosis (≥25%) in 4 out of 34 CRLM patients receiving irinotecan.23 With irinotecan, steatosis and steatohepatitis seemed to be the predominant lesions.24,25 Caution is advised when using irinotecan-based therapies in steatosic patients (high body mass index, diabetes mellitus, metabolic syndrome) who can develop steatohepatitis with the risk of progressing to fibrosis and cirrhosis. Assessment of the underlying liver is critical in the selection of surgical resection type.19 PVE may provide information about functional hepatic reserves. FRL hypertrophy after PVE predicts the outcome from hepatectomy.9 If adequate hypertrophy occurs, hepatectomy may be safe. The use of either a FOLFIRI- or a FOLFOX-based regimen as NC in CRLM patients is guided by response rates; however, associated liver injuries, steatohepatitis from the former regimen with the risk of hepatic failure and veno-occlusion from the latter regimen without hepatic failure risk, should also be considered. In our present experience with irinotecan, no significant vascular or steatosic lesions were observed in comparison to NC− patients.
Like other authors,13,26 we conclude that NC did not alter non-embolized liver hypertrophy. However, Beal et al.27 reported reduced liver hypertrophy after NC. Caution should be taken in the interpretation of these data which contained few patients who received a variety of chemotherapy regimens. Notably, structural data were not published in their study.13,26,27 Whether structural alteration-related NC reduces liver hypertrophy after PVE remains unknown. FRL hypertrophy after PVE varied between 20% and 42%.13,26 Reduced hypertrophy rates have been noted in diabetic, jaundiced, fibrotic and cirrhotic patients.9,28 However, Giraudo et al.29 noted that diabetes mellitus, cirrhosis and chemotherapy had no effect on the degree of liver hypertrophy, but the time required to obtain adequate liver hypertrophy was significantly longer. We must emphasize that the variety of chemotherapy regimens in most series did not affect the degree of liver growth, but a prospective study is necessary to compare different chemotherapy regimens with evaluation of histopathological alterations.
The liver is a quiescent organ, and the hepatocyte replication rate does not exceed 0.01%.30 After PVE, hepatic cell apoptosis and atrophy appear on the embolized side, around the periportal and pericentrolobular areas in association with sinusoidal dilatation which may be a consequence of cellular regression.31 In the non-embolized side, PVE evokes hepatocyte proliferation for 2 weeks with increased nuclear density and global hepatic mass.31 A few studies have reported the impact of chemotherapy on liver proliferation. In rats, 5-fluorouracil suppressed DNA synthesis and liver cell division when administered within 24 h of hepatectomy but not later.32 In humans, Hewes et al. investigated isolated hepatocytes from liver specimens after CRLM resection. Prior hepatectomy, oxaliplatin and 5-fluorouracil did not impair the function or culture integrity of isolated hepatocytes.33 This observation may be explained by the fact that 5-fluorouracil-related toxicity is of short duration, and oxaliplatin-related toxicity is sinusoidal and not hepatocytic. With irinotecan, the results could be different as steatosis is the predominant toxicity-related lesion. More investigations should be undertaken including irinotecan as well.
Curative resection may be achieved in only 63–75% of CRLM patients undergoing PVE3,16,29,34 because of the incidental discovery of peritoneal carcinomatosis or CRLM progression. In our study, planned liver resection was cancelled because of CRLM progression in two NC− patients. The FRL hypertrophy rate of structural alteration-related NC livers has to be confirmed in a large series. NC should be used to control CRLM proliferation, as it may not deprive patients of FRL hypertrophy after PVE; however, prospective trials are needed.13,35,36
Summary
Progress in CRLM management is partly as a result of peri-operative chemotherapy. In our experience and despite a limited number of patients, we conclude that NC with a FOLFIRI-based regimen represses tumoral progression and does not alter FRL hypertrophy after PVE.
Acknowledgments
The authors thank Ovid Da Silva, Research Support Office, Research Centre, CHUM, for editing this paper; and Assia Belblidia, MD, Department of Radiology, CHUM, for the liver volumetric calculations.
Conflicts of interest
None declared.
References
- 1.Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 2.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1051. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–658. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupinacci R, Penna C, Nordlinger B. Hepatectomy for resectable colorectal cancer metastases indicators of prognosis, definition of resectability, techniques and outcomes. Surg Oncol Clin N Am. 2007;16:493–506. doi: 10.1016/j.soc.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 7.Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin N Am. 2004;84:659–671. doi: 10.1016/j.suc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1064. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist. 2007;12:825–839. doi: 10.1634/theoncologist.12-7-825. [DOI] [PubMed] [Google Scholar]
- 11.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 12.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 13.Goéré D, Farges O, Leporrier J, Sauvanet A, Vilgrain V, Belghiti J. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10:365–370. doi: 10.1016/j.gassur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, et al. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–630. doi: 10.1148/radiol.2342031996. [DOI] [PubMed] [Google Scholar]
- 15.Massicotte L, Lenis S, Thibeault L, Sassine MP, Seal RF, Roy A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transpl. 2006;12:117–123. doi: 10.1002/lt.20559. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahajpal A, Vollmer CM, Jr, Dixon E, Chan EK, Wei A, Cattral MS, et al. Chemotherapy for colorectal cancer prior to liver resection for colorectal cancer hepatic metastases does not adversely affect peri-operative outcomes. J Surg Oncol. 2007;95:22–27. doi: 10.1002/jso.20632. [DOI] [PubMed] [Google Scholar]
- 19.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 20.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 22.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 23.Parikh AA, Gentner B, Wu TT, Curley SA, Ellis LM, Vauthey JN. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451–455. doi: 10.1097/SLA.0b013e31815ed693. [DOI] [PubMed] [Google Scholar]
- 27.Beal IK, Anthony S, Papadopoulou A, Hutchins R, Fusai G, Begent R, et al. Portal vein embolisation prior to hepatic resection for colorectal liver metastases and the effects of periprocedure chemotherapy. Br J Radiol. 2006;79:473–478. doi: 10.1259/bjr/29855825. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg. 2007;31:367–374. doi: 10.1007/s00268-006-0526-2. [DOI] [PubMed] [Google Scholar]
- 29.Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Black DM, Behrns KE. A scientist revisits the atrophy-hypertrophy complex: hepatic apoptosis and regeneration. Surg Oncol Clin N Am. 2002;11:849–864. doi: 10.1016/s1055-3207(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 31.Harada H, Imamura H, Miyagawa S, Kawasaki S. Fate of the human liver after hemihepatic portal vein embolization: cell kinetic and morphometric study. Hepatology. 1997;26:1162–1170. doi: 10.1053/jhep.1997.v26.pm0009362357. [DOI] [PubMed] [Google Scholar]
- 32.Kohno H, Inokuchi K. Effects of postoperative adjuvant chemotherapy on liver regeneration in partially hepatectomized rats. Jpn J Surg. 1984;14:515–523. doi: 10.1007/BF02469796. [DOI] [PubMed] [Google Scholar]
- 33.Hewes JC, Riddy D, Morris RW, Woodrooffe AJ, Davidson BR, Fuller B. A prospective study of isolated human hepatocyte function following liver resection for colorectal liver metastases: the effects of prior exposure to chemotherapy. J Hepatol. 2006;45:263–270. doi: 10.1016/j.jhep.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Elias D, Ouellet JF, De Baère T, Lasser P, Roche A. Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. 2002;131:294–299. doi: 10.1067/msy.2002.120234. [DOI] [PubMed] [Google Scholar]
- 35.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, De Baere T, Roche A, Ducreux M, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–788. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]


