Abstract
Objectives:
This study aimed to illustrate the indications for, and types and outcomes of surgical portosystemic shunt (PSS) and/or Rex bypass in a single centre.
Methods:
Data were collected from children with a PSS and/or Rex bypass between 1992 and 2006 at Mount Sinai Medical Center, New York.
Results:
Median age at surgery was 10.7 years (range 0.3–22.0 years). Indications included: (i) refractory gastrointestinal bleeding in portal hypertension associated with (a) compensated cirrhosis (n= 12), (b) portal vein thrombosis (n= 10), (c) hepatoportal sclerosis (n= 3); (ii) refractory ascites secondary to Budd–Chiari syndrome (n= 3), and (iii) familial hypercholesterolaemia (n= 4). There were 20 distal splenorenal, four portacaval, three Rex bypass, two mesocaval, two mesoatrial and one proximal splenorenal shunts. At the last follow-up (median 2.9 years, range 0.1–14.1 years), one shunt (Rex bypass) was thrombosed. Two patients had died and two had required a liver transplant. These had a patent shunt at last imaging prior to death or transplant.
Conclusions:
Portosystemic shunts and Rex bypass have been used to manage portal hypertension with excellent outcomes. In selected children with compensated liver disease, PSS may act as a bridge to liver transplantation or represent an attractive alternative.
Keywords: Extrahepatic portal hypertension, compensated cirrhosis, surgical shunt, outcome
Introduction
Portal hypertension is defined by a pathological increase in portal pressure in which the pressure gradient between the portal vein and inferior vena cava (portal pressure gradient) is increased above the upper normal limit of 5 mmHg.1 An evidence-based approach for the management of portal hypertension in children does not exist. A panel of paediatric experts recently reviewed the most recent Baveno statement on management of portal hypertension in adults and modified it to be applicable to the paediatric setting.2 Portosystemic shunt (PSS) and Rex bypass have been performed in children with extrahepatic portal vein thrombosis when variceal bleeding is refractory to medical or endoscopic therapy. When there is intrinsic liver disease, the selection of candidates for PSS as opposed to transjugular intrahepatic portosystemic shunt (TIPS) or liver transplantation (LTx) requires careful consideration.
Portosystemic shunt can be non-selective (decompressing all portal hypertension; e.g. side-to-side portacaval, mesocaval and proximal splenorenal shunts) or selective (decompressing gastro-oesophageal varices through the short gastric veins, spleen and splenic vein to left renal vein, but maintaining the superiormesenteric vein flow to the liver, described as distal splenorenal shunt [DSRS] by Warren et al.3). The TIPS is a side-to-side PSS placed radiographically in the liver parenchyma joining the portal and hepatic veins. This technique emerged in the early 1990s as an alternative to surgical shunts. The encephalopathy rate after TIPS appears to be similar to that after non-selective surgical shunts.4 Although expertise in TIPS in adults is not uncommon, the procedure can be technically difficult in children, especially in children aged <5 years. The smaller size of the venous structures and liver and the presence of anatomic variants are limiting factors for success in paediatric patients.5
The superior mesenteric vein to left portal vein (Rex) bypass is described as re-directing the portal flow into the liver, and therefore should not, either anatomically or physiologically, be considered a shunt procedure.6,7 This operation restores portal flow by bringing mesenteric venous and splenic blood around the extrahepatic portal obstruction into a patent intrahepatic portal venous system. This restoration of the physiological hepatopedal flow may result in both developmental and neurological benefits in children.7,8 The present study reviews a single-centre experience of surgical PSS and Rex bypass procedures in children with respect to diagnosis, selection of candidates, type of surgical procedure, management and outcome.
Materials and methods
A retrospective chart review of patients presenting from January 1992 to December 2006 was undertaken after institutional review board approval. Data were collected from all patients seen in the paediatric liver programme who received a PSS and/or Rex bypass at Mount Sinai Medical Center, New York. Data collected included age, gender, diagnosis, type of surgical procedure and outcome. The aetiology of portal hypertension in children who received a PSS and/or Rex bypass was documented. In children with intrinsic liver disease, details of liver function tests were obtained to document the indication for PSS as opposed to LTx. In children with familial hypercholesterolaemia, information regarding ongoing treatment with lipid-lowering agents was also obtained. At last follow-up, information was collected regarding patency of shunt and need for transplantation. A shunt noted to be patent on imaging studies was considered to be a functioning shunt.
Surgical techniques and postoperative care
Surgical techniques for the various shunt procedures have been published in detail before.9–12 In the Rex bypass, the internal jugular vein of the patient was used as a conduit (Fig. 1). In one case where the internal jugular vein was not available, an 8F gortex graft was used. Rex bypass was performed from December 2004 onwards in this series. For mesoatrial shunt cases, 12F ring gortex grafts were used as conduits. In all surgical shunts, special care was taken to avoid kinking or twisting at the anastomosis. Interrupted sutures were used to avoid anastomotic stricture. Patients were anticoagulated postoperatively with low molecular weight heparin and aspirin in the short-term and aspirin alone in the longterm. Those with a pro-coagulant status were continued on low molecular weight heparin longterm. Children with familial hypercholesterolaemia were not given aspirin in the longterm as they were anticoagulated regularly as part of ongoing apheresis therapy.
Figure 1.
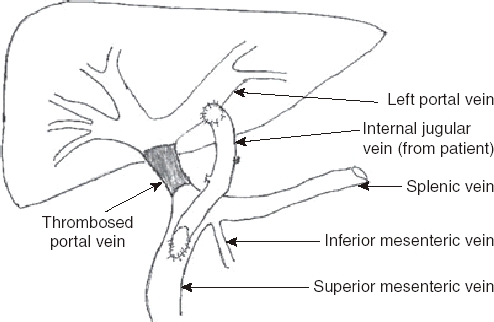
Schematic view of mesenterico–left portal vein (Rex) bypass using the patient's right internal jugular vein
Results
Thirty-two patients including two following LTx received a PSS and/or Rex bypass in our paediatric liver programme between January 1992 and December 2006. Indications included: (i) refractory gastrointestinal bleeding in portal hypertension associated with (a) compensated cirrhosis (n= 12), (b) portal vein thrombosis (n= 10), (c) hepatoportal sclerosis (n= 3); (ii) refractory ascites secondary to Budd–Chiari syndrome (n= 3), and (iii) familial hypercholesterolaemia (n= 4). There were 20 distal splenorenal, four portacaval, three Rex bypass, two mesocaval, two mesoatrial and one proximal splenorenal shunts. Median patient age at surgery was 10.7 years (range 0.3–22 years) and 22 patients were male. Fifteen patients were aged <10 years at surgery and eight were aged <5 years.
Twelve (patients 1–12) with intrinsic liver disease underwent PSS and 10 underwent DSRS (Table 1). The cause of liver disease and demographics of the shunt recipients are displayed in Table 1. Surgical shunts were performed electively in all but one patient. Patient 4 presented with uncontrollable upper gastrointestinal bleeding which failed sclerotherapy and Sengstaken–Blakemore tube placement. An emergency mesocaval shunt was performed using the iliac vein of a deceased donor between the superior mesenteric vein and the inferior vena cava. Patient 12, who had autoimmune hepatitis, inflammatory bowel disease and associated lymphoma, developed ascites and thrombocytopenia. He underwent a proximal splenorenal shunt with splenectomy at the request of the haemoncology team to facilitate chemotherapy. Liver function at the time of surgical shunting is displayed in Table 2. All shunt recipients in this category, other than patients 4 and 12, had stable compensated liver disease with preserved synthetic function. Low albumin was noted acutely in association with gastrointestinal bleeding and was >3 mg/dl preceding the bleeding episode in all patients except 4 and 12.
Table 1.
Demographics, diagnoses, types of portosystemic shunt and outcomes in children with intrinsic liver disease
| Patient | Sex | Diagnosis | Age at shunt, years | Type of shunt | Follow-up, years | Outcome |
|---|---|---|---|---|---|---|
| 1 | M | Biliary atresia | 2.8 | DSRS | 1.2 | Patent shunt |
| 2 | M | Biliary atresia | 3.9 | DSRS | 0.2 | Patent shunt |
| 3 | F | Biliary atresia | 5.5 | DSRS | 0.2 | Patent shunt |
| 4 | M | Biliary atresia | 8.8 | Mesocaval | 14.1 | Liver transplant |
| 5 | M | Congenital hepatic fibrosis | 16.7 | DSRS | 11.7 | Liver transplant |
| 6 | M | Congenital hepatic fibrosis | 6.4 | DSRS | 1.1 | Patent shunt |
| 7 | M | Cryptogenic cirrhosis | 18.0 | DSRS | 5.8 | Patent shunt |
| 8 | F | Cryptogenic cirrhosis | 22.1 | DSRS | 3.6 | Patent shunt |
| 9 | F | Autoimmune hepatitis | 18.6 | DSRS | 6.1 | Death (non-adherence) |
| 10 | M | Wilson's disease | 10.7 | DSRS | 0.3 | Patent shunt |
| 11 | F | Cystic fibrosis | 12.9 | DSRS | 4.7 | Patent shunt |
| 12 | M | Lymphoma | 17.9 | Proximal splenorenal | 0.2 | Death secondary to lymphoma |
M, male; F, female; DSRS, distal splenorenal shunt
Table 2.
Liver function tests in children with intrinsic liver disease who received a surgical portosystemic shunt
| Patient | Diagnosis | INR | Albumin, g/dl | Sodium, mmol/l | Total bilirubin, mg/dl | Direct bilirubin, mg/dl | Platelets × 103/µl |
|---|---|---|---|---|---|---|---|
| 1 | Biliary atresia | 1.2 | 1.8a | 137 | 1.0 | 0.4 | 42 |
| 2 | Biliary atresia | 1.2 | 4.3 | 136 | 0.4 | 0 | 93 |
| 3 | Biliary atresia | 1.5 | 2.1a | 135 | 0.6 | 0.2 | 97 |
| 4 | Biliary atresia | 1.3 | 2.5b | 141 | 9.8b | N/A | 81 |
| 5 | Congenital hepatic fibrosis | 1.2 | 4.2 | 138 | 1.7 | 0.4 | 110 |
| 6 | Congenital hepatic fibrosis | 1.2 | 3.8 | 138 | 0.2 | 0.4 | 81 |
| 7 | Cryptogenic cirrhosis | 1.3 | 3.7 | 139 | 2.9 | 1.7 | 59 |
| 8 | Cryptogenic cirrhosis | 1.6 | 2.9a | 141 | 1.5 | 0.7 | 37 |
| 9 | Autoimmune hepatitis | 1.3 | 4.3 | 130 | 1.4 | 0.3 | 49 |
| 10 | Wilson's disease | 1.5 | 3 | 137 | 2.5 | 0.7 | 80 |
| 11 | Cystic fibrosis | 1.3 | 3.3 | 140 | 0.6 | 0.2 | 102 |
| 12 | Lymphoma | 1.3 | 2.1c | 137 | 5.4c | 5.2 | 50 |
Low albumin was noted acutely in association with gastrointestinal bleeding and was >3 mg/dl preceding the bleed and during follow-up after the surgical shunt procedure
Patient 4 had an emergency mesocaval shunt for a gastrointestinal bleed that could not be controlled medically
Patient 12 had a portosystemic shunt to facilitate chemotherapy for lymphoma
INR, international normalized ratio; N/A, not available
Extrinsic portal hypertension
Ten patients had portal vein thrombosis (PVT) and required PSS or Rex bypass secondary to gastrointestinal bleeding refractory to medical treatment including sclerotherapy or band ligation. Two developed thrombosis of the portal vein after LTx and required DSRS secondary to hypersplenism with or without gastrointestinal bleeding post-LTx. All children had magnetic resonance imaging (MRI) to assess vascular anatomy before surgery. Three children, all <10 years of age, with PVT in whom the intrahepatic portion of the portal vein was patent on MRI received a Rex bypass. One of the 10 had pro-coagulant status and his shunt thrombosed despite anticoagulation; splenic artery embolization was subsequently performed. The remaining nine children were stable at last follow-up, with no recurrence in gastrointestinal bleeding.
Three patients with tense refractory ascites were referred to our centre. The first had acute onset abdominal swelling and was ultimately diagnosed with Budd–Chiari syndrome (post-sinusoidal portal hypertension) after imaging and liver biopsy locally. Magnetic resonance studies showed clotting of hepatic veins and the inferior vena cava, but the portal vein and hepatic artery were patent. This patient was also noted to have recurrent mouth ulcers, low protein C and was later diagnosed with Behçet's disease. Serum transaminase levels were <50 IU/l, total bilirubin was 1.5 mg/dl and prothrombin time 17 s. A mesoatrial shunt was performed for relief of refractory ascites and immunosuppressants administered for treatment of Behçet's disease. A young woman, with a history of taking herbal medications and oral contraceptives, presented with increasing abdominal distension and was diagnosed with Budd–Chiari syndrome. She had normal synthetic function with no cholestasis and received a mesocaval shunt. The last patient with Budd–Chiari syndrome was noted to have high-grade stenosis in the superior portion of the intrahepatic vena cava and hepatic veno-occlusive disease. He had recurrence of stenosis despite angioplasty twice locally and was referred to our centre for surgical shunt. A mesoatrial shunt was performed and his problems with ascites have resolved. Three children with hepatoportal sclerosis had DSRS secondary to gastrointestinal bleeding (two failed sclerotherapy and the third had massive gastric varices). All three were stable at last follow-up with no recurrence in bleeding or need for LTx.
Four patients with familial hypercholesterolaemia and absent low-density lipoprotein receptor had a portacaval shunt.11 The time required to reach a plateau in cholesterol lowering of 30–50% after shunt was approximately 6 months. The children also received apheresis, and all but one are on oral lipid-lowering agents (atorvastatin and ezetimibe). One has required replacement of her aortic valve and is on the heart and liver transplant list. Another has uncontrolled hypertension with asymptomatic single vessel coronary disease and the remaining two have minimal coronary artery disease.
None of the shunt recipients showed evidence of overt encephalopathy and all were attending school normally. No detailed testing had been performed to detect low-grade or subclinical encephalopathy in the longterm and it is possible that subtle neurocognitive or fine motor defects were missed. At last follow-up, at a median of 2.9 years (range 0.1–14.1 years), 27 of 32 were alive with a functioning shunt and had not required LTx after shunt surgery. Only one shunt was thrombosed. Two patients required LTx, of whom one had congenital hepatic fibrosis and required LTx secondary to recurrent cholangitis 8 years after the shunt, and the other, five days after undergoing emergency mesocaval shunt for life threatening gastrointestinal bleeding secondary to portal hypertension associated with biliary atresia. There were two deaths unrelated to PSS. One subject (patient 12) succumbed to lymphoma and the second (patient 9) to complications of end-stage liver disease. The latter was extremely non-adherent and not considered to be a suitable candidate for LTx. All four were noted to have a patent shunt at the last imaging study before death or transplant.
Discussion
This is one of the largest single-centre series illustrating the important role of surgical PSS and Rex bypass in paediatrics. Over a period of 15 years, 32 paediatric patients have undergone surgical procedures excluding LTx for complications related to portal hypertension and familial hypercholesterolaemia. The types of surgical procedures included DSRS, portacaval, proximal splenorenal, mesocaval and mesoatrial shunts and, more recently, the Rex bypass. The outcome has been excellent, with shunt occlusion in one and LTx in two patients.
The most important indication for PSS in our series was intrinsic liver disease with gastrointestinal haemorrhage refractory to medical treatment, endoscopic banding or sclerotherapy. Typically, these children are listed for and receive LTx in many centres. Avoiding or postponing LTx, especially in the current climate of organ shortage, is extremely valuable. The 12 children with intrinsic liver disease who had a surgical shunt rather than being listed for LTx had compensated liver disease. They were selected carefully based on the results of their liver function tests. Liver transplant was the preferred option for children with ascites, increasing cholestasis or any other evidence of decompensation of liver disease. All but one shunt recipient had good synthetic function of the liver and 10 of the 12 patients had serum direct bilirubin <2 mg/dl. Miga and colleagues reported that patients with biliary atresia and a serum bilirubin concentration <4 mg/dl at the first episode of variceal bleeding had a transplant-free survival rate of >80% for 4 years after this episode, and that 50% of those with bilirubin levels of 4–10 mg/dl were alive at 1 year, whereas survival in those with bilirubin levels >10 mg/dl was 50% at 4 months.13 Thus, the bilirubin level is of prognostic significance in biliary atresia: children with total bilirubin of <4 mg/dl are candidates for shunts, whereas those with higher bilirubin levels might be better served by transplant. The two patients with bilirubin >4 mg/dl who received a PSS in our series were not conventional shunt candidates.
In paediatric practice, a child with gastrointestinal bleeding secondary to portal hypertension despite endoscopic treatment is most likely to require shunt surgery. Traditionally, children with extrahepatic portal hypertension secondary to PVT have been the most common recipients of PSS. Two children in this series received PSS after LTx as they developed PVT-related complications after LTx. Medical treatment for variceal bleeding potentially includes beta-blockers and sclerotherapy/band ligation. When this fails, available treatment options include TIPS, shunt surgery and LTx. Henderson and colleagues reported the results of DIVERT, a multicentre study with 140 enrolled adult patients that compared TIPS with DSRS. Although survival rates were similar in both groups, rates of re-intervention, stenosis and thrombosis were significantly higher in the TIPS group compared with the DSRS group.14 However, TIPS can be technically difficult to perform in small children;5 in another paediatric series,15 only two of nine children in whom TIPS was attempted had a functioning shunt at last follow-up. In our series, 15 children were aged <10 years at the time of the shunt surgery and eight of 15 were aged <5 years. In young children with PVT, shunt surgery was therefore considered the treatment of choice and TIPS was not performed in any children in this series. Hepatoportal sclerosis in childhood mimics extrahepatic portal vein obstruction and is a cause of non-cirrhotic portal hypertension. The portal pressure measurements are suggestive of a pre-sinusoidal block, but a patent portal vein is demonstrated on angiography. Typically, there is abrupt narrowing of the intrahepatic portal branches, giving a ‘withered tree’ appearance.16 Characteristically, patients have symptoms of portal hypertension, but have preserved synthetic function of the liver.17 The three children in our series with hepatoportal sclerosis in whom surgical PSS was performed have not required LTx at last follow-up. Budd–Chiari syndrome is defined as non-cardiogenic hepatic venous outflow obstruction that results in ascites and liver enlargement.18 Medical therapy includes diuresis and treatment of predisposing factors. Percutaneous transhepatic angioplasty and venous stent placement have been successful in a small number of patients. Although TIPS has been used in adults, experience with the procedure in children is limited.19 Surgical portosystemic shunting to decompress portal hypertension caused by hepatic venous obstruction is well tolerated.20 Two patients in this series required mesoatrial shunting and one had a mesocaval shunt. All three had good synthetic function of the liver and were therefore not considered suitable candidates for LTx.
Familial hypercholesterolaemia is a historical indication for PSS in our centre and portacaval shunts were performed in four children before the year 2000. In the early 1980s, Starzl et al. reported that portacaval shunts performed in subjects with familial hypercholesterolaemia reduced the total serum cholesterol by 20–55%, and resulted in the reduction or disappearance of xanthomas and improvement in the growth of children, but data on the reversal of cardiovascular lesions were inconclusive.12 Subsequently, other forms of therapy have evolved, including plasmapheresis,21 statins, ezetimibe and LTx.22 Although portacaval shunts have an immediate hypolipaemic effect,23 this effect may be insufficient. Previous shunting can make LTx technically more difficult later in life;24 however, this has not been our experience.
The decision to perform a certain type of shunt depended on the indication for surgical shunt, the vascular anatomy of the subject and the time-frame. The DSRS was the procedure of choice in children with complications related to portal hypertension, in whom a surgical shunt was planned, as it allowed selective decompression of the variceal bed and preservation of the spleen. Mesocaval shunt was performed emergently in only one child with uncontrollable bleeding secondary to portal hypertension as a life-saving procedure. The first Rex bypass was performed in this series in children with extrahepatic portal hypertension in December 2004; three children received a Rex bypass. Only children who had a patent intrahepatic branch of the portal vein on MRI were considered suitable candidates for the Rex bypass. The decision about whether the Rex bypass procedure was feasible was made according to portal venography carried out in the operating room. Regardless of the diameter of the intrahepatic portal vein branches, if both right and left portal branches were patent, Rex bypass was performed. The Rex bypass is not indicated in children with intrinsic liver disease and patent portal vein. Proximal splenorenal shunt was performed in one child with lymphoma in whom splenectomy had been requested by the referring haemoncologist to facilitate chemotherapy. Portacaval shunts were performed only for hypercholesterolaemia in this series. The procedure of choice in Budd–Chiari syndrome was mesocaval shunt. Mesoatrial shunt had to be performed in two patients with Budd–Chiari syndrome in whom mesocaval shunt was technically not feasible as there was thrombosis in the vena cava.
In conclusion, PSS and the Rex bypass have important roles in the management of severe portal hypertension in children. Both DSRS and the Rex bypass can be used successfully to manage extrahepatic portal hypertension, particularly in small children in whom TIPS may be technically difficult. The outcome has been excellent in this series, with no mortality associated with the procedure and shunt occlusion in only one recipient at last follow-up. Portosystemic shunt was palliative in candidates with cirrhosis and portal hypertension who were not transplant candidates secondary to non-adherence, alcohol or drug addiction. Of note, in our centre, surgical PSS was used successfully in children with compensated cirrhosis to delay or avoid transplantation. Near-term morbidity and mortality from the underlying liver disease must be carefully considered in the decision-making process. In light of the organ shortage, PSS should continue to be considered as a component in the management strategy of children with compensated cirrhosis and may act as a bridge to LTx.
Conflicts of interest
None declared.
References
- 1.Shneider BL. Portal hypertension. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. New York: Cambridge University Press; 2007. pp. 138–162. [Google Scholar]
- 2.Shneider B, Emre S, Groszmann R, Karani J, McKiernan P, Sarin S, et al. Expert paediatric opinion on the Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. Pediatr Transplant. 2006;10:893–907. doi: 10.1111/j.1399-3046.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 3.Warren WD, Zeppa R, Fomon JJ. Selective trans-splenic decompression of gastroesophageal varices by distal splenorenal shunt. Ann Surg. 1967;166:437–455. doi: 10.1097/00000658-196709000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP, III, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20:46–55. doi: 10.1016/0270-9139(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 5.Fasulakis S, Rerksuppaphol S, Hardikar W, Vrazas J, Brooks M. Alternative technique for transjugular intrahepatic portosystemic shunt in a young child. Australas Radiol. 2006;50:447–450. doi: 10.1111/j.1440-1673.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 6.de Ville de Goyet J, Gibbs P, Clapuyt P, Reding R, Sokal EM, Otte JB. Original extrahilar approach for hepatic portal revascularization and relief of extrahepatic portal hypertension related to later portal vein thrombosis after pediatric liver transplantation. Longterm results. Transplantation. 1996;62:71–75. doi: 10.1097/00007890-199607150-00015. [DOI] [PubMed] [Google Scholar]
- 7.de Ville de Goyet J, Alberti D, Clapuyt P, Falchetti D, Rigamonti V, Bax NM, et al. Direct bypassing of extrahepatic portal venous obstruction in children: a new technique for combined hepatic portal revascularization and treatment of extrahepatic portal hypertension. J Pediatr Surg. 1998;33:597–601. doi: 10.1016/s0022-3468(98)90324-4. [DOI] [PubMed] [Google Scholar]
- 8.Mack CL, Zelko FA, Lokar J, Superina R, Alonso EM, Blei AT, Whitington PF. Surgically restoring portal blood flow to the liver in children with primary extrahepatic portal vein thrombosis improves fluid neurocognitive ability. Pediatrics. 2006;117:e405–12. doi: 10.1542/peds.2005-1177. [DOI] [PubMed] [Google Scholar]
- 9.Souba WW, Fink MP, Jurkovich GJ, Kaiser LR, Pearce WH, Pemberton JH, Soper NJ. Portal hypertension. In: Cho SC, Rikkers LF, editors. ACS Surgery. New York: WebMD; 2006. pp. 518–530. [Google Scholar]
- 10.de Ville de Goyet J, Clapuyt P, Otte JB. Extrahilar mesenterico. left portal shunt to relieve extrahepatic portal hypertension after partial liver transplant. Transplantation. 1992;53:231–232. [PubMed] [Google Scholar]
- 11.Botha JF, Campos BD, Grant WJ, Horslen SP, Sudan DL, Shaw BW, Jr, Langnas AN. Portosystemic shunts in children: a 15-year experience. J Am Coll Surg. 2004;199:179–185. doi: 10.1016/j.jamcollsurg.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Chase HP, Ahrens EH, Jr, McNamara DJ, Bilheimer DW, Schaefer EJ, et al. Portacaval shunt in patients with familial hypercholesterolaemia. Ann Surg. 1983;198:273–283. doi: 10.1097/00000658-198309000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miga D, Sokol RJ, Mackenzie T, Narkewicz MR, Smith D, Karrer FM. Survival after first oesophageal variceal haemorrhage in patients with biliary atresia. J Pediatr. 2001;139:291–296. doi: 10.1067/mpd.2001.115967. [DOI] [PubMed] [Google Scholar]
- 14.Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643–1651. doi: 10.1053/j.gastro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Heyman MB, LaBerge JM, Somberg KA, Rosenthal P, Mudge C, Ring EJ, Snyder JD. Transjugular intrahepatic portosystemic shunts (TIPS) in children. J Pediatr. 1997;131:914–919. doi: 10.1016/s0022-3476(97)70043-x. [DOI] [PubMed] [Google Scholar]
- 16.Carson JA, Tunell WP, Barnes P, Altshuler G. Hepatoportal sclerosis in childhood: a mimic of extrahepatic portal vein obstruction. J Pediatr Surg. 1981;16:291–296. doi: 10.1016/s0022-3468(81)80682-3. [DOI] [PubMed] [Google Scholar]
- 17.Isabel Fiel M, Thung SN, Hytiroglou P, Emre S, Schiano TD. Liver failure and need for liver transplantation in patients with advanced hepatoportal sclerosis. Am J Surg Pathol. 2007;31:607–614. doi: 10.1097/01.pas.0000213425.76621.f1. [DOI] [PubMed] [Google Scholar]
- 18.Dilawari JB, Bambery P, Chawla Y, Kaur U, Bhusnurmath SR, Malhotra HS, et al. Hepatic outflow obstruction (Budd. Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore) 1994;73:21–36. doi: 10.1097/00005792-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Molmenti EP, Segev DL, Arepally A, Hong J, Thuluvath PJ, Rai R, Klein AS. The utility of TIPS in the management of Budd. Chiari syndrome. Ann Surg. 2005;241:978–981. doi: 10.1097/01.sla.0000164180.77824.12. discussion 982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng LS, Peng QP, Li K, Ma XX, Zhao YF, Ye XX, et al. Management of severe Budd. Chiari syndrome: report of 147 cases. Hepatobiliary Pancreat Dis Int. 2004;3:522–525. [PubMed] [Google Scholar]
- 21.Gordon BR, Hudgins LC. Antman EM. Cardiovascular Therapeutics. Philadelphia, PA: WB Saunders; 2007. The steps beyond diet and drug therapy for severe hypercholesterolaemia; pp. 555–566. [Google Scholar]
- 22.Marais AD, Firth JC, Blom DJ. Homozygous familial hypercholesterolaemia and its management. Semin Vasc Med. 2004;4:43–50. doi: 10.1055/s-2004-822985. [DOI] [PubMed] [Google Scholar]
- 23.Cabre E, Navarro E, de Ramon M, Klaassen J, Planas R, Mingorance MD, et al. Impact of portacaval anastomosis on plasma fatty acid profile in cirrhosis: a randomized 24-month follow-up study. JPEN J Parenter Enteral Nutr. 1996;20:198–205. doi: 10.1177/0148607196020003198. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Santamaria M, Migliazza L, Gamez M, Murcia J, Diaz-Gonzalez M, Camarena C, et al. Liver transplantation in patients with homozygotic familial hypercholesterolaemia previously treated by end-to-sideportocaval shunt and ileal bypass. J Pediatr Surg. 2000;35:630–633. doi: 10.1053/jpsu.2000.0350630. [DOI] [PubMed] [Google Scholar]


