Abstract
The inflammasome is a cytoplasmic multiprotein complex that has recently been identified in immune cells as an important sensor of signals released by cellular injury and death. Analogous to immune cells, hepatic stellate cells (HSC) also respond to cellular injury and death. Our aim was to establish whether inflammasome components were present in HSC and could regulate HSC functionality. Monosodium urate (MSU) crystals (100 μg/ml) were used to experimentally induce inflammasome activation in LX-2 and primary mouse HSC. Twenty-four hours later primary mouse HSC were stained with α-smooth muscle actin and visualized by confocal microscopy, and TGF-β and collagen1 mRNA expression was quantified. LX-2 cells were further cultured with or without MSU crystals for 24 h in a transwell chemotaxis assay with PDGF as the chemoattractant. We also examined inhibition of calcium (Ca2+) signaling in LX-2 cells treated with or without MSU crystals using caged inositol 1,4,5-triphosphate (IP3). Finally, we confirmed an important role of the inflammasome in experimental liver fibrosis by the injection of carbon tetrachloride (CCl4) or thioacetamide (TAA) in wild-type mice and mice lacking components of the inflammasome. Components of the inflammasome are expressed in LX-2 cells and primary HSC. MSU crystals induced upregulation of TGF-β and collagen1 mRNA and actin reorganization in HSCs from wild-type mice but not mice lacking inflammasome components. MSU crystals inhibited the release of Ca2+ via IP3 in LX-2 cells and also inhibited PDGF-induced chemotaxis. Mice lacking the inflammasome-sensing and adaptor molecules, NLRP3 and apoptosis-associated speck-like protein containing CARD, had reduced CCl4 and TAA-induced liver fibrosis. We concluded that inflammasome components are present in HSC, can regulate a variety of HSC functions, and are required for the development of liver fibrosis.
Keywords: apoptosis-associated speck-like protein containing CARD, NLRP3
the inflammasome is a cytoplasmic multiprotein complex originally identified in immune cells. Inflammasome activation is required for immune cell recognition of signals released by pathogens, as well as signals released by local tissue injury and death (2). The inflammasome is composed of a sensory molecule that is a member of the family of nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (3). These sensory molecules interact with an intermediary adaptor molecule termed apoptosis-associated speck-like protein containing CARD (ASC), which in turn can activate cellular proteases. The sensory molecules are cytoplasmic pattern-recognition receptors that recognize a range of molecules ranging from pathogen-associated molecular patterns (PAMPs) to danger signals, such as uric acid and a variety of others. Members of this family of sensory molecules include NODs, NTPases implicated in apoptosis and multihistocompatability complex transcription, leucine-rich repeat, and pyrin-domain-containing proteins (NLRPs), and interleukin-1β-converting enzyme-protease activating factor (NLRC4).
Among the known inflammasome sensory molecules, NLRP3 has been identified as responsible for activation of immune cells in response to signals released by tissue injury and death. Monosodium urate (MSU) is known to be produced when cellular death occurs, and MSU crystals have been identified as potent activators of the inflammasome via the sensory molecule NLRP3. In fact, the well-known ability of MSU to cause experimental inflammation and the disease gout is dependent on its ability to activate the inflammasome via NLRP3. Analogous to immune cells, hepatic stellate cells (HSC) are known to undergo functional changes in response to tissue injury and death, but the pathways responsible for this are poorly understood (4). There are no data on the presence of inflammasome components in HSC and whether activation of the inflammasome can regulate HSC functions (4, 5).
In this study, we used MSU crystals for experimental inflammasome activation and demonstrate that in vitro inflammasome activation has a variety of functional consequences for HSC, including inhibition of chemotaxis, induction of actin reorganization, and enhanced collagen1 and TGF-β expression. The mechanism of chemotaxis inhibition is the downregulation of intracellular responses to the secondary messenger inositol 1,4,5-triphosphate (IP3), with reduction in PDGF-mediated increase in cytosolic Ca2+. In the absence of inflammasome components, the development of experimental liver fibrosis is also significantly impaired.
MATERIALS AND METHODS
Cell culture and reagents.
LX-2 cells are immortalized human stellate cells and were cultured in RPMI 1640 plus 5% FBS and 1% penicillin and streptomycin (23). Primary HSCs were cultured in M199, 10% FBS, 1% penicillin/streptomycin, 2% gentamycin, and fungizone in a 1:1,000 density, with a media change 24 h after isolation and then every 3 days. Histodenz, DNase, pronase, collagenase, TRIZOL reagent, fungizone, trypsin-EDTA, M199, RPMI 1640, DMEM, HBSS, and F-12 (HAM) were purchased from GIBCO/Invitrogen (Grand Island, NY). All reagents were of the highest quality grade commercially available. LX-2 cells were used between passages 4 and 7 and were used 3 days after passage. MSU crystals were produced as previously described (18). Briefly, 4 mg/ml uric acid (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.1 M borate buffer by continuously adjusting the pH to 8.0. The solution was filtered, and the crystals precipitated after 7 days were washed twice with absolute alcohol and once with acetone and air dried in a tissue culture hood before use.
Animals and HSC isolation.
All experiments were approved by the Yale University Institutional Animal Use Committee, were performed according to the Yale University Institutional Animal Care and Use Committee guidelines, and conformed to NIH guidelines for the care and use of animals. Primary mouse HSCs were isolated from the livers of 6–8-wk-old mice. The method described previously was modified per Liu et al.(10). Mice were anesthetized, and in situ liver perfusion and digestion were performed with pronase E (2.4 mg/ml; Roche Molecular Biochemicals, Chicago, IL) and collagenase B (0.3–0.45 mg/ml; Roche Molecular Biochemicals, Branchburg, NJ), and the resulting liver cell suspension was purified via density gradient centrifugation using 9–11% Nycodenz (Sigma-Aldrich). HSCs were plated on standard tissue culture plastic dishes in M199 medium with 10% FBS at a density of 1 × 106 per 1-cm plate and maintained in M199/10% FBS supplemented with glutamine and antibiotics [penicillin, 100 U/ml; streptomycin, 100 U/ml; gentamicin, 0.1 mg/ml; and fungizone, 2.5 g/ml (Invitrogen, Carlsbad, CA)]. Primary cells were used for experiments between days 6 and 7 postisolation. ASC−/− were back crossed for 10 successive generations onto a C57BL/6 background, and control C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME).
RT-PCR and Western blotting for expression of NLRP3 and ASC.
Complementary DNA was prepared from LX-2 HSC line and day 7 primary mouse HSC and amplified with primers of ASC (human, 5′-gttcaagctgaagctgctgtcggt-3′; murine, 5′-gcgagcagcagcaagagtaaaa-3′ and human, 5′-ttccaggctggtgtgaaactgaaga-3′; murine, 5′-tacttcagctctgctccaggt-3′), NLRP3 (human, 5′-ttgaagaggagtggatgggtt-3′; murine, 5′-ctgaagatgacgagtgtccgtt-3′ and human, 5′-gatgggtgggtttgggtc-3′; murine, 5′-ctcgggctcaaacagcagctccagcttaa-3′), and β-actin primer (human, 5′-atctggcaccacaccttctacaatgagctgcg-3′; murine, 5′-tggaatcctgtggcatccatgaaac-3′ and human, 5′-cgtcatactcctgcttgctgatccacatctgc-3′; murine, 5′- atctggcaccacaccttctacaatgagctgcg-3′). The PCR was performed with 1 μl of the complementary DNA, 1× buffer, 1 mM MgCl2, 200 μM of each eoxyribonucleotide triphosphate, 2.5 U Taq polymerase, and 0.2 μM of each ASC- and β-actin-specific primers. Western blotting for ASC and NLRP3 was performed using anti-ASC (1:200; Alexis Biochemicals, Lausen, Switzerland) and anti-NLRP3 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) in LX-2 cells using standard techniques.
Real-time PCR for mRNA expression.
Primary mouse HSCs and LX-2 cells were cultured in the presence of MSU (100 μg/ml). Twenty-four hours after culture, complementary DNA was prepared, and quantitative real-time PCR was performed for TGF-β1 and collagen1 using commercial primer-probe sets (Applied Biosystems, Framingham, MA) and the Applied Biosystems 7500 real-time PCR system. Expression of glyceraldehyde 3-phosphate dehydrogenase was used to standardize the samples, and the results were expressed as a ratio compared with untreated HSCs. After we confirmed that there were no significant differences in TGF-β and collagen1 mRNA levels, between control NLRP3−/−, control ASC−/−, and control wild-type mice, the controls in each mouse strain were set a one.
Chemotaxis assay.
The migration of LX-2 cells and primary mouse HSCs from C57BL/6 strain mice and ASC−/− mice was studied using transwell inserts equipped with 8-μm-pore polycarbonate-free filters as described previously (5). For the primary HSCs, two mice were used per isolation, and 2 × 104 HSCs were plated per well. Cells were treated with MSU (100 μg/ml). PDGF was added to the lower chamber 2 h afterward. After 24 h, the lower surface of the membrane was stained using hematoxylin-eosin, photographed, and analyzed. All experiments were repeated in triplicate. For each experimental group, a total of 40 high-power field images were taken. Cells per high-power field were counted, with results expressed as the average number of cells per high-power field. Statistical analysis was performed using the Student's t-test; a P value < 0.05 was considered significant.
Analysis of intracellular sensitivity to IP3 via measurement of changes in cytosolic Ca2+ concentration.
LX-2 cells were cultured with or without MSU (100 μg/ml) on glass coverslips for 20 min and then incubated with a solution containing 3 M caged IP3 (Alexis Biochemicals), 5 M Fluo-4 (Molecular Probes), 19.7 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid, 130 mM NaCl, 5 mM KCl, 1 mM MgSO4, and 1.25 mM CaCl2 for 30 min. These cells were placed in a perfusion chamber on the stage of a Zeiss S10NLO microscope; IP3 was then photoreleased using a custom-built system that couples a mercury lamp to a 1-mm quartz fiber optic cable through a high-speed shutter and filter wheel while cells were observed using time-lapse confocal microscopy.
Experimental liver fibrosis.
Liver fibrosis was induced in C57BL/6 mice, NLRP3−/− mice, and ASC−/− mice via intraperitoneal injection for CCL4 twice a week for 8 wk (each injection contained a CCl4 dose of 850 μl/kg, diluted 1:4 in corn oil). Control mice received injections of corn oil. After 8 wk, mice were euthanized, and liver protein was isolated for Western blot analysis. Liver samples were fixed for histology. A second model of thioacetamide induced (TAA) liver fibrosis was employed in ASC−/− mice. TAA was given at 0.2 g/kg ip three times a week for 10 wk.
Western blotting for α-smooth muscle actin.
Western blotting was performed for α-smooth muscle actin (α-SMA) (1: 1,000; Santa Cruz Biotechnology) and β-actin (1:1,000; Santa Cruz Biotechnology) using standard techniques. Radiographs were scanned and analyzed using TL100 software from the Total Lab range (Nonlinear Dynamics, Newcastle upon Tyne, UK). After confirming that there were no significant differences in the densitometry for α-SMA by Western blot between control NLRP3−/−, control ASC−/−, and control wild-type mice, the controls in each mouse strain were set as one.
Sirius red staining.
Liver samples were incubated in xylene (J. T. Baker, Phillipsburg, NJ) for 15 min and washed with ethanol (95% for 5 min, 90% for 5 min, 80% for 10 min, and 70% for 5 min). Samples were placed in 0.2% phosphomolybdic acid for 2 min and then incubated with 0.4% sirius red (Polysciences, Warrington, PA) for 110 min and washed with 0.01 N HCl and ethanol (95% and 100%, respectively). After being washed with CitriSolv three times for 3 min each time (FISHERbrand, Pittsburgh, PA), samples were covered by mounting media with a coverslip. Imaging was performed using nonpolarized light microscopy. The Sirius red-positive area was calculated as the percentage of pixels above the threshold value with respect to the total pixels per field using by Image J version 1.38x (http://rsbweb.nih.gov/ij).
Immunohistochemistry for α-SMA.
LX-2 cells were stained with anti-α-SMA primary antibody (1:650, Sigma) in PBS with 0.1% bovine serum albumin for 45 min at 37°C. Washing was performed with PBS with 0.1% bovine serum albumin three times for 3 min each time. Goat anti-mouse immunoglobulin G Alexa 488 was added as the secondary antibody (1:200) in PBS with 0.1% bovine serum albumin for 45 min at 37°C. Washing was performed again with PBS with 0.1% bovine serum albumin three times for 3 min each time. Samples were mounted with mounting media (Vector, Burlingame, CA). Primary mice HSCs were stained with anti-α-SMA primary antibody (1:650) and TO-PRO-3 iodide (1;500, Invitrogen) for 45 min at 37°C. Washing was performed with PBS with 0.1% bovine serum albumin three times for 3 min each time. Goat anti-mouse immunoglobulin G Alexa 488 was added as the secondary antibody (1:200) in PBS with 0.1% bovine serum albumin for 45 min at 37°C. Washing was performed again with PBS with 0.1% bovine serum albumin three times for 3 min each time. Samples were mounted with mounting media and placed on the glass slide with mounting media. The cells were imaged using a Zeiss microscope.
Statistics.
For the chemotaxis experiments, 40 high-power fields were counted, and for each experimental group the average number of cells per high-power field was calculated. The Student's t-test was performed, with P < 0.05 considered significant. For quantitative real-time PCR, data samples were run in groups of 12 for each sample, generating a ratio compared with the control sample.
RESULTS
MSU upregulates TGF-β and collagen1 in HSCs, not in ASC−/− mice.
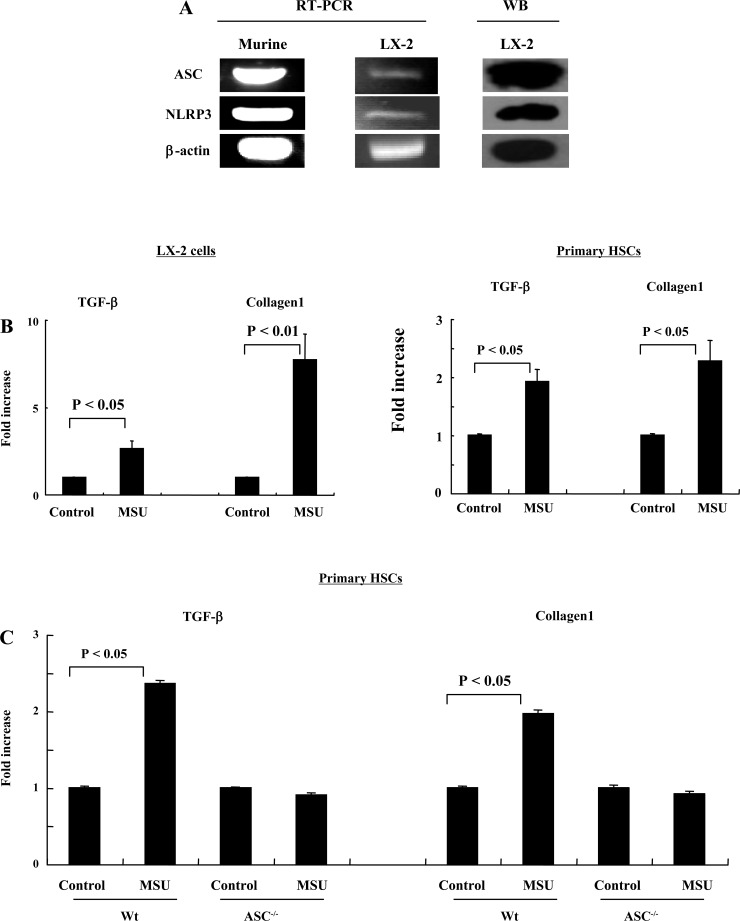
Transcripts and protein for human and mouse ASC and NLRP3 are present in the HSC line LX-2 and primary mouse HSCs (Fig. 1A). Upregulation of messenger RNA (mRNA) for TGF-β and collagen1 is an indicator of an increase in the activation state of HSCs (4). We therefore tested whether MSU crystals induce upregulation of the mRNA transcript for TGF-β and collagen1. Twenty-four hours after adding MSU crystals, the levels of TGF-β and collagen1 in LX-2 cells were significantly greater than those of controls (TGF-β, P < 0.05; collagen1, P < 0.01) and primary mouse HSCs (TGF-β, P < 0.05; collagen1, P < 0.05) (Fig. 1, B and C). No upregulation was seen in ASC−/− mice (Fig. 1C).
Fig. 1.
Monosodium urate (MSU) (100 μg/ml) upregulates TGF-β and collagen1 in LX-2 human and primary mouse hepatic stellate cells (HSC), but not apoptosis-associated speck-like protein containing CARD (ASC)−/− mice. A: mRNA for the NLRP3 and ASC are expressed in the human HSC cell line LX-2 and in primary mouse HSC by RT-PCR and Western blotting. B: MSU increases production of TGF-β and collagen1 mRNA by LX-2 cells and primary mouse HSC (P < 0.05, and P < 0.01). C: MSU upregulates TGF-β and collagen1 in primary HSC from wild-type (Wt) mice, but not from ASC−/− mice (P < 0.05).
MSU induces actin reorganization and stellation of HSCs.
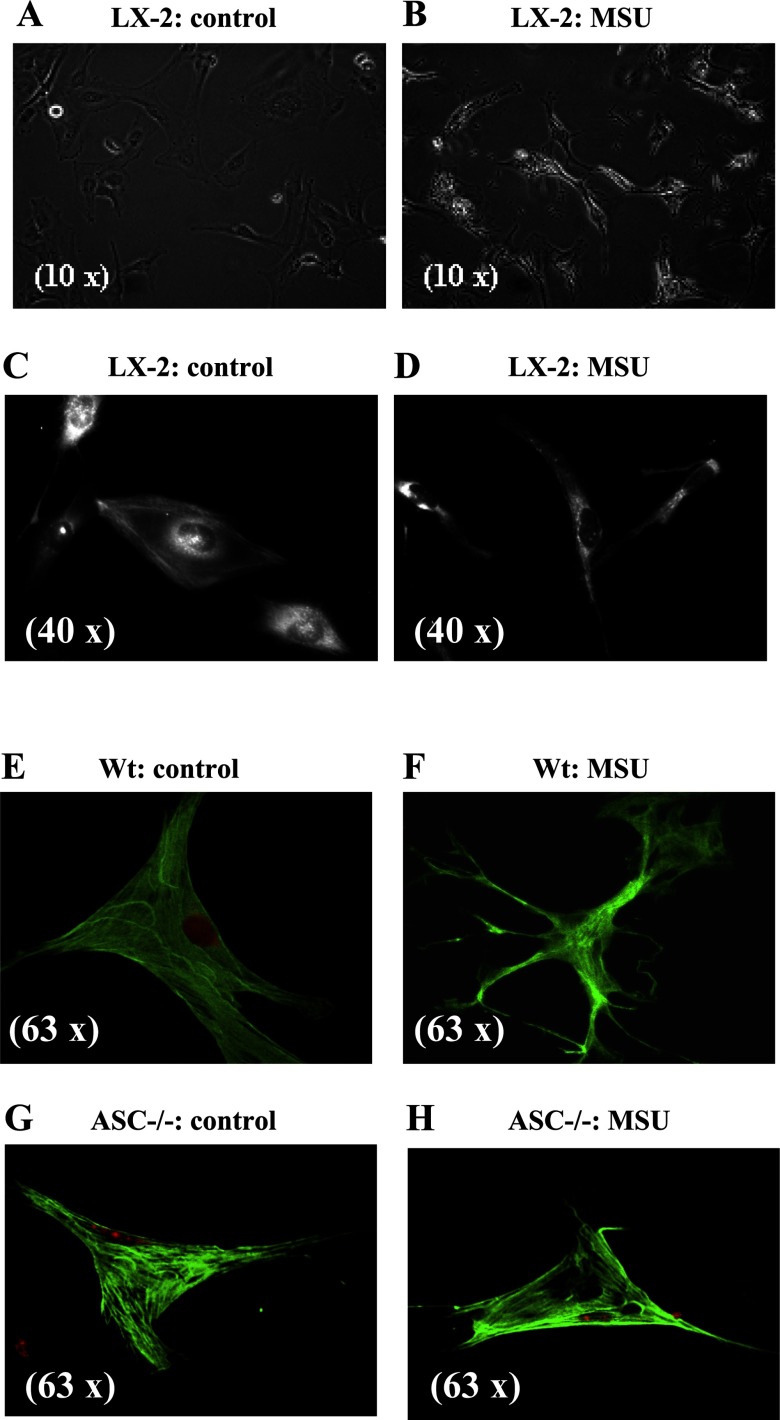
Activation of HSCs is associated with changes in cell shape, typically into a more stellate morphology (4). LX-2 cells have a flat, polygonal morphology as shown in Fig. 2, A and C, by phase contrast or by α-SMA staining, respectively, and 24 h after exposure to MSU crystals, morphological change is seen as shown in Fig. 2, B and D. Primary HSCs from wild-type mice also underwent morphological change in response to MSU crystals as shown in α-SMA-stained images in Fig. 2F. The morphological change in response to MSU was specific to HSC from wild-type mice and did not occur in HSC from ASC−/− mice (Fig. 2H).
Fig. 2.
MSU (100 μg/ml) induces actin reorganization and stellation of LX-2 cells and primary mice HSC from wild-type but not ASC−/− mice. A: phase-contrast images of LX-2 cells in culture have a flat cuboidal shape. B: phase-contrast images of LX-2 cells with MSU that induces actin reorganization and stellation. C: α-smooth muscle actin (SMA) staining of LX-2 cells shows a flat cuboidal shape. D: after exposure to MSU, this becomes more elongated. E and F: images of primary HSC from wild-type mice with α-SMA staining show actin reorganization after exposure to MSU. G and H: images of primary HSC from ASC−/− mice with α-SMA staining show absence of actin reorganization after exposure to MSU.
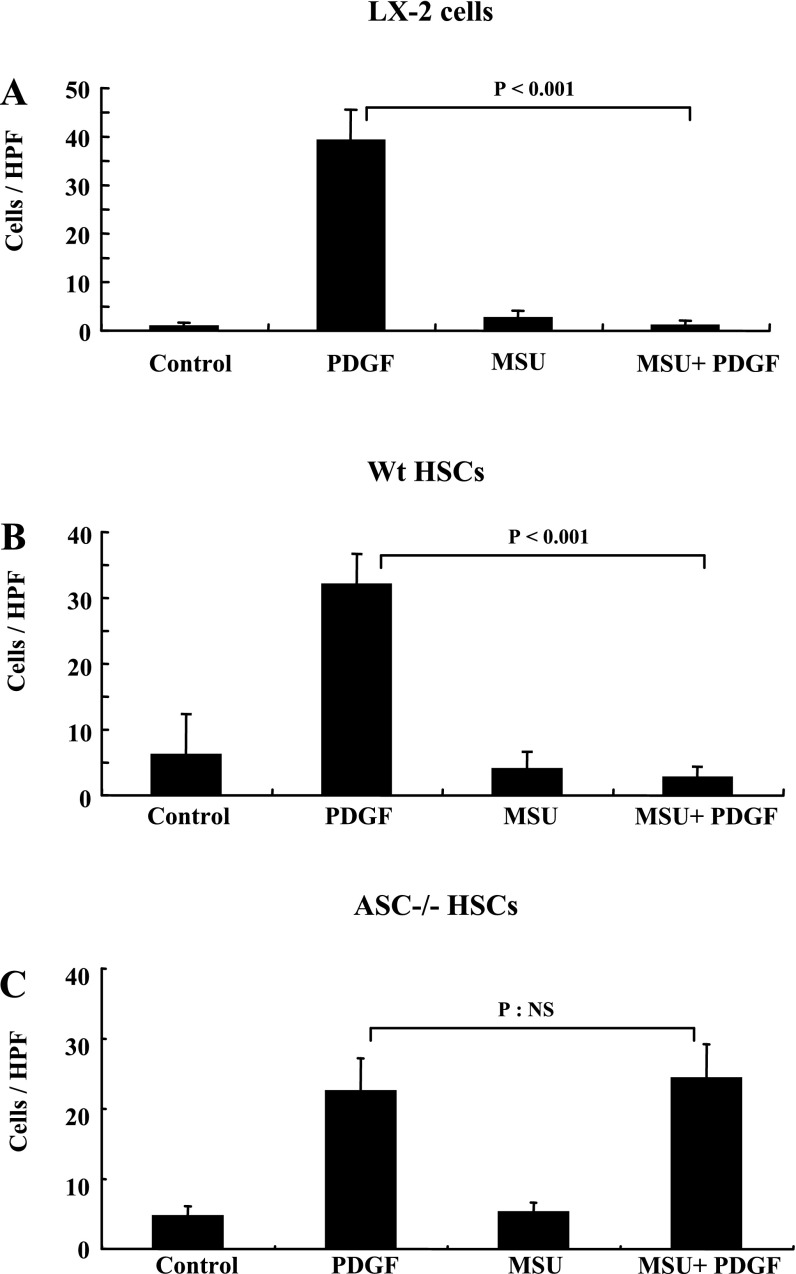
MSU crystals inhibit PDGF-mediated HSC chemotaxis in wild-type, not in ASC−/− mice.
We have shown that adenosine and mammalian DNA, which are produced in areas of cellular apoptosis, inhibits HSC chemotaxis induced by PDGF (5, 21). We tested whether MSU can also inhibit PDGF-mediated chemotaxis. Figure 3A shows transwell chemotaxis data for LX-2 HSCs. PDGF induces chemotaxis of LX-2 HSCs, and this is significantly inhibited by pretreatment with MSU (P < 0.001). Primary mouse HSCs have a very similar response, with MSU inhibiting PDGF-mediated chemotaxis (*P < 0.001) (Fig. 3B). As seen in Fig. 3C, PDGF-induced chemotaxis in primary HSCs from ASC−/− mice demonstrated that they are viable and able to respond to PDGF. In contrast to HSCs from wild-type mice, MSUs were unable to inhibit PDGF-induced chemotaxis.
Fig. 3.
MSU (100 μg/ml) inhibits PDGF-mediated chemotaxis of LX-2 cells and primary mouse HSC. A: MSU inhibits PDGF-mediated chemotaxis of LX-2 HSC across a transwell with 8-μm pores (P < 0.001). B: MSU inhibits PDGF-mediated chemotaxis of primary mouse HSC across a transwell with 8-μm pores (P < 0.001). C: MSU does not inhibit PDGF-mediated chemotaxis of primary mouse HSC from ASC−/− mice across a transwell with 8-μm pores (P: NS). NS; not significant; HPF, high-power field.
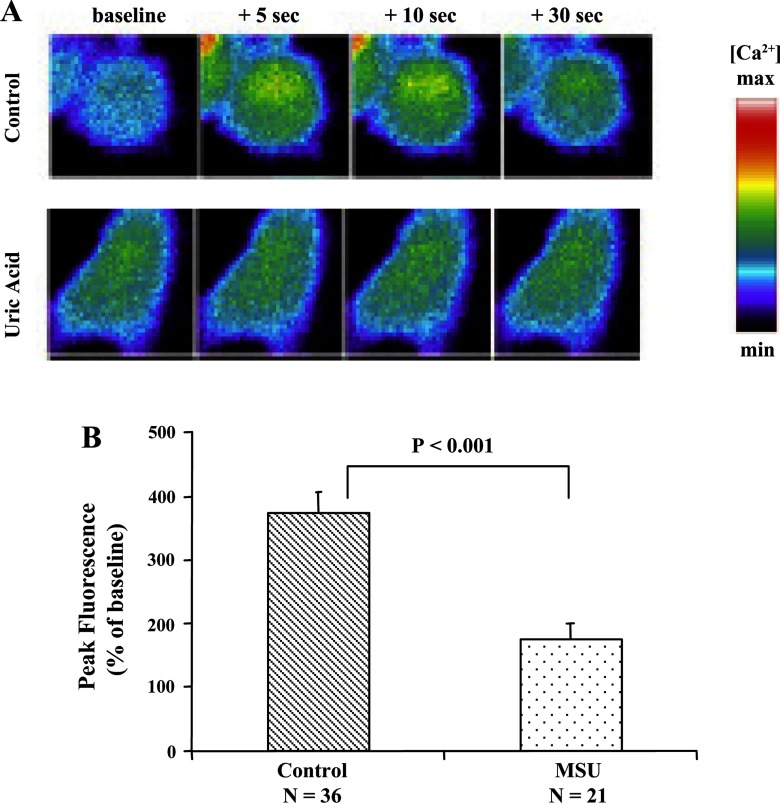
MSU crystals inhibit IP3-mediated Ca2+ signaling in HSCs.
LX-2 HSCs loaded with the Ca2+ fluorophore Fluo-4 and cell-permeant caged IP3ester (C-iso IP3, Alexis) were imaged during uncaging of IP3. In control cells, IP3 uncaging results in a rapid increase in [Ca2+]i, which persists for ∼30 s. In LX-2 HSCs pretreated with MSU crystals, there is minimal increase in [Ca2+]i in response to uncaging IP3 (Fig. 4, A and B). This demonstrates reduced sensitivity of IP3Rs to an increase in cytosolic IP3. By Western blot analysis, we identified that LX-2 cells express IP3 type 1R and IP3 type 3R, but neither of these underwent degradation in response to MSU (data not shown).
Fig. 4.
MSU (100 μg/ml) inhibits inositol 1,4,5-triphosphate (IP3)-mediated increase in cytosolic Ca2+. A: uncaging of caged IP3 LX-2 cells loaded with a Ca2+-sensing dye (fluorophore fluo-4/AM) results in a detectable increase in cytosolic Ca2+. B: summation of data from a number of control MSU-treated LX-2 cells shows a very clear inhibition of the increase in cytosolic Ca2+ in response to uncaging IP3 (P < 0.001).
Mice lacking ASC and NLRP3 have reduced collagen deposition and HSC activation in two models of experimental fibrosis.
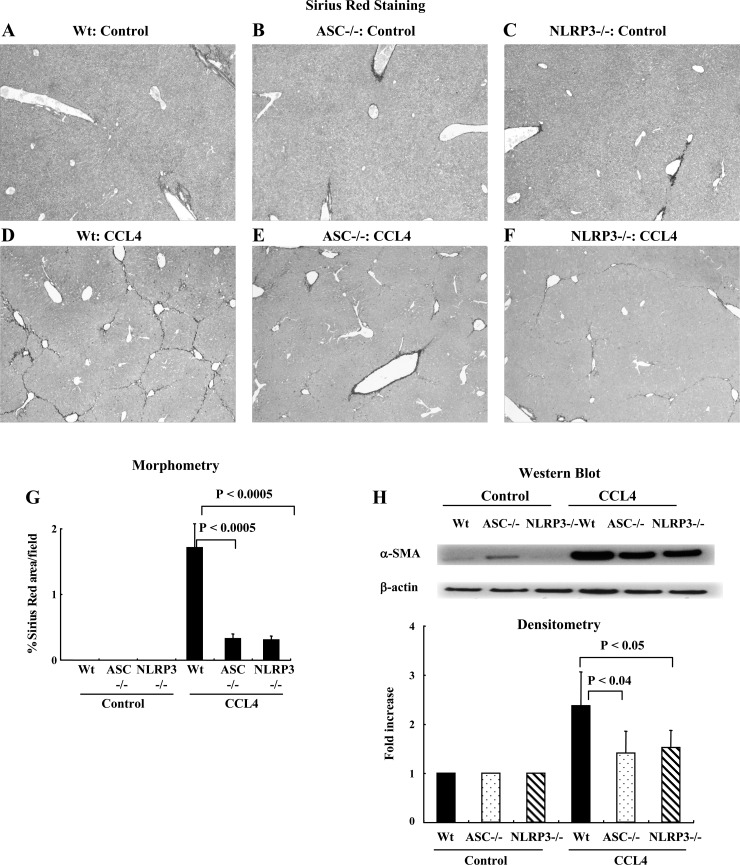
As expected, wild-type mice (n = 2), ASC−/− mice (n = 2), and NLRP3−/− (n = 2) mice receiving corn oil had no liver fibrosis (Fig. 5, A–C). Wild-type mice (n = 10) receiving CCl4 developed significant fibrosis as shown by a representative Sirius red-stained image (Fig. 5D), and the degree of fibrosis was significantly less in ASC−/− (n = 10) and NLRP3−/− mice (n = 5) (Fig. 5, E and F). The reduced liver fibrosis in ASC−/− and NLRP3−/− mice was confirmed by morphometric quantification, and the data is shown in Fig. 5G. The reduction in liver fibrosis was further demonstrated by examination of HSC activation using Western blotting for α-SMA from total liver protein. There was significant upregulation of α-SMA in the livers of wild-type mice after treatment with CCL4 and significantly less upregulation in the livers of ASC−/− and NLRP3−/− mice (Fig. 5H).
Fig. 5.
Mice lacking ASC (ASC−/−) and NLRP3 (NLRP3 −/−) have reduced collagen deposition in a CCL4 model of liver fibrosis. A–C: nonpolarized light Sirius red images of wild-type mice, ASC−/− mice, and NLRP3−/− mice receiving corn oil. D–F: wild-type mice receiving CCl4 developed significant fibrosis, but the degree of fibrosis was significantly less in ASC−/− and NLRP3−/− mice. G: quantification of differences in Sirius red staining by morphometry showing significantly less fibrosis in ASC−/− and NLRP3−/− mice (P < 0.0005). H: Western blotting was performed using the protein from whole liver tissue. The densitometry of this data is consistent with the Sirius red staining data.
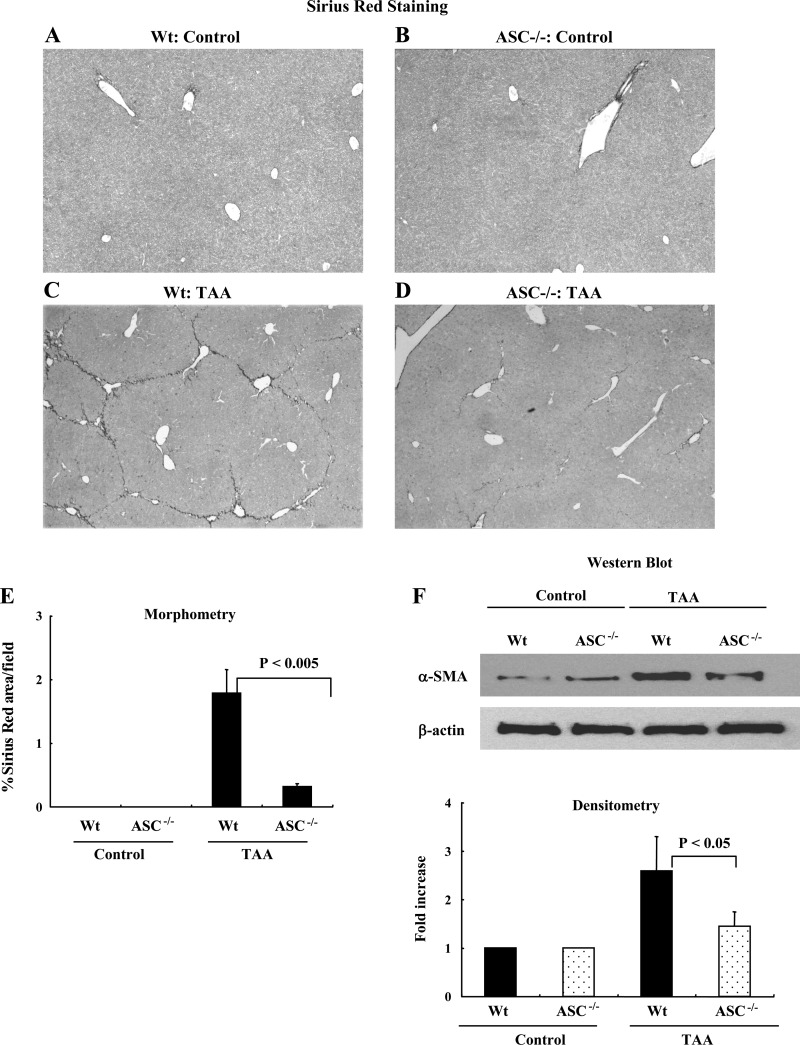
To ensure that the reduced fibrosis was not limited to the CCl4 model of liver fibrosis, we tested the ability of TAA to induce liver fibrosis in control and ASC−/− mice. As can be seen from Fig. 6, wild-type mice (n = 2) and ASC−/− (N = 2) mice receiving PBS had no liver fibrosis (Fig. 6, A and B). Wild-type mice (n = 5) receiving TAA developed significant fibrosis as shown by a representative Sirius red-stained image (Fig. 6C), and the degree of fibrosis was significantly less in ASC−/− mice (n = 5) (Fig. 6D). The reduced liver fibrosis in ASC−/− and NLRP3−/− mice was confirmed by morphometric quantification, and the data is shown in Fig. 5E. The reduction in liver fibrosis was further demonstrated by examination of HSC activation using Western blotting for α-SMA from total liver protein. There was significant upregulation of α-SMA in the livers of wild-type mice after treatment with TAA and significantly less upregulation in the livers of ASC−/− mice (Fig. 6F).
Fig. 6.
Mice lacking ASC (ASC−/−) have reduced collagen deposition in a thioacetamide (TAA) model of liver fibrosis. A and B: nonpolarized light Sirius red images of wild-type mice and ASC−/− mice receiving PBS. C and D: wild-type mice receiving TAA developed significant fibrosis, but the degree of fibrosis was significantly less in ASC−/− mice. E: quantification of differences in Sirius red staining by morphometry showing significantly less fibrosis in ASC−/− and NLRP3−/− mice (P < 0.005). F: Western blotting was performed using the protein from whole liver tissue. The densitometry of this data is consistent with the Sirius red staining data with reduced α-SMA in liver tissue from ASC−/− mice (P < 0.05).
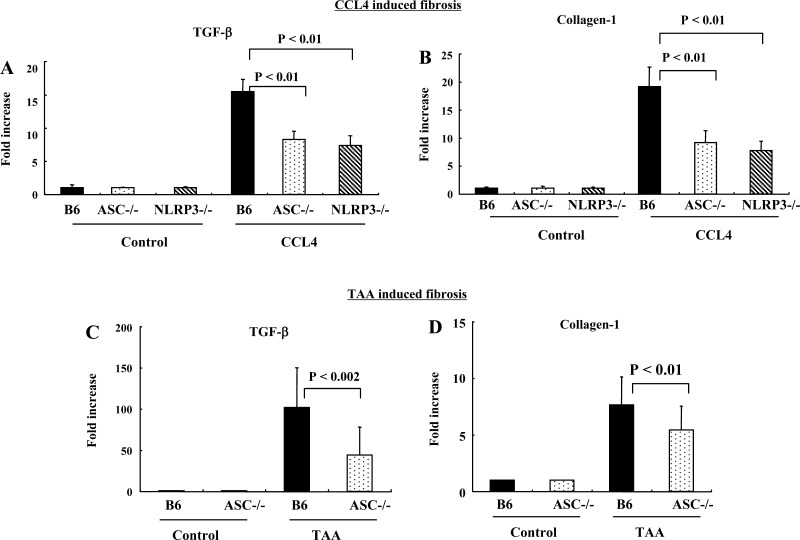
To further evaluate the induction of liver fibrosis in ASC−/− and NLRP3−/− mice, the levels of collagen1 and TGF-β mRNA transcripts in whole livers were quantified. As can be seen from Fig. 7, A and B, there were significantly lower transcripts for TGF-β and collagen mRNA in whole livers of ASC−/− and NLRP3−/− mice compared with wild-type. Similar results were obtained from analysis of TGF-β mRNA and collagen transcripts from livers of mice treated with TAA (Fig. 7, C and D).
Fig. 7.
Mice lacking ASC (ASC−/−) and NLRP3 (NLRP3−/−) had significantly less upregulation of TGF-β and collagen transcripts after experimental liver fibrosis. A and B: whole liver mRNA transcripts for TGF-β and collagen are significantly less in ASC−/− and NLRP3−/− mice than wild-type after CCl4-induced liver fibrosis (P < 0.01). C and D: whole liver mRNA transcripts for TGF-β and collagen are significantly less in ASC−/− mice than wild-type after TAA-induced liver fibrosis (P < 0.002 and P < 0.01).
DISCUSSION
The ability of hepatic stellate cells to respond to tissue injury and death in their immediate vicinity is shared by a number of other cells including cells of the immune system such as dendritic cells (19). Recently novel molecular pathways required for immune cells to respond to tissue injury and death were identified. These pathways are initiated by the activation of one of a family of cytosolic sensory molecule termed NLRs. The signals that activate these sensory molecules are varied, and include PAMPs as well as products of cellular apoptosis such as MSU (8). NLRP3 is one such sensory molecule and, along with the adaptor protein ASC, forms a cytosolic protein complex termed the NLRP3 inflammasome. NLRP3 was chosen among the NLRs for this study because it is required for detection of tissue death and injury by immune cells and is activated by MSU, which is known to be produced at sites of tissue injury and death (13).
We initially demonstrated expression of NLRP3 and the adaptor protein ASC in the human LX-2 HSC line and in freshly isolated primary mouse HSC (Fig. 1A). MSU crystals are known to require NLRP3 and other inflammasome components for their effects on immune cells. Conversely MSU crystals are also an established tool for in vitro activation of the inflammasome (18). It is of note that there is a requirement for the crystal form of MSU for inflammasome activation, which is consistent with the ability of other nonsoluble molecules such as silica and asbestos to activate the inflammasome. MSU crystals are now a well-established reagent for inducing NLRP3 inflammasome activation in vitro and were used by us for this purpose. Activation of the NLRP3 inflammasome by MSU crystals induced upregulation of TGF-β and collagen1 in LX-2 cells and in primary HSC (Fig. 1B). This occurred within 24 h of activation of the NLRP3 inflammasome and did not occur in primary HSC from ASC-deficient mice (Fig. 1C). This demonstrates that the NLRP3 inflammasome is functional in HSC, and its activation induces upregulation of genes associated with HSC matrix deposition.
Another key feature of HSC is a change in morphology from a flat fibroblast-like shape to a more angular or stellate shape (16). Such a change in HSC morphology is seen in response to a variety of stimuli including in vitro culture on plastic and in vivo after liver injury. We demonstrate that activation of the NLRP3 inflammasome in LX-2 cells by MSU crystals results in a change in morphology from a flat fibroblast-like shape to an angular stellate shape. This is visible in phase-contrast microscopy (Fig. 2, A and B) and also by immunohistochemistry for α-smooth muscle actin (Fig. 2, C and D). HSC stellation in response to inflammasome activation also occurred in primary HSC from wild-type mice (Fig. 2, E and F). To confirm that this effect was dependent on the inflammasome, it was demonstrated that control primary HSC from ASC-deficient mice did not undergo stellation (Fig. 2, G and H).
In established liver fibrosis, HSC are not distributed randomly within the parenchyma, but rather are present in very characteristic patterns or zones of fibrosis. For this to occur, a mechanism of localization must be present, and the cellular basis of this is thought to be the ability of HSC to undergo chemotaxis to a range of stimuli including PDGF (9, 12, 15). The ability to undergo chemotaxis explains movement toward a source of the chemokine but does not entirely explain the ability of cells to localize at a site of injury. We have recently shown that stimuli associated with tissue injury and death can inhibit PDGF-induced chemotaxis. Such stimuli include adenosine, which signals via its G protein-coupled A2a receptor, and DNA from apoptosing hepatocytes, which signals via the TLR9 receptor. The ability of signals from cellular apoptosis to inhibit HSC chemotaxis is proposed to provide a stop signal to HSC at sites of cellular apoptosis where HSC would localize. We tested the ability of inflammasome activation with MSU crystals to inhibit chemotaxis of LX-2 cells to PDGF and found very strong inhibition (Fig. 3A). The ability of uric acid to inhibit PDGF-induced chemotaxis was confirmed in primary HSC from wild-type mice (Fig. 3B). Control primary HSC from ASC-deficient mice were able to undergo PDGF-induced chemotaxis, but this was not inhibited by MSU crystals (Fig. 3C).
Next we examined the mechanism of the inhibitory effect of inflammasome activation on PDGF-induced chemotaxis. The important role of an increase in the free cytosolic Ca2+ concentration [Ca2+]i in chemotaxis prompted us to examine the effect of inflammasome activation on [Ca2+]i. The increase in cytosolic [Ca2+]i in response to PDGF is mediated by the secondary messenger IP3, which activates IP3 receptors (3). These receptors are a family of three proteins (types 1, 2, and 3) that form tetrameric ion channels in the endoplasmic reticulum (ER) membranes (2). On binding of IP3, the channels open and Ca2+ stored within the ER flows into the cytoplasm. The most direct way to induce changes in cytosolic Ca++ is by increasing the cytosolic concentration of the secondary messenger IP3. This results in activation of IP3 receptors on the ER and releases ER Ca++ into the cytosol. The direct increase in cytosolic IP3 has advantages over using PDGF because it removes the possibility of changes in PDGF receptor function as being interpreted as changes in Ca++ signaling machinery. Using LX-2 cells loaded with a Ca++-sensing dye (fluorophore fluo-4/AM) and caged IP3, propagation of a Ca++ wave was detected after uncaging of caged IP3 (Fig. 4A, upper). In LX-2 cells treated with uric acid, there was no change in [Ca2+]i after uncaging of IP3 (Fig. 4A, bottom). This data is summarized in Fig. 4B.
The results from our experiments using photorelease of caged IP3 clearly demonstrate inhibition of IP3-mediated signaling in response to activation of the inflammasome. This finding suggests that inflammasome activation results in a decrease in IP3R function and has been previously reported in response to a number of membrane receptors, including cholecystokinin and angiotensin II. One mechanism of a reduction in IP3R function is by a reduction in total cellular IP3R content, which can decrease by 90% in neuroblastoma cells in response to PLC-linked cell surface receptors (1). Increased ubiquination of IP3Rs in response to cholecystokinin with subsequent degradation by proteasome-dependent and proteasome-independent pathways has also been reported (22). IP3 type 3R degradation can occur in a caspase-3-dependent manner although we did not detect any degradation of the IP3 type 1R and IP3 type 3R (data not shown) (6). Finally IP3Rs have several phosphorylation sites that can alter the response of IP3Rs to IP3 (11). Identification of the mechanisms responsible for inflammasome-mediated reduction in the response of IP3R to IP3 is of great interest and deserves further study.
We propose that the inhibition of PDGF-induced chemotaxis provides a stop signal to activated HSCs when they have reached an area of hepatocyte apoptosis. In common with the broader field of chemotaxis, several molecules have been identified that induce HSC chemotaxis and explain movement of HSCs toward their source (17). However, there is little explanation of the mechanism for stopping cells when they arrive at the source of chemokines, and where they need to undergo effector function. A chemotaxis stop signal, produced at the site of cellular apoptosis, is an attractive mechanism for localizing HSCs to the site requiring matrix remodeling. Naive HSCs do not express the PDGF receptor, and responsiveness to PDGF is an indicator of at least a minimum level of HSC stimulation, which is consistent with our use of LX-2 cells cultured on plastic, and also day 7 primary HSC (9). We therefore propose that inflammasome activation from apoptotic cells acts as a late differentiation signal inducing a change from very mobile, partially activated HSCs to immobile effector HSCs. This places inflammasome activation along with adenosine 2a receptor and TLR9 as providing a stop signal for HSC chemotaxis and functions to provide a late differentiation step for HSC (5, 21).
Having identified multiple consequences of inflammasome in vitro, it was important to confirm in vivo that inflammasome activation is important in HSC biology and liver fibrosis. We therefore compared the development of experimental liver fibrosis in mice lacking the inflammasome components NLRP3 and ASC with wild-type mice. As can be seen from Fig. 5, A–F, mice lacking NLRP3 and ASC have significantly reduced liver collagen after 8 wk of CCl4. Differences in HSC responses were confirmed by Western blot analysis of α-SMA content in the liver tissues, and this was quantified by densitometry (Fig. 5G). To further confirm the low level of HSC responses to CCl4-induced liver injury in ASC−/− and NLRP3−/− mice, we quantified mRNA from whole liver for collagen and TGF-β, and this is shown in Fig. 5H. To exclude the possibility that reduced liver fibrosis in mice lacking inflammasome components was limited to the CCl4 model of liver fibrosis, a second model of TAA-induced fibrosis was used, with very similar results (Fig. 6).
A large number of stimuli have been identified as inducing inflammasome activation. These include products of infectious agents such as gram-negative bacteria and endogenous molecules released on cell death. The sensory NLR molecules provide the specificity for this response. For example, inflammasome activation by gram-negative bacteria requires NLRC4, and in contrast inflammasome activation by noninfectious signals including many endogenous signals requires NLRP3 (19). Presently known endogenous signals able to activate NLRP3 include MSU crystals and ATP. Asbestos and silica are two noninfectious exogenous signals that can also activate NLPR3. Although MSU crystals are a well-described reagent for inducing NLRP3 inflammasome activation in vitro, and are known to be important in the development of gout, their role in activation of HSC inflammasomes in vivo remains to be determined.
In conclusion, we have demonstrated that inflammasome activation can induce a number of well-characterized functional changes in HSC such as upregulation of TGF-β and collagen, as well as the more recently described inhibition of chemotaxis. The identification of a new molecular pathway that regulates HSC function has important implications including the development of strategies for reducing collagen deposition. MSU crystals are a well-described signal for NLRP3 inflammasome activation and were used in this study. It is, however, not clear whether MSU crystals are in vivo regulators of HSC function or whether additional signals for inflammasome activation are present in the liver. It is also of great interest to determine whether other NLR-sensing molecules that can induce inflammasome activation are present in HSC. For example, NLRC4 can sense components of gram-negative bacteria and may be a pathway in addition to TLR4 by which such organisms regulate HSC function.
GRANTS
This work was supported by the Ellison Foundation Grant (R. Flavell), NIH KO8 AI065517 (F. Sutterwala), NIH R01DK076674-01A2 (W. Mehal), and (P30) DK34989.
Supplementary Material
Acknowledgments
We are grateful to Dr. Kenneth Rock for expert advice on preparation of uric acid crystals and to A. Coyle, J. Bertin, and E. Grant (Millennium Pharmaceuticals) for providing NLRP3−/− and ASC−/− mice.
REFERENCES
- 1.Bokkala S, Joseph SK. Angiotensin II-induced down-regulation of inositol trisphosphate receptors in WB rat liver epithelial cells. Evidence for involvement of the proteasome pathway. J Biol Chem 272: 12454–12461, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Bosanac I, Michikawa T, Mikoshiba K, Ikura M. Structural insights into the regulatory mechanism of IP3 receptor. Biochim Biophys Acta 1742: 89–102, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Dawson AP Calcium signalling: how do IP3 receptors work? Curr Biol 7: R544–R547, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL Stellate cells: a moving target in hepatic fibrogenesis. Hepatology 40: 1041–1043, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Hashmi AZ, Hakim W, Kruglov EA, Watanabe A, Watkins W, Dranoff JA, Mehal WZ. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 292: G395–G401, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirota J, Furuichi T, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J Biol Chem 274: 34433–34437, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Johnston JB, Rahman MM, McFadden G. Strategies that modulate inflammasomes: insights from host-pathogen interactions. Semin Immunopathol 29: 261–274, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Kaparakis M, Philpott DJ, Ferrero RL. Mammalian NLR proteins: discriminating foe from friend. Immunol Cell Biol 85: 495–502, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest 80: 697–707, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Gaca MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem 278: 11721–11728, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Malathi K, Kohyama S, Ho M, Soghoian D, Li X, Silane M, Berenstein A, Jayaraman T. Inositol 1,4,5-trisphosphate receptor (type 1) phosphorylation and modulation by Cdc2. J Cell Biochem 90: 1186–1196, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology 29: 140–148, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell 126: 659–662, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Pinzani M PDGF and signal transduction in hepatic stellate cells. Front Biosci 7: d1720–d1726, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Reeves HL, Friedman SL. Activation of hepatic stellate cells—a key issue in liver fibrosis. Front Biosci 7: d808–d826, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol 285: G949–G958, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol 82: 259–264, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Takehara T, Tatsumi T, Suzuki T, Rucker EB 3rd, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology 127: 1189–1197, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology 46: 1509–1518, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Wojcikiewicz RJ, Ernst SA, Yule DI. Secretagogues cause ubiquitination and down-regulation of inositol 1, 4,5-trisphosphate receptors in rat pancreatic acinar cells. Gastroenterology 116: 1194–1201, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 54: 142–151, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.