Abstract
Molecular mechanisms controlling the assembly of cartilage-specific types II, IX and XI collagens into a heteropolymeric network of uniformly thin, unbanded fibrils are not well understood, but collagen XI has been implicated. The present study on cartilage from the homozygous chondrodysplasia (cho/cho) mouse adds support to this concept. In the absence of α1(XI) collagen chains, thick, banded collagen fibrils are formed in the extracellular matrix of cho/cho cartilage. A functional knock-out of the type XI collagen molecule has been assumed. We have re-examined this at the protein level to see if rather than a complete knock-out, alternative type XI chain assemblies were formed. Mass spectrometry of purified triple helical collagen from the rib cartilage of cho/cho mice identified α1(V) and α2(XI) chains. These chains were recovered in roughly equal amounts based on Coomassie blue staining of SDS-PAGE gels, in addition to α1(II)/α3(XI) collagen chains. Using telopeptide-specific antibodies and Western blot analysis, it was further shown that type V/XI trimers were present in the matrix cross-linked to each other and to type II collagen molecules to form heteropolymers. Cartilage from heterozygous (cho/+) mice contained a mix of α1(V) and α1(XI) chains and a mix of thin and thick fibrils on transmission electron microscopy. In summary, the results imply that native type XI collagen molecules containing an α1(XI) chain are required to form uniformly thin fibrils and support a role for type XI collagen as the template for the characteristic type II collagen fibril network of developing cartilage.
Keywords: Cartilage, type XI collagen, type V collagen, fibrils, chondrodysplasia, cross-links, type II collagen
INTRODUCTION
Type XI collagen, although a minor structural protein of cartilage, is essential for normal embryonic skeletal development and the cohesive properties of cartilage. This collagen accounts for 3% to 10% of the collagenous protein of adult and fetal cartilage (Eyre, 1991; Eyre et al., 2002a). In fetal cartilage, type XI collagen consists of molecules containing three genetically distinct chains, α1(XI), α2(XI) and α3(XI) in a 1:1:1 ratio. However, from mature articular cartilage, purified type XI collagen also includes about equal amounts of α1(V) and α1(XI) chains (Eyre and Wu, 1987; Eyre et al., 2002b), suggesting the existence of type V/XI hybrid molecules in the matrix. Type XI collagen molecules are cross-linked by lysyl oxidase-mediated bonds (Wu and Eyre, 1995) and a homopolymer of type XI collagen can form in vitro (Bruckner and van der Rest, 1994; Blaschke et al., 2000). Type XI collagen molecules are also cross-linked to type II collagen presumably as co-components of heterofibrils (Wu and Eyre, 1995). Since type XI collagen is proposed to be within the core of type II collagen fibrils (Mendler et al., 1989), it is becoming clear that type XI collagen may act as both the template for type II collagen fibrillogenensis and as a regulator of fibril diameter in cartilage (Blaschke et al., 2000; Holmes and Kadler, 2006; Wu and Eyre, 1995).
Three heritable type XI collagenopathies have been defined in humans: type II Stickler syndrome, dominant, and recessive otospondylo megaepiphyseal dysplasia (OSMED). Mutations in COL11A1 and/or COL11A2 genes have been implicated in all three diseases (reviewed in Spranger, 1998). Mutations in the α1(XI) andα2(XI) collagen genes have also been linked to early-onset osteoarthritis (Jakkula et al., 2005).
The neonatal lethal chondrodysplasia (cho) mouse is caused by a deletion of a cytidine residue 570 nucleotides downstream from the translation initiation codon in Col11a1, which results in a reading frame shift and the introduction of a premature stop codon (Li et al., 1995). The homozygous cho/cho (c/c) mouse develops a severe chondrodysplasia and dies at birth. The cho/+ (c/+) heterozygote survives, and develops early onset osteoarthritis (Rodriguez et al., 2004; Xu et al., 2003). The α1(XI) chain is not translated into protein in the homozygous mouse (cho/cho) cartilage, so a functional knock-out of type XI collagen seemed likely (Li et al., 1995). We have used the cho mouse to address whether this was the consequence at the protein level or that alternative type V/XI chain assemblies could form in the absence of α1(XI) chains and perhaps result in a misdirected collagen II fibril organization.
Here we report the effects of this mutation on the molecular properties of type XI collagen in the cartilage of chondrodysplasia mice.
RESULTS
Electron Microscopy
Transmission electron micrographs of homozygous cho/cho rib cartilage showed sparsely distributed, thick, banded collagen fibrils (greater than 80nm) as a characteristic feature of both the territorial and inter-territorial matrices surrounding the chondrocytes (Figure 1, right panel). The typical 67nm banded pattern of collagen was pronounced. This contrasted with the uniformly thin, unbanded collagen fibrils (20nm or less) forming a fine meshwork in the extracellular matrix of chondrocytes in normal rib cartilage (Figure 1, left panel).
Figure 1.
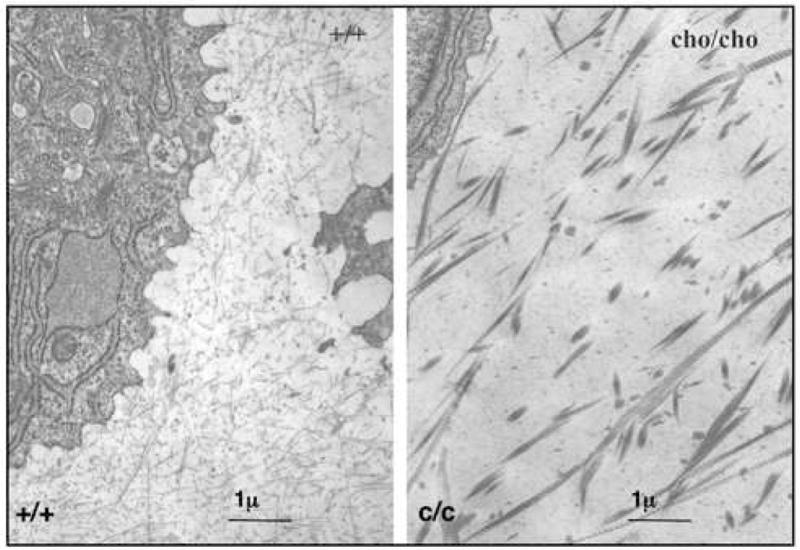
Electron Micrographs of collagen fibrils in rib cartilage extracellular matrix. When compared to normal (left panel) collagen fibrils in the cho/cho cartilage (right panel) have large diameters and are clearly banded. Such fibrils are unusual in fetal/young cartilage. Bar = 1 micron.
Collagen analysis
Collagen was solubilized from the rib cartilages of homozygous, heterozygous and normal mice by pepsin digestion, and compared by SDS-PAGE. As seen in Figure 2, electrophoresis of the pepsin-extracted collagen from the matrix of wild type (lane 2) and homozygous cho/cho mice (lane 3) clearly revealed a major pepsin-resistant band. This band co-migrated with the α1(II) collagen chain of human and rat type II collagen, seen in control lanes 1 and 4 respectively. Electrophoresis also resolved and detected pepsin-extracted α1(XI) and α2(XI) chains from wild-type cartilage (Figure 2, lane 2). The α3(XI) chain, which is the post-tranlationally overmodified product of the col2a1 gene, is not well resolved from the α1(II) collagen chain. From the cho/cho cartilage (from which the α1 type XI collagen chain is missing), two neighboring bands in a 1:1 ratio are seen running slower than the α1(II) chain. Band 1 (Figure 2, lane 3, open arrowhead), migrating slightly slower than α1(XI) (compare c/c and +/+ lanes in Figure 2), and band 2 migrating close to the position of α2(XI). These chains were identified incontrovertibly as mouse α1(V) and α2(XI) respectively by in-gel trypsin digestion and microbore LC/ion-trap mass spectrometry with database searching (Table 1). These chains ran slower presumably because of lysine overmodifications (Fernandes et al., 1998). Band 3 (Figure 2) was identified similarly as α1(II) (or 3(XI)), i.e., the product of the same gene. The band running slightly faster than the α1(II) band is a pepsin over-cleavage product of the α1(II) collagen chain and has been identified before (Fernandes et al., 1998).
Figure 2.

SDS-PAGE of collagen extracted from rib cartilage.
Pepsin-extracted collagen from wild type (+/+) lane 2, and homozygous cho (c/c) lane 3, mice. Pepsin-extracted collagen from Rat chondrosarcoma cell line, RCS-LTC, is in lane 4. Purified pepsin-extracted type II collagen from human cartilage is in lane 1. 10kDa Gibco BRL molecular weight ladder is in lane 4. Arrowhead points to a band migrating in the region of α1(XI) chains in the c/c cartilage. The numbered bands are identified in Table 1.
Table 1.
Tryptic peptides identified by in-gel trypsin digestion and mass spectrometry
| Band | Collagen chain | Sequence | Mass (M+H) |
|---|---|---|---|
| 1 | α1(V) | GPAGAAGPIGIP* | 1207.37 |
| GRGDP*GPSGPP*GIP*GDDGER | 1725.71 | ||
|
| |||
| 2 | α2(XI) | P*GATGQAGPP*GPVGPP*GLP*GLR | 2015.22 |
|
| |||
| 3 | α1(II) | VGPP*GANGNP*GPAGPP*GPAGK | 1814.94 |
| GEAGAQGPMGPSGPAGAR | 1568.70 | ||
Identities of bands 1, 2, and 3 from Figure 2 are shown. P*, Hydroxyproline
To address the possibility that these chains can form type V/XI hybrid molecules, pepsin-extracted triple-helical type XI collagen molecules were purified from the cartilage of heterozygous and homozygous cho mice by sequential salt precipitations.
As seen in Figure 3, triple-helical type II collagen was precipitated at 0.8M NaCl and triple-helical type XI collagen at 2.2M NaCl.
Figure 3.

Purification of type XI collagen molecules.
Collagen was extracted from the rib cartilage of heterozygous (c/+) and homozygous (c/c) mice. Sequential collagen precipitation at pH 3 resulted in type II collagen precipitating in the 0.8M NaCl precipitate and type XI collagen in the 2.2M NaCl precipitate. The identities of the numbered bands are shown in Table 2.
Mass spectroscopy of in-gel trypsin digests of selected bands from the gel shown in Figure 3 identified bands 1 and 2 as α1(II) chains (Table 2); band 3, from the heterozygote cartilage as a mixture of α1(V) and α1(XI) chains; band 4 as α2(XI). Bands 5 and 6 from the homozygote cartilage (present in 1:1 ratio) were identified as α1(V) and α2(XI) respectively.
Table 2.
Tryptic peptides identified by in-gel trypsin digestion and mass spectrometry
| Band | Collagen chain | Sequence | Mass (M+H) |
|---|---|---|---|
| 1, 2 | α1(II) | VGPP*GANGNP*GPAGPP*GPAGK | 1814.94 |
| GEAGAQGPMGPSGPAGAR | 1568.70 | ||
|
| |||
| 3 | α1(V) | GDP*GPSGPP*GIP*GDDGER | 1725.71 |
| α1(XI) | TGP*P*GPGGVVGPQGPTGETGPIGER | 2304.46 | |
|
| |||
| 4 | α2(XI) | P*GATGQAGPP*GPVGPP*GLP*GLR | 2015.22 |
| GDTGAQGLP*GPP*GEDGER | 1742.74 | ||
|
| |||
| 5 | α1(V) | GPAGAAGPIGIP*GR | 1207.37 |
| GDP*GPSGPP*GIP*GDDGER | 1725.71 | ||
|
| |||
| 6 | α2(XI) | GDTGAQGLP*GPP*GEDGER | 1742.74 |
Identities of bands 1–6 from Figure 3 are shown. Note the presence of peptides from both α1(XI) and α1(V) in band 3 from the heterozygote cartilage.
P*, Hydroxyproline
Thus, the mass spectrometric results confirmed that hybrid type V/XI molecules were present in the cartilage of cho/cho mice.
To examine whether these molecular forms were derived from cross-linked, polymeric collagen, we used Western blotting and antibodies that could detect different candidate cross-linked telopeptide adducts. Antibody 5892 was raised against the synthetic peptide GGDKGPVVAA, matching a sequence in the human α2(XI) collagen N-telopeptide. This antibody detects collagen chains that have this telopeptide stub (pepsin-cleaved) covalently cross-linked to the triple-helical domain. The Western blot in Figure 4A shows that it recognizes the α1(XI) chain from both heterozygotic cho and wild-type cartilages (lanes 1,2) and also from cartilage of the disproportionate micromelia mouse (lane 4), (Fernandes et al., 2003a). From the homozygote (lane 3), however, the antibody recognized two bands, which from the Coomassie Blue stained gel in Figure 2, are α1(V) and α2(XI). Using monoclonal antibody 1C10 (specific to the helix of α1(II)), Figure 4C shows where α1(II) chains migrate. Together the results indicate that in the matrix of homozygotic cho cartilage α2(XI) chains (N-telopeptide) were cross-linked to the helical domains of α1(V) and α2(XI) chains (triple helix) implying the presence of polymeric type V/XI molecules.
Figure 4.
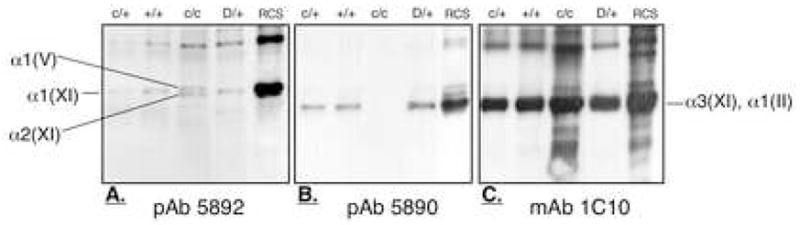
Type V/XI collagen polymer forms in homozygous cho cartilage.
Western blot of pepsin-extracted collagen from homozygous (c/c), wild-type (+/+) and heterozygous (c/+) mouse rib cartilage. A. In c/c cartilage, the pAb 5892 recognized the α1(V) and α2(XI) chain indicating that the α2(XI) N-telopeptide is cross-linked to these chains. B. In c/+ and +/+ cartilage the pAb 5890 recognized the α1(II) chain indicating the α1(XI) chain was cross-linked to it. However, in c/c cartilage, the pAb 5890 was unable to recognize the type II collagen because α1(XI) chains are not translated into protein here. C. mAb 1C10 recognized the α1(II) chains in all samples including pepsin extracted collagen from the matrix of RCS-LTC cell line containing type II and type XI collagen (RCS-LTC) and D/+, pepsin-extracted collagen from rib cartilage of the heterozygous disproportionate micromelia mouse.
The antibody 5890 was raised against the synthetic peptide DGSKGPTISA sequence within the α1(XI) N-telopeptide containing the cross-linking lysine. This antibody recognizes collagen chains to which α1(XI) N-telopeptide stubs cleaved by pepsin are covalently attached. As seen in Figure 4B, since the α1(XI) chain is missing from the homozygous cartilage, this antibody fails to detect any collagen chains (lane 3). This antibody does detect α3(XI) chains which have α1(XI) N-telopeptides attached from heterozygous and normal cartilage (lanes 1, 2).
To examine whether type II collagen was also cross-linked to the type XI collagen molecular isoforms, monoclonal antibody 10F2 was also applied to Western blots. This antibody recognizes cleaved α1(II) C-telopeptide cross-linked to pepsin-solubilized collagen chains. When α1(II) chains are absent, as seen in cartilage of the homozygous disproportionate micromelia mouse (Figure 5, lane 2), there is no reactivity. As expected, this antibody was strongly reactive with α1(II) chains from homozygous cho cartilage (Figure 5, lane 1), which contains polymeric type II collagen. A band running in the position of the α1(V) chain (identified in Figure 2) was also detected in homozygous cho cartilage (Figure 5, lane 3), consistent with an α1(II) C-telopeptide cross-linked to the helix of α1(V) and, hence, co-polymers of types II and XI/V in the cho/cho cartilage.
Figure 5.
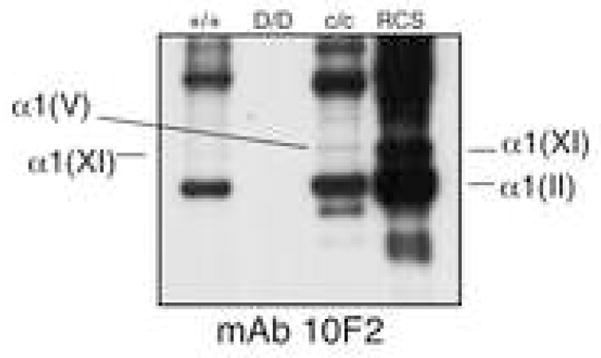
Type II-type V/XI heteropolymer forms in homozygous cho cartilage.
Western blot of type II and type XI collagen extracted from homozygous (c/c) and wild-type (+/+) mouse rib cartilage. In c/c cartilage, mAb 10F2 recognized the α1(V) chain indicating that the α1(II) C-telopeptide is cross-linked to this chain.
RCS, partially purified type II and type XI collagen from RCS-LTC cell line. D/D, pepsin-extracted collagen from rib cartilage of the homozygous disproportionate micromelia mouse. Stable type II collagen is not present in D/D cartilage (Fernandes et al., 2003a).
DISCUSSION
The results show that the chondrocytes in neonatal homozygous cho cartilage express a matrix containing sparsely distributed, thick, banded collagen type II fibrils. Even though the α1(XI) collagen chain is missing, native collagen V/XI hybrid molecules are present as cross-linked polymers in the matrix, presumably within and as a template for the collagen type II.
We hypothesized that in the absence of α1(XI) collagen chains, isoforms of type XI collagen molecules can assemble and form a template for type II collagen fibrils. We rationalized that substitution of α1(V) for α1(XI) collagen chains may be expected because they belong to the same clade of collagen gene products (Boot-Handford and Tuckwell, 2003).
Fibrillogenesis experiments have demonstrated that type II collagen molecules can be reconstituted in vitro into aggregates that resemble native type II collagen fibrils (Lee and Piez, 1983; Birk and Silver, 1984; Fertala et al., 1994; Yang et al., 1995; Fertala et al., 1996). There is evidence that type XI collagen plays a critical role in maintaining the thin diameters the type II collagen fibrils form in vivo (Keene et al., 1995; Blaschke et al,, 2000). The present and previous findings on the cho/cho mouse (in which the α1(XI) collagen chain is not synthesized) support this hypothesis. It was previously assumed that in the absence of the α1(XI) collagen chain, cho/cho cartilage lacked any type XI collagen molecules (Li et al., 1995) and the large diameter fibrils previously observed in the rib, tracheal, and limb cartilage matrix (Seegmiller, 1971; Seegmiller, 1972; Seegmiller, 1982) were homopolymers of type II collagen. The present biochemical data (Figure 2, Table 1,2), however, show that there is a significant amount of an aberrant form of type XI collagen in the matrix, which shows similarities to a chain composition observed in adult articular cartilage (Eyre and Wu, 1987). The findings suggest that in the absence of α1(XI) collagen chains, α1(V) collagen chains are expressed and can trimerize with α2(XI) and α3(XI) chains forming stable (pepsin resistant) type V/XI hybrid molecules (Figure 3). Since COL11A1, COL11A2 and COL5A1 are all members of the same B clade of collagen genes (Boot-Handford and Tuckwell, 2003), it is reasonable that they can substitute for each other in assembling into mature collagen molecules. As noted above, the α1(V) chain has been found in increasing amounts with tissue maturity as a component of the type XI collagen fraction from articular cartilage. Hybrid molecules composed of α1(XI) and α2(V) chains are also in the primary V/XI component, together with type II collagen in vitreous of the eye (Mayne et al., 1993). Related molecular isoforms have also been found in nucleus pulposus and annulus fibrosus of the intervertebral disc (Wu et al., 2005) and recently in fetal tendon (Wenstrup et al., 2006). In mature bone, the α1(XI) collagen chain appears and increases with age as a component of type V collagen, presumably in the form of hybrid molecules trimerized with α1(V) and α2(V) chains (Niyibizi and Eyre, 1989). Recent studies on the osteoblast-like cell line, SAOS-2, show expression of α1(XI) and α2(XI) chains in addition to collagen V chains (Fernandes et al., 2007).
The functional significance of such tissue-specific isoforms based on different chain combinations of type V collagen and type XI collagen gene products is not clear. But the findings imply a role in regulating the overall fibril form and network architecture of the extracellular matrix. It is notable that as adult cartilages age and the proportion of α1(V) collagen chain in the type XI collagen fraction increases, the mean diameter of collagen fibrils in the matrix increases and their banded periodicity becomes more distinct (Keene et al., 1995). This resembles the situation in cho/cho mouse cartilage in which the collagen fibrils are unusually broad and distinctly banded compared with wild-type cartilage fibrils (Figure 1). To speculate, the α1(XI) collagen chain and in particular its N-propeptide domain may play a regulatory role in restricting the diameter of type II collagen fibrils assembled in developing cartilages. Exactly how the N-propeptide domain may affect fibril diameter is unclear, but recent structural analysis (Fallahi et al., 2005) and microfibril modeling predictions suggest that the N-propeptide globular domains are excluded from the microfibril interior, encircle the fibril and prevent further lateral aggregation of collagen molecules (Holmes and Kadler, 2006). The absence of α1(XI) chains from type V/XI hybrid molecules in cho/cho cartilage (Figure 2, tables 1, 2) may be responsible at least in part for an impaired regulation of type II collagen fibril diameters. The fibrils observed in cho/cho cartilage matrix are thick and clearly banded compared with the thin fibrillar meshwork of wild-type cartilage (Figure 1). The findings support a hypothesis that normal type XI collagen containing the α1(XI) chain is required in the assembly of the thin heterotypic collagen fibrillar meshwork characteristic of young cartilage matrix. Presumably neither α2(XI) nor α1(V) can substitute in this role. Variations in sequence, domain architecture and possibly processing of the different N-propeptide domains may account for this (Thom and Morris, 1991; Fallahi et al., 2005). In addition, the protein interaction properties involved in interaction with fibril-associated molecules (collagen type IX, small leucine-rich proteoglycans, etc.) may also differ.
From electron microscopic studies type XI collagen was seen as an integral component of type II collagen fibrils buried in the interior (Mendler et al., 1989). Blaschke et al. (2000) showed that type XI collagen in vitro could nucleate and promote the self–assembly of type II collagen into fibrils typical of cartilage. Type XI collagen therefore can be viewed as a template for type II collagen assembly as type V collagen is for type I (Wenstrup et al., 2004). From the present results (Figure 1, Table 2) we conclude that a form of type XI collagen assembled with a chain composition of α1(V), α2(XI), α3(XI), but other chain combinations are possible. The Western blotting results indicate that the α2(XI) chain is cross-linked to α1(V) and α2(XI) chains in polymers of these molecules in the matrix (Figure 4). In addition, the V/XI hybrid molecules are cross-linked to type II collagen (Figure 5), consistent with a cross-linked heteropolymer. Though not presented here, collagen IX is also present in the cho/cho cartilage, but whether linked to type II collagen as in normal cartilage is not clear.
The results of analysis of type XI collagen prepared from rib cartilage of heterozygote cho mice (Figure 3 and Table 2) showed α1(XI) and α1(V) chains in about equal amount. This would be consistent with two forms of molecule, normal type XI [α1(XI) α2(XI) α3(XI)] and the apparent molecular form of homozygous cho cartilage [α1(V) α2(XI) α3(XI)]. This may be related to the observations of Xu et al. (2003) of two populations of collagen fibrils coexisting in the heterozygote cartilage matrix. Thin fibrils of wild-type morphology and thick, banded fibrils with a mean diameter three times that of the wild-type-fibrils were about equally abundant. One possible explanation is that polymers of normal type XI collagen molecules provide a template for the thin fibrils and polymers of the type V/XI hybrid provide a template for the thick collagen fibrils.
The question remains whether homotypic type II collagen fibrils can form in vivo. If, for example, chondrocytes had been unable to assemble any V/XI molecules as a template, would this have prevented embryogenesis to term? In the analogous situation with col5a1 −/− mice (Wenstrup et al., 2004), the lack of an α1(V) chain results in a functional knock-out of type V collagen since stable molecules cannot form from α2(V) chains. As a result, collagen fibrils were absent from the mesenchyme of col5a1 −/− embryos, which did not survive past day E10.
The findings emphasize the complexity in molecular assembly responsible for the characteristic collagen network of cartilage matrix. The exact molecular contribution of different type V/XI isoforms in helping regulate the assembly of distinctive collagen networks in different kinds of cartilage and other related tissues will be a challenge to understand.
METHODS
Tissue acquisition and genotype determination
Neonatal fetuses were obtained from matings of heterozygous mice. The genotype of the fetuses was determined by PCR amplification of a region of the Col11a1 gene followed by restriction enzyme analysis as described before (Fernandes et al., 2003a; Rodriguez et al., 2004).
Electron Microscopy
Rib plates from homozygous mutant (c/c) and wild-type mice (+/+) were fixed in 3% glutaraldehyde in 0.1M phosphate buffer (pH7.3, 20°C), post-fixed in 2% osmium tetroxide in 0.1 M phosphate buffer, and flat-embedded in Epon 812 as described before (Seegmiller et al., 1971). Sections at 2μ were cut on a Porter-Blum MT-1 microtome. Thin sections, mounted on 200-mesh uncoated grids or formvar-coated slotted grids, were stained with 2% uranyl acetate and Reynold’s lead citrate for examination with an RCA EMU-3 or a Philips-300 electron microscope.
Collagen extraction
Rib plates from wild-type (+/+) mice and from mice homozygous (c/c) and heterozygous (c/+) for the cho mutation were minced and extracted with 4 M GuHCl. Collagen in the residue was solubilized by pepsin digestion (Fernandes et al., 1998). Pepsin digests were fractionated into collagen types II, and IX by precipitation at 0.8M and 2.2M NaCl, respectively (Wu and Eyre, 1995).
Electrophoresis and Western blotting
Pepsin solubilized collagen chains were resolved by Laemmli SDS-PAGE and stained with Coomassie Blue. Collagen chains transferred to PVDF membrane were probed by three different antibodies designed to probe their heterotypic cross-linking interactions in the tissue. Polyclonal antibody (pAb) 5892 (raised against the synthetic peptide GGDKGPVVAA, which is a sequence in the α2(XI) collagen N-telopeptide domain) specifically recognizes an α2(XI) N-telopeptide proteolytic stub found cross-linked to the triple-helical region of the α1(XI) chain in normal cartilage (Wu and Eyre 1995). Thus, this antibody normally detects an α1(XI) chain, which is the primary site of interaction for the α2(XI) N-telopeptide. Polyclonal antibody (pAb) 5890 (raised against the synthetic peptide DGSKGPTISA, which is a sequence in the α1(XI) collagen N-telopeptide) specifically recognizes a proteolytic stub of the α1(XI) N-telopeptide, which is normally cross-linked to the triple-helical region of the α3(XI)/α1(II) chain (Wu and Eyre, 1995). Thus this antibody normally detects the α3(XI)/α1(II) chain or any other collagen chain cross-linked to the α1(XI) N-telopeptide. In addition, the monoclonal antibody (mAb) 10F2, which recognizes the C-telopeptide of α1(II) collagen in cross-linkage with other collagen chains (Fernandes et al., 2003b), was used to detect type II-type XI cross-linkages. Types II and XI collagens from pepsin-solubilized matrix of a rat chondrosarcoma cell line (RCS-LTC) and rib cartilage from homozygous or heterozygous disproportionate micromelia, D/D or D/+) mouse were used as standards. mAb 1C10 was used to identify α1(II) chains of type II collagen (Fernandes et al., 2003a).
Mass Spectrometry
A ThermoFinnnigan LCQ Deca XP mass spectrometer with electrospray ionization source and in-line C8 RP-HPLC was used. After Coomassie Blue staining on SDS-PAGE, individual protein bands were digested in-gel by trypsin. The resulting peptides were subjected to microbore column liquid chromatography (μLC) interfaced directly to a tandem mass spectrometer equipped with a micro-electrospray ionization source (Fernandes et al., 2007). For protein identification, peptide fragments were compared with the NCBI non-redundant protein database using SEQUEST, an automated database search algorithm designed for use with tandem mass spectrometry data.
Acknowledgments
Funding for this work was provided by NIH grants AR52896(RJF) and AR37318(DRE). We gratefully acknowledge Crystal Lam for her technical expertise and Kae Ellingsen for her help in preparing this manuscript. RJF also acknowledges the Friday Harbor Laboratories of the University of Washington for providing a creative environment to envision this project and begin writing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birk DE, Silver FH. Collagen fibrillogenesis in vitro: comparison of types I, II, and III. Arch Biochem Biophys. 1984;235:178–185. doi: 10.1016/0003-9861(84)90266-2. [DOI] [PubMed] [Google Scholar]
- Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–10378. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- Boot-Handford RP, Tuckwell DS. Fibrillar collagen: The key to vertebrate evolution? A tale of molecular incest. Bioessays. 2003;25:142–151. doi: 10.1002/bies.10230. [DOI] [PubMed] [Google Scholar]
- Bruckner P, van der Rest M. Structure and function of cartilage collagens. Microsc Res Tech. 1994;28:378–384. doi: 10.1002/jemt.1070280504. [DOI] [PubMed] [Google Scholar]
- Eyre DR. The collagens of articular cartilage. Semin Arthritis Rheum. 1991;21:2–11. doi: 10.1016/0049-0172(91)90035-x. [DOI] [PubMed] [Google Scholar]
- Eyre DR. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Wu JJ. Type XI or 1a2a3a collagen. In: Mayne R, Burgeson RE, editors. Structure and Function of Collagen Types. Academic Press; New York: 1987. pp. 261–281. [Google Scholar]
- Eyre DR, Wu JJ, Fernandes RJ, Pietka TA, Weis MA. Recent developments in cartilage research: matrix biology of the collagen II/IX/XI heterofibril network. Biochem Soc Trans. 2002;30:893–899. doi: 10.1042/bst0300893. [DOI] [PubMed] [Google Scholar]
- Fallahi A, Kroll B, Warner LR, Oxford RJ, Irwin KM, Mercer LM, Shadle SE, Oxford JT. Structural model of the amino propeptide of collagen XI alpha 1 chain with similarity to the LNS domains. Protein Sci. 2005;14:1526–1537. doi: 10.1110/ps.051363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes RJ, Harkey MA, Weis M, Askew JW, Eyre DR. The post-translational phenotype of collagen synthesized by SAOS-2 osteosarcoma cells. Bone. 2007;40:1343–1351. doi: 10.1016/j.bone.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes RJ, Seegmiller RE, Nelson WR, Eyre DR. Protein consequences of the Col2a1 C-propeptide mutation in the chondrodysplastic Dmm mouse. Matrix Biol. 2003a;22:449–453. doi: 10.1016/s0945-053x(03)00077-5. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Schmid TM, Eyre DR. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur J Biochem. 2003b;270:3243–3250. doi: 10.1046/j.1432-1033.2003.03711.x. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Wilkin DJ, Weis MA, Wilcox WR, Cohn DH, Rimoin DL, Eyre DR. Incorporation of structurally defective type II collagen into cartilage matrix in Kniest chondrodysplasia. Arch Biochem Biophys. 1998;355:282–290. doi: 10.1006/abbi.1998.0745. [DOI] [PubMed] [Google Scholar]
- Fertala A, Holmes DF, Kadler KE, Sieron AL, Prockop DJ. Assembly in vitro of thin and thick fibrils of collagen II from recombinant procollagen II. The monomers in the tips of thick fibrils have the opposite orientation from monomers in the growing tips of collagen I fibrils. J Biol Chem. 1996;271:14864–14869. doi: 10.1074/jbc.271.25.14864. [DOI] [PubMed] [Google Scholar]
- Fertala A, Sieron AL, Hojima Y, Ganguly A, Prockop DJ. Self-assembly into fibrils of collagen II by enzymic cleavage of recombinant procollagen II. Lag period, critical concentration, and morphology of fibrils differ from collagen I. J Biol Chem. 1994;269:11584–11589. [PubMed] [Google Scholar]
- Holmes DF, Kadler KE. The 10+4 microfibril structure of thin cartilage fibrils. Proc Natl Acad Sci U S A. 2006;103:17249–17254. doi: 10.1073/pnas.0608417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkula E, Melkoniemi M, Kiviranta I, Lohiniva J, Raina SS, Perala M, Warman ML, Ahonen K, Kroger H, Goring HH, Ala-Kokko L. The role of sequence variations within the genes encoding collagen II, IX and XI in non-syndromic, early-onset osteoarthritis. Osteoarthritis Cartilage. 2005;13:497–507. doi: 10.1016/j.joca.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Keene DR, Oxford JT, Morris NP. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: type XI collagen is restricted to thin fibrils. J Histochem Cytochem. 1995;43:967–979. doi: 10.1177/43.10.7560887. [DOI] [PubMed] [Google Scholar]
- Lee SL, Piez KA. Type II collagen from lathyritic rat chondrosarcoma: preparation and in vitro fibril formation. Coll Relat Res. 1983;3:89–103. doi: 10.1016/s0174-173x(83)80036-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- Mayne R, Brewton RG, Mayne PM, Baker JR. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993;268:9381–9386. [PubMed] [Google Scholar]
- Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyibizi C, Eyre DR. Identification of the cartilage alpha 1(XI) chain in type V collagen from bovine bone. FEBS Lett. 1989;242:314–318. doi: 10.1016/0014-5793(89)80492-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez RR, Seegmiller RE, Stark MR, Bridgewater LC. A type XI collagen mutation leads to increased degradation of type II collagen in articular cartilage. Osteoarthritis Cartilage. 2004;12:314–320. doi: 10.1016/j.joca.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Seegmiller R, Ferguson CC, Sheldon H. Studies on cartilage. VI. A genetically determined defect in tracheal cartilage. J Ultrastruct Res. 1972;38:288–301. doi: 10.1016/s0022-5320(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Seegmiller R, Fraser FC, Sheldon H. A new chondrodystrophic mutant in mice. Electron microscopy of normal and abnormal chondrogenesis. J Cell Biol. 1971;48:580–593. doi: 10.1083/jcb.48.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller RE. Ultrastructural and biochemical properties of limb cartilage in mice with chondrodysplasia. Prog Clin Biol Res. 1982;110(Pt B):193–201. [PubMed] [Google Scholar]
- Spranger J. The type XI collagenopathies. Pediatr Radiol. 1998;28:745–750. doi: 10.1007/s002470050459. [DOI] [PubMed] [Google Scholar]
- Thom JR, Morris NP. Biosynthesis and proteolytic processing of type XI collagen in embryonic chick sterna. J Biol Chem. 1991;266:7262–7269. [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Cooperative regulation of fibrillogenesis by collagen types V/XI. Matrix Biology. 2006;25:S7. [Google Scholar]
- Wu JJ, Eyre DR. Structural analysis of cross-linking domains in cartilage type XI collagen. Insights on polymeric assembly. J Biol Chem. 1995;270:18865–18870. doi: 10.1074/jbc.270.32.18865. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Weis MA, Eyre DR. Transactions of the Orthopaedic Research Society. Vol. 30. Washington, DC: 2005. A distinctive type V/XI collagen phenotype in the intervertebral disc; p. 107. [Google Scholar]
- Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, Li Y. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48:2509–2518. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- Yang C, Notbohm H, Acil Y, Heifeng R, Bierbaum S, Muller PK. In vitro fibrillogenesis of collagen II from pig vitreous humour. Biochem J. 1995;306 (Pt 3):871–875. doi: 10.1042/bj3060871. [DOI] [PMC free article] [PubMed] [Google Scholar]


