Abstract
Background:
Liver transplantation involves a period of ischemia and reperfusion to the graft which leads to primary non-function and dysfunction of the liver in 5–10% of cases. Remote ischemic preconditioning (RIPC) has been shown to reduce ischemia reperfusion injury (IRI) injury to the liver and increase hepatic blood flow. We hypothesized that RIPC may directly modulate hepatic microcirculation and have investigated this using intravital microscopy.
Methods:
A rat model of liver IRI was used with 45 min of partial hepatic ischemia (70%) followed by 3 h of reperfusion. Four groups of animals (Sham, IRI, RIPC+IRI, RIPC+Sham) were studied (n= 6, each group). Intravital microscopy was used to measure red blood cell (RBC) velocity, sinusoidal perfusion, sinusoidal flow and sinusoidal diameter. Neutrophil adhesion was assessed by rhodamine labeling of neutrophils and cell death using propidium iodide.
Results:
RIPC reduced the effects of IRI by significantly increasing red blood cell velocity, sinusoidal flow and sinusoidal perfusion along with decreased neutrophil adhesion and cell death.
Conclusions:
Using intravital microscopy, this study demonstrates that RIPC modulates hepatic microcirculation to reduce the effects of IRI. HO-1 may have a key role in the modulation of hepatic microcirculation and endothelial function.
Keywords: ischemic preconditioning, remote ischemic preconditioning, hepatic ischemia reperfusion injury
Introduction
Liver transplantation involves a period of cold and warm ischemia followed by reperfusion which initiates an inflammatory cascade resulting in organ non-function and dysfunction.1–3 This is referred to as ischemia reperfusion injury (IRI).
IRI remains a major concern in liver surgery and transplantation as the incidence of primary non-function is 5–10% in liver transplantation and the problem is aggravated in fatty livers. The success and expansion of liver transplantation has resulted in a shortage of organ donors and the use of marginal grafts including steatotic livers. Preconditioning is an adaptive response which helps reduce the severity of IRI in an experimental setting and has the potential of increasing the donor pool by increasing tolerance of marginally fatty livers to IRI. Ischemic preconditioning (IPC) involves the interruption of blood flow to the liver for brief periods followed by reperfusion. Nitric oxide (NO)/adenosine release has been shown to regulate endothelial function and increase blood flow to the liver.4–8 However, direct IPC produces trauma to major vessels and direct stress to the target organ. Remote ischemic preconditioning (RIPC) is a novel method which entails brief periods of ischemia followed by reperfusion of one organ and results in protection of remote organs from ischemia without direct stress or trauma to blood vessels. It ispresumed that remote preconditioning acts by release of biochemical messengers into the circulation or by activation of nerve pathways which confer protection on the remote organ. Studies have been carried out to show the benefit to the heart, lung and kidney after brief ischemia in the mesentery,9–12 limb13–20 and kidney.
Previous studies (Menger et al.)21using intravital microscopy have shown that the hepatic microcirculation is a major target of hepatic IRI. Microvascular injury precedes parenchymal injury and is as a result of hypoxia secondary to lack of microvascular perfusion (no-reflow) and reperfusion-associated inflammatory response which includes activation of kupffer cells and neutrophils (reflow – paradox). No reflow in sinusoids is because of endothelial swelling and intravascular hemoconcentration. Reperfusion is associated with release of proinflammatory cytokines, oxygen radicals, up-regulation of endothelial and leukocyte adhesion molecules and interaction of leukocytes with endothelial lining of the hepatic microvasculature. Hepatic IR induces neutrophil adhesion in sinusoids and post-sinusoidal venules.22,23
Kanoria et al. demonstrated improved liver function using RIPC in hepatic IRI and increased hepatic blood flow by ICG and laser Doppler flow measurements.13 Lai et al. demonstrated improved liver function and the role of hemoxygenase in hepatic protection.24 Gustaffson demonstrated protection of hepatic function by remote preconditioning.25 None of these studies addressed the in vivo microcirculatory changes seen in hepatic IR and the effect of RIPC on hepatic IR. We focused our study on the hepatic microcirculatory changes in hepatic IR and the modulation of the hepatic microcirculation by RIPC. We hypothesized that RIPC directly modulates hepatic microcirculation in IR injury and applied intravital microscopy to investigate the effects of RIPC.
Material and methods
Animals and surgical procedures
All experiments were conducted under a project license from the UK home office in accordance with the Animals' Scientific procedures act 1986. Male Sprague–Dawley rats, weighing 250–300 g, were used. Animals were kept in a temperature-controlled environment with a 12 h light/dark cycle and allowed tap water as well as standard rat chew pellets ad libitium. Animals were anesthetized with 4 l/min of isoflurane (Baxter, Norfolk, UK) and maintained with 1–1.5 l/min of O2 and 0.5–1.0% isoflurane. They were allowed to breathe spontaneously through a concentric mask connected to an oxygen regulator and monitored with a pulse oximeter (Ohmeda biox 3740 pulse oximeter; Ohmeda, Louisville, KY, USA).
Polyethylene catheters (0.76-mm inner diameter; Portex, Kent, UK) were inserted into the right carotid artery for monitoring of mean arterial blood pressure and the left jugular vein (0.40-mm inner diameter; Portex) for administering normal saline (1 ml/100 g/h body weight to compensate for intra-operative fluid loss). The animals were placed in a supine position on a heating mat (Harvard apparatus Ltd., Kent, UK) to maintain their temperature.
Animal model
Hepatic I/R
A standard model of lobar hepatic ischemia of the left lateral and median lobes (70% of liver) was used.26 Laparotomy was carried out through a midline incision. The ligamentous attachments of the liver were cut and the liver was exposed. Ischemia was induced by clamping the corresponding vascular pedicle with an atraumatic microvascular clamp. This model avoids splanchnic congestion. Hepatic ischemia was for a period of 45 min followed by 3 h of reperfusion. All animals were given a bolus of heparin [20 U/kg, intravenously (i.v.)] prior to clamping to prevent hepatic artery thrombosis.
Limb preconditioning
The technique of remote ischemic preconditioning involved a hind limb tourniquet, which was applied around the thigh. Limb perfusion was monitored by a laser Doppler (Moor instruments, Surrey, UK); the tourniquet around the limb was tightened until no flow was detected. The procedure involved 5 min of ischemia followed by 5 min of reperfusion. This was repeated four times.27
Tissue and blood collection
After 3 h of reperfusion, the animals were killed by exsanguination and blood was collected in a BD vacutainer tube SSTmII advance 5.0 ml tubes for Serum, BD vacutainer tube LH 102 I.U> (6 ml) for citrated plasma and BD vacutainer K 2E 7.2 mg (4 ml) for ethylenediaminetetraacetic acid (EDTA) plasma and centrifuged at 1500 g for 10 min to sediment the red blood cells (RBC). Serum and plasma samples were snap frozen in liquid nitrogen and subsequently stored at −80°C. Liver tissue was also snap frozen and stored at −80°C. Tissues were also fixed in 10% formalin and stored for histology.
Experimental groups (n= 6 in each group)
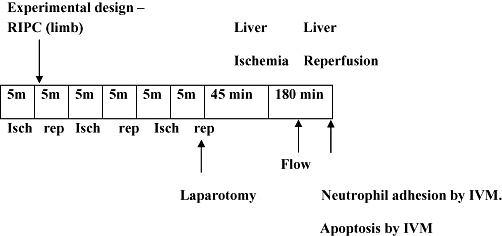
Four groups of animals were studied. Group one (Sham) in which animals were subjected to laparotomy only and underwent an identical experimental protocol without clamping. In group two (IRI) the animals were subjected to 45 min of liver ischemia followed by 3 hours of reperfusion. Ischemia was induced by a microvascular clamp applied across the vascular pedicle supplying the left and median lobes of the liver (70% ischemia). Group three were preconditioned immediately prior to the IRI (RIPC+IRI) group as shown in Fig. 1. In group four (RIPC+Sham) preconditioning was done in the Sham group. Standard protocols for preconditioning and inducing ischemia as described above were used.
Figure 1.
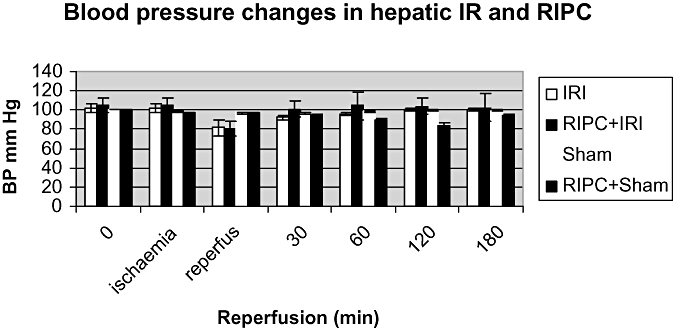
Changes in blood pressure seen in hepatic ischemia reperfusion (IR) and effect of preconditioning. Both IR and (RIPC)+IR show a similar fall in blood pressure; however, RIPC prior to IR rapidly restores blood pressure to baseline compared with IR only. Values expressed as mean + SEM. P < 0.05
 |
Intravital videofluorescence microscopy
The animals were maintained under anesthesia with isoflurane and oxygen. Their temperature was maintained by a warming mat (which was regulated by a thermostat).
The liver was exposed, placed upon a glass mount and covered with a cover slip. The liver was continuously irrigated with normal saline. A drop of saline was placed on the cover slip and the tip of the microscope lens was immersed in the saline drop.
A Nikon (Tokyo, Japan) microscope (Nikon epi-illumination system with filter block set suitable for Texas Red, FITC and DAPI dyes) coupled to a CCD camera [JVC TK-C1360β (Osaka, Japan) colour video camera] was used. The power of magnification was 10× and 40×. The microscopy images were recorded for offline analysis. Quantitative assessment of microcirculatory parameters was performed both during the experiment and offline using frame-by-frame analysis of the recorded images.
Microcirculation was assessed by evaluating acinar perfusion in 10 randomly chosen acini and leukocyte endothelial interaction in 10 post-sinusoidal venules.21,22,23,28 LUCIA (lab universal computer image analysis; Nikon) software was used to analyze the images.
RBC velocity (V)
FITC (Fluoroisothyocyanate; Sigma-Aldrich, Dorset, UK) labeled red cells were prepared from rat blood suspended in glucose saline buffer solution (20 mg FITC/ml of RBC).29 Labeled RBCs (0.5 ml) were administered i.v. to assess the velocity of RBC flow. Ten randomly chosen non-overlapping rappaport acini were assessed at each time point and the mean value was calculated. The RBC velocity was calculated by assessing the distance of RBC movement (microns) in each sinusoid in subsequent frames. Twenty-five frames were captured per second. The velocity was calculated using the formula = D × 25/number of frames moved.
Sinusoidal perfusion and perfusion index (PI)
The sinusoidal perfusion index was calculated as the ratio of perfused hepatic sinusoids. Continuous perfusion has been described as continuous flow of red cells through hepatic sinusoids for at least 1 min. Intermittent perfusion has been described as occasional flow of RBCs in sinusoids. Perfusion index = (Scp + Sip/Scp+Sip+Snp). [Continuous perfusion (Scp) + intermittent perfusion (Sip) to the total visible sinusoids which includes non perfused sinusoids (Snp)].22
Sinusoidal diameter (D)
Sinusoidal diameter was assessed by measuring the length across 10 randomly chosen hepatic sinusoids at 30, 60, 120 and 180 min of reperfusion and the mean value was expressed in microns.
Sinusoidal blood flow
The sinusoidal blood flow was calculated using the formula: (V) ×22/7× (D/2)2.
V = Velocity, D = diameter.
Neutrophil adhesion
Rhodamine 6 G (0.3 mg/kg; Sigma)30 was given i.v. after 180 min of reperfusion to assess neutrophil adhesion. The numbers of leukocytes adherent to the sinusoidal endothelium and venular endothelium were counted as those stationary for a period of 30 s under green filter light and expressed as leukocytes/mm2. The area of the vessels was calculated using the product of diameter and length assuming cylindrical geometry (3.14 × D × L).
Cell death
Hepatocellular death was assessed by i.v. injection of Propidium iodide (0.05 mg/kg i.v.)31 at 180 min of reperfusion prior to termination of the animal. The numbers of nuclei stained with the dye per high power field were counted. Ten randomly chosen high power fields were assessed.
Histology
At the end of the experiment, liver tissue was fixed in 10% formalin and embedded in paraffin in preparation for light microscopy analysis. Sections were cut at 5µ and stained with hematoxylin and eosin (H&E) for histological analysis. The histologic changes in the routine (H&E) stained sections were graded using the modified Suzuki's criteria.32 In this classification system, sinusoidal congestion, hepatocyte necrosis and hepatocyte vacuolation were graded on a score of 0–4. No changes were scored as 0 whereas severe congestion, extensive vacuolation and greater than 60% of lobular necrosis was awarded a score of 4.
| Numerical assessment | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Sinusoidal congestion | None | Minimal | Mild | Moderate | Severe |
| Vacuolation | None | Minimal | Mild | Moderate | Severe |
| Necrosis | None | Single cell | 30% | 60% | >60% |
Statistical analysis
All data are presented as mean and standard error of the mean (SEM). Differences were considered significant for P < 0.05. Comparisons between groups were performed using one-way analysis (anova). Bonferroni's correction was applied for anova.
Results
Hemodynamic parameters
Blood pressure (MAP)
There was no significant difference in the sham group between baseline and 180 min demonstrating a hemodynamically stable model. The blood pressure reduced transiently in the IR and RIPC+IR groups but this was insignificant. However, the recovery to baseline was more rapid in the RIPC group (Fig. 1).
Pulse rate
No significant difference was seen between the groups.
Oxygen saturations
The fall in saturations in both the IR group and RIPC+IR group was not statistically significant.
Hepatocellular injury (liver function tests)
After 180 min of reperfusion, the difference between the Sham and IRI groups was statistically significant (P < 0.05). Preconditioning prior to IRI reduced serum transaminase levels although the difference was not statistically significant. Preconditioning in Sham animals did not show any significant elevation in transaminases.
| IR | RIPC+IR | Sham | RIPC+Sham | |
|---|---|---|---|---|
| ALT (IU/ml) | 2079 ± 322.3 | 1398 ± 460.8 | 118 ± 22.37 | 132 ± 25.49 |
Histologic investigation
Histology of the liver tissue revealed that IR injury produced significant periportal congestion in association with severe necrosis in zones 2 and 3 and also sub capsular necrosis. The changes were diffuse. RIPC+IR showed less necrosis and damage compared with the IR group. Sham animals revealed minimal changes. Congestion was present in both portal vein and central veins. Hepatocytes showed vacuolation. RIPC+Sham animals showed minimal changes, some vacuolation, but no necrosis, similar to the Sham group (Fig. 2)
Figure 2.
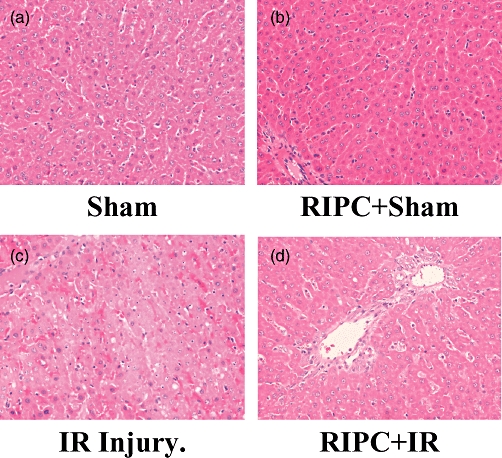
Histology (a) remote ischemic preconditioning+ischemia reperfusion (RIPC+IR): the hematoxylin and eosin (H&E) section shows sinusoidal congestion, some hepatocyte vacuolation but no significant necrosis. (b) IR: the H&E section shows large areas of necrosis and sinusoidal congestion, normal residual hepatocytes noted at bottom of the frame. (c) Sham: the H&E section reveals no significant damage. (d) RIPC+Sham: the H&E section reveals a congested central vein but no other significant change
Histological scoring table
| IR | RIPC+IR | Sham | RIPC+Sham | |
|---|---|---|---|---|
| Suzuki score | 8.83 ± 0.7 | 6.2 ± 0.58 | 4 ± 0.31 | 1.5 ± 0.34 |
The Suzuki score was significantly reduced in the preconditioned group compared with the IR group (P < 0.001).
Intravital microscopy results
Intravital microscopy demonstrated significant changes to the hepatic microcirculation after IR and modification by RIPC.
Velocity of RBC flow
The mean RBC velocity in the Sham group was constant throughout the time of observation. IRI led to decreased RBC velocity whereas preconditioning significantly increased RBC velocity (Fig. 3a).
Figure 3.
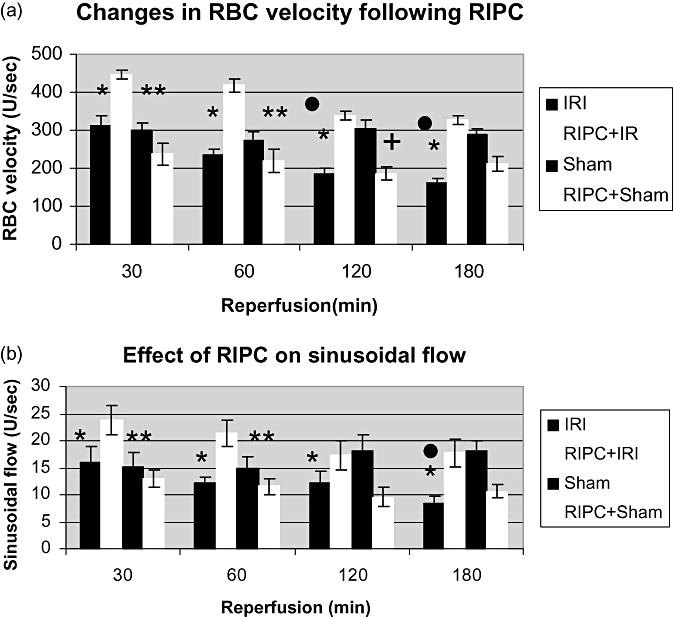
(a) Velocity of red blood cell (RBC) flow in Sham animals remained unchanged throughout 180 min of observation, although there was a significant increase in velocity in preconditioned animals prior to ischemia reperfusion injury (RIPC+IRI) as compared with IRI at 30, 60 and 120 min of reperfusion. Values expressed as mean ± SEM. *P < 0.05 (RIPC+IR/IR). **P < 0.05 (RIPC+IR/Sham). •, P < 0.05 (Sham/IR). +, P < 0.05 (Sham/RIPC+Sham). (b) Sinusoidal flow: V × (D/2)2 ×π= V is velocity of RBC, D is sinusoidal diameter. There was a significantly better flow in preconditioned animals (RIPC+IRI) as compared with non-preconditioned (IR). Values expressed as mean ± SEM. *P < 0.05 (RIPC+IR/IR). **P < 0.05 (RIPC+IR/Sham). •, P < 0.05 (Sham/IR)
Sinusoidal blood flow
The sinusoidal blood flow was significantly better in the RIPC+IRI group compared with the IRI group (Fig. 3b).
Diameter of sinusoids
The mean diameter showed no significant difference between the RIPC+IR group and the IR group. The difference between the Sham and RIPC+IR and between the Sham and IRI groups was not statistically significant.
Perfusion of sinusoids
Preconditioning significantly improved sinusoidal perfusion being significantly high compared with the IRI group. These data show no significant difference between RIPC+ IRI and Sham groups at all time points (Fig. 4).
Figure 4.
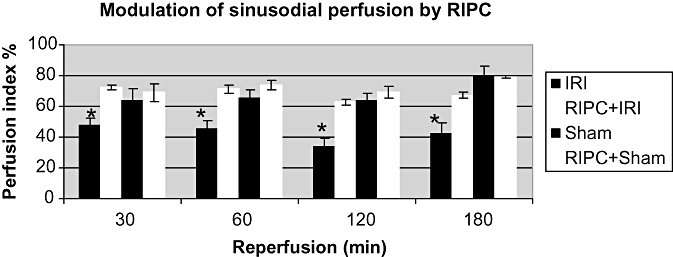
Sinusoidal perfusion index (PI): the PI remains unchanged in Sham animals throughout 180 min of observation. The PI in remote preconditioned animals (RIPC+IRI) is significantly higher than non-preconditioned animals (IRI). The PI in RIPC+IR is not different from Sham. Values expressed as mean ± SEM. *P < 0.05
Neutrophil adhesion
The number of adherent neutrophils was significantly lower in the RIPC+IRI group as compared with the IR group and similar to the Sham group. In the sinusoidal bed, neutrophil adhesion was significantly reduced in the preconditioned group as compared with the IR group (Figs 5a,b,6).
Figure 5.
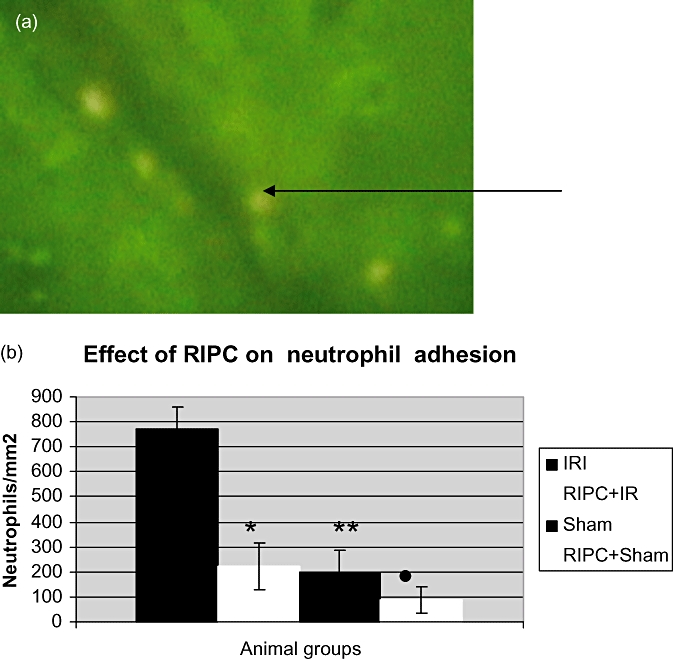
(a) Neutrophils stained using rhodamine are seen adherent to post-sinusoidal venular endothelium. The number of adherent neutrophils divided by the area of endothelial surface (π× D × L) gives the number of neutrophils/mm2. D, sinusoidal diameter, L, length of segment along adherent neutrophils. (b) Significantly reduced venular neutrophil adhesion in the preconditioned (RIPC+IRI) group compared with the non-preconditioned group (IRI). Values expressed as mean ± SEM. *P < 0.05 (IR/RIPC+IR), **P < 0.05 (IR/Sham), •, P < 0.05 (IR/RIPC+Sham)
Figure 6.
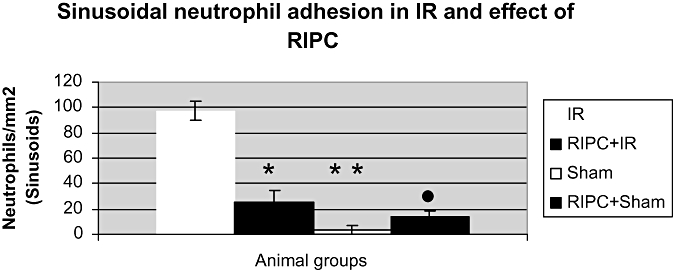
Significantly reduced sinusoidal neutrophil adhesion in the preconditioned group (RIPC+IRI) compared with non-preconditioned group (IRI). Values expressed as mean ± SEM. *P < 0.05 (IR/RIPC+IR), **P < 0.05 (IR/Sham), •, P < 0.05 (IR/RIPC+Sham)
Hepatocellular death
No significant difference was found between the Sham and RIPC groups but both had a significantly lower number of apoptotic cells compared with IRI (Fig. 7).
Figure 7.
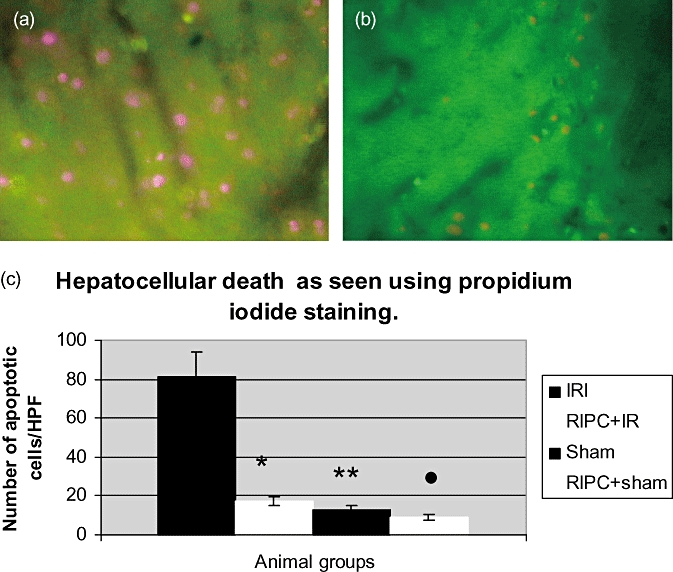
(a) Hepatocellular cell death in ischemia reperfusion injury (IRI) using propidium iodide staining (IVM). The dead cells appear pink stained by propidium iodide. The number of cells divided by the surface area of the field above gives the number of cells/mm2. (b) Hepatocellular cell death seen in remote ischemic preconditioning (RIPC)+IR using IVM. (c) Hepatocellular cell death in the preconditioned group (RIPC+IRI) was significantly less compared with the non-preconditioned (IRI) group. Values expressed as mean ± SEM. *P < 0.05 (IR/RIPC+IR), **P < 0.05 (IR/Sham), •, P < 0.05 (IR/RIPC+Sham)
Discussion
New findings
This study demonstrates that RIPC modulates microcirculatory disturbances in hepatic IR leading to increased RBC velocity, sinusoidal perfusion, decreased sinusoidal and venular neutrophil adhesion, improved hepatic microcirculation and decreased hepatocyte cell death. This is the first in vivo study to demonstrate microcirculatory effects of RIPC in hepatic IR by intravital microscopy and suggest that improvement of hepatic microcirculation is a key mechanism responsible for hepatic protection by RIPC in IRI. Recently RIPC has been shown to modulate pancreatic microcirculation and reduce pancreatic IRI.33
Adequacy of model
The experimental model used is a previously well-described hepatic lobar ischemia model of warm IR. In the model 45 min of partial ischemia (70% of the liver) was induced followed by 3 h of reperfusion.26 Intravital microscopy has shown that IRI significantly reduced velocity of flow and perfusion as compared with Sham animals. Therefore the model was considered suitable to study the effects of RIPC. As this study was limited to investigating the effects of warm IR and the effects of RIPC in warm IR, a partial hepatic IR model was chosen as against a transplant model. RIPC was produced using a tourniquet around the hind limb for four cycles of 5-min ischemia followed by reperfusion. This technique of RIPC has previously been used in the modulation of cardiac IRI after transplantation.25 Three or more cycles of preconditioning have been shown to be more effective than a single cycle in previous experimental studies27,34,35 and this formed the basis for four cycles of RIPC used in the current study.
Systemic hemodynamics
Shams were hemodynamically stable throughout the course of the experiments. Limb preconditioning in Sham animals caused a transient insignificant fall in blood pressure during preconditioning with rapid recovery to baseline levels suggesting no major effects of Sham laparotomy, anesthesia or RIPC alone. A transient fall in blood pressure and oxygen saturations associated with onset of reperfusion was seen in both the IR and RIPC+IR groups. However, recovery of blood pressure to normal levels was rapid in the RIPC+IR group as compared with the IR group only. These observations have been observed in preconditioning models of lung IRI.36,37 Kanoria et al. have demonstrated a fall in blood pressure after hepatic IR in a rabbit model and rapid recovery of blood pressure in the group which was preconditioned.13
Histologic findings and limitations of the scoring system
The RIPC+IR group showed less necrosis, vacuolation and congestion compared with the IR group and objective scoring showed a significantly lower score. However, it was difficult to arrive at an objective score if the changes noted were focal/patchy. There were several sections with only sub capsular infarction/necrosis. Scoring these sections were difficult using the criteria.
Hepatic microcirculatory changes
Sham
The Sham group showed constant velocity, flow and perfusion over the 180 min of observation. This suggests a stable model for the study.
Effects of IRI on hepatic microcirculation
Our study shows a significant fall in sinusoidal perfusion and velocity of flow in the IRI group which was observed at all time points. Increased venular neutrophil adhesion, hepatocyte cell death, sinusoidal neutrophil stasis in the IR group with significantly increased serum transaminase levels in IRI and a significantly lower perfusion index compared with Sham animals were observed. Leukocyte adhesion causes endothelial injury, RBC stasis, modulation of sinusoidal tone and perfusion in hepatic IR.22,23 Mechanisms which contribute to failure of perfusion include endothelial swelling and injury, leukocyte endothelial interaction, leukostasis, alteration in RBC flow and increased viscosity of blood.22,23,38–40 Both Perfusion failure and venular adhesion have been shown to correlate with hepatic dysfunction, reduced bile flow and increased transaminases.39 These studies support our observations in the hepatic IR model in this study. Reduced RBC velocity seen in hepatic IR in our study is explained by previous studies which suggest that free radical release during IRI leads to erythrocyte damage, increased deformity and sluggishness of RBC causing reduced RBC velocity, erythrocyte flux and increased viscosity of blood.39
Effects of RIPC on hepatic microcirculation in IRI
This study demonstrates that RIPC increases RBC velocity and erythrocyte flux across sinusoids, significantly reduced hepatocyte cell death, increased sinusoidal perfusion and sinusoidal flow as compared with the IR group. The serum transaminase levels in the RIPC+IR group were lower than IRI. These observations suggest an improvement in liver function in the RIPC+IR group as compared with IR group. Interestingly there is a significant initial rise in RBC velocity and flow in the RIPC+IR group as compared with Sham and IR although the initial velocities in Sham and IR are not significantly different. However, at 120-min reperfusion a significant drop in velocity is seen in the IR group as compared with Sham whereas the velocity in RIPC+IR is similar to the Sham group. These observations suggest that the initial rise in baseline velocity in the RIPC+IR group acts as a protective mechanism against the deleterious effects of IR and prevents a fall in velocity and sinusoidal perfusion below Sham values as reperfusion injury progresses over a period of time. The initial molecular pathway responsible for the increased baseline velocity is unclear but it is likely to be induced by RIPC in the hepatic ischemia period itself as the velocity of flow is seen to be high at 30 min of reperfusion.
A significant reduction in both venular neutrophil adhesion and sinusoidal adhesion implies modulation of neutrophil activation by RIPC leading to decreased oxidative stress, IRI and perfusion failure. Previous studies41 have shown ischemic preconditioning to reduce hepatic IR-induced perfusion failure and preservation of mitochondrial redox state but none have demonstrated the role of RIPC in hepatic IR. Studies have shown that reduction in RBC velocity occurs because of leukocyte adhesion, endothelial injury and perfusion impairment (no reflow phenomenon) and increased RBC damage as a result of free radical release during IR. The observations in our study suggest that RIPC modulates hepatic microcirculation and may induce protective mechanisms which protect RBC against free radical induced damage; however, this needs to be investigated further.
Effects of RIPC on sham
Remote ischemic preconditioning in the normal Sham group showed a reduced RBC velocity and sinusoidal flow at 120 min as compared with 30 min and recovery to baseline at 180 min. This change in velocity was not statistically significant. This maybe as a result of initial preconditioning induced oxidative stress followed by development of a tolerance to the low-grade oxidative stress. This observation is supported by Chen et al.20 who demonstrated that limb RIPC produced an increase in free radical levels in the blood of preconditioned animals which served as a trigger to induce heat shock protein expression in remote skeletal tissues and confer protection against skeletal IR. In comparison with the Sham group, the velocity of flow and sinusoidal flow in RIPC+ Sham was less at 120 min and 180 min as a result of low-grade oxidative stress induced in a normal liver.
Potential pathway and mechanisms in remote ischemic preconditioning of the liver
Studies have shown RIPC-induced NO in the heart to reduce myocardial IRI which was not abolished by neurogenic blockade,19 increase in plasma free radicals after brief limb RIPC which induced heat shock proteins in remote skeletal muscle20 and increased hemoxygenase (HO-1) in hepatic macrophages after limb preconditioning the source of which was not peripheral lymphocytes.24 These data suggest that brief limb ischemia leads to release of biochemical messengers such as free radicals in the blood which may induce oxidative stress in the remote organ and HO-1 expression. HO-1 is known to promote degradation of hem, increase CO production and scavenge free radicals resulting in improved hepatic function. In vivo studies of the liver have shown cells, which are hepatic sinusoid-associated pericytes primarily responsible for CO-mediated blood flow regulation in sinusoids.42,43 The role of free radicals as messengers in inducing HO-1 in the liver, the pathway for HO-1 induction in the liver after brief limb ischemia and the role of HO-1 in modulation of hepatic microcirculation and endothelial function needs to be investigated further. The findings in this study suggest decreased neutrophil activation, decreased endothelial adhesion and injury resulting in improved perfusion and flow in the hepatic microcirculation. These data suggest that RIPC may induce a decrease in endothelial adhesion molecule expression through HO-1. HO-1 is known to reduce endothelial ICAM expression30,42,44 and this needs further investigation in animal models of remote ischemic preconditioning.
Conclusion
RIPC protects the liver from IRI by modulation of the hepatic microcirculation. Our study using intravital microscopy demonstrates that increased hepatic blood flow after RIPC is as a result of increased RBC velocity, sinusoidal perfusion and decreased neutrophil adhesion and cell death. Future animal studies need to focus on the role of RIPC in animal transplant models and the use of HO-1 knockout models to clarify the role of HO-1 pathways in the regulation of endothelial function. Clinical trials need to investigate the role of RIPC in reducing morbidity and mortality in patients undergoing liver transplant and resection surgery.
Acknowledgments
This study was funded by the Peter Samuel grant (Royal Free NHS trust, UK).
Conflicts of interest
None declared.
References
- 1.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J Invest Surg. 2003;16:127–140. [PubMed] [Google Scholar]
- 3.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 4.Koti RS, Seifalian AM, McBride AG, Yang W, Davidson BR. The relationship of hepatic tissue oxygenation with nitric oxide metabolism in ischemic preconditioning of the liver. FASEB J. 2002;16:1654–1656. doi: 10.1096/fj.01-1034fje. [DOI] [PubMed] [Google Scholar]
- 5.Peralta C, Prats N, Xaus C, Gelpi E, Rosello-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481–1489. doi: 10.1002/hep.510300622. [DOI] [PubMed] [Google Scholar]
- 6.Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpi E, et al. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126–132. doi: 10.1002/hep.510290104. [DOI] [PubMed] [Google Scholar]
- 7.Peralta C, Hotter G, Closa D, Gelpi E, Bulbena O, Rosello-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934–937. doi: 10.1002/hep.510250424. [DOI] [PubMed] [Google Scholar]
- 8.Peralta C, Closa D, Hotter G, Gelpi E, Prats N, Rosello-Catafau J. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264–270. doi: 10.1006/bbrc.1996.1790. [DOI] [PubMed] [Google Scholar]
- 9.Wolfrum S, Nienstedt J, Heidbreder M, Schneider K, Dominiak P, Dendorfer A. Calcitonin gene related peptide mediates cardioprotection by remote preconditioning. Regul Pept. 2005;127:217–224. doi: 10.1016/j.regpep.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34:1317–1323. doi: 10.1006/jmcc.2002.2072. [DOI] [PubMed] [Google Scholar]
- 11.Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc Res. 2002;55:583–589. doi: 10.1016/s0008-6363(02)00408-x. [DOI] [PubMed] [Google Scholar]
- 12.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 13.Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762–768. doi: 10.1002/bjs.5331. [DOI] [PubMed] [Google Scholar]
- 14.Liem DA, Verdouw PD, Duncker DJ. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circ. 2003;107:e218–e219. doi: 10.1161/01.CIR.0000077520.36997.F9. [DOI] [PubMed] [Google Scholar]
- 15.Addison PD, Neligan PC, Ashrafpour H, Khan A, Zhong A, Moses M, et al. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1435–H1443. doi: 10.1152/ajpheart.00106.2003. [DOI] [PubMed] [Google Scholar]
- 16.Xia Z, Herijgers P, Nishida T, Ozaki S, Wouters P, Flameng W. Remote preconditioning lessens the deterioration of pulmonary function after repeated coronary artery occlusion and reperfusion in sheep. Can J Anaesth. 2003;50:481–488. doi: 10.1007/BF03021061. [DOI] [PubMed] [Google Scholar]
- 17.Kuntscher MV, Kastell T, Sauerbier M, Nobiling R, Gebhard MM, Germann G. Acute remote ischemic preconditioning on a rat cremasteric muscle flap model. Microsurgery. 2002;22:221–226. doi: 10.1002/micr.10041. [DOI] [PubMed] [Google Scholar]
- 18.Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707–H1712. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- 19.Chen XG, Wu BY, Wang JK, Bai T. Mechanism of the protective effects of noninvasive limbs preconditioning on myocardial ischemia-reperfusion injury. Chin Med J (Engl) 2005;118:1723–1727. [PubMed] [Google Scholar]
- 20.Chen YS, Chien CT, Ma MC, Tseng YZ, Lin FY, Wang SS, et al. Protection ‘outside the box’ (skeletal remote preconditioning) in rat model is triggered by free radical pathway. J Surg Res. 2005;126:92–101. doi: 10.1016/j.jss.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepatogastroenterology. 1999;46(Suppl.)(2):1452–1457. [PubMed] [Google Scholar]
- 22.Vollmar B, Menger MD, Glasz J, Leiderer R, Messmer K. Impact of leukocyte-endothelial cell interaction in hepatic ischemia-reperfusion injury. Am J Physiol. 1994;267:G786–G793. doi: 10.1152/ajpgi.1994.267.5.G786. [DOI] [PubMed] [Google Scholar]
- 23.Vollmar B, Glasz J, Menger MD, Messmer K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery. 1995;117:195–200. doi: 10.1016/s0039-6060(05)80085-6. [DOI] [PubMed] [Google Scholar]
- 24.Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–1317. doi: 10.1097/01.tp.0000203555.14546.63. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson BI, Friman S, Wallin M, Heiman J, Delbro DS. Effect of remote preconditioning on mild or severe ischemia-reperfusion injury to rat liver. Transpl Proc. 2006;38:2708–2709. doi: 10.1016/j.transproceed.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 26.Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182–1191. doi: 10.1053/jlts.2002.36846. [DOI] [PubMed] [Google Scholar]
- 27.Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, et al. Remote preconditioning reduces ischemia-reperfusion injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol. 2004;288:H1252–1256. doi: 10.1152/ajpheart.00207.2004. [DOI] [PubMed] [Google Scholar]
- 28.Vajdova K, Heinrich S, Tian Y, Graf R, Clavien PA. Ischemic preconditioning and intermittent clamping improve murine hepatic microcirculation and Kupffer cell function after ischemic injury. Liver Transpl. 2004;10:520–528. doi: 10.1002/lt.20126. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerhackl B, Parekh N, Brinkhus H, Steinhausen M. The use of fluorescent labeled erythrocytes for intravital investigation of flow and local hematocrit in glomerular capillaries in the rat. Int J Microcirc Clin Exp. 1983;2:119–129. [PubMed] [Google Scholar]
- 30.Wunder C, Brock RW, McCarter SD, Bihari A, Harris K, Eichelbronner O, et al. Inhibition of haem oxygenase activity increases leukocyte accumulation in the liver following limb ischaemia-reperfusion in mice. J Physiol. 2002;540:1013–1021. doi: 10.1113/jphysiol.2001.015446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brock RW, Carson MW, Harris KA, Potter RF. Microcirculatory perfusion deficits are not essential for remote parenchymal injury within the liver. Am J Physiol. 1999;277:G55–G60. doi: 10.1152/ajpgi.1999.277.1.G55. Pt. [DOI] [PubMed] [Google Scholar]
- 32.Fondevila C, Katori M, Lassman C, Carmody I, Busuttil RW, Bach FH, et al. Biliverdin protects rat livers from ischemia/reperfusion injury. Transpl Proc. 2003;35:1798–1799. doi: 10.1016/s0041-1345(03)00720-6. [DOI] [PubMed] [Google Scholar]
- 33.Oehmann C, Benz S, Drognitz O, Pisarski P, Hopt UT, Obermaier R. Remote preconditioning reduces microcirculatory disorders in pancreatic ischemia/reperfusion injury. Pancreas. 2007;35:e45–e50. doi: 10.1097/mpa.0b013e318073d1b7. [DOI] [PubMed] [Google Scholar]
- 34.Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, Zair M, et al. Mitochondrial KATP channels in hind limb remote ischemic preconditioning of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol. 2004;288:H559–567. doi: 10.1152/ajpheart.00845.2004. [DOI] [PubMed] [Google Scholar]
- 35.Addison PD, Neligan PC, Ashrafpour H, Khan A, Zhong A, Moses M, et al. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1435–H1443. doi: 10.1152/ajpheart.00106.2003. [DOI] [PubMed] [Google Scholar]
- 36.Harkin DW, Barros D'Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia – reperfusion protects against acute lung injury. J Vasc Surg. 2002;35:1264–1273. doi: 10.1067/mva.2002.121981. [DOI] [PubMed] [Google Scholar]
- 37.Waldow T, Alexiou K, Witt W, Albrecht S, Wagner F, Knaut M, et al. Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia. Transpl Int. 2005;18:198–205. doi: 10.1111/j.1432-2277.2004.00005.x. [DOI] [PubMed] [Google Scholar]
- 38.Vollmar B, Richter S, Menger MD. Leukocyte stasis in hepatic sinusoids. Am J Physiol. 1996;270:G798–G803. doi: 10.1152/ajpgi.1996.270.5.G798. [DOI] [PubMed] [Google Scholar]
- 39.Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421–1431. [PMC free article] [PubMed] [Google Scholar]
- 40.Vollmar B, Glasz J, Post S, Menger MD. Role of microcirculatory derangements in manifestation of portal triad cross-clamping-induced hepatic reperfusion injury. J Surg Res. 1996;60:49–54. doi: 10.1006/jsre.1996.0009. [DOI] [PubMed] [Google Scholar]
- 41.Glanemann M, Vollmar B, Nussler AK, Schaefer T, Neuhaus P, Menger MD. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol. 2003;38:59–66. doi: 10.1016/s0168-8278(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, et al. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;15:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 43.Suematsu M, Wakabayashi Y, Ishimura Y. Gaseous monoxides: a new class of microvascular regulator in the liver. Cardiovasc Res. 1996;32:679–686. [PubMed] [Google Scholar]
- 44.Wunder C, Scott JR, Lush CW, Brock RW, Bihari A, Harris K, et al. Heme oxygenase modulates hepatic leukocyte sequestration via changes in sinusoidal tone in systemic inflammation in mice. Microvasc Res. 2004;68:20–29. doi: 10.1016/j.mvr.2004.03.003. [DOI] [PubMed] [Google Scholar]


