Abstract
Background:
The majority of patients with pancreatic cancer are non-resectable and jaundiced at presentation. Methods of palliation in such patients with locally advanced disease comprise endoscopic placement of a biliary endoprosthesis or surgical bypass.
Methods:
This retrospective study compared morbidity, mortality, hospital stay, readmission rate and survival in consecutive patients with incurable locally advanced pancreatic ductal adenocarcinoma.
Results:
We identified a total of 56 patients, of whom 33 underwent endoscopic stenting and 23 underwent a surgical bypass consisting of a hepaticojejunostomy-en-Y and a gastrojejunostomy. There were no significant differences in complication or mortality rates between patients undergoing palliative stenting and those undergoing palliative surgery. However, after excluding admissions for chemotherapy-related problems, the number of readmissions expressed as a percentage of the group population size was greater in stented patients compared with biliary bypass patients (39.4% vs. 13.0%, respectively; P < 0.05). Overall survival amongst patients undergoing palliative bypass was significantly greater than in stented patients (382 days vs. 135 days, respectively; P < 0.05).
Conclusions:
On analysis of these data and the published literature, we conclude that surgical bypass represents an effective method of palliation for patients with locally advanced pancreatic cancer. Patients need to be carefully selected with regard to both operative risk and perceived overall survival.
Keywords: pancreatic cancer, survival, surgical bypass, biliary bypass, biliary stent
Introduction
Up to 80% of ductal adenocarcinomas of the head of the pancreas are not resectable at presentation.1,2 As 70–90% of patients with carcinomas of the head of the pancreas and ampullary region have jaundice at presentation,3–5 palliation that ensures biliary drainage represents a large proportion of the hepatobiliary surgeon's workload.
Various therapeutic options have been described. Some centres advocate resection surgery even in the palliative setting, arguing that it may offer a survival advantage or better palliation and has equivalent morbidity and mortality rates to bypass surgery,6–8 but these results are disputed by other groups.9 Other options include surgical biliary bypass incorporating either an hepatojejunostomy (choledochojejunostomy),10–12 cholecystojejunostomy12,13 or choledochoduodenostomy10,12 with or without a concomitant gastrojejunostomy.14 More recently, these procedures have been undertaken laparoscopically, with reportedly low morbidity and mortality.15,16
In addition, biliary endoprostheses can be employed to relieve biliary stasis. Internal drainage of the biliary tree via the transhepatic route was first described in 1978.17,18 The introduction of endoscopically placed stents enabled the use of larger-calibre endoprostheses.19,20 The advantage of biliary stents is that their positioning is a minimally invasive procedure which is well tolerated by patients. However, their palliative potential is limited by the recurrence of jaundice secondary to stent migration or accretion. Tumour progression and duodenal invasion may render repeated stenting impossible.
The aim of this study was to compare morbidity and mortality rates and effectiveness of palliation between patients matched for tumour size and clinical stage who underwent surgical bypass or endoscopic stenting for locally advanced pancreatic adenocarcinoma.
Materials and methods
Study methodology
Case notes were analysed retrospectively for all patients who presented consecutively to the University Hospitals of Leicester from January 2003 to April 2004 with primary cancer of the head of the pancreas, requiring palliation of their malignant obstructive jaundice. Data were divided into two treatment groups consisting of, respectively, patients who underwent stenting +/− chemotherapy and patients who underwent palliative surgery +/− chemotherapy. All patients with metastatic disease proven by radiological or surgical assessment were excluded from the final analyses. Patients with histological diagnoses other than adenocarcinoma were also excluded.
Date of diagnosis was the first computed tomography (CT) scan date. Tumour size was defined by surgical or radiological staging. In patients for whom no histology was available, the diagnosis of pancreatic cancer was based on information from the clinical history, radiology reports and tumour marker levels. CA19.9 was considered significant if it was above the upper normal limit of our local laboratories (>37 kU/l). Patients with normal CA19.9 values were excluded from the final analyses.
Initial hospital stay duration was defined as the time spent in hospital after the patient's surgical or endoscopic procedure. Data on short- and longterm morbidity in terms of postoperative complications and hospital readmissions were collected. Perioperative complications were defined as occurring within 30 days after stent insertion or surgery. Thirty-day mortality for each type of treatment was calculated. Longterm disease-specific survival data were collected by telephoning the appropriate general practice surgery or from hospital records. The selection of patients for palliative surgery over endoscopic palliation was based on patient preference and judged fitness for operative intervention.
Data were analysed using spss Version 11.5 (SPSS Inc., Chicago, IL, USA). Median survival was analysed by the log rank test and represented by Kaplan–Meier graphs. Other continuous data of normal distribution were analysed by descriptive analysis with means compared by independent t-test. Categorical variables were analysed using Fisher's exact test. A P-value of <0.05 was considered statistically significant.
Endoscopic technique
Patients undergoing therapeutic stenting received a plastic endoprosthesis using a side-viewing endoscope under fluoroscopic guidance. The stent position was confirmed by its anatomic location, visualization of bile drainage and injection of contrast into the stent following placement to ensure its position above the bile duct stricture.
Surgical technique
All patients undergoing palliative surgery underwent a double bypass comprising a hepatojejunostomy-en-Y and gastrojejunostomy (Fig. 1).
Figure 1.
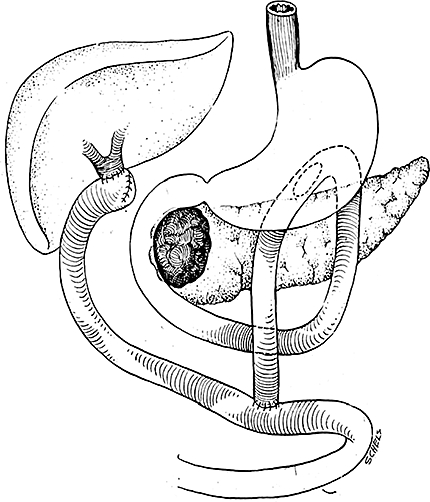
Surgical bypass procedure, final reconstruction
Results
Within the study period, a total of 56 eligible patients were identified. Of these, 33 underwent endoscopic stenting and 23 underwent palliative surgery. Follow-up ranged from 1 to 86 weeks. Baseline demographic and haematological values are displayed in Table 1. Patients undergoing palliative surgery had significantly lower CA19.9, despite having equivalent tumour sizes. No other values differed statistically between the groups.
Table 1.
Demographic variables among patients undergoing endoscopic stenting and palliative surgery
| Variable | Palliative surgery group(n= 23) | Endoscopic stent group(n= 33) | Significance |
|---|---|---|---|
| Gender | |||
| Male | 57% | 49% | NS |
| Female | 43% | 51% | |
| Median age, years | 65.2 | 69.0 | NS |
| Median CA19.9, kU/l | 1153.00 | 3878.50 | <0.05 |
| Median bilirubin, kU/l | 189 | 290 | NS |
| Median white cell count, ×109/l | 8.00 | 8.00 | NS |
| Median C-reactive protein, mg/l | 19.00 | 31.50 | NS |
| Median tumour size, mm | 30.00 | 32.50 | NS |
NS, not significant
There were no significant differences in immediate complication or mortality rates between patients who underwent palliative stenting and those who underwent palliative surgery (Table 2). In the surgical group, the one death was attributed to respiratory infection complicated by multiple organ failure. In the stent group, causes of death were cholangitis (n= 1), acute renal failure (n= 2), upper gastrointestinal haemorrhage (n= 1) and respiratory complications (n= 2). After excluding admissions for chemotherapy-related problems (diarrhoea, skin rashes, anaemia or vomiting occurring within 48 hours of treatment) the number of readmissions expressed as a percentage of the group population size was greater in stented patients compared with biliary bypass surgery patients (39.4% vs. 13.0%, respectively) (Fig. 2). The total length of stay in hospital per patient was also lower in the surgical bypass group, even including their postoperative stay (34.1 days vs. 10.2 days). The main reasons for readmission were jaundice and sepsis, which accounted for 76.9% of all readmissions. Two patients in the endoscopic stent group developed intractable vomiting, which persisted despite discontinuing their chemotherapy, and had radiological evidence of gastric outlet obstruction. Neither individual proceeded to intervention as they quickly succumbed from advanced disease.
Table 2.
Hospital stay, complications and mortality within 30 days after palliative surgery or endoscopic stenting
| Variable | Palliative surgery group(n= 23) | Endoscopic stent group(n= 33) | P-value | |
|---|---|---|---|---|
| Complications | Sepsis | 10 | 7 | NS |
| 43.5% | 21.2% | |||
| Bleeding | 8 | 5 | NS | |
| 34.9% | 15.1% | |||
| Stent blockage | NA | 2 | NA | |
| 6.1% | ||||
| 30-day mortality | 1 | 6 | NS | |
| 4.3% | 18.1% | |||
NS, not significant; NA, not applicable
Figure 2.
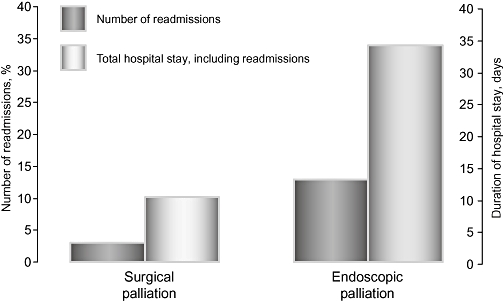
Number of readmissions and total length of hospital stay following palliative surgery and endoscopic stenting, respectively (P < 0.05)
Overall survival amongst patients undergoing palliative bypass was significantly greater than in stented patients at 382 days vs. 135 days, respectively (Fig. 3). Preoperative CA19.9 also appeared to influence survival: in both groups, patients with CA19.9 values <1000 survived significantly longer than those with CA19.9 >1000 (Fig. 4).
Figure 3.
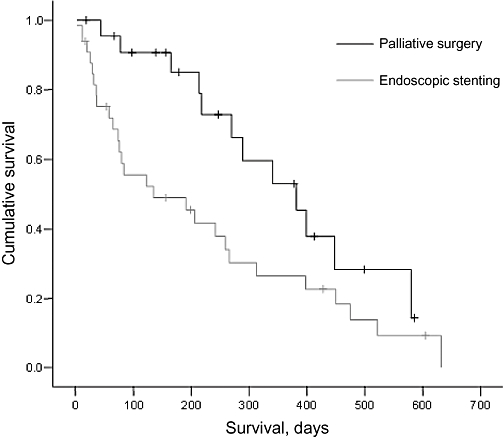
Survival following palliative surgery and endoscopic stenting, respectively (P < 0.05)
Figure 4.
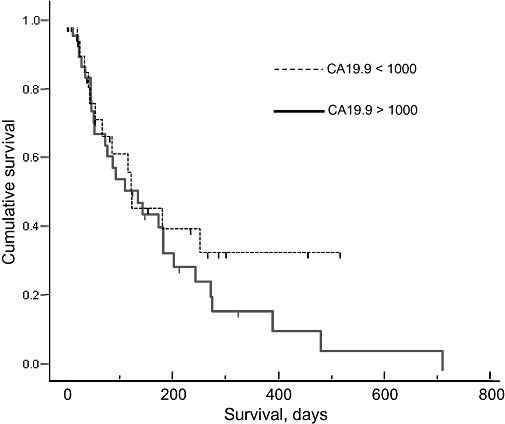
Survival and preoperative CA19.9 values (P < 0.05)
Discussion
Overall, these results suggest that surgical bypass can be performed with equivalent morbidity and mortality rates to biliary stenting in selected patients, but with a significantly lower risk of readmission. Hence, better palliation can be achieved in patients who are able to tolerate biliary bypass surgery. However, quality of life data are needed to add further strength to this observation. The increased survival observed in the surgical bypass cohort is probably the result of a better overall state of fitness, reflected in the lower median age of patients undergoing surgery. In addition, patients who underwent bypass surgery had significantly lower CA19.9 levels than those who underwent endoscopic stenting (Table 1). In this study, we found CA19.9 levels >1000 to be associated with significantly lower survival than levels <1000 (Fig. 4). Hence, despite our attempts to match patients using radiological imaging, it is possible that patients who underwent endoscopic stenting had a higher occult tumour burden than those who underwent bypass surgery, which would doubtless have negatively impacted on their longterm survival.
The survival of patients who underwent bypass may also be influenced by our unit's relatively conservative policy regarding the selection of patients for pancreatic adenocarcinoma resection21 and the fact that no patients with distant pancreatic metastases were included in the study. Despite these considerations, it is conceivable that by avoiding recurrent admissions for jaundice and concurrent sepsis, surgical bypass may contribute to the increased survival in this group.
There are relatively few data examining the relative merits of surgical bypass vs. endoscopic stenting in the literature: we found only three randomized trials and a number of largely retrospective studies, which are summarized in Table 3.22–33 In a review of the randomized trials, Schwarz et al. concluded that an endoprosthesis should be placed if there was evidence of hepatic, peritoneal or pulmonary metastases or if the patient had significant co-morbidity precluding surgery.34 However, it is worth noting that a later meta-analysis noted that the evidence did not allow for a definitive conclusion on which treatment was preferable.35 Despite this, the overall pattern would appear to indicate that, for the most part, surgical bypass can be performed with similar morbidity and mortality rates to endoprosthesis, but with a longer initial hospital stay. Rates of late complications and readmissions are greater in patients who undergo biliary stenting than in those who undergo biliary bypass.
Table 3.
Summary of available literature comparing endoscopic biliary endoprosthesis with surgical palliation
| Study, author(s), year | Study type | Number of patients | Findings | Study conclusions |
|---|---|---|---|---|
| Sunpawervong et al., 200522 | Retrospective | 116 | No difference in survival time, morbidity or cost-effectiveness; surgical palliation resulted in significantly less common late complications (jaundice) | In favour of surgical palliation |
| Nuzzo et al., 200423 | Retrospective | 84 | Higher incidence of complications in stented group, with frequent hospital admissions and lower quality of life | In favour of surgical palliation |
| Santagati et al., 200324 | Retrospective | 107 | Higher complication rate, mortality rate and hospital stay in surgically palliated patients | In favour of endoscopic palliation |
| Maosheng et al., 200125 | Retrospective | (Metallic stents) | Higher rate of late complications in metallic stent group, but shorter hospital stay and lower cost | In favour of surgical palliation in patients expected to live >6 months |
| Wagner et al., 200026 | Retrospective | 348 | – | In favour of surgical palliation |
| Raikar et al., 199627 | Retrospective | 66 | Endoscopic treatment resulted in shorter hospital stays at reduced cost, with equivalent survival | In favour of endoscopic palliation |
| Smith et al., 199428 | Randomized | 204 | Lower mortality and complication rates with stenting, but higher rate of late complications | Both effective palliative treatments |
| van den Bosch et al., 199429 | Retrospective | 148 | Higher early morbidity and mortality in surgical bypass, higher late complications with stenting | Surgical palliation if life expectancy >6 months |
| Anderson et al., 198930 | Randomized | 50 | No differences in survival or palliation | In favour of endoscopic palliation |
| Shepard et al., 198831 | Randomized | 52 | No difference in overall survival, more readmissions in the stented group, but total time in hospital still shorter than in those undergoing surgical bypass | Endoscopic palliation is a good alternative to surgery |
| Sonnenfeld et al., 198632 | Retrospective | 41 | Major complications more common in surgical bypass group with longer hospital stays; no difference in mortality or survival | – |
| Bornman et al., 198633 | Randomized | 53 | Shorter hospital admission in the stented group, but higher rate of readmissions longterm; no difference in survival | – |
These published data would suggest that biliary endoprosthesis should be reserved for those with a shorter expected survival and that surgical palliation should be offered to those patients who are expected to live longer and who are able to tolerate surgical intervention. The cut-off in survival determining these two treatment modalities has often been quoted at 6 months.22,23,25,29,34,36 Outwith anaesthetic considerations, tumour-related determinants of poor survival in the palliation of pancreatic cancer include C-reactive protein levels,37 leucocytosis,37 duodenal invasion,38 liver metastases,38 peritoneal dissemination,38 high bilirubin,39 low haemoglobin,39 low albumin,39 presence of ascites40 and the Karnofsky index of performance status.41 These considerations are useful when selecting cases appropriate for bypass procedures vs. endoscopic stenting.
During surgery, it is our policy to perform a gastrojejunostomy at the time of a biliary bypass. It is probably worth noting that one patient who underwent endoscopic palliation of a biliary obstruction re-presented with gastric outlet obstruction. The use of a routine gastrojejunostomy is in keeping with current literature showing that gastrojejunostomies can be performed with no increase in morbidity and mortality and recommending that a gastrojejunostomy and biliary bypass be undertaken as a single procedure.14,36,42–46 The fact that two patients in the endoscopic stent group in the present study developed gastric outlet obstruction also supports the use of prophylactic gastrojejunostomy in patients undergoing operative intervention. It has been reported that patients with liver metastases and carcinomatosis do not survive long enough to develop gastric outlet obstruction;10 however, these patients would make poor surgical candidates and are best treated using endoscopic means.
It remains to be seen what impact metallic stents will have on surgical bypass procedures. Although they are more expensive than conventional stents, metallic stents are purported to have longer patency, but may still become occluded by tumour ingrowth or overgrowth.47 Only one study, to date, has compared surgical bypass with metallic stents; it concluded that metallic stents were cost-effective when compared with surgical bypass but had a higher rate of late complications.25 The use of self-expanding metal stents to treat malignant gastroduodenal outlet obstruction raises the possibility of using endoscopic stents solely to palliate advanced pancreatic cancer without the need for bypass,48,49 although it would appear that even these gastrointestinal stents are susceptible to tumour ingrowth.50 More data are needed to evaluate this further.
Major limitations of this study refer to its retrospective nature and small sample size. In conclusion, we tentatively suggest that, based on evidence from our own data and the published literature, the surgical double bypass is an efficacious means of palliating patients with locally advanced pancreatic adenocarcinoma. The published literature suggests that surgical bypass offers a lower rate of late complications, but involves a higher rate of initial complications and a longer hospital stay than stenting. By contrast, we have shown that surgical bypass can be performed with equivalent morbidity and mortality rates and length of initial hospital stay as endoscopic stenting, and that it results in a lower rate of late complications. Surgical bypass in this series is associated with prolonged survival compared with palliative stenting. Although the precise reasons for this remain unclear, it should be an important consideration when considering palliation for patients with pancreatic cancer.
Conflicts of interest
None declared.
References
- 1.Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Haematol. 2006;58:213–241. doi: 10.1016/j.critrevonc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Watanape P, Williamson RCN. Surgical palliation for pancreatic cancer: developments during the past two decades. Br J Surg. 1992;79:8–20. doi: 10.1002/bjs.1800790105. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RGH, Wormsley KG. Cancer of the pancreas. Gut. 1977;18:580–596. doi: 10.1136/gut.18.7.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moossa AR. Pancreatic cancer: approach to diagnosis, selection for surgery and choice of operation. Cancer. 1982;50:2689–2698. [PubMed] [Google Scholar]
- 5.Singh SM, Longmire WP, Reber HA. Surgical palliation for pancreatic cancer. Ann Surg. 1990;212:132–139. doi: 10.1097/00000658-199008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kymionis GD, Konstadoulakis MM, Leandros E, Manouras A, Apostolou A, Alexiou D, et al. Effect of curative versus palliative surgical treatment for stage III pancreatic cancer patients. J R Coll Surg Edinb. 1999;44:231–235. [PubMed] [Google Scholar]
- 7.Lillemoe KD, Cameron JL, Yeo CJ, Sohn TA, Nakeeb A, Sauter PK, et al. Pancreaticoduodenectomy. Does it have a role to play in the palliation of pancreatic cancer? Ann Surg. 1996;223:718–7125. doi: 10.1097/00000658-199606000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakkevold KE, Kambestad B. Palliation of pancreatic cancer. A prospective multicentre study. Eur J Surg Oncol. 1995;21:176–182. doi: 10.1016/s0748-7983(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 9.Vanhooser R, Organ CH. Is the Whipple procedure a better palliative option for pancreatic cancer? J Natl Med Assoc. 1991;83:405–408. [PMC free article] [PubMed] [Google Scholar]
- 10.Di Fronzo LA, Cymerman J, Egrari S, O'Connell TX. Unresectable pancreatic carcinoma: correlating length of survival with choice of biliary bypass. Am Surg. 1999;65:955–958. [PubMed] [Google Scholar]
- 11.Lesurtel M, Dehni N, Tiret E, Pare R, Paye F. Palliative surgery for unresectable pancreatic and periampullary cancer: a reappraisal. J Gastrointest Surg. 2006;10:286–291. doi: 10.1016/j.gassur.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Deziel DJ, Wilhemli B, Staren JB, Doolas A. Surgical palliation for ductal adenocarcinoma of the pancreas. Am Surg. 1996;62:582–588. [PubMed] [Google Scholar]
- 13.Gouma DJ, van Geenan R, van Gulik R, de Wilt LT, Obertop H. Surgical palliative treatment in bilio-pancreatic malignancy. Ann Oncol. 1999;10:269–272. [PubMed] [Google Scholar]
- 14.Lillemoe KD, Cameron JL, Hardacre JM, Sohn TA, Sauter PK, Coleman J, et al. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322–328. doi: 10.1097/00000658-199909000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AZ, Miles WF, Singh KK. Initial experience with laparoscopic bypass for upper gastrointestinal malignancy: a new option for palliation of patients with advanced upper gastrointestinal tumours. J Laparoendosc Adv Surg Tech. 2005;15:374–378. doi: 10.1089/lap.2005.15.374. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem AM, Hamade AM, Sheen AJ, Owera A, Al-Baharani AZ, Ammori BJ. Laparoscopic gastric and biliary bypass: a single-centre cohort prospective study. J Laparoendosc Adv Surg Tech. 2006;16:21–26. doi: 10.1089/lap.2006.16.21. [DOI] [PubMed] [Google Scholar]
- 17.Pererias RV, Rheingold OJ, Hutson D. Relief of malignant obstructive jaundice by percutaneous insertion of a permanent prosthesis in the biliary tree. Ann Intern Med. 1978;89:589–593. doi: 10.7326/0003-4819-89-5-589. [DOI] [PubMed] [Google Scholar]
- 18.Burcharth F. A new endoprosthesis for non-operative intubation of the biliary tree in malignant obstructive jaundice. Surg Gynaecol Obstet. 1978;146:76–78. [PubMed] [Google Scholar]
- 19.Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;5:412–417. doi: 10.1016/s0016-5107(88)71407-8. [DOI] [PubMed] [Google Scholar]
- 20.Huitbretes K, Tytgat GN. Palliative treatment of obstructive jaundice by transpapillary introduction of a large bore bile duct endoprosthesis. Gut. 1982;23:371–375. doi: 10.1136/gut.23.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcea G, Dennison AR, Ong SL, Pattenden CJ, Neal CP, Sutton CD, et al. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of the pancreas. Eur J Surg Oncol. 2007;33:892–897. doi: 10.1016/j.ejso.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Sunpaweravong S, Ovarlarnporn B, Khwo-ean U, Soontraprnchai P, Charoonratan V. Endoscopic stenting versus biliary bypass in advanced malignant bile duct obstruction: cost-effectiveness analysis. Asian J Surg. 2005;28:262–265. doi: 10.1016/S1015-9584(09)60357-2. [DOI] [PubMed] [Google Scholar]
- 23.Nuzzo G, Clemente G, Cadeddu F, Giovannini I. Palliation of unresectable periampullary neoplasms, ‘surgical’ versus ‘non-surgical’ approach. Hepatogastroenterology. 2004;51:1282–1285. [PubMed] [Google Scholar]
- 24.Santagati A, Ceci V, Donatelli G, Pasqualini MJ, Silvestri F, Pitasi E, et al. Palliative treatment for malignant jaundice: endoscopic versus surgical approach. Eur Rev Med Pharmacol Sci. 2003;7:175–180. [PubMed] [Google Scholar]
- 25.Maosheng D, Ohtsuka T, Ohuchida J, Inoue K, Yokohata K, Yamaguchi K, et al. Surgical bypass versus metallic stent for unresectable pancreatic cancer. J Hepatobiliary Pancreat Surg. 2001;8:367–373. doi: 10.1007/s005340170010. [DOI] [PubMed] [Google Scholar]
- 26.Wagner M, Egger B, Kulli C, Redaelli CA, Krahenbuhl L, Seiler CA, et al. Stent of surgical bypass as palliative therapy in obstructive jaundice. Swiss Surg. 2000;6:283–288. doi: 10.1024/1023-9332.6.5.283. [DOI] [PubMed] [Google Scholar]
- 27.Raikar GV, Melin MM, Ress A, Lettieri SZ, Poterucha JJ, Nagornery DM, et al. Cost-effective analysis of surgical palliation versus endoscopic stenting in the management of unresectable pancreatic cancer. Ann Surg Oncol. 1996;3:470–475. doi: 10.1007/BF02305765. [DOI] [PubMed] [Google Scholar]
- 28.Smith AC, Dowsett JF, Russell JC, Hatfield AR, Cotton PB. Randomized trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344:1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 29.van den Bosch RP, van der Schelling GP, Klinkenbijl JH, Mulder PG, van Blankenstein M, Jeekel J. Guidelines for the application of surgery and endoprosthesis in the palliation of obstructive jaundice in advanced cancer of the pancreas. Ann Surg. 1994;219:18–24. doi: 10.1097/00000658-199401000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson JR, Sorenson SM, Kruse A, Rokjaer M, Matzen P. Randomized trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132–1135. doi: 10.1136/gut.30.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepard HA, Royle G, Ross AP, Diba A, Arthur M, Colin-Jones D. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg. 1988;75:1166–1168. doi: 10.1002/bjs.1800751207. [DOI] [PubMed] [Google Scholar]
- 32.Sonnenfeld T, Gabrielsson N, Granqvist S, Perbeck L. Non-resectable malignant bile duct obstruction. Surgical bypass or endoprosthesis? Acta Chir Scand. 1986;152:297–300. [PubMed] [Google Scholar]
- 33.Bornman PC, Harries-Jones EP, Tobias R, Van Stiegmann G, Terblanche J. Prospective controlled trial of transhepatic biliary endoprosthesis versus bypass surgery for incurable carcinoma of the head of the pancreas. Lancet. 1986;1:69–71. doi: 10.1016/s0140-6736(86)90719-1. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz A, Beger HG. Biliary and gastric bypass or stenting in non-resectable periampullary cancer: analysis on the basis of controlled trials. Int J Pancreatol. 2000;27:51–58. doi: 10.1385/IJGC:27:1:51. [DOI] [PubMed] [Google Scholar]
- 35.Taylor MC, McLeod RS, Langer B. Biliary stenting versus bypass surgery for the palliation of malignant distal bile duct obstruction: a meta-analysis. Liver Transpl. 2000;6:302–308. doi: 10.1053/lv.2000.5196. [DOI] [PubMed] [Google Scholar]
- 36.Isla AM, Worthington T, Kakkar AK, Williamson RCN. A continuing role for surgical bypass in the palliative treatment of pancreatic carcinoma. Dig Surg. 2000;17:143–146. doi: 10.1159/000018817. [DOI] [PubMed] [Google Scholar]
- 37.Engelken FJ, Bettschart V, Rahman MQ, Parks RW, Garden OJ. Prognostic factors in the palliation of pancreatic cancer. Eur J Surg Oncol. 2003;29:368–373. doi: 10.1053/ejso.2002.1405. [DOI] [PubMed] [Google Scholar]
- 38.Fujino Y, Suzuki Y, Ajiki T, Tanioka Y, Ku Y, Kuroda Y. Predicting factors for survival of patients with unresectable pancreatic cancer: a management guideline. Hepatogastroenterology. 2003;50:250–253. [PubMed] [Google Scholar]
- 39.Shirahatti RG, Alphons N, Joshi RM, Prasad KV, Wagle PK. Palliative surgery in malignant obstructive jaundice. J R Coll Surg Edinb. 1997;42:238–243. [PubMed] [Google Scholar]
- 40.Wade TP, Neuberger TJ, Swope TJ, Virgo KS, Johnson FE. Pancreatic cancer palliation: using tumour stage to select appropriate palliation. Am J Surg. 1994;167:208–212. doi: 10.1016/0002-9610(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 41.Bakkevold KE, Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing short-term results. Ann Surg. 1993;217:356–358. doi: 10.1097/00000658-199304000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesurtel M, Dehni N, Tiret E, Parc R, Paye F. Palliative surgery for unresectable pancreatic and periampullary cancer: a reappraisal. J Gastrointest Surg. 2006;10:286–291. doi: 10.1016/j.gassur.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Shyr YM, Su CH, Wu CW, Lui WY. Prospective study of gastric outlet obstruction in unresectable periampullary adenocarcinoma. World J Surg. 2000;24:60–64. doi: 10.1007/s002689910012. [DOI] [PubMed] [Google Scholar]
- 44.van Wagensveld BA, Coene PP, van Gulik TM, Rauws EA, Obertop H, Gouma DJ. Outcome of palliative biliary and gastric bypass surgery for pancreatic head carcinoma in 126 patients. Br J Surg. 1997;84:1402–1406. [PubMed] [Google Scholar]
- 45.Neuberger TJ, Wade TP, Swope TJ, Virgo KS, Johnson FE. Palliative operations for pancreatic cancer in the hospitals of U S Department of Veterans Affairs from 1987–1991. Am J Surg. 1993;166:626–636. doi: 10.1016/s0002-9610(05)80669-5. [DOI] [PubMed] [Google Scholar]
- 46.Huguier M, Baumel H, Maderscheid JC, Houry S, Fabre JM. Surgical palliation for unresected cancer of the exocrine pancreas. Eur J Surg Oncol. 1993;19:342–347. [PubMed] [Google Scholar]
- 47.Costamagna G, Pandolfi M. Endoscopic stenting for biliary and pancreatic malignancies. J Clin Gastroenterol. 2004;38:59–67. doi: 10.1097/00004836-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Lindsay JO, Andreyev HJ, Vlavianos P, Westaby D. Self-expanding metal stents for the palliation of malignant gastroduodenal obstruction in patients unsuitable for surgical bypass. Aliment Pharmacol Ther. 2004;19:901–905. doi: 10.1111/j.1365-2036.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 49.Schiefke I, Zabel-Langhennig A, Wiedmann M, Huster D, Witzigmann H, Mossner J, et al. Self-expandable metallic stents for malignant duodenal obstruction caused by biliary tract cancer. Gastrointest Endosc. 2003;58:213–219. doi: 10.1067/mge.2003.362. [DOI] [PubMed] [Google Scholar]
- 50.Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc. 2004;60:1010–1017. doi: 10.1016/s0016-5107(04)02276-x. [DOI] [PubMed] [Google Scholar]


