Abstract
LXRα is a member of a nuclear receptor superfamily that regulates transcription. LXRα forms a heterodimer with RXRα, another member of this family, to regulate the expression of cholesterol 7α-hydroxylase by means of binding to the DR4-type cis-element. Here, we describe a function for LXRα as a cAMP-responsive regulator of renin and c-myc gene transcriptions by the interaction with a specific cis-acting DNA element, CNRE (an overlapping cAMP response element and a negative response element). Our previous studies showed that renin gene expression is regulated by cAMP, at least partly, through the CNRE sequence in its 5′-flanking region. This sequence is also found in c-myc and several other genes. Based on our cloning results using the yeast one-hybrid system, we discovered that the mouse homologue of human LXRα binds to the CNRE and demonstrated that it binds as a monomer. To define the function of LXRα on gene expression, we transfected the renin-producing renal As4.1 cells with LXRα expression plasmid. Overexpression of LXRα in As4.1 cells confers cAMP inducibility to reporter constructs containing the renin CNRE. After stable transfection of LXRα, As4.1 cells show a cAMP-inducible up-regulation of renin mRNA expression. In parallel experiments, we demonstrated that LXRα can also bind to the homologous CNRE in the c-myc promoter. cAMP promotes transcription through c-myc/CNRE:LXRα interaction in LXRα transiently transfected cells and increases c-myc mRNA expression in stably transfected cells. Identification of LXRα as a cAMP-responsive nuclear modulator of renin and c-myc expression not only has cardiovascular significance but may have generalized implication in the regulation of gene transcription.
LXRα is a member of a superfamily of nuclear receptors. LXRα has been shown to form heterodimer with RXR, bind to a sequence (5′-GGTTTAAATAAGTTCA-3′) called DR4-type LXR-responsive element (DR4/LXRE) (1, 2), and induce transcription. The DR4/LXRE is located in the cholesterol 7α-hydroxylase gene, which encodes the rate-limiting enzyme in the bile acid synthesis pathway, and an in vivo study using mouse suggests that LXRα plays a role in the regulation of cholesterol metabolism (3–5).
Expression of renin gene, an important cardiovascular factor, is controlled in a tissue-specific and developmentally regulated manner (6). We have previously documented that renin gene expression depends on the complex interaction of two transcriptional factors (a cAMP-inducible transcription activator and a repressor of transcription) with a cis-acting DNA element in the promoter region of the renin gene, known as the CNRE (an overlapping cAMP response element and a negative response element) region (TACCTAACTTGGTCTCACAGGCTAGAATTTATC; −619 to −588 of the transcriptional start site of mouse Ren-1d gene) (7–9). The core binding sequence for the cAMP-inducible transcription activator is identified as TCTCACAG (−607 to −600), and exhibits a low homology to the classic consensus cAMP response element (CRE) (TGACGTCA) described in other cAMP-responsive genes (10). The human and rat renin genes also contain similar sequences, suggesting that their tissue expression may be regulated by similar cis–trans interactions (11). Further analysis of sequence homology revealed that CNRE is also present in the promoters of several cAMP-responsive genes, such as c-myc (12, 13), uPA (14), tyrosine aminotransferase (15), and PRL (16). In this paper, we report the successful cloning of a CNRE binding protein using the yeast one-hybrid approach by screening a mouse kidney cDNA library. Sequence analysis of the CNRE binding protein revealed that the cloned CNRE binding protein is the mouse homologue of human LXRα. The results of our functional assays demonstrate a role for LXRα as a cAMP-responsive regulator of renin and c-myc gene expressions.
Materials and Methods
Electrophoretic Mobility Shift Assay (EMSA).
mLXRα was synthesized in an in vitro transcription/translation system (Promega). EMSA was performed as described previously (8, 9). Anti-LXRα antibody was provided by J. M. Lehmann (Glaxo Wellcome Research and Development, Research Triangle Park, NC) (4). Purified recombinant CRE binding protein (CREB) and antibodies for CREB and ATF were purchased from Santa Cruz Biotechnology. Double-stranded oligonucleotides corresponding to mouse Ren-1d CNRE, mutated human renin CNRE (mCNRE, 5′-TTCACCCACCTAGCTCTGATGTTTCTTGAGATTTA-3′, mutated nucleotides are shown in bold italics), human c-myc CNRE (5′-ATGATTTATACTCACAGGACAAGG-3′) (12), and human fibronectin CRE (FN/CRE, 5′-CTAGCTTACCCACAGTCCCCCGTGACGTCACCCGGC-3′) (17) were synthesized by GIBCO/BRL.
Stable Transfections.
The mouse renin-expressing cell line (As4.1, ATCC CRL2193) was cultured as described previously (18). Establishment of stable transfectants of As4.1 cells expressing GFP/mLXRα and GFP alone was performed as described previously (19).
Plasmid.
A three tandem-repeated segment of the human fibronectin promoter region (−192 to −160) carrying a consensus sequence of CREB/ATF binding site (FN/CRE) was cloned upstream of pTK-luc to construct pFN/CRE-TK-luc plasmid. The human renin promoter (−580 to +16)-luciferase plasmid (phuREN-luc) was kindly provided by K. W. Gross (Roswell Park Cancer Institute, Buffalo, NY). The human c-myc promoter-luciferase plasmid (phuMYC-luc) was constructed by inserting a human c-myc promoter fragment (−2290 to +510 relative to the major transcription start site) excised from pPLF-CAT into the upstream of luciferase gene (21). The expression vectors for CREB (pc-CREB) and mouse RXRα (pc-mRXRα) were generous gifts from M. R. Montiminy (Joslin Diabetes Center, Boston, MA) and W. Chin (Brigham and Women's Hospital, Boston, MA), respectively.
Site-Directed Mutagenesis of mLXRα.
mLXRα cDNA was cloned into eukaryotic expression vector pcDNA3.1 (Invitrogen), and this mLXRα expression plasmid (pc-mLXRα) was used as a template to construct mutations in the mLXRα by oligonucleotide-directed mutagenesis (22).
Transient Transfections.
As4.1 cells were cotransfected with 1.0 μg of pc-mLXRα, 1.0 μg of luciferase reporter plasmid, and 0.1 μg of β-galactosidase expression plasmid by Fugene-6 (Roche Molecular Biochemicals) when cells reached 80% confluence in six-well cell culture plates. Twenty-four hours after transfection, cells were treated for 12 h with vehicle or 1 mM 8-Br-cAMP, and cell extracts were prepared with cell lysis buffer (Promega). Cell extracts were assayed for luciferase activity and for β-galactosidase activity. The luciferase activity of each sample was normalized against β-galactosidase activity.
Results
Yeast One-Hybrid Cloning of a CNRE Binding Protein and Identification as LXRα.
We created a cDNA library from DBA/2J mouse kidney cortex in the pGAD10 fusion vector (CLONTECH) that drives the expression of chimeric proteins containing the amino-terminal transcriptional activator domain of GAL4. cDNA library plasmids were transfected into a yeast strain bearing a three tandem-repeat segment of mouse Ren-1d CNRE upstream of HIS3 reporter gene (23, 24). The plasmid DNAs were isolated from positive clones in the first screening among more than two million primary colonies screened, and then transfected into a second yeast strain containing a trimerized CNRE sequence upstream of the LacZ gene from each individual colony to identify double-positive cDNA plasmids.
One positive clone, KND9, contains a 1.5-kb insert including a zinc-finger DNA binding domain and shares high homology to RLD-1, a rat nuclear receptor, that binds to the thyroid hormone-induced elements (25). Because the KND9 cDNA lacks the first ATG and the 5′-end of this clone is identical to the 3′-end of a mouse expressed sequence tag (EST) clone (aa415858) of cDNA bank at Washington University, we performed RT-PCR using total RNA from mouse kidney to obtain the full-length KND9 cDNA. Sequencing of this determined that the protein is 91% identical to human LXRα and 97% identical to rat RLD-1 (data not shown) (1, 25), indicating that this clone is the mouse homologue of human LXRα (mLXRα).
Specific and Preferential Binding of LXRα to CNRE as a Monomer.
We performed EMSA with 32P-labeled renin CNRE and mLXRα prepared by an in vitro transcription/translation reaction to determine whether mLXRα specifically binds to the CNRE. Incubation of mLXRα with radiolabeled CNRE probe produced a shifted band, which was dramatically reduced by the addition of a monoclonal antibody to LXRα (Fig. 1A). To examine the characteristics of mLXRα binding, we compared the binding affinity of mLXRα to CNRE with that to the classical CRE (FN/CRE). The binding affinity of mLXRα with CNRE was stronger than that with FN/CRE, whereas the affinity of CREB with CNRE was weaker than that with FN/CRE (Fig. 1B). These results indicate that mLXRα preferentially binds to CNRE as a CNRE binding protein.
Figure 1.
Specific and preferential binding of mLXRα to CNRE as a monomer. (A) EMSA with mLXRα produced by in vitro transcription/translation and 32P-labeled CNRE probe. Anti-LXRα monoclonal antibody abolished the complexes formed by mLXRα with CNRE. (B) EMSA using mLXRα or CREB with or without the monoclonal antibodies to LXRα or CREB. CNRE and the classical CRE (FN/CRE) were used as probes. mLXRα preferentially bound to CNRE and the binding affinity of CREB to CNRE was very weak. (C) EMSA using mLXRα and 32P-labeled full CNRE probe with different fragments of the full CNRE as cold competitors. The whole consensus sequence (del/CNRE-6) is required for the efficient binding of mLXRα to the CNRE. (D) EMSA with mLXRα and N-terminal deletion mutant of mLXRα (delN-mLXRα). DR4/LXRE and del/CNRE-6 were used as probes. Although DR4/LXRE:mLXRα interaction is homodimeric, mLXRα binds to CNRE as a monomer.
Previously, our studies identified a consensus sequence in the CNRE as 5′-TNN(T/G)TC(C/T)CA(C/G)AGG-3′ (−611 to −599 of the transcriptional start site of mouse Ren-1d gene), which contains the 8-bp core binding sequence [TC(C/T)CA(C/G)AG, −607 to −600] (7–9, 26). To determine the minimal binding site required for binding of mLXRα to the CNRE, we performed EMSA using different fragments of the full CNRE as cold competitors. The mLXRα binding could be competed efficiently by unlabeled full CNRE (−619 to −588), del/CNRE-1 (−619 to −599), del/CNRE-2 (−611 to −588), del/CNRE-3 (−614 to −596), del/CNRE-4 (−613 to −597), -5 (−612 to −598), and -6 (−611 to −599), but only slightly by del/CNRE-7 (−610 to −599), del/CNRE-8 (−611 to −600), and -9 (−610 to −600) (Fig. 1C). Because del/CNRE-6 corresponds to the whole consensus CNRE sequence, these results indicate that the 8-bp core binding sequence is not sufficient but the 13-bp consensus binding sequence is required for the efficient binding of mLXRα to the CNRE.
It has been reported that deletion of the N-terminal (A/B) domain in the nuclear receptor does not affect its DNA binding and dimerization characteristics (27). Therefore, to clarify whether mLXRα binds as a monomer or homodimer to the CNRE, we constructed a mutant in which the N-terminal domain of mLXRα was deleted (named as delN-mLXRα lacking the first 43 amino acids). To compare the mode of mLXRα binding to DR4/LXRE and CNRE, we performed EMSA with 32P-labeled DR4/LXRE or the minimal binding site of CNRE (del/CNRE-6) as the probes. mLXRα and delN-mLXRα were prepared by in vitro transcription/translation. delN-mLXRα bound to both binding sites with affinities similar to those of mLXRα and formed protein-DNA complexes with a different mobility than that of the wild-type receptor (Fig. 1D; compare lane 1 with lane 3 and lane 4 with lane 6). A previous finding suggested that mLXRα can bind to the DR4/LXRE as a homodimer (4). As expected, the mixing of mLXRα and delN-mLXRα with DR4/LXRE resulted in the formation of three shifted bands, DR4/LXRE-bound mLXRα/delN-mLXRα heterodimers, which migrated with a mobility intermediate between those of the homodimeric mLXRα and delN-mLXRα complexes (Fig. 1D, lane 2). In contrast, the 32P-labeled del/CNRE-6 did not give rise to a complex with an intermediate mobility, indicating that both mLXRα and delN-mLXRα bind to CNRE as monomers (Fig. 1D, lane 5). Moreover, consistent with previous findings (1, 4, 25), mLXRα could form heterodimers with mRXRα that bind to the DR4/LXRE. With CNRE as the probe, mixing of mRXRα with mLXRα had no effect on the formation of DNA-bound mLXRα monomer (data not shown), providing further evidence that mLXRα binds to the CNRE as a monomer.
Stably Expressed LXRα Increases Nuclear Binding Activity to CNRE and Renin mRNA Levels.
We next used a clonal renin-expressing cell line, As4.1 cells, which is derived from a kidney juxtaglomerular tumor of a mouse renin promoter/SV40 T antigen transgenic mouse (18). We constructed stable transfectants of As4.1 cells expressing mLXRα (LXRα/As4.1 cells) to examine a possible effect of mLXRα on renin gene expression. Western blot analysis confirmed that mLXRα protein was expressed in LXRα/As4.1 cells but not in GFP-mocked stable As4.1 cells (GFP/As4.1 cells) (Fig. 2A). We incubated radiolabeled CNRE with nuclear extracts from LXRα/As4.1 cells. Nuclear extracts isolated from control GFP/As4.1 cells failed to produce a shifted band, whereas nuclear extracts from LXRα/As4.1 cells produce a strong DNA binding activity. This binding could be competed efficiently by unlabeled CNRE but only slightly by FN/CRE (Fig. 2B). The shifted band was significantly reduced by anti-LXRα antibody but not by anti-CREB or anti-ATF antibodies, thereby suggesting that the complex was mainly derived from mLXRα binding to CNRE. Interestingly, basal renin mRNA expression is higher in LXRα/As4.1 cells than in GFP/As4.1 cells, suggesting a positively activating property of mLXRα on renin gene expression (Fig. 2C).
Figure 2.
Stably expressed mLXRα increases nuclear binding activity to CNRE and renin mRNA levels. (A) Western blot analysis with nuclear extracts from GFP/As4.1 and LXRα/As4.1 cells. LXRα protein was detected only in LXRα/As4.1 cells. (B) EMSA with nuclear extracts (NE) from GFP/As4.1 and LXRα/As4.1 cells, using CNRE as the probe. The unlabeled competitors and antibodies (5 μg) are indicated at the top of the gel. Stable transfection of mLXRα significantly increased nuclear binding activity to CNRE, which was inhibited by unlabeled CNRE probes and by anti-LXRα antibody. (C) Northern blot analysis with total RNA from GFP/As4.1 and LXRα/As4.1 cells. Basal renin mRNA expression is higher in LXRα/As4.1 cells than in GFP/As4.1 cells. (D) Northern blot analysis with total RNA from GFP/As4.1 and LXRα/As4.1 cells treated with 1 mM 8-Br-cAMP for 0, 1, 6, 12, and 24 h. The relative renin mRNA levels were measured by using a densitometer, normalized by 18S expression, and expressed as mean ± SE (n = 3, GFP/As4.1 without cAMP as 100%). cAMP increased renin mRNA levels in LXRα/As4.1 cells but not in GFP/As4.1 cells. (E) Northern blot analysis with total RNA from six independent LXRα-stably transfected As4.1 cell lines (from LA-1 to LA-6) treated with 1 mM 8-Br-cAMP for 12 h. These isolated clones show a similar up-regulation of renin mRNA in response to cAMP.
Next, we examined the possibility that LXRα is a cAMP-inducible transcription activator that interacts with CNRE to stimulate renin gene expression in response to cAMP. Previous studies showed that cAMP or forskolin did not increase renin mRNA level and secretion in As4.1 cells (28, 29), and interestingly, whereas LXRα was highly expressed in kidney, we did not detect mLXRα expression in As4.1 cells. Therefore, we postulate that the absence of mLXRα expression in As4.1 cells is responsible for the lack of cAMP responsiveness of renin gene expression in these cells. To test this hypothesis, we treated LXRα/As4.1 cells with cAMP and examined the renin mRNA expression. The levels of renin mRNA were increased by treatment with 8-Br-cAMP in a time-dependent manner in LXRα/As4.1 cells (Fig. 2D). On the other hand, the levels of renin mRNA in GFP/As4.1 cells were unaffected by cAMP. Six independent LXRα-stably transfected As4.1 cell lines show a similar up-regulation of renin mRNA in response to cAMP (Fig. 2E).
Transient Transfection Assays Confirming the CNRE:LXRα Interaction.
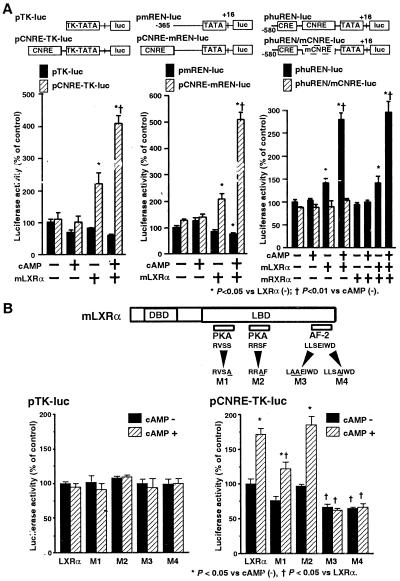
To examine the effect of overexpression of mLXRα on transcriptional activity directed by CNRE, we inserted a three tandem-repeated segment of CNRE upstream of the HSV-TK promoter-driven luciferase plasmid (pCNRE-TK-luc). Transient cotransfection of pc-mLXRα with the pCNRE-TK-luc into As4.1 cells increased luciferase activity (Fig. 3A, Left). Exposure to 8-Br-cAMP further elevated CNRE-dependent transcriptional activity. On the other hand, transcriptional activity directed by the HSV-TK promoter (pTK-luc) was not affected by either LXRα or cAMP. We also made a chimeric construct containing three tandem repeats of CNRE upstream of the endogenous mouse Ren-1c promoter (−365 to +16) (a fragment lacking the CNRE), and luciferase gene (pCNRE-mREN-luc). Overexpression of mLXRα increased luciferase expression from the pCNRE-mREN-luc construct (Fig. 3A, Middle), which could be further increased with cAMP. In contrast, no induction by mLXRα or cAMP was observed with pmREN-luc construct.
Figure 3.
Transient transfection assays confirming the CNRE:LXRα interaction. (A) Luciferase assay using As4.1 cells transiently cotransfected with pcDNA3.1, pc-mLXRα, or pc-mRXRα in combination with pCNRE-TK-luc, pCNRE-mREN-luc, phuREN-luc, and phuREN/mCNRE-luc plasmids. After DNA transfection, the cells were incubated with vehicle or 1 mM 8 Br-cAMP for 12 h. The luciferase activities were expressed in relative units (mean ± SE, n = 6, pcDNA3.1 without cAMP as 100%) normalized by β-gal activity as an internal standard for the transfection efficiency. Expression of mLXRα increased basal luciferase activity and conferred cAMP inducibility only to constructs containing the CNRE. mLXRα conferred the cAMP-inducible transactivation property to CNRE both in the native renin promoter and in the heterologous TK promoter contexts. (B) Luciferase assay using As4.1 cells cotransfected with either wild-type pc-mLXRα or mutant LXRα expression plasmids in combination with pTK-luc and pCNRE-TK-luc plasmids. Altered amino acids of mLXRα mutants (M1, M2, M3, and M4) were designated by underline. DBD, DNA-binding domain; LBD, ligand-binding domain; pKa, potential pKa phosphorylation sites; AF-2, AF-2 domain. The luciferase activities were expressed as in A. Mutations in the AF-2 domain of mLXRα abolished cAMP-inducible transactivation by CNRE:mLXRα interaction.
Furthermore, we examined whether CNRE:mLXRα mediates cAMP-mediated activation of the human renin promoter. Although the human renin promoter contains the classical CRE in addition to the CNRE, expression of the classical CREB protein was very low (data not shown) and the human renin promoter did not exhibit cAMP-inducibility in As4.1 cells (Fig. 3A, Right). However, cotransfection of pc-mLXRα with human renin promoter (−580 to +16)-driven luciferase plasmid (phuREN-luc) (a fragment containing both the consensus CRE and the CNRE) into As4.1 cells also resulted in cAMP-inducible increase in promoter activity. To further examine the functional role of CNRE:mLXRα interaction, we mutated the 8-bp core binding sequence of CNRE in the endogenous human renin promoter by PCR-directed mutagenesis. We confirmed that in vitro transcribed/translated mLXRα cannot efficiently bind to this mutated CNRE in EMSA (data not shown). In transient transfection assay, this CNRE mutation almost abolished cAMP-mediated stimulation of endogenous human renin promoter-luciferase gene (phuREN/mCNRE-luc) expression (Fig. 3A, Right). These results indicate that the functional CNRE:mLXRα interaction is involved in the activation of endogenous human renin promoter in response to cAMP.
We also examined a possible effect of transient expression of mRXRα on mLXRα-mediated transactivation of CNRE in As4.1 cells. Consistent with the results of EMSA, mLXRα, but not mRXRα, increased endogenous human renin promoter-luciferase gene (phuREN-luc) expression (Fig. 3A, Right). Moreover, 22(R)-hydroxycholesterol, which is a putative ligand for DR4/LXRE:LXRα/RXRα interaction (3, 4), did not have apparent additive effect on cAMP-mediated stimulation of phuREN-luc expression in As4.1 cells transiently cotransfected with pc-mLXRα (data not shown), providing further evidence for a potential mechanism of CNRE:mLXRα interaction, which is distinct from that of DR4/LXRE:mLXRα/mRXRα interaction.
Previous studies showed that the phosphorylation sites of PKA mediate transcriptional regulation of several nuclear receptors by cAMP (30, 31). In addition, nuclear receptors, including LXRα, are known to contain a transactivation domain (referred to as AF-2) within the carboxy-terminal region of the receptors (1, 2). To test the role of these regions to cAMP-inducible transactivation by mLXRα, mutations were introduced in the potential pKa phosphorylation sites (M1, M2) and AF-2 domain (M3, M4) of mLXRα. Nuclear extracts of transiently transfected As4.1 cells were used in EMSA, and no significant differences in DNA binding were observed when comparing wild-type and mutant mLXRα proteins (data not shown). It was therefore concluded that mutant LXRα proteins retained their ability to bind DNA. Neither the wild-type nor mutant mLXRα expression plasmids influenced expression from pTK-luc. However, significant differences in transactivation were obtained when comparing wild-type and mutant mLXRα in cells cotransfected with pCNRE-TK-luc (Fig. 3B). Mutant M1 showed a moderate decrease in the cAMP-induced activity, whereas mutant M2 retained complete basal and cAMP-inducible activation. On the other hand, mutants M3 and M4 affected the AF-2 domain and showed significantly lower basal activity and no cAMP-inducible ability to transactivate pCNRE-TK-luc.
Additive Effect of CREB and LXRα on cAMP-Mediated Transactivation of Human Renin Promoter Containing the Classical CRE and CNRE.
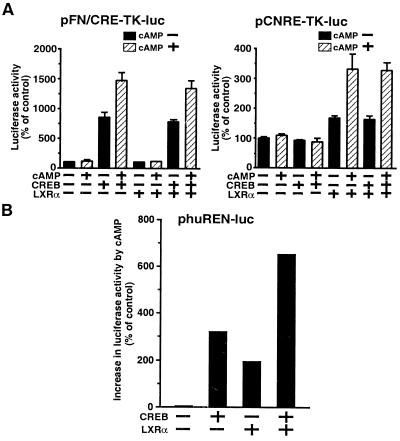
Because the human renin promoter contains the classical CRE in addition to the CNRE, we examined the relative ability of CREB-1 and LXRα to transactivate phuREN (−580 to +16)-luc in As4.1 cells. Neither CREB-1 nor mLXRα expression affected the luciferase expression from pTK-luc (data not shown). Expression of CREB was able to transactivate expression of pFN/CRE-TK-luc but had no effect on expression of the pCNRE-TK-luc reporter gene. In contrast, expression of mLXRα transactivated expression of pCNRE-TK-luc but had no effect on expression of pFN/CRE-TK-luc (Fig. 4A). Consistent with the presence of both the “classical” CRE and the CNRE in the human renin promoter, both CREB and mLXRα were able to increase the basal and cAMP-inducible expression of luciferase from phuREN-luc (Fig. 4B). Both CREB and mLXRα mediated a two times increase in expression following cAMP treatment. Coexpression of CREB and LXRα resulted in an additive increase in expression, suggesting that CREB and LXRα were independently involved in the cAMP-induced transactivation of the human renin promoter in As4.1 cells.
Figure 4.
Additive effect of CREB and LXRα on cAMP-mediated transactivation of human renin promoter containing the classical CRE and CNRE. Luciferase assay using As4.1 cells cotransfected with pcDNA3.1, pc-CREB, or pc-mLXRα in combination with pFN/CRE-TK-luc, pCNRE-TK-luc (A); and huREN-luc plasmids (B). The luciferase activities were expressed as in Fig. 3A. The classical CRE:CREB and CNRE:LXRα interactions could confer cAMP-inducible transactivation property to human renin promoter containing the consensus CRE and CNRE in an additive manner.
Potential Role of LXRα on c-myc Expression Through CNRE.
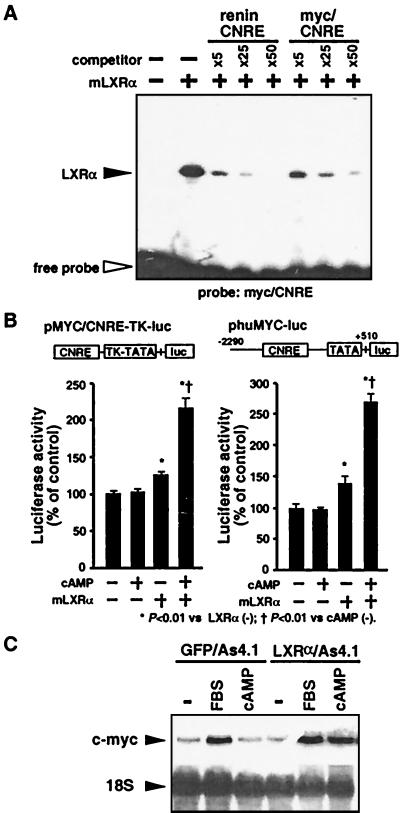
Renin CNRE shares homology with the promoter element in the c-myc gene (myc/CNRE), whose expression is also regulated by cAMP (12, 13). To examine whether LXRα can interact with the myc/CNRE to modulate c-myc gene expression, we first performed EMSA with 32P-labeled myc/CNRE and mLXRα. Incubation of mLXRα with the myc/CNRE probe produced a shifted band, which was competed effectively by unlabeled myc/CNRE as well as by unlabeled renin CNRE (Fig. 5A). We next inserted a three tandem-repeated segment of myc/CNRE (−336 to −313) upstream of the HSV-TK promoter-driven luciferase plasmid (pMYC/CNRE-TK-luc). Transient cotransfection of pc-mLXRα with the pMYC/CNRE-TK-luc into As4.1 cells slightly increased luciferase activity, and exposure to 8-Br-cAMP further elevated myc/CNRE-dependent transcriptional activity (Fig. 5B). Furthermore, cotransfection of pc-mLXRα with human c-myc promoter (−2290 to +510)-driven luciferase plasmid (phuMYC-luc) into As4.1 cells also resulted in cAMP-inducible increase in promoter activity. Finally, we used LXRα/As4.1 cells to examine a possible effect of mLXRα on endogenous c-myc gene expression. The levels of c-myc mRNA were increased by treatment with 8-Br-cAMP in LXRα/As4.1 cells (Fig. 5C). On the other hand, the levels of c-myc mRNA in GFP/As4.1 cells were unaffected by cAMP.
Figure 5.
Potential role of LXRα on c-myc expression through CNRE. (A) EMSA with mLXRα and 32P-labeled myc/CNRE probe. mLXRα specifically binds to the myc/CNRE as well as the renin CNRE. (B) Luciferase assay using As4.1 cells cotransfected with either pcDNA3.1 or pc-mLXRα in combination with pMYC/CNRE-TK-luc and phuMYC-luc plasmids. The luciferase activities were expressed as in Fig. 3A. mLXRα conferred the cAMP-inducible transactivation property to myc/CNRE. (C) Northern blot analysis with total RNA from GFP/As4.1 and LXRα/As4.1 cells. The cells were cultured in low serum medium (1% FBS) for 24 h, and then treated with 5% FBS or 1 mM 8-Br-cAMP for 2 h. cAMP increased c-myc mRNA levels in LXRα/As4.1 cells but not in GFP/As4.1 cells.
Effect of cAMP on DR4/LXRE:LXRα/RXRα Interaction.
To examine a possible effect of cAMP on transcription directed by DR4/LXRE through LXRα/RXRα heterodimer, we inserted a three tandem-repeated segment of DR4/LXRE (5′-GATCCCTTAGTTCACTCAAGTTCAAGTGGATC-3′) (4) upstream of the HSV-TK promoter-driven luciferase plasmid (pDR4/LXRE-TK-luc). We performed transient cotransfection of pc-mLXRα and pc-mRXRα with the pDR4/LXRE-TK-luc into As4.1 cells. Interestingly, exposure to 8-Br-cAMP enhanced luciferase expression in the presence or absence of 22(R)-hydroxycholesterol (Fig. 6). This result indicates that cAMP activates transcription through DR4/LXRE:LXRα/RXRα interaction as well as through CNRE:LXRα interaction.
Figure 6.
Effect of cAMP on DR4/LXRE:LXRα/RXRα interaction. Luciferase assay using As4.1 cells cotransfected with pc-mLXRα and pc-mRXRα in combination with the pDR4/LXRE-TK-luc plasmid. The cells were treated with 1 mM 8-Br-cAMP, 5 μM 22(R)-hydroxycholesterol (22-R-CHO), or vehicle for 12 h, and luciferase activities were expressed as in Fig. 3A. Note that cAMP also activates DR4/LXRE:LXRα/RXRα interaction.
Discussion
Until recently, the function(s) of LXRα was undefined. Willy et al. (1, 2) reported that LXRα heterodimerized with RXRα and is involved in the transcriptional regulation of cholesterol 7α-hydroxylase by binding to the DR4/LXRE. In this study, we identified a binding mechanism and function of LXRα as a regulator of gene transcription through its binding as a monomer to a specific sequence first described in the mouse renin gene, termed CNRE. The functional consequences of CNRE:LXRα binding indicate that LXRα play a role in the regulation of renin and c-myc gene expression in response to cAMP.
The activity of the circulating renin-angiotensin system depends on the expression and release of renin by the juxtaglomerular cells in the kidney. Multiple systemic and local factors regulate juxtaglomerular renin, and recent data suggest that many positive stimulatory effects are likely to be finally mediated by means of the cAMP-signaling pathway (32–34). We believe that further study is necessary to characterize cellular localization and function of LXRα in the kidney.
Evidence supports the notion that cAMP may regulate transactivation by means of several members of the nuclear receptor superfamily. For example, previous studies showed that the phosphorylation of RARα and HNF4 by pKa mediates transcriptional regulation by cAMP (30, 31). Because the cAMP-dependent transactivation by mLXRα requires pKa and LXRα contains two potential pKa phosphorylation sites [RVSS and RRSF in the ligand-binding domain, with a consensus sequence for pKa: R-R/K-X-S/T > R-X-X-S/T = R-X-S/T] (35), it is possible that phosphorylation of mLXRα at these potential pKa sites is involved in the mLXRα-mediated activation of renin gene by cAMP. In addition, recent studies reported that SF-1, a nuclear receptor that is important for the proper development and function of steroid hormone-producing cells, also mediates cAMP-induced transcription of its target genes (36–38) and showed that the AF-2 domain is essential for the activation of SF-1 by pKa-dependent signaling pathway (39). In the present study, a mutation of serine to alanine in a potential pKa phosphorylation site (from RVSS to RVSA) of LXRα caused a moderate inhibition of cAMP responsiveness, whereas mutation in the AF-2 domain resulted in an almost complete inhibition of cAMP-mediated transactivation. These results suggest that cAMP-mediated transactivation of CNRE by mLXRα depends mainly on an intact AF-2 domain. Indeed, the AF-2 domain of nuclear receptors have been shown to interact with coactivators to mediate transactivation property (40, 41).
In this study, we also demonstrated that mLXRα can bind to the CNRE sequence in the c-myc promoter and confer cAMP responsiveness to c-myc gene expression in As4.1 cells. It is known that c-myc is involved in cellular proliferation control and that the prolonged stimulation of renin expression in the kidney is often accompanied by juxtaglomerular cell hyperplasia (42–44). Thus, considering that cAMP has a major influence on the expression and secretion of renin in juxtaglomerular cells as a common positive regulator, the cAMP-LXRα pathway can be a potential link between stimulation of juxtaglomerular renin expression and juxtaglomerular cell hyperplasia in response to various stimuli. We also asked whether the effects of cAMP on transactivation by LXRα was only mediated by CNRE or whether other elements may also participate. Our results demonstrated that not only does LXRα confer cAMP dependence to the CNRE but to the DR4/LXRE as well, suggesting that LXRα may mediate cAMP responsiveness of a wide range of genes. Indeed, the DR4/LXRE is located in the promoter region of cholesterol 7α-hydroxylase gene, an important enzyme in cholesterol metabolism, and expression of cholesterol 7α-hydroxylase gene is modulated not only by hormones but also by signal transduction pathways including pKa (45).
The data obtained from this study demonstrate that LXRα activates renin and c-myc gene expressions through a cAMP-responsive element, CNRE. Characterization of the cellular and molecular mechanism of LXRα regulatory renin and c-myc transcriptions should have important cardiovascular pathophysiological and therapeutic implications.
Acknowledgments
This work was supported by National Institutes of Health Grant HL35610. K.T. is supported by a Postdoctoral Fellowship for Research Abroad of the Japan Society for the Promotion of Science, and Y.E.C. is supported by an American Heart Association Fellowship. V.J.D. is a recipient of National Institutes of Health MERIT Award HL35610.
Abbreviations
- DR4/LXRE
DR4-type LXR-responsive element
- EMSA
electrophoretic mobility shift assay
- pKa
protein kinase A
- GFP
green fluorescent protein
- CRE
cAMP response element
- CNRE
an overlapping CRE and a negative response element
- CREB
CRE binding protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence of mouse LXRα reported in this paper has been deposited in the GenBank database (accession no. AF085745).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100519097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100519097
References
- 1.Willy P J, Umesono K, Ong E S, Evans R M, Heyman R A, Mangelsdorf D J. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 2.Willy P J, Mangelsdorf D J. Genes Dev. 1997;11:289–298. doi: 10.1101/gad.11.3.289. [DOI] [PubMed] [Google Scholar]
- 3.Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 5.Peet D J, Turley S D, Ma W, Janowski B A, Lobaccaro J M, Hammer R E, Mangelsdorf D J. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 6.Dzau V J, Burt D W, Pratt R E. Am J Physiol. 1988;255:F563–F573. doi: 10.1152/ajprenal.1988.255.4.F563. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura N, Burt D W, Paul M, Dzau V J. Proc Natl Acad Sci USA. 1989;86:56–59. doi: 10.1073/pnas.86.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horiuchi M, Nakamura N, Tang S S, Barrett G, Dzau V J. J Biol Chem. 1991;266:16247–16254. [PubMed] [Google Scholar]
- 9.Horiuchi M, Pratt R E, Nakamura N, Dzau V J. J Clin Invest. 1993;92:1805–1811. doi: 10.1172/JCI116770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montminy M. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 11.Burt D W, Nakamura N, Kelley P, Dzau V J. J Biol Chem. 1989;264:7357–7362. [PubMed] [Google Scholar]
- 12.Hay N, Takimoto M, Bishop J M. Genes Dev. 1989;3:293–303. doi: 10.1101/gad.3.3.293. [DOI] [PubMed] [Google Scholar]
- 13.Hultgardh-Nilsson A, Querol-Ferrer V, Jonzon B, Krondahl U, Nilsson J. Exp Cell Res. 1994;214:297–302. doi: 10.1006/excr.1994.1261. [DOI] [PubMed] [Google Scholar]
- 14.Nagamine Y, Sudol M, Reich E. Cell. 1983;32:1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- 15.Shinomiya T, Scherer G, Schmid W, Zentgraf H, Schutz G. Proc Natl Acad Sci USA. 1984;81:1346–1350. doi: 10.1073/pnas.81.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke N E, Baxter J D. Nature (London) 1982;297:603–606. doi: 10.1038/297603a0. [DOI] [PubMed] [Google Scholar]
- 17.Bowlus C L, McQuillan J J, Dean D C. J Biol Chem. 1991;266:1122–1127. [PubMed] [Google Scholar]
- 18.Sigmund C D, Okuyama K, Ingelfinger J, Jones C A, Mullins J J, Kane C, Kim U, Wu C Z, Kenny L, Rustum Y, et al. J Biol Chem. 1990;265:19916–19922. [PubMed] [Google Scholar]
- 19.Liu X, Sun Y, Constantinescu S N, Karam E, Weinberg R A, Lodish H F. Proc Natl Acad Sci USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomita S, Tomita N, Yamada T, Zhang L, Kaneda Y, Morishita R, Ogihara T, Dzau V J, Horiuchi M. Circ Res. 1999;84:1059–1066. doi: 10.1161/01.res.84.9.1059. [DOI] [PubMed] [Google Scholar]
- 21.Pietenpol J A, Holt J T, Stein R W, Moses H L. Proc Natl Acad Sci USA. 1990;87:3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi R, Krummel B, Saiki R K. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J J, Herskowitz I. Science. 1993;262:1870–1874. doi: 10.1126/science.8266075. [DOI] [PubMed] [Google Scholar]
- 24.Wang M M, Reed R R. Nature (London) 1993;364:121–126. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]
- 25.Apfel R, Benbrook D, Lernhardt E, Ortiz M A, Salbert G, Pfahl M. Mol Cell Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett G, Horiuchi M, Paul M, Pratt R E, Nakamura N, Dzau V J. Proc Natl Acad Sci USA. 1992;89:885–889. doi: 10.1073/pnas.89.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangelsdorf D J, Evans R M. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 28.Laframboise M, Reudelhuber T L, Jutras I, Brechler V, Seidah N G, Day R, Gross K W, Deschepper C F. Kidney Int. 1997;51:104–109. doi: 10.1038/ki.1997.13. [DOI] [PubMed] [Google Scholar]
- 29.Jensen B L, Lehle U, Muller M, Wagner C, Kurtz A. Pflugers Arch. 1998;436:673–678. doi: 10.1007/s004240050688. [DOI] [PubMed] [Google Scholar]
- 30.Rochette-Egly C, Oulad-Abdelghani M, Staub A, Pfister V, Scheuer I, Chambon P, Gaub M P. Mol Endocrinol. 1995;9:860–871. doi: 10.1210/mend.9.7.7476969. [DOI] [PubMed] [Google Scholar]
- 31.Viollet B, Kahn A, Raymondjean M. Mol Cell Biol. 1997;17:4208–4219. doi: 10.1128/mcb.17.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner C, Jensen B L, Kramer B K, Kurtz A. Kidney Int Suppl. 1998;67:S78–S83. doi: 10.1046/j.1523-1755.1998.06716.x. [DOI] [PubMed] [Google Scholar]
- 33.Schnermann J. Am J Physiol. 1998;274:R263–R279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz A, Gotz K H, Hamann M, Wagner C. Proc Natl Acad Sci USA. 1998;95:4743–4747. doi: 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennelly P J, Krebs E G. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 36.Zhang P, Mellon S H. Mol Endocrinol. 1996;10:147–158. doi: 10.1210/mend.10.2.8825555. [DOI] [PubMed] [Google Scholar]
- 37.Lopez D, Sandhoff T W, McLean M P. Endocrinology. 1999;140:3034–3044. doi: 10.1210/endo.140.7.6846. [DOI] [PubMed] [Google Scholar]
- 38.Zeitoun K, Takayama K, Michael M D, Bulun S E. Mol Endocrinol. 1999;13:239–253. doi: 10.1210/mend.13.2.0229. [DOI] [PubMed] [Google Scholar]
- 39.Jacob A L, Lund J. J Biol Chem. 1998;273:13391–13394. doi: 10.1074/jbc.273.22.13391. [DOI] [PubMed] [Google Scholar]
- 40.Feng W, Ribeiro R C, Wagner R L, Nguyen H, Apriletti J W, Fletterick R J, Baxter J D, Kushner P J, West B L. Science. 1998;280:1747–1749. doi: 10.1126/science.280.5370.1747. [DOI] [PubMed] [Google Scholar]
- 41.Westin S, Kurokawa R, Nolte R T, Wisely G B, McInerney E M, Rose D W, Milburn M V, Rosenfeld M G, Glass C K. Nature (London) 1998;395:199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- 42.Gomez R A, Chevalier R L, Everett A D, Elwood J P, Peach M J, Lynch K R, Carey R M. Am J Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 43.Sugaya T, Nishimatsu S, Tanimoto K, Takimoto E, Yamagishi T, Imamura K, Goto S, Imaizumi K, Hisada Y, Otsuka A, et al. J Biol Chem. 1995;270:18719–18722. doi: 10.1074/jbc.270.32.18719. [DOI] [PubMed] [Google Scholar]
- 44.Bartter F C, Pronove P, Gill J R, Jr, MacCardle R C. J Am Soc Nephrol. 1998;9:516–528. doi: 10.1681/ASN.V93516. [DOI] [PubMed] [Google Scholar]
- 45.Crestani M, Stroup D, Chiang J Y. J Lipid Res. 1995;36:2419–2432. [PubMed] [Google Scholar]