Abstract
Disturbed blood flow promotes atherosclerosis mainly by inducing inflammatory gene expression in endothelial cells. Flow stimulates the proinflammatory transcription factor NF-κB through integrin- and Rac-dependent production of reactive oxygen species (ROS). Previous work demonstrated that NF-κB activation by flow is matrix-specific, occurring in cells on fibronectin but not collagen. Activation of p21-activated kinase (PAK) followed the same matrix-dependent pattern. We now show that inhibiting PAK in cells on fibronectin blocked NF-κB activation by both laminar and oscillatory flow in vitro and at sites of disturbed flow in vivo. Constitutively active PAK rescued flow-induced NF-κB activation in cells on collagen. Surprisingly, PAK was not required for flow-induced ROS production. Instead, PAK modulated the ability of H2O2 to activate the NF-κB pathway. These data demonstrate that PAK controls NF-κB activation by modulating cells’ sensitivity to ROS.
Introduction
Atherosclerosis, a chronic inflammatory disease of the artery wall, is highly affected by risk factors such as hyperlipidemia, smoking, and diabetes. These factors, however, are relatively uniform throughout the vasculature, whereas atherosclerosis occurs mainly at vessel curvatures, branch points, and bifurcations that show disturbances in blood flow2, 3. Endothelial cells (ECs) in these regions show decreased flow-induced nitric oxide release and enhanced inflammatory gene expression, so called endothelial cell dysfunction4. Systemic risk factors stimulate these sites to develop into fatty streaks, regions of lipid-laden tissue macrophages, and subsequently into atherosclerotic plaques.
Flow patterns critically regulate endothelial cell function in vitro. Applying laminar flow to endothelial cell monolayers triggers transient activation of signaling events including increased integrin affinity and activation of Rho family GTPases, NF-κB and JNK5. However, these events are downregulated at later times as cells adapt. Prolonged laminar flow decreases oxidative stress, endothelial cell turnover, and inflammatory gene expression6. By contrast, disturbed flow stimulates sustained activation of inflammatory events and endothelial turnover7–9.
The NF-κB family of transcription factors is an important component of the endothelial inflammatory response. NF-κB consists of heterodimeric protein complexes, the most studied involving the p65 and p50 subunits (hereafter referred to as NF-κB), that stimulate anti-apoptotic and pro-inflammatory gene expression10. Inactive p65 is held in the cytoplasm by inhibitory IκB proteins10. When activated, the upstream IKK kinases phosphorylate IκB, leading to its ubiquitination and degradation, thereby allowing p65 to translocate to the nucleus. IKKs also phosphorylate p65 on a critical serine (S536) that modulates transcriptional activity11. Multiple atherogenic stimuli, including disturbed flow, inflammatory cytokines and reactive oxygen species (ROS) activate NF-κB12. Atherosclerosis-prone arterial regions show chronic NF-κB activation and NF-κB-dependent gene expression, including adhesion molecules and inflammatory cytokines13, 14. Therefore, endothelial NF-κB is thought to contribute to atherogenesis by modulating inflammatory gene expression.
The pathway by which flow stimulates NF-κB has been studied extensively. Flow appears to act directly on a complex of proteins at cell-cell junctions, resulting in stimulation of PI 3-kinase and conversion of integrins to a high affinity state15, 16. Newly activated integrins bind ECM proteins, which initiates intracellular signals that include activation of the small GTPase Rac15. Rac activates the NADPH oxidase complex to produce ROS17, which stimulates NF-κB-inducing kinase (NIK) and IKKβ18, 19, critical kinases in the classical NF-κB activation pathway. All of these components are required for NF-κB activation by flow15, 18, 20, 21.
The composition of the subendothelial ECM dictates which of the many EC integrins bind ligand following flow-induced activation22. The subendothelial ECM strongly influences signaling in response to flow through the distinct signaling properties of different integrins. For example, flow activates NF-κB in ECs on fibronectin (FN) and fibrinogen (FG), which are found mainly at sites of injury and inflammation, but not on collagen (Coll) or laminin, components of the normal basement membrane23. Importantly, there is little FN or FG beneath the endothelium in most of the vasculature but these proteins are found are at sites of disturbed flow in vivo23. This matrix remodeling correlates closely with endothelial inflammatory markers such as ICAM-1 and VCAM-1. Interestingly, deletion of an alternatively spliced domain of FN that reduces its assembly into matrix decreases atherosclerosis in hypercholesterolemic ApoE−/− mice24. Taken together, these data support a role for matrix remodeling in endothelial cell dysfunction and atherosclerosis.
PAKs 1–3 are a group of highly homologous Ser/Thr protein kinases that serve as effectors for Rac and Cdc4225. PAK1 and 2 are found in endothelial cells whereas PAK3 is found largely in the brain. PAK is maintained in an inactive state by its N-terminal autoinhibitory domain (AID), which binds and blocks the kinase domain. Activation results in dissociation of the AID-kinase domain complex and phosphorylation of residues further block autoinhibition. Over 25 substrates for PAKs have been identified, including many cytoskeletal proteins, MAP kinase pathway compontents and regulators of cell survival. PAK also regulates NF-κB activation in a few systems26, 27. However, this control is by no means universal28 and constitutively active PAK does not activate NF-κB28, 29. Flow activates PAK in endothelial cells, and active PAK regulates junctional integrity and monolayer or vessel permeability30. Interestingly, PAK shows the same matrix-dependence as NF-κB, occurring in endothelial cells on FN or FG but not on Coll or basement membrane protein. Furthermore, PAK activation in mouse arteries correlates with areas of FN deposition and inflammatory gene expression30.
These findings prompted us to investigate the relationship between matrix-specific activation of PAK and NF-κB in this system. These studies identified a novel role for PAK in matrix-specific NF-κB activation by modulating the ability of ROS to activate NF-κB.
Materials and Methods
Cell Culture, Transfection, and Shear Stress
Bovine aortic endothelial (BAE) cells (gift of Dr. Joanne Murphy-Ullrich, University of Alabama-Birmingham) were cultured as previously described23. Human umbilical vein endothelial cells (HUVEC) were maintained in DMEM:F12 media containing 10% FBS, 1% bovine brain extract, 60 µg/mL heparin, 10 U/ml penicillin, and 10 µg/ml streptomycin. Endothelial cells were plated onto glass slides and exposed to laminar flow (12 dynes/cm2) as previously described23. Oscillatory flow was generated using an infusion-withdrawal pump (New Era) combined with a peristaltic pump to superimpose a 1 dyne/cm2 laminar flow to promote nutrient and gas exchange. Transient transfection of HA-PAK AID, Myc-PAK p21 binding domain (PBD), Myc-PAK2, and Myc-PAK T423E was performed using Lipofectamine 2000 per the manufacturer’s instructions. The control and PAK-Nck blocking peptides31 were produced by EZBiolab.
Immunoblotting
Cell lysis and immunoblotting was performed as previously described32. Rabbit anti-phospho-Ser536 p65, rabbit anti-phospho-p38 (Cell Signaling Technologies), rabbit anti-p65, rabbit anti-ICAM, rabbit anti-ERK, goat anti-PAK2, and rabbit anti-phospho-NIK (Santa Cruz) were all used at 1:1000 dilutions. Rabbit anti-phospho-Ser141 PAK (Biosource) was used at a 1:5000 dilution.
Immunocytochemistry
Cells were processed for immunocytochemistry as previously descrbed23. Primary antibodies included rabbit anti-p65 (1:200; Santa Cruz) and mouse anti-HA (1:500; Covance). Primary antibody binding was visualized using Alexa488-conjugated goat anti-rabbit and Alexa568-conjugated goat anti-mouse secondary antibodies. Coverslips were mounted using Fluoromount G (Southern Biotechnology) and images were taken using the 60× oil immersion objective on a Nikon DiaPhot Microscope equipped with a Photometrics CoolSnap video camera using the Inovision ISEE software program.
ROS quantification
BAE cells were preincubated with the dye 2,7-dichlorodihydrofluorescein diacetate (H2-DCFDA)33 (10 µM) for 30 minutes prior to the onset of flow. Shear stress was applied to the cells in the continued presence of dye for varying times. Cells were rinsed with PBS and lysed in PBS containing 0.2% Triton X-100 and 1 mM N-acetylcystein. Fluorescence was measured using the 485 excitiation/530 nm emission filter in a Fluorostar plate reader. Fluorescence was normalized to total protein in the lysates (Bradford assay, Pierce).
Quantitative RT-PCR
To quantify mRNA levels, we extracted total RNA using TRIzol (Invitrogen) and made cDNAs using the iScript cDNA Synthesis kit (Biorad). Real time RT-PCR was performed using the BioRad iCycler and Sybr Green Master Mix kit. Primers used were as follows: 18S forward 5’-CGGCTACCACATCCAAGGAA, 18S reverse 5’-AGCTGGAATTACCGCGGC, ICAM forward 5’-TGTCCCCCTCAAAAGTCATC, ICAM reverse 5’- TAGGCAACGGGGTCTCTATG, IL-8 forward 5’-CTGCGCCAACACAGAATTTA, IL-8 reverse 5’- TGAATTCTCAGCCCTCTTCAA. Results were normalized to 18S levels and are shown as a ratio of target mRNA to 18S mRNA.
Animals and Vessel Harvest
Eight male C57Bl/6 mice from Jackson Laboratories (Bar Harbor, ME), 8–12 weeks old, and weighing 18–20 g were used for this experiment. Mice were maintained on a chow diet for 28 weeks. Mice were injected intraperitoneally with 0.1 ml of either the control or PAK-Nck inhibitory peptide (10 mg/ml) daily for three days. Mice were perfused with 4% paraformaldehyde and the carotid sinuses were excised and processed for paraffin embedding.
Immunohistochemistry (IHC)
5 µm sections were cut, deparaffinized and rehydrated, then processed with antigen retrieval solution (Vector Labs). Sections were blocked in either 10% goat serum or 10% donkey serum in PBS/ fish skin gelatin solution for 1h and incubated with anti-p65 (Chemicon, 1 µg/100 µl) pre-labeled with Alexa-546 (Molecular Probes) overnight in 1% BSA at 4°C. All sections were stained with TOTO-3 (Molecular Probes) and mounted with anti-fading mounting gel.
Analysis of Nuclear NF-κB
Image analysis was performed to assess the relative intensity of nuclear NF-κB in the endothelium. Confocal images of dual stained NF-κB and TOTO-3 were imported into MetaMorph Imaging software (Molecular Devices). Positive TOTO-3 staining was used to define nuclei. These regions were transferred to the NF-κB stained image and NF-κB intensity was measured for each nucleus.
Results
PAK is required for NF-κB activation by onset of flow
To determine if PAK is required for flow-induced NF-κB activation in cells on FN, we first used a previously described cell-permeant peptide corresponding to the Nck-binding, proline rich sequence of PAK31. This peptide prevents the interaction between PAK and Nck, and blocks PAK-dependent changes in endothelial monolayer permeability, migration and angiogenesis30, 31. Activation of the classical NF-κB pathway involves IKK-dependent phosphorylation and degradation of the inhibitor IκB, as well as Ser536 phosphorylation and nuclear translocation of p6510. Pretreatment of ECs with this peptide completely blocked p65 nuclear translocation in response to flow compared to inactive control peptide (Fig. 1A/B). Results with control peptide were indistinguishable from untreated cells (not shown). Flow-induced p65 phosphorylation was also substantially reduced by the PAK-Nck peptide (Fig. 1C).
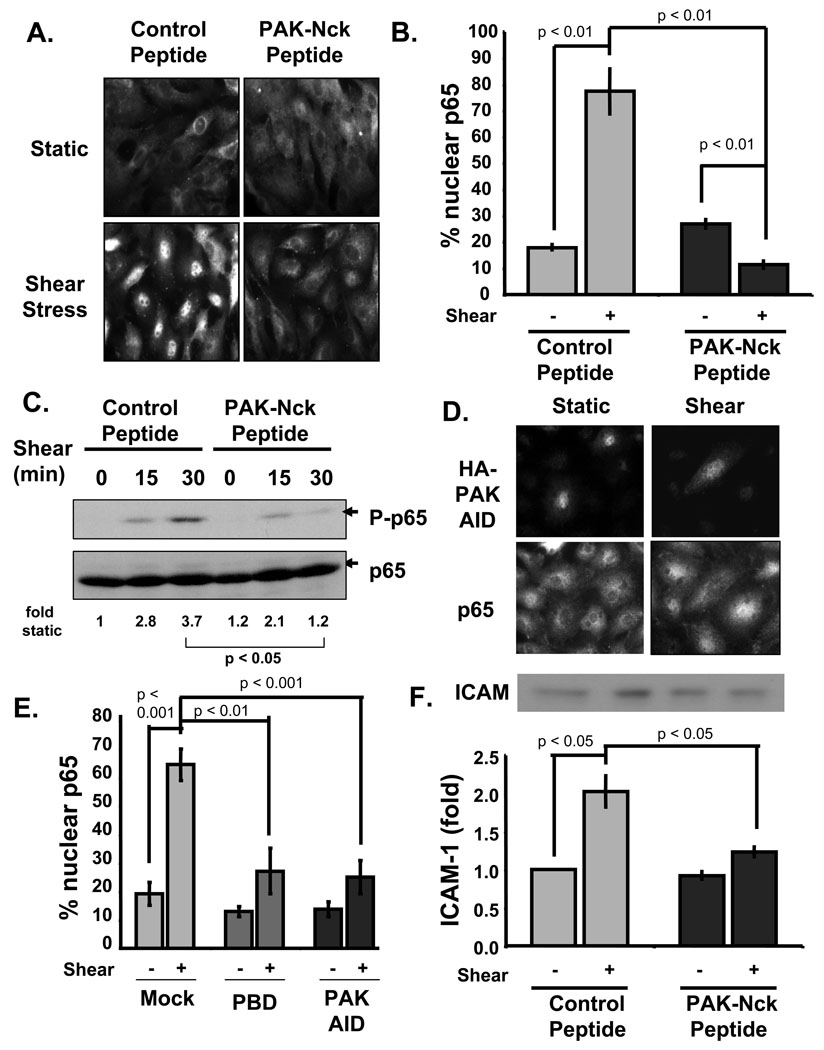
Figure 1. PAK is required for NF-κB activation by onset of flow on FN.
BAE cells plated on FN for 4h were pretreated with either control or PAK-Nck inhibitory peptides (20 µg/ml for 1 h) and sheared for 30 minutes. (A) Cells were fixed and stained for the NF-κB p65 subunit. (B) Cells from A were scored for nuclear p65 (percent positive cells). 100 cells were counted per condition. Values are means ± S.D. n = 3. (C) Cells were lysed and immunoblotted for p65 phosphorylation on Ser536 or total p65, n = 4. (D) Flow-induced p65 nuclear translocation was assessed as in A in BAE cells transfected with HA-tagged PAK AID. Cells expressing the AID construct were identified by staining for the HA tag. (E) Nuclear translocation of p65 was scored (as in B) in cells expressing the PAK AID from (D) or with the PAK PBD. n = 3. (F) Cells sheared for 4 h were lysed and ICAM-1 expression determined by immunoblotting. Values are means ± S.D., n = 3.
To confirm these results, we examined two other PAK inhibitors. Expression of a PAK AID construct that blocks kinase activity did not affect basal NF-κB activity but flowinduced p65 nuclear translocation was significantly inhibited (Fig. 1D). Transfecting cells with the PAK PBD, which binds and inhibits the upstream GTPases Rac and Cdc42, also blocked flow-induced NF-κB nuclear translocation (Fig. 1E). Finally, the induction of the NF-κB target gene ICAM-1 in response to acute onset of flow was significantly inhibited by pretreatment with the PAK-Nck peptide (Fig. 1F).
Disturbed flow in vitro and in vivo
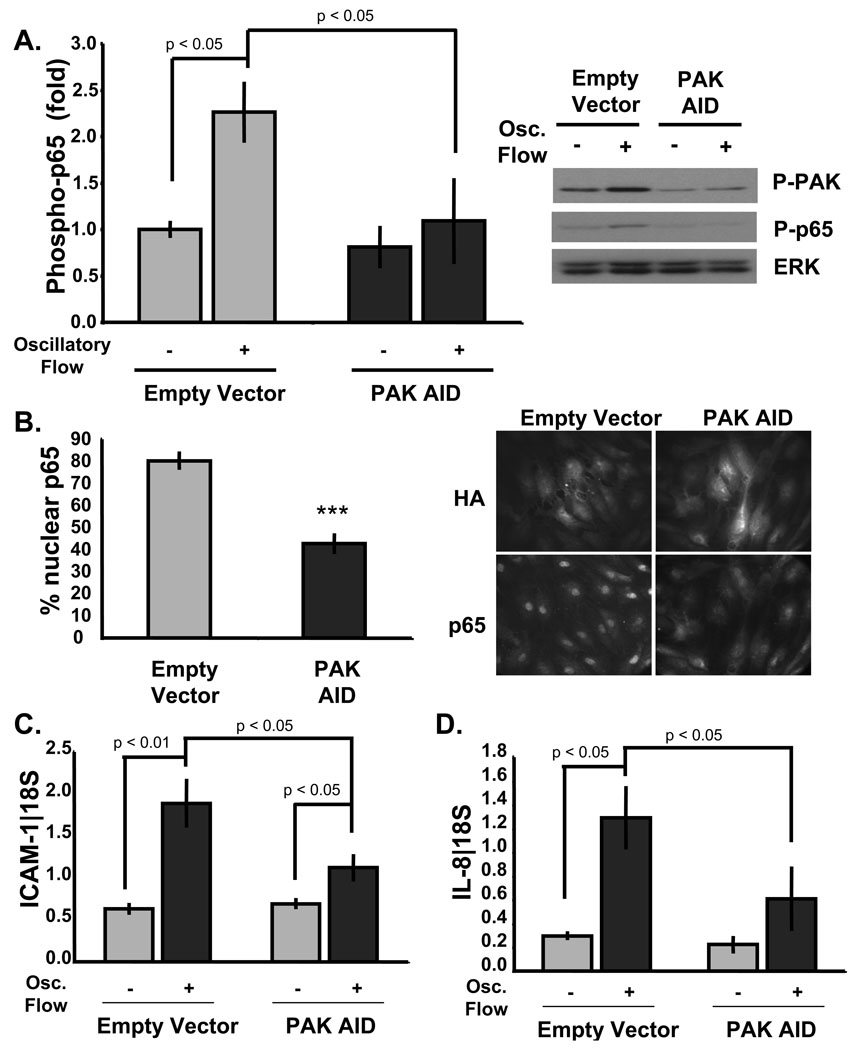
Both PAK and NF-κB are activated transiently by acute onset of flow30, 34 but in a sustained manner under disturbed flow in vitro9, 30 and at sites of disturbed flow in vivo13, 30. To test whether PAK is required for sustained NF-κB activation by disturbed flow, HUVECs transfected with the PAK AID construct were plated on FN and stimulated with oscillatory flow for 18 hours. The PAK AID completely inhibited both basal (49% reduction, p < 0.01) and flow-induced PAK activation (67% reduction, p < 0.01), as expected (Fig. 2A). AID expression also blocked the increase in NF-κB p65 phosphorylation (Fig 2A) and nuclear translocation (Fig. 2B). Oscillatory flow-induced expression of the proinflammatory genes ICAM-1 and IL-8 were inhibited as well (Fig. 2C/D). Thus, PAK is required for both transient activation of NF-κB in laminar shear and sustained activation in oscillatory shear.
Figure 2. PAK is required for sustained NF-κB activation in oscillatory flow.
HUVECs transfected with either empty pcDNA3.1 or HA-PAK AID were plated on FN for 4 h and exposed to oscillatory flow for 18 hrs. (A) Cells were lysed and phosphorylation of p65 (Ser536) and PAK (Ser141) were determined by immunoblotting using phosphospecific antibodies. Quantified values for p65 phosphorylation were normalized to total protein and shown as fold change compared to static cells. Values are means ± S.D, n = 3–4. (B) Fixed cells were stained for p65 and HA to determine p65 nuclear localization in cells expressing the empty HA vector or HA-tagged PAK AID. The percentage of cells showing p65 in the nucleus was scored. Values are means ± S.D., 100 cells per condition. n = 4. *** p < 0.001. (C) and (D) Inflammatory gene expression was determined by measuring mRNA levels of ICAM-1 (C) and IL-8 (D) using quantitative RT-PCR. Target gene levels were normalized to 18S. Values are means ± S.D., n = 3. Results are representative of 3 independent experiments.
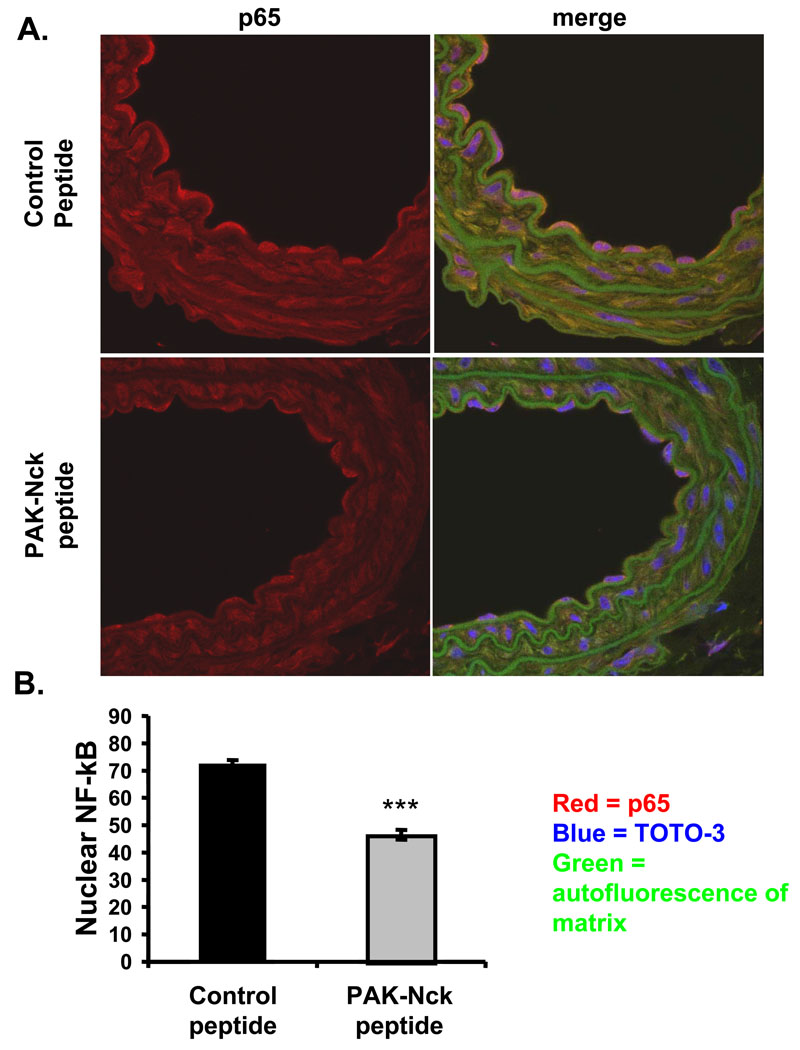
We next asked whether PAK is required for NF-κB activation at regions of disturbed flow in arteries in vivo. These regions show FN in the subendothelial ECM23 and we previously found that injecting mice with the PAK-Nck peptide reduced vascular permeability at these sites30. C57Bl/6 mice have modest PAK and NF-κB activation at locations of disturbed flow in the absence of other markers of atherosclerosis (refs). Mice therefore received injections of control or PAK-Nck peptide for three days, at which time arteries were examined by immunohistochemistry. Similar to untreated mice13, mice treated with control peptide showed nuclear NF-κB in ECs at the expected sites, which was decreased in PAK-Nck peptide treated mice (Fig. 3A/B). Taken together, these results show that PAK is critical for flow-induced NF-κB activation in vitro and in vivo.
Figure 3. PAK inhibitors reduce nuclear p65 at sites of disturbed flow in vivo.
C57Bl/6 mice at 36 weeks received intraperitoneal injections of either control or PAK-Nck blocking peptides daily for three days. Mice were then sacrificed and the carotid sinuses stained for anti-p65 (red) and the nucleus (TOTO-3; blue). Autofluorescence of the elastic lamina is green. (A) Representative images. (B) Images were analyzed by confocal microscopy and mean nuclear intensity of p65 staining was compared between the treated and untreated samples. n = 8. *** p < 2 × 10−28.
Rescue by active PAK
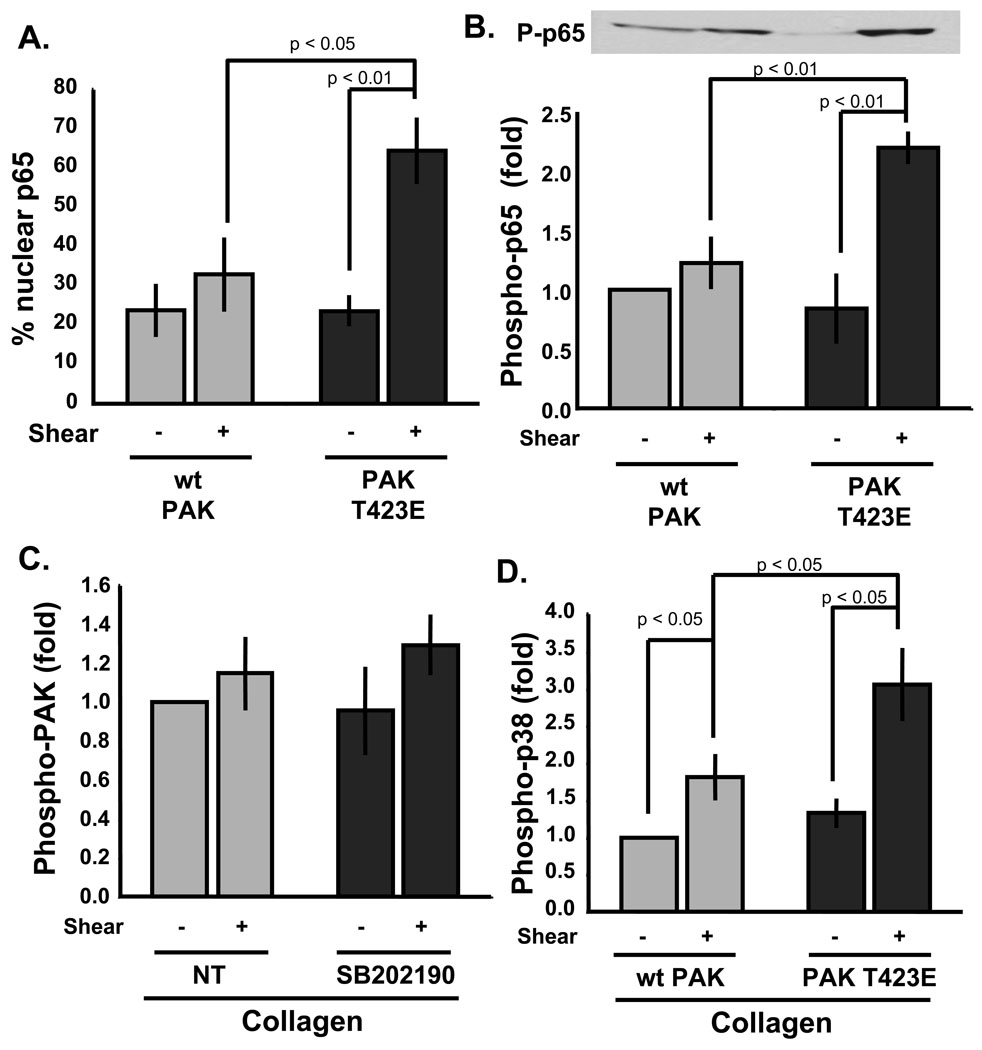
ECs plated on basement membrane proteins, such as Coll and laminin, do not activate either PAK or NF-κB23, 30. To test whether low PAK activity is rate limiting for NF-κB activation under these conditions, cells were transfected with WT or active T423E PAK. Active PAK did not directly activate NF-κB in cells on Coll but rescued both p65 nuclear translocation (Fig. 4A) and p65 phosphorylation on Ser 536 (Fig. 4B) in response to flow, compared to cells transfected with wildtype PAK. These data provide strong evidence suggest that differential PAK activation mediates matrix-specific NF-κB activation by flow.
Figure 4. PAK activation restores flow-induced NF-κB activation on Coll.
BAE cells expressing wild type or active T423E PAK were plated on Coll for 4h and sheared for 30 minutes. (A). Cells were stained for myc to identify cells expressing PAK constructs and for p65. Expressing cells were then scored for nuclear p65. Values are percents from 100 cells/condition ± S.D. n = 3. (B) Phosphorylation of p65 Ser536 was determined by immunoblotting cell lysates using phosphospecific antibodies. Values were quantified and normalized to total protein. n = 3 (C) Cells on Coll were pretreated with the p38 inhibitor SB202190 (1 µM) and flow-induced PAK phosphorylation was determined as previously described. n = 4. (D) Phosphorylation of p38 was determined as in A for phospho-p65, except that antibodies specific for activated and total p38 were used for immunoblotting. n = 3.
Relationship to p38 MAP kinase
Previous results demonstrated that p38 MAP kinase was preferentially activated in cells on Coll and that blocking p38 partially restored NF-κB activation by flow23. We therefore investigated the relationship between p38 and PAK signaling in this system. Flow does not activate PAK in cells on Coll, suggesting that Coll-specific p38 activation could prevent NF-κB activation by inhibiting PAK. However, inhibiting p38 in cells on Coll did not increase flow-induced PAK activation (Fig. 4C). To test the converse hypothesis, that PAK stimulates NF-κB activation in cells on FN by suppressing p38, we transfected cells with active PAK and plated them on Coll. While active PAK is sufficient to rescue NF-κB activation in cells on Coll, active PAK increased rather than p38 activation both with and without flow (Fig. 4D). Thus, PAK cannot promote NF-κB by inhibiting p38; rather, the data suggest that the inhibitory effect of p38 cannot overcome the effect of active PAK. Therefore, these results show that the matrix-specific regulation of PAK and p38 are independent events, with PAK being the major determinant of matrix-specific NF-κB activation.
Role of NF-κB -inducing kinase (NIK)
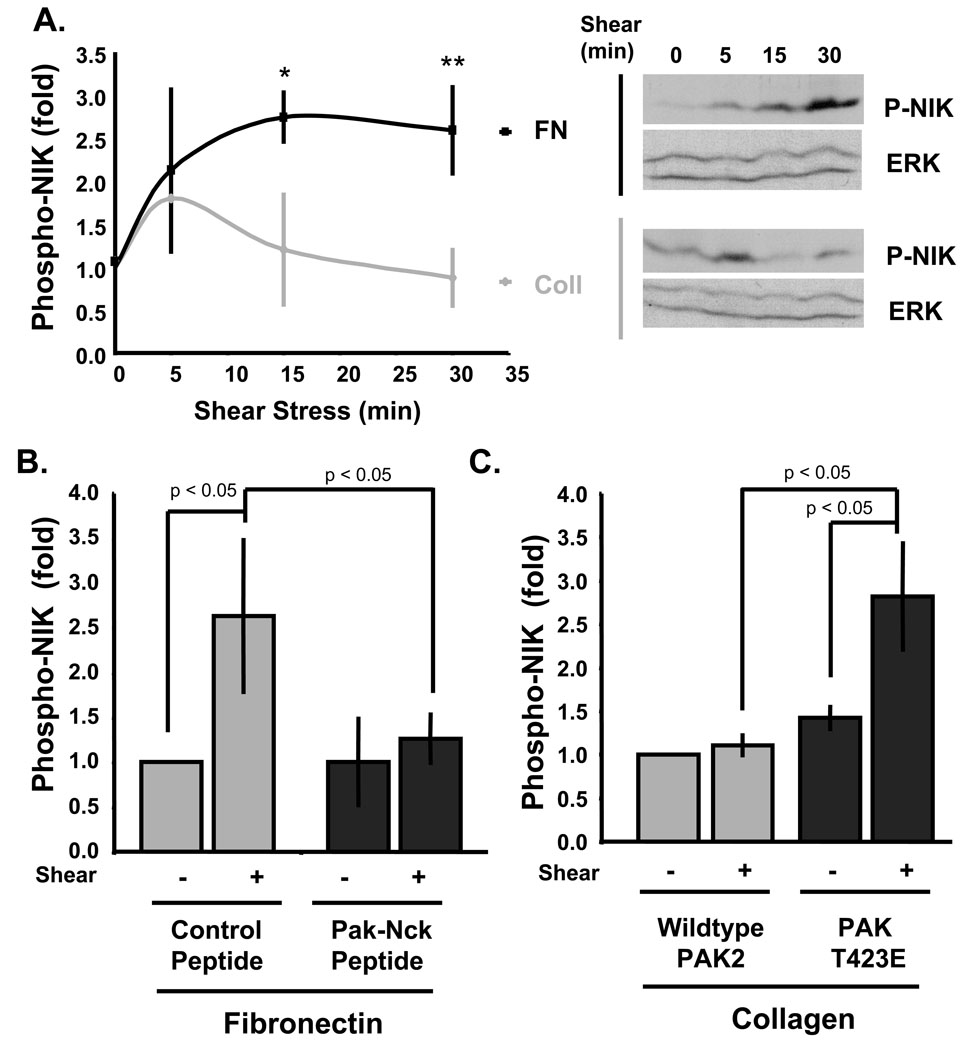
We next turned our attention to the mechanism by which PAK regulates flow-induced NF-κB activation. We previously found that activation of IKKβ by flow is matrix-specific, occurring in cells on FN but not Coll23. NIK phosphorylates and activates IKKα and IKKβ35, is activated by ROS19, and is required for NF-κB activation by both flow18, 20 and constitutively active Rac27, 29. To test the involvement of NIK, cells plated on Coll or FN were stimulated with flow and NIK activation assayed using a phosphorylation-specific antibody to Thr559, a key phosphorylation site in NIK’s activation loop that regulates NIK kinase activity36. In cells on FN, flow induced a sustained 2.5–3-fold increase in NIK phosphorylation, whereas cells on Coll showed only a slight and transient response (Fig 5A). Inhibiting PAK in cells on FN with the PAK-Nck peptide reduced flow-mediated NIK phosphorylation (Fig 5B) and expression of constitutively active T423E PAK in cells on Coll rescued flow-induced NIK phosphorylation (Fig. 5C). Thus, NIK activation is matrix-specific and PAK-dependent. These data indicate that PAK regulates NF-κB activation either at or upstream of NIK.
Figure 5. Matrix-specific NIK activation by flow requires PAK.
(A) BAE cells on either Coll or FN for 4 h were sheared for the indicated times and NIK phosphorylation on Thr559 was assessed by Western blotting using a phospho-specific antibody. Bands were quantified and normalized to Erk2 as a loading control. Values are means ± S.D., n = 3–4. * p < 0.05, ** p < 0.01. (B) Endothelial cells on FN were treated with control or PAK-Nck blocking peptides prior to onset of flow. NIK phosphorylation was assessed after 30 min of flow. n = 4. (C) BAE cells expressing wild type or constitutively active PAK (T423E) were plated on Coll and flow-induced NIK phosphorylation was determined as in B. Values are means ± S.D, n = 3.
Role of ROS
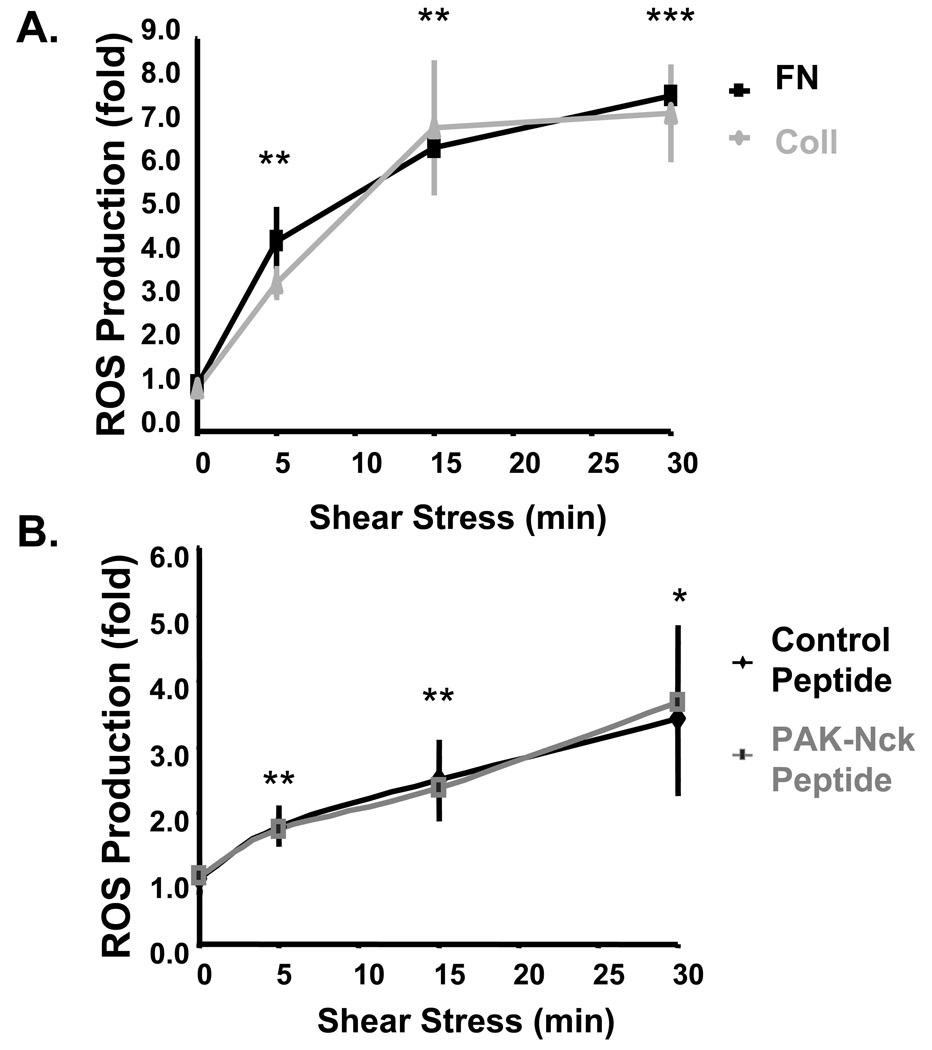
Flow-induced NF-κB activation depends on the production of ROS, as both antioxidants and genetic deletion of p47phox prevent flow-induced NF-κB activation21, 37. PAK regulates the neutrophil NADPH oxidase complex through phosphorylation of both the p67phox38 and p47phox subunits39, suggesting that PAK might regulate flow-induced ROS production in ECs. To test this idea, we measured ROS production in ECs on either Coll or FN using the cell-permeant redox sensitive compound H2-DCFDA. H2-DCFDA is oxidized primarily by H2O2, a metabolite of superoxide33. Surprisingly, the ability of shear stress to increase H2-DCFDA fluorescence was matrix-independent (Fig. 6A). Additionally, the inhibitory PAK peptide had no effect on flow-induced ROS production in cells on FN (Fig. 6B). Thus, PAK does not act by controlling ROS production.
Figure 6. Neither matrix nor PAK regulate flow-induced ROS production.
(A) BAE cells were plated on Coll or FN for 4 h and loaded with 2,7-H2DCFDA (10 µM for 30 minutes). Cells were then stimulated with shear stress for the indicated times, lysed, and fluorescence measured using a plate reader. Fluorescence was normalized to total protein levels in the lysates. Values are means ± S.D, normalized to static conditions, n = 3–4 ** p < 0.01, *** p < 0.001 compared to static. (B) BAE cells on FN received control or PAK-Nck inhibitory peptide for 1 h, then loaded with 2,7-H2DCFDA (as in A), and stimulated with shear stress for the indicated times. Fluorescence was quantified as in (A), n = 3–4. * p < 0.05, ** p < 0.01 compared to static.
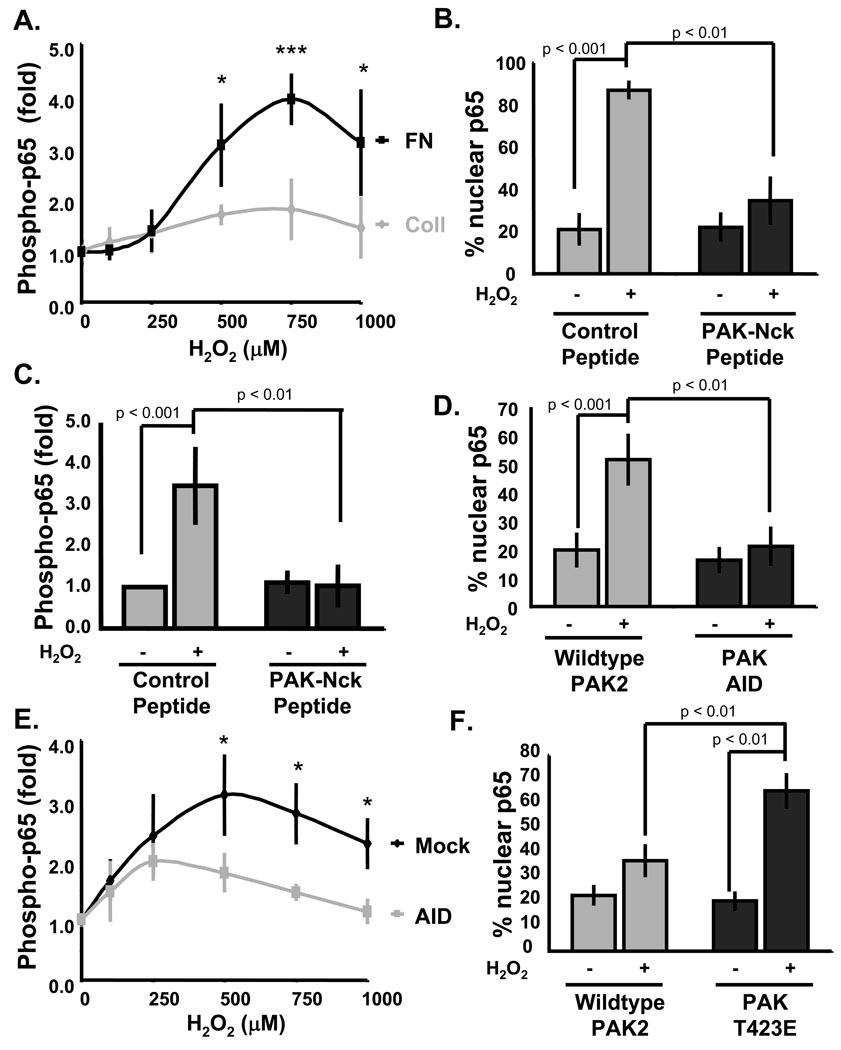
The H2O2 scavenger catalase blocks both flow-induced NF-κB activation40 and atherosclerosis in vivo41, and addition of exogenous H2O2 is sufficient to activate NF-κB42. The lack of flow-induced NF-κB activation in cells on Coll, despite the production of ROS, suggests that matrix regulates cellular sensitivity to ROS. To test this, ECs on Coll or FN were stimulated by addition of H2O2 and activation of NF-κB assayed. Cells on FN showed much higher H2O2-induced p65 phosphorylation compared to cells on Coll (Fig. 7A). Similar to flow-induced NF-κB activation, blocking PAK in cells on FN with the inhibitory peptide abolished both H2O2-induced p65 nuclear translocation (Fig. 7B) and phosphorylation (Fig. 7C). This effect is not due to enhanced antioxidant activity on Coll or in response to the peptide inhibitors since neither treatment affected the oxidation of H2-DCFDA by flow. Similar to the peptide inhibitors, the PAK AID construct also diminished both H2O2-induced p65 nuclear translocation (Fig. 7D) and phosphorylation (Fig. 7E) in cells on FN. Finally, expression of the constitutively active T423E PAK construct in cells on Coll rescued H2O2-induced p65 nuclear translocation (Fig. 7F).
Figure 7. Matrix-specific PAK signaling regulates NF-κB activation by H2O2.
(A) BAE cells on Coll or FN for 4 h were treated for 15 minutes with indicated doses of H2O2. Phosphorylation of p65 was determined as in Fig 1. Values are means ± S.D., normalized to total p65, n = 4. * p < 0.05, *** p < 0.01. (B and C) BAE cells on FN were treated with control or PAK-Nck inhibitory peptide and p65 nuclear translocation (B) and phosphorylation (C) were determined as in Fig 1, n = 3. (D and E) BAE cells transfected with wild type or PAK AID were plated on FN for 4 hours, and the ability of H2O2 to induce p65 nuclear translocation (D) and phosphorylation (E) were assessed. Approximately 100 cells were counted for each condition per experiment, n = 3. * p < 0.05. (F) BAE cells transfected with wild type or T423E PAK were plated on Coll for 4 hours, and the ability of H2O2 to induce p65 nuclear translocation was assessed, n =3.
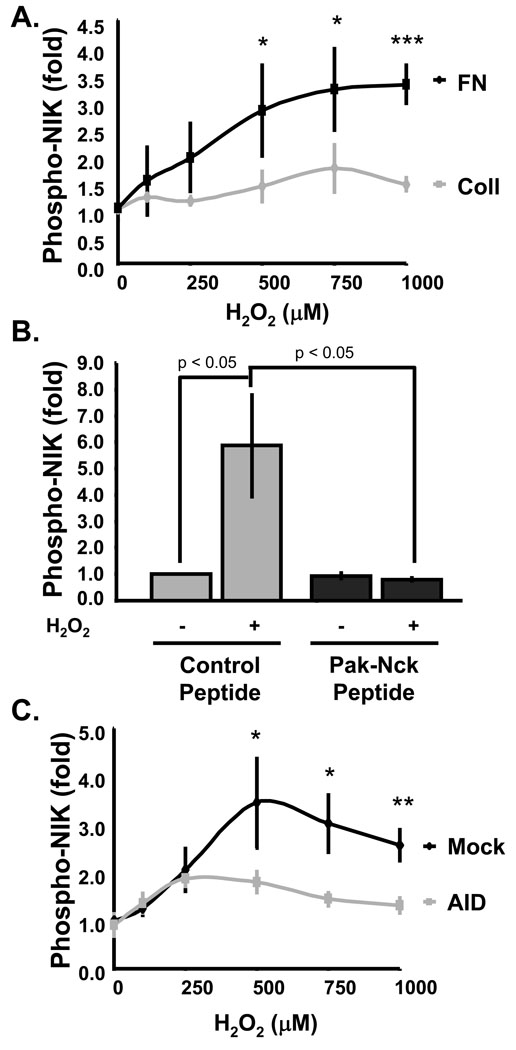
To confirm these results, we also examined NIK activation. ECs on FN showed much higher H2O2-induced NIK activation compared to cells on Coll (Fig. 8A). The PAK-Nck peptide abolished H2O2-induced NIK phosphorylation in cells on FN (Fig. 8B) and expression of T423E PAK rescued H2O2-induced NIK phosphorylation in cells on Coll (Fig. 8C). Taken together, these data provide strong evidence that matrix-specific PAK activation regulates flow-induced NF-κB activaty by modulating the ability of ROS to activate NIK and NF-κB.
Figure 8. Matrix-specific NIK activation by H2O2 requires PAK.
Cells were lysed and NIK phosphorylation determined by Western blotting as in Fig 1. Values are means ± S.D. relative to untreated cells. (A) BAE cells on Coll or FN for 4 h were treated for 15 minutes with the indicated doses of H2O2 prior to analysis of NIK phosphorylation, n = 3. * p < 0.05. *** p < 0.001. (B) BAE cells on FN were treated with control or PAK-Nck inhibitory peptide and NIK phosphorylation determined, n = 3. (C) BAE cells transfected with wild type PAK or PAK AID were plated on FN for 4 h, H2O2 added and NIK phosphorylation assayed, n = 3. * p < 0.05. ** p < 0.01.
Discussion
The current work defines PAK as a critical upstream mediator of matrix-specific NF-κB activation by flow. This conclusion is based on results showing that PAK inhibitors blocked NF-κB activation by both acute onset of flow and oscillatory flow in cells on FN; conversely, active PAK restored activation of NF-κB by flow in cells on Coll. Blocking PAK also decreased NF-κB activation in atherosclerosis prone regions of the mouse carotid sinus in vivo. Active PAK did not, however, affect basal NF-κB activity in the absence of flow. These data suggest that PAK-dependent regulation of NF-κB activation is highly specific and demonstrate that NF-κB activation in this system requires multiple inputs.
Previous work showed that activation of NF-κB by flow21, 37 or Rac43 requires ROS. PAK can regulate the NADPH oxidase complex in neutrophils, where NOX2 is a critical NADPH oxidase subunit38, 39. However, neither matrix composition nor PAK inhibition affected flow-induced ROS production in ECs. These cells utilize mainly NOX1 to generate ROS in response to flow40. Thus, a distinct requirement for PAK is not surprising. Instead, we found that activation of both NF-κB and NIK by exogenous H2O2 was higher in cells on FN compared to Coll. Furthermore, the response to H2O2 was decreased by PAK inhibitors in cells on FN and increased by activating PAK in cells on Coll. Taken together, these data provide strong evidence that PAK modulates the pathway by which H2O2 triggers NF-κB activation.
Relatively little is currently known concerning the role of PAK in the inflammatory response. Migration of leukocytes to CXCL144 and CXCL1245 requires PAK1, and the PAK-Nck inhibitory peptide reduces neutrophil activation and infiltration in LPS–induced lung injury in mice46. Active PAK can stimulate the activation of the JNK and p38 MAP kinase pathways, both of which are implicated in proinflammatory gene expression47. However, reports of PAK involvement in NF-κB activation have been inconsistent. Constitutively active Rac activates NF-κB through production of ROS43, which is blocked by dominant negative NIK and IKKβ27, 29. Active Rac mutants incapable of activating PAK still activate NF-κB28, and the active T423E PAK construct is insufficient to activate NF-κB28, 29. Thus, PAK is not a central component of the pathway linking Rac to NF-κB. However, dominant negative PAK inhibits NF-κB activation by some stimuli, including expression of activated Rac26, 27 . These data can be reconciled by a model in which PAK sensitizes the NIK/IKKβ pathway to activation by ROS. As in other signaling networks, the relative importance of PAK would then depend on both the strength and the nature of the upstream signal48.
In addition to flow and atherosclerosis, oxidant-induced activation of NF-κB has been implicated in responses to cigarette smoke, proinflammatory cytokines such as IL-1β, aging, ischemia-reperfusion injury, myocardial infarction, cancer and diabetic renal failure49, 50. The ability of PAK to regulate oxidant-dependent NF-κB activation may therefore be important in multiple pathologies and suggests that PAK is a potential therapeutic target. However, long-term global PAK inhibition is likely to be deleterious, as strong immunosuppression increases the risk of infection and cancer51. Furthermore, PAK3 is important in brain function and PAK inhibition using a different cell-permeable peptide inhibitor results in symptoms resembling Alzheimer’s disease in mice52. However, multiple endogenous proteins can inhibit PAK signaling, including nischarin, hPIP, POPX1/2, and PKA25. These endogenous negative feedback mechanisms, especially those that primarily affect the vasculature, could be useful therapeutic targets in limiting endothelial activation and atherosclerosis.
Acknowledgements
The authors acknowledge Andy Pryor for assistance with the immunohistochemistry, Lynn Hedrick and Brian Wamhoff (University of Virginia) for RT-PCR primers, and David Scott (University of Virginia) for assistance with plasmid production and useful discussions.
Sources of Funding
This work was supported by USPHS grants RO1 HL75092 to M.A.S., American Heart Association Scientist Development Grant 0735308N to A.W.O., and NIH RO1 HL082836 to B.R.B.
Footnotes
Subject Codes: [138] Cell signaling/signal transduction, [137] Cell biology/structural biology, [134] Atherosclerosis Pathophysiology,
References
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223(211):1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 3.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis--an inflammatory disease.[see comment] New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98(2):176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 6.Garin G, Berk BC. Flow-mediated signaling modulates endothelial cell phenotype. Endothelium. 2006;13(6):375–384. doi: 10.1080/10623320601061599. [DOI] [PubMed] [Google Scholar]
- 7.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9(1):27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 8.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82(10):1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 9.Mohan S, Mohan N, Sprague EA. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol. 1997;273((2 Pt 1)):C572–C578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 10.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nature Reviews Molecular Cell Biology. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 11.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. Journal of Immunology. 2003;170(11):5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 12.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? Journal of Clinical Investigation. 2001;107(3):255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. Journal of Clinical Investigation. 1996;97(7):1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arteriosclerosis, Thrombosis & Vascular Biology. 1998;18(5):842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 15.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO Journal. 2002;21(24):6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 17.Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, Irani K, Alevriadou BR. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol. 1999;276((4 Pt 1)):C838–C847. doi: 10.1152/ajpcell.1999.276.4.C838. [DOI] [PubMed] [Google Scholar]
- 18.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappaB in endothelial cells. Am J Physiol Cell Physiol. 2007;292(1):C362–C371. doi: 10.1152/ajpcell.00535.2005. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Huang X, Cang H, Gao F, Yamamoto T, Osaki T, Yi J. The endogenous reactive oxygen species promote NF-kappaB activation by targeting on activation of NF-kappaB-inducing kinase in oral squamous carcinoma cells. Free Radic Res. 2007;41(9):963–971. doi: 10.1080/10715760701445045. [DOI] [PubMed] [Google Scholar]
- 20.Hay DC, Beers C, Cameron V, Thomson L, Flitney FW, Hay RT. Activation of NF-kappaB nuclear transcription factor by flow in human endothelial cells. Biochim Biophys Acta. 2003;1642((1–2)):33–44. doi: 10.1016/s0167-4889(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 21.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2-from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278(47):47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 22.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17(11):4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169(1):191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104(1):11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 25.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 26.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120((Pt 20)):3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 27.Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NF-kappa B activity by multiple signaling pathways requires PAK1. J Biol Chem. 2000;275(26):19693–19699. doi: 10.1074/jbc.M909860199. [DOI] [PubMed] [Google Scholar]
- 28.Reddig PJ, Xu D, Juliano RL. Regulation of p21-activated kinase-independent Rac1 signal transduction by nischarin. J Biol Chem. 2005;280(35):30994–31002. doi: 10.1074/jbc.M502546200. [DOI] [PubMed] [Google Scholar]
- 29.Cammarano MS, Minden A. Dbl and the Rho GTPases activate NF-kappa B by I kappa B kinase (IKK)-dependent and IKK-independent pathways. J Biol Chem. 2001;276(28):25876–25882. doi: 10.1074/jbc.M011345200. [DOI] [PubMed] [Google Scholar]
- 30.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176(5):719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circulation Research. 2002;90(6):697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- 32.Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi-and phosphoinositide 3-kinase-dependent ERK activation. Journal of Biological Chemistry. 2002;277(23):20453–20460. doi: 10.1074/jbc.M112091200. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, King MA, Meyer EM. alpha7 nicotinic receptor-mediated protection against ethanol-induced oxidative stress and cytotoxicity in PC12 cells. Brain Res. 2000;861(1):165–167. doi: 10.1016/s0006-8993(99)02457-9. [DOI] [PubMed] [Google Scholar]
- 34.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochemical & Biophysical Research Communications. 1994;201(2):950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 35.Israel A. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 2000;10(4):129–133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 36.Lin X, Mu Y, Cunningham ET, Jr, Marcu KB, Geleziunas R, Greene WC. Molecular determinants of NF-kappaB-inducing kinase action. Mol Cell Biol. 1998;18(10):5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohan S, Mohan N, Valente AJ, Sprague EA. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am J Physiol. 1999;276((5 Pt 1)):C1100–C1107. doi: 10.1152/ajpcell.1999.276.5.C1100. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed S, Prigmore E, Govind S, Veryard C, Kozma R, Wientjes FB, Segal AW, Lim L. Cryptic Rac-binding and p21(Cdc42Hs/Rac)-activated kinase phosphorylation sites of NADPH oxidase component p67(phox) J Biol Chem. 1998;273(25):15693–15701. doi: 10.1074/jbc.273.25.15693. [DOI] [PubMed] [Google Scholar]
- 39.Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein--coupled receptors. Science. 1995;269(5221):221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 40.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95(8):773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95(11):1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. Identification of hydrogen peroxide as the relevant messenger in the activation pathway of transcription factor NF-kappaB. Adv Exp Med Biol. 1996;387:63–68. doi: 10.1007/978-1-4757-9480-9_9. [DOI] [PubMed] [Google Scholar]
- 43.Sulciner DJ, Irani K, Yu ZX, Ferrans VJ, Goldschmidt-Clermont P, Finkel T. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol Cell Biol. 1996;16(12):7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry. 2002;41(22):7100–7107. doi: 10.1021/bi025902m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rousseau S, Dolado I, Beardmore V, Shpiro N, Marquez R, Nebreda AR, Arthur JS, Case LM, Tessier-Lavigne M, Gaestel M, Cuenda A, Cohen P. CXCL12 and C5a trigger cell migration via a PAK1/2-p38alpha MAPK-MAPKAP-K2-HSP27 pathway. Cell Signal. 2006;18(11):1897–1905. doi: 10.1016/j.cellsig.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Reutershan J, Stockton R, Zarbock A, Sullivan GW, Chang D, Scott D, Schwartz MA, Ley K. Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med. 2007;175(10):1027–1035. doi: 10.1164/rccm.200612-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270(47):27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 48.White MA, Anderson RG. Signaling networks in living cells. Annu Rev Pharmacol Toxicol. 2005;45:587–603. doi: 10.1146/annurev.pharmtox.45.120403.095807. [DOI] [PubMed] [Google Scholar]
- 49.Kutuk O, Basaga H. Inflammation meets oxidation: NF-kappaB as a mediator of initial lesion development in atherosclerosis. Trends Mol Med. 2003;9(12):549–557. doi: 10.1016/j.molmed.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Christman JW, Blackwell TS, Juurlink BH. Redox regulation of nuclear factor kappa B: therapeutic potential for attenuating inflammatory responses. Brain Pathol. 2000;10(1):153–162. doi: 10.1111/j.1750-3639.2000.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin Cancer Biol. 2006;16(1):80–88. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]