Abstract
Background/aims:
Distal pancreatectomy (DP) is performed for a range of benign and malignant lesions. Accurate pre-operative diagnosis can be unreliable and morbidity remains high. This study evaluates a 12-year, single-centre experience with open DP to review indications, diagnoses and associated morbidity.
Methods:
Retrospective review of patients who underwent DP at a UK-based tertiary referral centre between 1994 and 2006.
Results:
Sixty-five patients (mean age 49.9 years) had final diagnoses of chronic pancreatitis ± pseudocyst (n= 22), benign cystadenoma (n= 15), neuroendocrine tumour (n= 8), primary pancreatic carcinoma (n= 6) and 14 other conditions. DP performed for presumed cystic neoplasm (n= 24) revealed a correct pre-operative diagnosis in 71% of patients. Histological examination confirmed that 59% of resected cystic tumours were either malignant or had malignant potential. When DP was undertaken for presumed pseudocyst (n= 12), 83% of cases were correctly diagnosed pre-operatively. Overall mortality and morbidity rates were 3% and 39%, respectively, with five patients (8%) developing a clinically significant pancreatic fistula. Ten (17%) patients developed diabetes mellitus and nine (14%) required long-term pancreatic exocrine supplementation.
Conclusions:
Open DP can be performed with acceptable morbidity, low mortality and preservation of pancreatic function in the majority of cases, setting the standard for laparoscopic techniques.
Keywords: distal pancreatectomy, laparoscopic, postoperative pancreatic fistula
Introduction
Distal pancreatectomy (DP), involving resection of the pancreas to the left of the superior mesenteric vessels, is performed for a range of benign and malignant lesions, trauma and inflammatory disorders. In recent years, the procedure has been increasingly performed by laparoscopic means, although published series are small and report comparatively high morbidity.1,2 Accurate pre-operative diagnosis of pancreatic lesions, especially differentiating malignant and non-malignant cystic neoplasms, can be unreliable and is often only possible after detailed pathological examination.3 However, the reported high incidence of malignant tumours and tumours with malignant potential often favours resection over conservative management.4
Distal pancreatectomy was first described over a century ago, however, resection rates have shown little increase over this time despite the introduction of laparoscopic approaches to treatment.1,5 This may relate to the relative infrequency of potentially resectable distal pancreatic lesions, the complexity of the procedure and high post-operative morbidity, especially pancreatic fistula.6 Over recent years, attempts to reduce post-operative pancreatic fistula (POPF) have focussed on methods of distal pancreatic remnant closure (staple, suture, duct ligation or acombination) and the use of somatostatin analogues to inhibit exocrine secretion of the pancreas although results are inconclusive.6,7
This study aims to review a 12-year, single-centre experience with DP, providing a background on which to discuss the emerging technique of laparoscopic DP.
Patients and methods
A retrospective review of cases coded as distal pancreatectomy in the Lothian surgical audit database was undertaken. The Lothian Surgical Audit recorded all surgical activity at a UK-based tertiary referral centre, between August 1994 and August 2006. Admission records, operation details, pathology records and clinic notes were reviewed for each available case and the presenting complaint, pre-operative investigations, operative technique, final diagnosis and post-operative outcome were recorded.
Definitions
The ISGPF definition (International study group: pancreatic fistula, 2005) of POPF was used i.e. drain amylase concentration three times serum amylase on or after post-operative day three.8 The ISGPF definition further categorizes fistulae according to a range of parameters into ‘Grade A’– a transient fistula of no clinical consequence, ‘Grade B’– requiring deviation from routine management and ‘Grade C’– requiring major deviation from routine management and aggressive intervention (Table 1). Only clinically significant POPF grade B and C were recorded in this study.
Table 1.
The main parameters for post-operative pancreatic fistula (POPF) grading according to ISGPF definition8
| Grade | A | B | C |
|---|---|---|---|
| Clinical conditions | Well | Often well | Ill appearing/bad |
| Specific treatment | No | Yes/no | Yes |
| US/CT | Negative | Negative/positive | Positive |
| Persistent drainage | No | Usually yes | Yes |
| Reoperation | No | No | Yes |
| Death related to POPF | No | No | Possibly yes |
| Signs of infection | No | Yes | Yes |
| Sepsis | No | No | Yes |
| Readmission | No | Yes/no | Yes/no |
US, ultrasound; CT, computed tomography; POPF, post-operative pancreatic fistula
Post-operative morbidity was defined as any complication arising in the 30-day post-operative period. Pancreatic endocrine and exocrine deficiencies were defined as the requirement for endocrine or exocrine supplementation during the follow-up period where pancreatic deficiency was not present pre-operatively.
Statistical analysis
Data analysis was performed using SPSS version 11 (SPSS Inc., Chicago, IL, USA). Student's t-test was used to compare differences between length of stay. Chi-square analysis assessed differences in complication rates between procedural variations (i.e. stump closure technique). The level of significance was set at P < 0.05.
Results
Seventy-six cases were coded as ‘distal pancreatectomy’ in the Lothian Surgical Audit database. Eleven were excluded either because of miscoding (n= 5) or incomplete case details (n= 6). The final group of 65 patients (40 females and 25 males) had a mean age of 49.9 years (range 15–88).
Presentation
The mode of presentation to the surgical team included abdominal pain (n= 37), pancreatic lesion noted on CT for evaluation of suspected non-pancreatic intra-abdominal malignancy (n= 8), symptoms of hypoglycaemia (n= 6), incidental finding on CT (n= 5), trauma (n= 3), pleural effusion (n= 2), gastrointestinal bleed (n= 2), painless jaundice (n= 1) and abdominal mass (n= 1). Computed tomography (CT) scanning was performed in all patients. Other investigations included endoscopic retrograde cholangiopancreatography (ERCP) (n= 8), exploratory laparoscopy +/− ultrasound (n= 6), endoscopic ultrasound (n= 4) and magnetic resonance imaging (MRI) (n= 1). The principal indications for DP included presumed cystic neoplasia (n= 23), presumed non-cystic neoplasia (n= 19) and presumed pseudocyst/chronic pancreatitis (n= 15) (Fig. 1).
Figure 1.
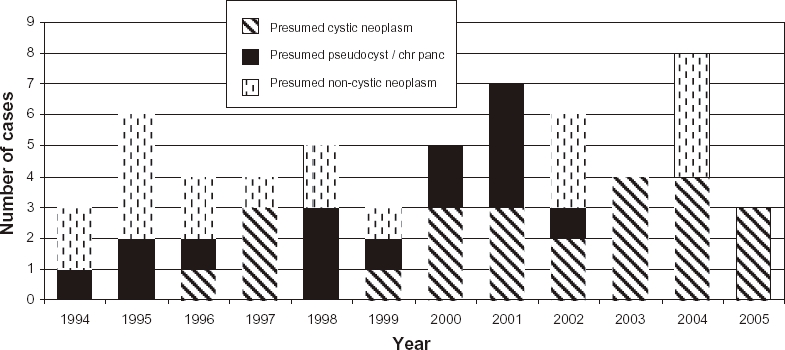
Main indications for distal pancreatectomy (DP) over the study period (miscellaneous indications excluded). NB ‘Year’ represents August to August
Operative details
During the study period, 65 procedures were performed. Intra-operative ultrasound of the pancreas was used to assess the cystic lesions in 31 patients. Closure of the pancreatic stump involved suture plus duct ligation (n= 34), suture alone (n= 19) or staple plus suture (n= 12). Additional procedures included splenectomy (n= 40), splenectomy plus cholecystectomy (n= 5), splenectomy plus partial gastrectomy (n= 5), left hemicolectomy (n= 3) and cholecystectomy alone (n= 2). Spleen preserving DP without additional procedure was performed in eight patients. Peri-operative octreotide was administered in five cases, based on individual clinician decision, with dosing regimens ranging from 100–250 µg (three times a day) for 3 to 5 days.
Pancreatic pathology
Pancreatic pathology included chronic pancreatitis and/or pseudocyst (n= 22), benign cystadenoma (n= 15), neuroendocrine tumour (n= 8), primary pancreatic carcinoma (n= 5), no abnormalities (n= 4), trauma (n= 3), acute pancreatitis (n= 2), metastatic carcinoma (n= 2), simple cyst (n= 2), gastrointestinal stromal tumour (GIST) (n= 1) and malignant local invasion (n= 1). Of the neuroendocrine tumours, three showed malignant change.
DP performed for presumed cystic neoplasia (n= 24) revealed a correct pre-operative diagnosis in 71% of patients with other diagnoses including pseudocyst (n= 6) and chronic pancreatitis (n= 1). Histological examination confirmed that 10 out of the 17 (59%) resected cystic tumours were either malignant or possessed malignant potential. Ten out of the 12 (83%) presumed pseudocysts were diagnosed correctly pre-operatively. The pathological findings in those patients with incidental distal pancreatic lesions included mucinous cystadenoma (n= 2), primary adenocarcinoma (n= 1), serous cystadenoma (n= 1) and chronic pancreatitis (n= 1). In the four cases where no histological pancreatic abnormality was identified, the reasons for resection included known colonic carcinoma with presumed pancreatic invasion (n= 2), presumed neoplasm noted on CT (n= 1) and presumed chronic pancreatitis (n= 1).
Post-operative course
The median post-operative hospital stay was 10 days (range 5–64 days). The morbidity and length of stay increased significantly when procedures unrelated to DP (cholecystectomy/gastrectomy/colectomy) were performed (P= 0.049). There was no significant difference in length of stay between spleen preserving DP and DP with splenectomy (P= 0.346).
Overall mortality and morbidity rates were 3% and 39%, respectively. Mortality (n= 2) was related to post-operative intra-abdominal haemorrhage (n= 1) and acute pancreatitis (n= 1). Five patients (8%) developed POPF grade B with ultrasound +/− CT evidence of a pancreatic fistula (n= 3) or peripancreatic collection (n= 2). Additional procedures (n= 4) included splenectomy alone (n= 2), left hemicolectomy (n= 1) and splenectomy plus partial gastrectomy (n= 1). Diagnoses in these cases included mucinous cystadenoma (n= 2), trauma (n= 2) and normal pancreas (n= 1). After prolonged intra-abdominal drainage, all resolved spontaneously without further intervention (median length of drainage 18 days; range 10–56 days). There was no difference in length of hospital stay between the POPF B and non-POPF group (t= 1.05; P= 0.342). There was no association between pancreatic stump closure (staple and suture; suture and duct ligation; suture alone) and POPF B (P= 0.814). No patients developed POPF grade C. Non-pancreatic complications included pulmonary embolus (n= 4), wound infection (n= 3), pneumonia (n= 2), ruptured spleen requiring splenectomy (n= 1) and miscellaneous (n= 8).
The median duration of follow up was 8.5 months (range 2–64 months). Nine patients required readmission for pain control [DP indication: intra-abdominal malignancy (n= 4), pseudocyst (n= 4) and chronic pancreatitis (n= 1)] and two patients were readmitted for treatment of a wound infection. During the follow-up period, pancreatic endocrine deficiency developed in 11 patients (17%), requiring either insulin (n= 9) or an oral hypoglycaemic agent (n= 2). Pancreatic exocrine deficiency developed in nine patients (14%) requiring pancreatic exocrine supplementation. Four patients developed both endocrine and exocrine deficiencies. In six cases of post-operative pancreatic insufficiency, the resected specimen revealed chronic pancreatitis and/or pseudocyst.
Discussion
This study represents a comprehensive long-term review of distal pancreatectomy at a UK-based tertiary referral centre, providing a background for which the development of laparoscopic techniques in DP may be compared. While advances in preoperative imaging, surgical technique and peri-operative management over recent years may have enhanced resection rates for other pancreatic procedures,5 DP rates have remained static. The reason is likely to be multifactorial with limiting factors including the relative infrequency of distal pancreatic lesions and delayed presentation of distal pancreatic neoplasia.9 There is a suggestion of changing indications for DP possibly reflecting trends towards more conservative management of chronic pancreatitis with an increased number of cystic lesions noted using modern CT imaging.10 Indeed distal pancreatectomy is now rarely performed for chronic pancreatitis given the high rate of pain recurrence.
The increasing number of incidental pancreatic lesions reported by some centres, often in young individuals, has lead to a greater demand for laparoscopic DP, minimizing surgical intervention and targeting tumour eradication.11 In our series, the incidental finding of suspicious pancreatic lesions on CT imaging represented an important minority, with three out of five incidental lesions later shown to be premalignant or malignant. Accurate pre-operative diagnosis is vital in identifying appropriate surgical candidates and it is important that the potential benefits of minimal access surgery do not influence previous operative indications.11 In this series, approximately one-third of presumed cystic neoplasms were found to be non-neoplastic, suggesting that this subgroup may benefit from more invasive pre-operative investigation. Endoscopic ultrasound in combination with fine-needle biopsy and analysis of cystic fluid represents the most accurate pre-operative technique to differentiate benign from premalignant or malignant cystic lesions.12 In a recent multicentre trial, Brugge et al. demonstrated that carcinoembryonic antigen (CEA) was the most accurate indicator of pre-malignant or malignant cystic lesions using an optimal CEA cut-off level of 192 ng/ml.13 Cystic fluid CEA is significantly more accurate than cytology, morphology or any other combination of cystic fluid markers.13 Endoscopic ultrasound of distal pancreatic lesions is now being performed more regularly and may prove a valuable adjunct to current pre-operative assessment.
Despite the improved post-operative course and minimal cosmetic impact of laparoscopic techniques, the adequacy of laparoscopic procedures to assess, stage and resect malignant pancreatic lesions is uncertain. This has resulted in high rates of conversion to open DP when suspicious lesions have been noted intra-operatively.14,15 Previously, pancreatic malignancy has been regarded as a contraindication to laparoscopic resection, however, Fernandez-Cruz et al. demonstrated adequate resection margins in a small series of pancreatic neuroendocrine malignancies.15 Despite these promising results, long-term outcome data are still not available. In addition, it is not yet possible to quantify laparoscopic-specific risks in DP, such as port site metastasis, which have limited laparoscopic resection of other intra-abdominal cancers.16 Given the number of incidental lesions detected by modern imaging modalities and the difficulties in pre-operative diagnosis, a minimally invasive alternative for resection is certainly attractive and is more commonly being undertaken.
POPF simply represents a leak from the pancreatic remnant; however, more than 26 individual centre definitions have been reported making inter-centre comparison problematic.8 This distal pancreatectomy series presents incidence of POPF using the ISGPF definition with an overall grade B fistula rate of 8% (5% if trauma cases are excluded). The recent standardization of POPF is an important development in the assessment of pancreatic complications and should now form the accepted method of classification, allowing meaningful comparison between laparoscopic and open techniques. The ISGPF definition has received criticism relating to its dependence on subjective opinion and broad categorization.17 However, the definition provides a concise system to differentiate so-called ‘transient fistulae’ with no clinical sequelae and fistulae resulting in deviation from routine management.18 Previous studies report POPF rates of between 5% and 34% after open DP, depending on the definition used.19 In a recent multicentre series of 46 laparoscopic DP, POPF developed in 15%, requiring either prolonged (n= 5) or percutaneous drainage (n= 2).14 The authors of the largest multicentre laparoscopic DP series to date (n= 82) commented on their high rate of pancreatic complications when compared with open procedures, especially pancreatic fistula.20 The current study is limited by the exclusion of POPF Grade A (clinically insignificant fistulae) and this relates to retrospective data collection. As no association has been demonstrated between transient POPF and morbidity, it is questionable whether a clinically inconsequential finding should be included in morbidity data.
Selecting the most efficacious closure method of the pancreatic stump is limited by the lack of large randomized controlled trials. While no clear favourite has been established, a recent meta-analysis reported a trend in favour of staple closure.6 Identification and ligation of the pancreatic duct may not always be feasible but is the only significantly beneficial technique reported to date.21 In laparoscopic DP, the pancreatic stump is generally stapled as a result of the technical challenge of duct identification and ligation, possibly accounting for the high fistula rates reported.20 This series showed no difference between remnant closure techniques as reported in other retrospective studies.22 The low POPF rate may reflect the authors aim to routinely ligate the pancreatic duct and oversew the stump. Regardless of closure method, careful surgical technique is perhaps the major factor in minimizing POPF.
The use of somatostatin analogues to reduce POPF by reducing exocrine pancreatic secretion remains controversial. A recent meta-analysis by Connor et al. identified three small trials (n < 70) where somatostatin analogues were evaluated in DP showing no significant benefit over placebo.7 These trials did not categorize DP by indication or pathology and this may prove useful in targeting specific subgroups. Assessing the efficacy of somatostatin analogues in this study was not possible owing to the subjective indications for their administration; however, the continuing lack of evidence may limit their use further. Large multicentre trials are clearly indicated.
A recent small observational study (n= 9) suggested that pre-operative stenting of the pancreatic duct with the aim of reducing intraductal pressure may prevent POPF.23 This feasibility study was not sufficient to draw conclusions although the risks of endoscopic stenting, such as pancreatitis and stent blockage, may outweigh potential benefits.
Evaluating pancreatic remnant exocrine and endocrine function has received limited attention with only two studies assessing post DP pancreatic function. In a US study of 235 patients who underwent DP, 8% developed diabetes over an unspecified period.5 Hutchins et al. looked specifically at DP for chronic pancreatitis and using objective exocrine and endocrine assessment reported deficiency rates of 60% and 43%, respectively.24 This study reported a 14% exocrine and 17% endocrine deficiency rate based on clinical evaluation at follow up. The variation is clearly accounted for by methodological variance and patient group, resulting in difficulties with study comparison. Evaluating pancreatic remnant dysfunction may be beneficial to provide improved pre-operative information to patients; however, further research is required in this area.
In conclusion, distal pancreatectomy can be performed for a wide range of pancreatic disorders with low mortality, acceptable morbidity and preservation of pancreatic function in the majority of cases. The management of distal pancreatic cystic lesions may benefit from pre-operative endoscopic ultrasound and evaluation of aspirate CEA levels. Laparoscopic techniques have the potential to further improve the post-operative course, although specific indications should be considered and the comparatively high pancreatic fistula rates need to be addressed.
Conflicts of interest
None declared.
References
- 1.Pierce RA, Spitler JA, Hawkins WG, Strasberg SM, Linehan DC, Halpin VJ, et al. Outcomes analysis of laparoscopic resection of pancreatic neoplasms. Surg Endo. 2007;21:579–586. doi: 10.1007/s00464-006-9022-x. [DOI] [PubMed] [Google Scholar]
- 2.D'Angelica M, Are C, Jarnagin W, DeGregoris G, Coit D, Jaques D, et al. Initial experience with hand-assisted laparoscopic distal pancreatectomy. Surg Endo. 2006;20:142–148. doi: 10.1007/s00464-005-0209-3. [DOI] [PubMed] [Google Scholar]
- 3.Goh BK, Tan YM, Chung YF, Chow PK, Ong HS, Lim DT, et al. Non-neoplastic cystic and cystic-like lesions of the pancreas: may mimic pancreatic cystic neoplasms. ANZ J Surg. 2006;76:325–331. doi: 10.1111/j.1445-2197.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- 4.Ooi LL, Ho GH, Chew SP, Low CH, Soo KC. Cystic tumours of the pancreas: a diagnostic dilemma. ANZ J Surg. 1998;68:844–846. doi: 10.1046/j.1440-1622.1998.01481.x. [DOI] [PubMed] [Google Scholar]
- 5.Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–700. doi: 10.1097/00000658-199905000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knaebel HP, Diener MK, Wente MN, Büchler MW, Seiler CM. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–546. doi: 10.1002/bjs.5000. [DOI] [PubMed] [Google Scholar]
- 7.Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg. 2005;92:1059–1067. doi: 10.1002/bjs.5107. [DOI] [PubMed] [Google Scholar]
- 8.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes and prognostic factors. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melotti G, Butturini G, Piccoli M, Casetti L, Bassi C, Mullineris B, et al. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. 2007;246:77–82. doi: 10.1097/01.sla.0000258607.17194.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SJ, Alasadi R, Wayne JD, Rao S, Rademaker A, Bell R, et al. Preoperative evaluation of pancreatic cystic lesions: cost-benefit analysis and proposed management algorithm. Surgery. 2005;138:672–680. doi: 10.1016/j.surg.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Taylor C, O'Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, et al. Laparoscopic distal pancreatectomy: the Brisbane experience of forty-six cases. HPB. 2008;10:38–42. doi: 10.1080/13651820701802312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Cruz L, Blanco L, Cosa R, Rendon H. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumours? World J Surg. 2008;32:904–917. doi: 10.1007/s00268-008-9467-2. [DOI] [PubMed] [Google Scholar]
- 16.Curet MJ. Port site metastases. Am J Surg. 2004;186:705–712. doi: 10.1016/j.amjsurg.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 17.DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–939. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM. Clinical and economic validation of the international study group of pancreatic fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, et al. Pancreatic fistula rate after pancreatic resection: the importance of definitions. Dig Surg. 2004;21:54–59. doi: 10.1159/000075943. [DOI] [PubMed] [Google Scholar]
- 20.Mabrut JY, Fernandez-Cruz L, Azagra JS, Bassi C, Delvaux G, Weerts J, et al. Laparoscopic pancreatic resection: Results of a multicenter European study of 127 patients. Surgery. 2005;137:597–605. doi: 10.1016/j.surg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg. 2003;90:190–196. doi: 10.1002/bjs.4032. [DOI] [PubMed] [Google Scholar]
- 22.Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837–842. doi: 10.1016/j.gassur.2005.01.287. [DOI] [PubMed] [Google Scholar]
- 23.Abe N, Sugiyama M, Suzuki Y, Yamaguchi Y, Yanagida O, Masaki T, et al. Preoperative stenting for prophylaxis of pancreatic fistula development after distal pancreatectomy. Am J Surg. 2006;191:198–200. doi: 10.1016/j.amjsurg.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Hutchins RR, Hart RS, Pacifico M, Bradley NJ, Williamson RC. Long-term results of distal pancreatectomy for chronic pancreatitis in 90 patients. Ann Surg. 2002;236:612–618. doi: 10.1097/00000658-200211000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


