INTRODUCTION1–6
Hereditary angioedema (HAE) is a rare genetic disorder resulting from an inherited deficiency or dysfunction of the C1 inhibitor (C1-INH), a molecule that inhibits kallikrein and other serine proteinases. HAE is characterized by unpredictable and recurrent attacks of inflammation affecting the hands, feet, face, abdomen, urogenital tract, and larynx. Three distinctive types of HAE have been identified:
Type I affects 85% of HAE patients and results from low plasma levels of a normal C1-INH protein.
Type II affects 15% of HAE patients and occurs in the presence of normal or elevated but dysfunctional C1-INH levels.
Type III occurs when the angio-edema is characterized by normal, functional, and quantitative levels of C1-INH as estrogen-dependent and/or as an estrogen-associated inherited type.
Approximately 6,000 people (1 in 50,000) in the U.S. are affected by HAE either as a result of deficient C1-INH concentrations (type I) or dysfunctional C1-INH (type II). The incapacitating and occasionally life-threatening inflammation is believed to be caused by mutations of the C1-INH gene, located in the q12-q13.1 subregion of chromosome 11, causing dysfunctional levels of C1-INH. HAE is not associated with any predilection for race, age, or sex. C1-INH is the major inhibitor of:
two serine proteases in the complement pathway, C1s and C1r
the mannan-binding lectin-associated serine proteases (MASPs)
the contact (coagulation) system proteases, factor XIa, factor XIIa (Hageman factor), and kallikrein
Type III HAE typically affects women, but case reports involving men have been identified. The exact way in which estrogen leads to angioedema is not clear. A potential mechanism may involve up-regulation of bradykinin or a mutation in factor XII, resulting in activation of a kinin surge.
Symptoms of C1-INH deficiency can occur during any stage of life. The deficiency may be diagnosed at birth or later in life. Typical manifestations of HAE include acute, episodic self-limited attacks of painless, nonpruritic, angio edema of the skin affecting various parts of the body. HAE can be life-threatening when angioedema involves the larynx directly. The clinical presentation of a patient with HAE is illustrated in Figure 1.
Figure 1.
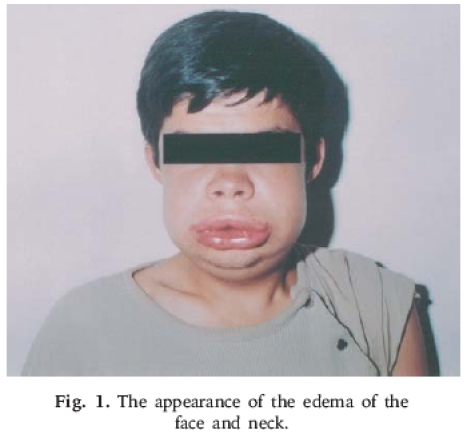
Appearance of edema of the face and neck. (Courtesy of Turk J Pediatr. Available at: http://tjp.dergisi.org/images/xml/figure-85-001.png.)
Current research indicates that deficient or dysfunctional C1-INH levels may amplify key steps in the complement and contact systems. Basically, increased production of C1s and Clr serine proteases helps to activate complement factors, amplify coagulation factors XIa and XIIa, and disrupt inhibition of the kallikrein–kinin system. This disruption leads to abnormally high amounts of kallikrein in plasma. High-molecular-weight kininogen, when cleaved, produces high levels of bradykinin.
Bradykinin, a known potent mediator of vasodilation through calcium-regulated signaling pathways, produces three important vasodilatory mediators: endothelium-derived hyperpolarizing factor (EDHF), nitric oxide (NO), and prostacyclin (PGI2). In conditions with excess bradykinin, such as HAE, this potent vasodilatory action of bradykinin promotes increased vascular permeability, mediating angioedema. Despite consistently elevated bradykinin levels, attacks occur at unpredictable rates. However, certain triggers can lead to a HAE attack. Trauma or surgery, dental procedures, infection, pregnancy, emotional stress, menstruation, and oral contraceptive use are all considered to be potential triggers. However, HAE attacks can also occur without an exogenous trigger or without autoactivation of the contact or complement system.
This article describes the human plasma-derived C1 esterase inhibitor (Cinryze, Lev Pharmaceuticals), which was approved in October 2008 for the prevention of HAE attacks.
DIAGNOSIS AND TREATMENT
The diagnosis of HAE is based on the patient’s family history, clinical presentation, and laboratory results (Table 1). Treatment is aimed at stabilizing the patient, treating the acute angioedema attack, and preventing future attacks. Long-term prophylactic therapies are warranted for patients with a history of multiple episodes of edema (more than one episode per month). Several therapeutic options exist for this indication, such as attenuated androgens like stanozolol (Winstrol, Winthrop) or danazol (Danocrine); antifibrinolytic agents (aminocaproic acid, tranexamic acid); and the exogenous serine proteinase inhibitor C1-INH.
Table 1.
Laboratory Findings in Hereditary Angioedema
| Type I | Type II | Type III |
|---|---|---|
| Low C1-INH | High or low C1-INH; however, noted as dysfunctional | Normal C1-INH |
| Low C4 and C2 | Low C4 and C2 | C1-INH functional assay and C4 level normal |
| Normal C1q | Normal C1q |
INDICATION AND USAGE7
As the first in its class to be approved for use in the U.S., Cinryze is indicated for routine prophylaxis against HAE attacks in adolescents older than nine years of age and in adults with a history of HAE.
CLINICAL PHARMACOLOGY7
Cinryze provides active exogenous C1-INH. The angioedema is thought to be caused by increased vascular permeability resulting from events that originate from activation of the contact system. C1-INH helps to suppress contact system activation, thus correcting vascular permeability and preventing the clinical manifestations of HAE.
PHARMACOKINETICS AND PHARMACODYNAMICS7
The pharmacokinetic properties of Cinryze were analyzed in a randomized, parallel-group, open-label study of patients with nonsymptomatic HAE. Patients received Cinryze either as a single dose of 1,000 units or as 1,000 units, followed by a second dose of 1,000 units 60 minutes later. The pharmacokinetic parameters of Cinryze, as reported from this study, are listed in Table 2. This is the only study that investigated the pharmacokinetics of this agent. No additional pharmacokinetic studies have evaluated specific patient populations (geriatric or pediatric), sex, race, or the presence of renal or hepatic impairment.
Table 2.
Pharmacokinetic Profile of C-1 Esterase Inhibitor (Cinryze)
| Parameters | Single Dose | Double Dose |
|---|---|---|
| Cbaseline (units/mL) | 0.31 ± 0.20 (n = 12) | 0.33 ± 0.20 (n = 12) |
| Cmax (units/mL) | 0.68 ± 0.08 (n = 12) | 0.85 ± 0.12 (n = 13) |
| Tmax (hours) | 3.9 ± 7.3 (n = 12) | 2.7 ± 1.9 (n = 13) |
| AUC(0–t) (units * hour/mL) | 74.5 ± 30.3 (n = 12) | 95.9 ± 19.6 (n = 13) |
| Clearance (mL/minute) | 0.85 ± 1.07 (n = 7) | 1.17 ± 0.78 (n = 9) |
| Half-life (hours) | 56 ± 36 (n = 7) | 62 ± 38 (n = 9) |
AUC = area-under-the-curve concentration; Cmax = peak concentration; Tmax = time to peak concentration.
Data from Cinryze package insert.7
CLINICAL TRIALS7
Cinryze as a therapy for preventing HAE attacks was evaluated in the 24-week, multicenter, double-blind, placebo-controlled, crossover CHANGE Trial (C1-Inhibitor in Hereditary Angioedema Nanofiltration Generation Evaluating Efficacy). The primary efficacy outcome was the total number of angioedema episodes in each treatment period in adults and adolescents. An attack of HAE was defined as swelling in any location on the patient’s body following a report of no swelling on the previous day.
Secondary efficacy outcomes included the average severity of attacks, as determined by the investigators and as reported by the patients as mild (a score of 1), moderate (a score of 2), or severe (a score of 3). The total severity score was calculated by multiplying the total number of mild attacks by 1, moderate attacks by 2, and severe attacks by 3. Secondary outcome measurements also included the average duration of HAE attacks, the average duration of swelling, the number of patients withdrawing from the study protocol, and the number of open-label C1-INH infusions.
Monitoring of adverse events included only effects resulting from treatment. Adverse events were defined as those that started on or after the time of the first infusion of Cinryze and whose severity worsened on or after the time of infusion and up to 30 days after the last Cinryze infusion. Other parameters included in the safety analysis were vital signs, physical findings, and laboratory results.
Patients received blinded Cinryze 1,000 units via an intravenous (IV) infusion twice weekly for 12 weeks at the study site and then crossed over to receive twice-weekly IV infusions of placebo for 12 weeks at the study site, or vice versa. Open-label, Cinryze was permitted for managing acute HAE attacks; however, if an open-label infusion was given, a period of 24 hours was required before the next prophylactic infusion could be administered. Outcome data were collected at the time of infusion at the study site, and safety data were collected before and 60 minutes after each infusion.
Investigators determined the sample size by assuming angioedema attack rates for patients in the placebo and treatment arms. It was assumed that there would be one attack every two weeks in the placebo phase and one attack every 12 weeks in the prophylactic treatment phase. It was reported that 10 subjects per sequence, for a total of 20 patients, provided more than 90% power to detect the treatment effect.
The investigators enrolled 26 patients; 24 were included in the safety analysis, 23 patients received the study drug and placebo, and 20 patients completed the entire study. Of the four patients who withdrew from the study, two withdrew consent, one withdrew at the investigators’ discretion, and one discontinued for “other” reasons.
Patients were enrolled in this study if they had a history of HAE and had experienced at least two or more acute HAE attacks per month. The patients were six years of age or older (mean age, 38.1 years, range, 9–73 years). Most patients were female (90.9%), and 95.5% were Caucasian. Patients were excluded from the study if they had a history of B-cell malignancy or a previous allergic reaction to C1-INH or other blood products; if they were addicted to narcotics; if they had received blood or blood products within the past 90 days; if they were pregnant or lactating; or if they had any clinically significant medical condition that would interfere with their ability to participate in the study.
The mean number of HAE attacks in each treatment period was significantly lower for patients receiving Cinryze (6.1 ± 5.4 attacks) than for those receiving placebo (12.7 ± 4.8 attacks) (P < 0.0001). Significant reductions were also noted in the mean severity of HAE attacks (1.3 ± 0.85 with Cinryze, 1.9 ± 0.35 with placebo; P < 0.0006), the mean duration of attacks (2.1 ± 1.13 days, 3.4 ± 1.39 days; P < 0.0004), and the mean duration of swelling (10.1 ± 10.73 days, 29.6 ± 16.9 days; P < 0.0001). During the study period, fewer open-label rescue C1-INH infusions were required with Cinryze (4.7 ± 8.66) than with placebo (15.4 ± 8.41) (P < 0.0001).
Of the 24 patients included within the safety population, 21 reported one or more treatment-emergent adverse event. The most common treatment-emergent events were sinusitis, rash (in five subjects, 21.7%), headache, upper respiratory tract infection (in four subjects, 17.4%), and viral upper respiratory tract infection (in three subjects, 13%), gastro-esophageal reflux disease, pruritus, and vomiting (in two subjects, 8.7%). No events were reported to have led to death.
The investigators concluded that Cinryze, at 1,000 units per dose twice weekly by IV injection, was effective in preventing or reducing the frequency of HAE attacks in patients with the disease. Ongoing clinical trials (CHANGE 2 and CHANGE 3) are under way in an effort to evaluate Cinryze in relieving symptoms within a four-hour time period.
ADVERSE DRUG REACTIONS7
In the clinical trial, Cinryze was well tolerated, with most observed adverse reactions reported as upper respiratory infection, sinusitis, rash, and headache. Additional events were considered to be more serious and occurred with the use of Cinryze, although they were determined to be unrelated to the study drug. These events included death caused by non–catheter-related foreign-body embolus, pre-eclampsia resulting in emergency cesarean section, stroke, and exacerbation of HAE attacks.
PREGNANCY7
Cinryze is classified as a pregnancy Category C drug.
DRUG INTERACTIONS1,10
Interactions with the coadministration of Cinryze and other potential agents continue to be evaluated. Information about potential drug interactions is limited. However, agents similar to angiotensin-converting enzyme (ACE) inhibitors (i.e., angiotensin-receptor blockers), which increase plasma levels of bradykinin, may delay response or may precipitate HAE attacks. It is possible that other agents, including estrogen-containing oral contraceptives and estrogen-replacement therapy, also induce attacks.
CONTRAINDICATIONS7
Cinryze is contraindicated in patients with any known immediate hypersensitivity reactions (e.g., hives, chest tightness, wheezing, shortness of breath, hypo tension, and anaphylaxis) to any of its constituents. An interesting, and perhaps complicating, concern arises when hypersensitivity reactions present as angioedema. It may be difficult to determine whether the reaction is evidence of treatment failure or whether it is truly a hypersensitivity reaction. Careful clinical judgment is warranted because symptoms of hypersensitivity may be analogous to those of angioedema.
PRECAUTIONS AND WARNINGS7
Because Cinryze is derived from human plasma, its use carries a risk of viral transmission, including Creutzfeldt–Jakob disease. If a patient has an infection that was believed to have been transmitted by Cinryze, the manufacturer recommends calling Lev Pharmaceuticals (877-945-1000) or reporting the event directly to the FDA (800-FDA-1088 or www.fda.gov/medwatch). To date, there have been no reports of viral transmission, but the potential exists.
There have been reports of thrombo-embolic events associated with C1-INH products when used in an off-label fashion at high doses. These events have also been documented in animal studies involving C1-INH. Caution is needed with patients with risk factors for thrombosis.
DOSAGE AND ADMINISTRATION7
Cinryze is intended for IV use only. The recommended dose for routine prophylaxis against HAE attacks is 1,000 units at an initial infusion rate of 1 mL/minute (10 minutes of infusion time) every three to four days. The sterile, lyophilized preparation of C1-INH is derived from human plasma that is purified by filtration and chromatographic procedures.
Following reconstitution of the freeze-dried powder with 5 mL of Sterile Water for Injection, USP, each vial contains approximately 500 units of functionally active C1-INH. One unit of Cinryze corresponds to the mean quantity of C1-INH present in 1 mL of normal fresh plasma.
COST8
The average wholesale price for each 500-unit sterile, single-use glass vial of Cinryze is $2,340.
CONCLUSION
Cinryze is the first FDA-approved C1 esterase inhibitor therapy for preventing HAE attacks in the U.S. Before this approval, available products for treating HAE were limited to anabolic steroids. Although HAE is a rare event, patients with this genetic deficiency experience significant medical, psychiatric, and economic consequences. With the introduction of Cinryze, patients may have a chance to regain a normal lifestyle.
Other investigational agents for HAE are undergoing clinical trials. Safety and efficacy data remain to be determined; however, agents like icatibant (Fiazyr, Jerini AG), a bradykinin receptor (B2) inhibitor; ecallantide (DX-88, Dyax), a kallikrein inhibitor; and Berinert-P (CSL Behring), a plasma-derived C1-inhibitor, may have a role in treating this debilitating disease based on preliminary data and based on the inimitable mechanism of action each agent possesses. As of April 2008, the FDA issued a nonapprovable letter for icatibant, requiring further evaluation.
Footnotes
Disclosure: The authors have no commercial or industrial relationships to report in regard to this article.
REFERENCES
- 1.Nzeako UC, Frigas E, Tremaine W. Hereditary angioedema: A broad review for clinicians. Arch Intern Med. 2001;161:2417–2429. doi: 10.1001/archinte.161.20.2417. [DOI] [PubMed] [Google Scholar]
- 2.Davis AE. C1-inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson VH, Bissler JJ. C1-inhibitors and their genes: An update. J Lab Clin Med. 1992;119:330–333. [PubMed] [Google Scholar]
- 4.Agostoni A, Ygoren-Pursun E, Binkley KE, et al. Hereditary and acquired angioedema: Problems and progress. Proceedings of the Third C1 Esterase Inhibitor Deficiency Workshop and beyond. J Aller gy Clin Immunol. 2004;114:S131. doi: 10.1016/j.jaci.2004.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bas M, Adams V, Suvorava T, et al. Non-allergic angioedema: Role of bradykinin. Allergy. 2007;62:842–856. doi: 10.1111/j.1398-9995.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 6.FDA Product approval informationAvailable at: www.fda.gov/cber/approvltr/cinryze101008L.htm Accessed October 10, 2008.
- 7.New York: Lev Pharmaceuticals, Inc; 2009. Cinryze (C1-inhibitor, human), package insert. [Google Scholar]
- 8.Drug Topics Red Book(Update)Montvale, NJ: Thomson Reuters; February200928225 [Google Scholar]
- 9.Gompels MM, Lock RJ, Morgan JE, et al. Short report: Multicentre evaluation of the diagnostic efficiency of serological investigations for C1 inhibitor deficiency. J Clin Pathol. 2002;55:145–147. doi: 10.1136/jcp.55.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bork K, Fischer B, Dewald G. Recurrent episodes of skin angioedema and severe attacks of abdominal pain induced by oral contraceptives or hormone replacement therapy. Am J Med. 2003;114(4):294–298. doi: 10.1016/s0002-9343(02)01526-7. [DOI] [PubMed] [Google Scholar]


