Abstract
Background:
Utilizing laparoscopy for major surgeries such as hepatectomy is a relatively new concept. Initially, benign pathologies dominated indications for resection. Our experience in an Australian setting with primarily malignant diagnoses is described.
Methods:
A review of patients' profiles, pathology, surgery and outcome was performed on 35 patients between December 2005 and August 2008. Data were collected and analysed retrospectively from medical records on a pre-designed datasheet.
Results:
Commonest indication for resection was colorectal metastasis (54%), 71% of all resections were for malignancy. Average operating time was 2 h 31 min (range 30 min–7 h, 15 min). Major morbidity consisted of one bile leak, two subphrenic abscesses and one pulmonary embolus. There were no deaths. Conversion to open was required in 20% and two patients required intra-operative blood transfusions. Average length of stay overall was 6.1 days (range 1–27), but as low as 2 days for some left lateral sectionectomies. Cessation of parenteral analgesia, return to normal diet and full mobility were achieved on average at 2.4, 2.3 and 2.8 days. Significant post-operative liver dysfunction was seen in two patients, which returned to normal by discharge. One patient died of disease progression 4 months after surgery. There were two involved margins in 35 patients (6%).
Conclusions:
Laparoscopic hepatectomy is a developing and safe technique in a select group of patients including those with malignancies, resulting in short hospital stays, rapid return to normal diet, full mobility and minimal morbidity with acceptable oncological parameters. This study is not comparative in nature, but provides evidence to support further investigation and establishment of this new technique for liver resection.
Keywords: laparoscopy, hepatectomy, oncology
Introduction
Laparoscopy has fundamentally changed general and particularly upper gastrointestinal surgery since its inception in the late 1980s.1 Gradually this approach has been applied to a wider range of operations and now many complex procedures initially beyond the skill of laparoscopists are being successfully completed.2–4 Liver resection is one area of recent development, and we report here our initial experience in performing laparoscopic liver resections. In contrast to most series, the majority of our patients had a resection for a malignant diagnosis. We describe our case mix and operative procedures, as well as post-operative outcome data including complications, return of function and length of stay and pathological resection margin.
Methods
We retrospectively identified patients who had undergone laparoscopic liver resection by querying a prospective case load database of one of the surgeons (V.U.), and by searching for medicare item number coding for the other surgeon in the group (P.E.) at each of the public and private hospitals in which these surgeons operate. A datasheet outlining clinical details, operative approach andpost-operative course was designed prospectively. Pre-operative data including liver function tests, presence, absence and aetiology of chronic liver disease and indication for surgery were recorded. Intra-operative details such as extent of resection, method for parenchymal transection, intra-operative blood loss and need for transfusion, and whether the procedure was completed laparoscopically were recorded. Post-operative course including complications, peak international normalized ratio (INR) and bilirubin, and length of stay, as well as return to full diet, full mobility and cessation of parenteral analgesia was also extracted from these patients' medical histories. The data were collected and analysed on Microsoft Excel™ for Macintosh.
Results
A total of 35 patients were operated on in three tertiary referral centres in Melbourne between December 2005 and August 2008. There were 17 males and 18 females, average age was 56.6 years, (range 32–79 years). Indication for surgery was most commonly colorectal carcinoma metastasis (19 cases, 54%). Three patients had resections for primary liver carcinoma [all hepatocellular carcinoma (HCC)], two for neuroendocrine metastases, one for metastatic rectal squamous cell carcinoma (SCC) and 10 resections for benign disease including five cases of focal nodular hyperplasia (thought pre-operatively to be hepatic adenomata), two haemangiomata (both thought pre-operatively to be colorectal metastases), one resection for hydatid disease, one for a hepatic cyst (also thought to be colorectal metastases), and one case of hepatic adenoma (see Tables 1,2). Overall, 25/35 (71%) cases had surgery for confirmed malignant diagnoses.
Table 1.
Demographics, resection and length of stay
| Resection | Age (years) median (range) | Gender (% male) | Length of Stay, (days) median (range) |
|---|---|---|---|
| Segmentectomy | 63 (45–74) | 100 | 4.5 (2–10) |
| Wedge resection | 66 (34–70) | 66 | 3 (1–7) |
| Left lateral sectionectomy | 52.5 (32–79) | 54 | 4 (2–8) |
| Right hemihepatectomy | 60 (32–74) | 33 | 11.5 (5–28) |
Table 2.
Resection by indication
| Indication | Resection | Count |
|---|---|---|
| Metastatic CRC (colon) | segmentectomy | 5 |
| wedge resection | 1 | |
| left lateral | 9 | |
| right hemihepatectomy | 4 | |
| HCC | segmentectomy | 1 |
| left lateral | 2 | |
| FNH | wedge resection | 1 |
| left lateral | 4 | |
| Neuroendocrine metastases | left lateral | 1 |
| right hemihepatectomy | 1 | |
| Haemangioma | wedge resection | 1 |
| left lateral & wedge caudate | 1 | |
| Hydatid | left lateral | 1 |
| Rectal SCC | left lateral | 1 |
| Hepatic cyst | left lateral | 1 |
| Hepatic adenoma | right hemihepatectomy | 1 |
CRC, colorectal cancer; HCC, hepatocellular carcinoma; FNH, focal nodular hyperplasia; SCC, squamous cell carcinoma.
The most common operation performed was the left lateral sectionectomy (20/35, 57%) but we also completed six right hemiheptatectomies, six segmental and three wedge resections. The harmonic scalpel (Harmonic ACE™; Ethicon Endosurgery, Johnson & Johnson, Langhorne, PA, USA) was used in all cases but three, and an average 4.8 stapler firings per patient were used in 28 cases where this method was also used. The stapler was used both for parenchymal transection and for control of vascular pedicles. At most, 14 stapler firings were used in one right hemihepatectomy (see Fig. 1 for frequency distribution of staple firings). Staplers were not used for the three wedge resections, three segmentectomies and one left lateral sectionectomy which were all completed with the harmonic scalpel and diathermy alone. All specimens were retrieved using an endoscopic pouch to minimize wound contamination. The average operating time (excluding right hemihepatectomies) was 2 h 4 min (range 30 min–4 h 10 min). The right hemihepatectomies took between 3 h and 7 h 15 min (average 4 h 39 min). Average time for all cases was 2 h 31 min. The Pringle manoeuvre was not used in any of the cases. Two cases required intra-operative blood transfusion, of two units during a left lateral sectionectomy where the staple line applied to the parenchyma was not haemostatic in a Child–Pugh A cirrhotic (the operation was completed at open approach) and one unit during a right hemihepatectomy. The open conversion rate was 20% (7 cases), because of uncontrolled bleeding in three cases (again from intolerable loss from the parenchymal surface that had been divided with staplers), one right hemihepatectomy was abandoned as access to the inferior vena cava (IVC) and caudate branches was not safely obtainable, in one patient with myasthenia gravis, pneumoperitoneum was not able to be sustained and the procedure converted to open, one tumour margin was encroached on laparoscopically and one fatty liver was unable to be safely manipulated. There were no intraoperative deaths.
Figure 1.
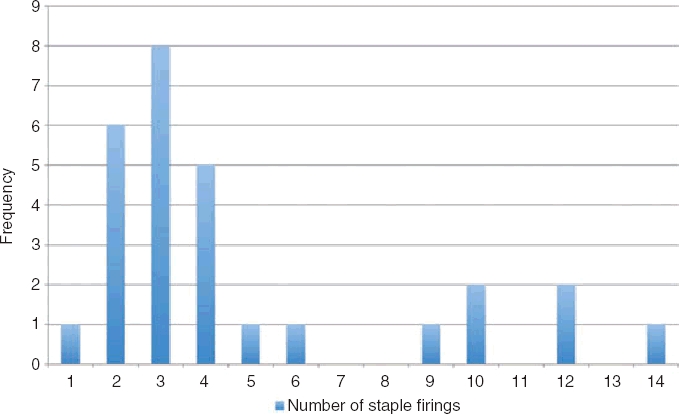
Frequency distribution of stapler firings
Post-operative liver dysfunction was seen to some degree after all the right hemihepatectomies. INR peaked at 1.8 in two cases both at day 3, whereas in the other cases, INR only reached a maximum of 1.4. Bilirubin peak was 125 micromol/l and 128 micromol/l at day 8 in the first and day 15 in the second case in which INR reached 1.8. Both these values had normalized prior to discharge. We only operated on one patient with pre-operative chronic liver disease (Child–Pugh A, secondary to hepatitis C) who had a left lateral sectionectomy for an HCC completed at an open approach after bleeding from the staple line (requiring transfusion as above). This patient's post-operative course was unremarkable without post-operative deterioration in his liver impairment. One patient who had a right hemihepatectomy developed a bile leak and subphrenic abscess on day 11, with an associated sympathetic effusion without respiratory compromise. Radiological drainage of the abscess, endoscopic retrograde cholangiopancreatography (ERCP) and a stent were performed with subsequent good resolution of abscess and control of the bile leak. Another patient also developed a subphrenic collection with pleural effusion and pneumonia (but did not have a bile leak), this also resolved with radiological drainage. There was one pulmonary embolus after a left lateral sectionectomy for a haemangioma. Two patients required respiratory support for 24 h after a segmentectomy for an HCC and a segmentectomy for a colorectal metastasis, secondary to co-morbid lung disease. One patient died with metastatic progression 4 months post-operatively, (see Table 3 complications).
Table 3.
Complications following laparoscopic resection
| Age | Resection | Indication | Complication |
|---|---|---|---|
| 47 | Left lateral & wedge caudate | haemangioma | pulmonary embolus |
| 52 | Segmentectomy | metastatic CRC | required respiratory support |
| 74 | Segmentectomy | HCC | required respiratory support |
| 74 | Right hemihepatectomy | metastatic rectal ca | liver dysfunction (INR 1.8, bili 128) |
| 54 | Right hemihepatectomy | metastatic CRC | bile leak, liver dysfunction (INR 1.8, bili 125), subphrenic collection, pleural effusion, required ERCP |
| 61 | Right hemihepatectomy | metastatic CRC | subphrenic collection, pleural effusion, pneumonia |
CRC, colorectal cancer; HCC, hepatocellular carcinoma.
Average length of stay was 4.6 days (range 1 to 10 days) excluding the right hemihepatectomies and 6.1 days overall (right hemihepatectomies length of stay was on average 13.1 days, range 5 to 27 days), (Table 1). On average, patients had returned to a normal diet on day 2.3, could mobilize fully without assistance on day 2.8, and had ceased opiate use on day 2.4.
The pathological specimens removed ranged from 12 mm to 200 mm in diameter, with a range of weights from 34 g to 1142 g (average 336 g). The average resection margin for malignant disease was 19.6 mm (median: 15 mm, range: involved – 80 mm), with one margin involved following a left lateral sectionectomy for a rectal SCC metastasis, and 1 margin involved following a right hemihepatectomy that was converted to the open approach as the dissection was felt to be impinging on the margin at laparoscopy.
Discussion
Laparoscopic liver resection has certainly been shown to be safe for benign conditions.5,6 Our series shows the laparoscopic approach can be applied to liver resections for malignant conditions, as it is a safe and effective procedure with acceptable pathological resection margins. Only six patients suffered 12 complications from our group of 35 (two patients suffered five and three complications, respectively, after right hemihepatectomies), this morbidity compares favourably with the open approach.7–11 Importantly, this group did not only represent patients with benign liver lesions in whom the indication for surgery was arguable. Criticism of the laparoscopic approach has often cited inappropriate liver resection in cases where open surgery would not otherwise be offered.12 There are certainly a number of series of resections for benign conditions,13–16 but this is one of the first reports in the literature of cases performed for primarily malignant conditions. In our series 71% of patients had a malignant diagnosis. Our margin was an average 19.6 mm (median 15 mm), with only two margins involved. One patient had a margin less than 5 mm, and four further patients had a margin of less than 10 mm, all other patients had a margin of 10 mm or more. In addition, margins are artificially lowered when the stapler is used as tissue is destroyed at the transection line.17 The laparoscopic approach does not compromise resection margins and achieves an adequate oncologic resection.
Patients recover rapidly from laparoscopic surgery.18 Our patients had ceased parenteral analgesia and returned to a full diet by about day 2, and full mobility by day 3.
Ours, and most series report primarily on left lateral sectionectomies, and this operation among all the liver resections is that most suitable for laparoscopic surgery.19,20 While right hemihepatectomy can also be performed laparoscopically, this is a much more technically demanding exercise,12,21 and our experience is limited at this stage. Certainly left lateral sectionectomy can be performed in an acceptable time period (average just over 2 h), and with acceptable disposable usage (75% of cases used four staplers or fewer) so as not to invalidate it as an expensive and time consuming waste of resources in the public sector despite earlier concerns about cost.22 A full cost analysis should, however, be undertaken, considering increased costs of disposables as well as reduced post-operative stay, in combination with return to work data. This is however beyond the scope of this report. This procedure has been shown in this and other series to be safe and well tolerated by patients, with acceptable oncologic parameters and applicable in most indications for liver resection.10,11,19,23 We only have one patient in our series with cirrhosis, and in that patient the stapler appeared to be less haemostatic necessitating conversion to the open approach. Laparoscopic hepatectomy has been successfully completed in patients with underlying liver disease24 but with some reports of high complication rates.25,26 It may be a good option if transplantation is considered an eventual option for patients with cirrhosis and HCCs.27,28 There are other reports that this approach may be superior to open surgery for cirrhotics, especially with respect to blood loss (from the effect of the hydrostatic pressure of the pneumoperitoneum against the transected parenchymal surface), and with less deterioration in ascites from less disruption of lymphatics in the abdominal wall.29
Short-term data on 2- and 5-year survival for laparoscopic hepatectomies has been reported.7,8,30 It appears survival and outcome is equivalent to the open approach.8 Our data here does not include follow up, but does demonstrate that oncologically the resection achieves adequate margins and that patients experience similar morbidity [if not less31] than the open approach. Consideration should be given to the laparoscopic approach to resection for malignant disease, particularly if in a technically easily accessible location.32 Specific issues related to laparoscopy such as port sites metastases and intraabdominal dissemination of tumour have not been widely proven in the literature.11 Advances in device technology such as improvements in endostaplers also promises increased safety in the future,17 and the harmonic scalpel is also an invaluable aid.33
Conclusion
Laparoscopic liver surgery is a viable approach to resection of most pathologies that affect the liver, and we have shown here it can be safely and acceptably performed for malignant diagnoses too. It is particularly suited to resection of the left lateral section,20 and can be done in a timely manner with minimal morbidity and good patient outcomes with early discharge and rapid return to a normal diet and mobility. It remains a procedure that should only be carried out by highly trained specialist hepatobiliary surgeons with appropriate laparoscopic training.34
Conflicts of interest
None declared.
References
- 1.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 2.Croce E, Azzola M, Russo R, Golia M, Angelini S, Olmi S. Laparoscopic liver tumour resection with the argon beam. Endosc Surg Allied Technol. 1994;2:186–188. [PubMed] [Google Scholar]
- 3.Rau HG, Buttler E, Meyer G, Schardey HM, Schildberg FW. Laparoscopic liver resection compared with conventional partial hepatectomy – a prospective analysis. Hepatogastroenterology. 1998;45:2333–2338. [PubMed] [Google Scholar]
- 4.Gugenheim J, Mazza D, Katkhouda N, Goubaux B, Mouiel J. Laparoscopic resection of solid liver tumours. Br J Surg. 1996;83:334–335. doi: 10.1002/bjs.1800830311. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242–248. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 6.Ardito F, Tayar C, Laurent A, Karoui M, Loriau J, Cherqui D. Laparoscopic liver resection for benign disease. Arch Surg. 2007;142:1188–1193. doi: 10.1001/archsurg.142.12.1188. discussion 1193. [DOI] [PubMed] [Google Scholar]
- 7.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs. open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 8.Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, et al. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–544. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 9.Mala T, Edwin B, Gladhaug I, Fosse E, Soreide O, Bergan A, et al. A comparative study of the short-term outcome following open and laparoscopic liver resection of colorectal metastases. Surg Endosc. 2002;16:1059–1063. doi: 10.1007/s00464-001-9176-5. [DOI] [PubMed] [Google Scholar]
- 10.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–242. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 11.Laurence JM, Lam VW, Langcake ME, Hollands MJ, Crawford MD, Pleass HC. Laparoscopic hepatectomy, a systematic review. ANZ J Surg. 2007;77:948–953. doi: 10.1111/j.1445-2197.2007.04288.x. [DOI] [PubMed] [Google Scholar]
- 12.Gayet B, Cavaliere D, Vibert E, Perniceni T, Levard H, Denet C, et al. Totally laparoscopic right hepatectomy. Am J Surg. 2007;194:685–689. doi: 10.1016/j.amjsurg.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, et al. Laparoscopic liver resection of benign liver tumors. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 14.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–811. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Berends FJ, Meijer S, Prevoo W, Bonjer HJ, Cuesta MA. Technical considerations in laparoscopic liver surgery. Surg Endosc. 2001;15:794–798. doi: 10.1007/s004640090094. [DOI] [PubMed] [Google Scholar]
- 16.Katkhouda N, Hurwitz M, Gugenheim J, Mavor E, Mason RJ, Waldrep DJ, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229:460–466. doi: 10.1097/00000658-199904000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko H, Otsuka Y, Takagi S, Tsuchiya M, Tamura A, Shiba T. Hepatic resection using stapling devices. Am J Surg. 2004;187:280–284. doi: 10.1016/j.amjsurg.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Dulucq JL, Wintringer P, Stabilini C, Berticelli J, Mahajna A. Laparoscopic liver resections: a single center experience. Surg Endosc. 2005;19:886–891. doi: 10.1007/s00464-004-2044-3. [DOI] [PubMed] [Google Scholar]
- 19.Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94:58–63. doi: 10.1002/bjs.5562. [DOI] [PubMed] [Google Scholar]
- 21.O'Rourke N, Fielding G. Laparoscopic right hepatectomy: surgical technique. J Gastrointest Surg. 2004;8:213–216. doi: 10.1016/j.gassur.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Hashizume M, Shimada M, Sugimachi K. Laparoscopic hepatectomy: new approach for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2000;7:270–275. doi: 10.1007/s005340070048. [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke N, Shaw I, Nathanson L, Martin I, Fielding G. Laparoscopic resection of hepatic colorectal metastases. HPB (Oxford) 2004;6:230–235. doi: 10.1080/13651820410023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim S, Chen CL, Wang SH, Lin CC, Yang CH, Yong CC, et al. Liver resection for benign liver tumors: indications and outcome. Am J Surg. 2007;193:5–9. doi: 10.1016/j.amjsurg.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90–97. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HY, Juan CC, Ker CG. Laparoscopic liver surgery for patients with hepatocellular carcinoma. Ann Surg Oncol. 2008;15:800–806. doi: 10.1245/s10434-007-9749-1. [DOI] [PubMed] [Google Scholar]
- 27.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 29.Dagher I, Lainas P, Carloni A, Caillard C, Champault A, Smadja C, et al. Laparoscopic liver resection for hepatocellular carcinoma. Surg Endosc. 2008;22:372–378. doi: 10.1007/s00464-007-9487-2. [DOI] [PubMed] [Google Scholar]
- 30.Teramoto K, Kawamura T, Takamatsu S, Nakamura N, Kudo A, Noguchi N, et al. Laparoscopic and thoracoscopic approaches for the treatment of hepatocellular carcinoma. Am J Surg. 2005;189:474–478. doi: 10.1016/j.amjsurg.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Buell JF, Rosen S, Yoshida A, Labow D, Limsrichamrern S, Cronin DC, et al. Hepatic resection: effective treatment for primary and secondary tumors. Surgery. 2000;128:686–693. doi: 10.1067/msy.2000.108220. [DOI] [PubMed] [Google Scholar]
- 32.Bryant R, Laurent A, Tayar C, van Nhieu JT, Luciani A, Cherqui D. Liver resection for hepatocellular carcinoma. Surg Oncol Clin N Am. 2008;17:607–633. ix. doi: 10.1016/j.soc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Dulucq JL, Wintringer P, Stabilini C, Mahajna A. Isolated laparoscopic resection of the hepatic caudate lobe: surgical technique and a report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2006;16:32–35. doi: 10.1097/01.sle.0000202183.27042.63. [DOI] [PubMed] [Google Scholar]
- 34.Cherqui D. Laparoscopic liver resection. Br J Surg. 2003;90:644–646. doi: 10.1002/bjs.4197. [DOI] [PubMed] [Google Scholar]


