Abstract
Corticotropin releasing factor (CRF), a mediator of stress response, alters gastrointestinal (GI) functions. Stress-related changes in colonic motility are blocked by selective CRF1 receptor antagonists. Our aim was to assess whether modulation of central and peripheral CRF1 receptors affects colonic transit and bowel function in female patients with diarrhea-predominant irritable bowel syndrome (D-IBS). This randomized, double-blind, placebo-controlled, 2-wk study evaluated the effects of oral pexacerfont (BMS-562086), a selective CRF1 receptor antagonist, 25 and 100 mg qd, on GI and colonic transit of solids [by validated scintigraphy with primary end point colonic geometric center (GC) at 24 h] and bowel function (by validated daily diaries) in 39 women with D-IBS. The 100-mg dose was comparable to a dose that inhibited colonic motility in stressed rats. Treatment effects were compared by analysis of covariance with baseline colonic transit as covariate. The study had 80% power (α = 0.05) to detect clinically meaningful (26%) differences in colonic transit. Thirty-nine of 55 patients fulfilled eligibility criteria (9 screen failures, 5 baseline GC24 outside prespecified range). At baseline, three treatment groups had comparable age, body mass index, and GC 24 h. Significant effects of pexacerfont relative to placebo were not detected on colonic GC24 (P = 0.53), gastric emptying, orocecal transit, ascending colon emptying half-time, and stool frequency, consistency, and ease of passage. No safety issues were identified. We conclude that in women with D-IBS, pexacerfont, 25 or 100 mg qd, does not significantly alter colonic or other regional transit or bowel function. The role of central and peripheral CRF1 receptors in bowel function in D-IBS requires further study.
Keywords: corticotropin releasing factor, pexacerfont, diarrhea-predominant irritable bowel syndrome, gastrointestinal transit, randomized trial
irritable bowel syndrome (IBS) is a very common (19) disorder characterized by recurrent symptoms of abdominal pain and/or discomfort associated with a change in stool consistency (30). The pathophysiology is likely multifactorial (10). An increased understanding of pathophysiology and potential mediators of bowel dysfunction provide the rationale for new therapeutic approaches in IBS (1).
Corticotropin releasing factor (CRF) is a key mediator of the stress response in the brain-gut axis (39); CRF1 and CRF2 receptor subtypes have been cloned. In animal models, stress activation of CRF receptors alters gastrointestinal functions (45), and stress-related changes in gastrointestinal motility such as delayed gastric emptying or accelerated colonic transit can be blocked by a selective CRF1 receptor antagonist, antalarmin, (24) and the nonselective-CRF receptor antagonist, α-helical CRF (37). There is an association of IBS with increased response to stress (51), and concomitant anxiety or depression is frequent in IBS. In a comprehensive study of 120 IBS patients, 46% of patients with diarrhea-predominant IBS (D-IBS) had accelerated colonic transit (10). If CRF antagonists inhibit colonic motility, they may be efficacious in the treatment of D-IBS. To ensure that the effects of the drug were tested in patients who did not have slow transit, all participants underwent baseline colonic transit study.
Pexacerfont (BMS-562086) is an orally active, potent, and selective antagonist of the CRF1 receptor. Preclinical studies showed that pexacerfont reduces colonic motility (measured as fecal pellet output with plasma concentrations of pexacerfont of ∼500 nM at the lowest effective dose) and the frequency of diarrhea in stressed rats or rats administered exogenous CRF (8). These observations are consistent with previous observations that acute environmental stress or exogenous CRF increases colonic motility and causes diarrhea (46). Preclinical data also support a role for CRF in causing hypersensitivity to visceral stimuli (46). Pharmacological antagonism of CRF attenuates or abolishes these changes (36).
Patients with IBS display an increased sensitivity to exogenous CRF and are more likely to suffer abdominal pain and hypermotility compared with healthy controls (22). Moreover, administration of a peptidic CRF antagonist alleviates visceral stimulation-induced abdominal pain and motility in patients with IBS (42).
The literature provides conflicting evidence in support of dysregulation of the hypothalamic-pituitary axis in IBS. Thus, whereas Dinan et al. (18) observed elevated cortisol levels and exaggerated ACTH and cortisol release to CRF infusion in 21 patients compared with 21 controls, a recent study by Chang et al. (14) in 41 patients showed lower ACTH and higher cortisol response to sigmoidoscopy. In the latter study, basal cortisol was positively correlated with anxiety, not with IBS severity and abdominal pain (14).
The study hypothesis was that antagonism of central and peripheral CRF-1 receptors influence colonic transit and bowel function in D-IBS. Even though upper gastrointestinal complaints are not part of the functional symptoms of IBS patients, stress impacts gastric as well as colonic motility, with delayed gastric emptying being the most common response evoked by acute stressors (40). Given that CRF may modulate stress-related changes in gastric emptying, the role of CRF antagonism on gastric emptying was also measured. The specific aim of this phase IIa trial was to evaluate the pharmacodynamic effects of pexacerfont, 25 and 100 mg once daily, compared with placebo in patients with D-IBS.
MATERIALS AND METHODS
Study design and participants.
We performed a double-blind, dose-ranging, multiple-dose, randomized, parallel-group, placebo-controlled study evaluating the effects of oral pexacerfont, 25 and 100 mg once daily, in 39 women, aged 18–65 yr, with persistent D-IBS, based on Rome II criteria (48). The study focused on women for several reasons. First, IBS is more prevalent in women and it is known that women are more likely to seek medical advice and therefore more willing to participate in studies (15, 43). Second, the inclusion of men in the study may have increased heterogeneity and decreased the ability to detect a meaningful effect. Finally, women were studied exclusively in view of the reports that the number of CRH-expressing neurons in the human hypothalamic paraventricular nucleus differs with gender and increases with age, but only in men (3). The study was approved by the Mayo Clinic Institutional Review Board, and all participants signed written, informed consent. Participants were allowed to continue stable doses of thyroid replacement, estrogen replacement, low-dose aspirin (81 mg/day), and birth control pills or depot estrogen injections.
Exclusion criteria included use of any IBS or antidiarrheal medication within 7 days prior to baseline transit measurement and throughout the course of the study, any structural or metabolic diseases or conditions that affect the GI system, and evidence of an evacuation disorder (28). Participants completed bowel disease symptom questionnaires (47) at baseline and Hospital Anxiety and Depression Inventory (52) at baseline and at the end of the treatment period.
Study drug.
The molecular formula, pharmacology, pharmacokinetics, and safety of pexacerfont are described in detail in the supplemental appendix (7).1 Pexacerfont is a potent and selective CRF1 antagonist that shows safety and no significant effects on serum ACTH or cortisol in human studies.
On the basis of plasma concentrations achieved in other studies, it was estimated that a 100-mg dose would yield steady-state concentrations in the targeted range for efficacy (i.e., >500 nM as determined from nonclinical studies). A dose of 25 mg was thought to be at the lower end of the potential therapeutic range (i.e., peak plasma concentrations would be near 500 nM but not necessarily throughout the full 24-h dosing interval).
In preclinical studies (7, 8), pexacerfont inhibited CRF-mediated ACTH release from rat pituitary cells and, in the absence of CRF stimulation, pexacerfont did not affect basal ACTH secretion or cortisol levels. Additionally, clinical studies revealed no significant effects on serum ACTH or cortisol in humans. Preclinical studies also showed that pexacerfont crosses the blood-brain barrier, although experiments to demonstrate this in humans have not been undertaken.
Formulation, randomization, treatment allocation, and study procedure.
Pexacerfont and matching placebo were provided by Bristol-Myers Squibb (Princeton, NJ) and were administered in the form of tablets in the morning on 14 consecutive days during the treatment period.
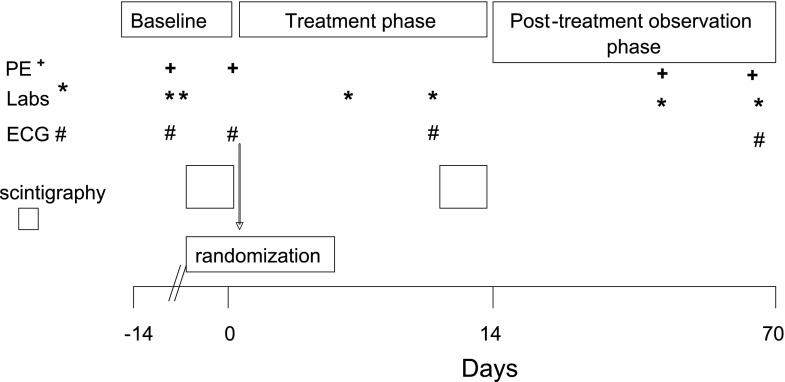
The study consisted of three periods (Fig. 1). During the baseline or pretreatment period, D-IBS patients underwent a screening visit and a 7-day baseline observation period. The observations were a baseline colonic transit test, as an abbreviated adaptation of Mayo Clinic's established scintigraphic method (6, 9, 12, 13, 16, 17, 41), assessment of bowel function with documentation daily of frequency of bowel movements, and Bristol Stool Form Scale (BSFS) scores (25, 29) for consistency of each stool passed, ease of stool passage scores (25), and completeness of evacuation. Pexacerfont plasma concentrations were taken at scheduled times during the course of the study.
Fig. 1.
Study design. The study consisted of 3 periods: baseline, treatment, and a posttreatment observation phase. Physical exam (PE, +), laboratory testing (Labs, *), electrocardiograms (ECG, #), and scintigraphy measurements were obtained at the indicated time periods.
To be eligible for randomization to treatment, the patient's baseline colonic transit measurement had to be above the median for healthy controls studied in the laboratory: geometric center (GC) ≥2.65 at 24 h or ≥3.90 at 48 h (17). This was to ensure that the colonic transit was not too slow to modify it with study drug. Patients who qualified on transit criteria and satisfied all other inclusion/exclusion criteria were randomized.
Randomization was 1:1:1 for placebo; 50 mg pexacerfont once daily on day 1 to day 7, followed by 25 mg pexacerfont once daily on day 8 to day 15; and 200 mg pexacerfont once daily on day 1 to day 7, followed by 100 mg pexacerfont once daily on day 8 to day 15. Because pexacerfont has a very long terminal half-life (2–4 wk), a loading phase (i.e., twice the maintenance dose) was instituted over the first week of dosing to yield near steady-state plasma concentrations earlier than would be achieved by daily administration of the maintenance dose. Subjects were administered the first dose of the loading phase of the study drug at the clinic on day 1 and were given the remainder of the loading-phase study drug to be taken at home on days 2 through 7. Subjects returned to the clinic on day 8 and were given the first dose of the treatment phase of the study medication and were given the remainder of the treatment phase medication to be taken at home on days 9 through 15.
The randomization code, generated by Bristol-Myers Squibb, was provided to the Mayo research pharmacist. All clinical and laboratory study personnel were blinded throughout the study, and treatment allocation was concealed until after all data were locked and analyzed by the study statistician (A. R. Zinsmeister).
Patients kept daily diaries for the pretreatment and treatment periods for all bowel movements. Patients returned to the clinic on day 14 after an 8-h overnight fast for gastric, small bowel, and colonic transit studies. Scans at 24 and 32 h after the first scan were obtained the following day (day 15). Subjects took their last dose of study medication at the clinic within 30 min prior to the 24-h scan and returned the next day (day 16) for the final scan at 48 h. Subjects followed up on days 42 and 70 for physical exam and routine laboratory tests.
The posttreatment period was defined as the 54 days (±1 day) after the last dose of study medication, when patients returned to the clinic site for final assessment. Safety monitoring was conducted throughout the study.
Gastrointestinal transit measurements.
An adaptation of our established scintigraphic method was used to measure gastrointestinal and colonic transit (6, 9, 12, 13, 16, 17, 41); indium-111 (111In) adsorbed on activated charcoal particles was delivered to the colon by means of a methacrylate-coated, delayed-release oral capsule. During baseline transit, only colonic transit was measured by means of images at 4, 24 and 48 h. During days 3 to 5 of the 5-day treatment period, the same colonic transit measurements were performed with the 111In capsule administered at the same time as the dose of drug. After the capsule emptied from the stomach (documented by its position relative to radioisotopic markers placed on the anterior iliac crests), a radiolabeled meal [technetium-99m (99mTc)-sulfur colloid labeling two scrambled eggs, one slice of whole wheat bread, and one glass of whole milk] was ingested to measure gastric and small bowel transit. Subjects ingested standardized meals for lunch and dinner at 4 and 8 h after the radiolabeled meal. Abdominal scans were obtained every hour for the first 6 h (the first 4 h for the assessment of gastric emptying) and at 8, 24, 32, and 48 h after ingestion of the 111In capsule. The performance characteristics of this test are summarized elsewhere (17).
Transit data analysis.
This was performed as in prior studies (9, 17) and details are provided in the supplemental appendix. The primary end points were the colonic GC at 24 h (GC 24) and AC emptying half-time (t1/2). Further scintigraphic assessments were the GC at 48 h, the gastric emptying t1/2, and the colonic filling at 6 h.
Daily stool diaries.
During 7 days of the baseline period and during 14 days of the treatment period, patients recorded each bowel movement with the exact time and with the description of stool consistency according to the BSFS (25, 29), ranging from 1 = “hard lumps” to 7 = “watery,” and the ease of passage, ranging from 1 = “manual disimpaction” to 7 = “incontinence.” They also answered whether or not they felt they had completely emptied their bowels [1 = “yes” and 0 = “no” (25)]. The diaries, therefore, contained the values for the time to first bowel movement after first drug intake (defined as the number of hours between administration of the first dose of study medication and occurrence of the first bowel movement thereafter), stool consistency, stool frequency, ease of passage, and sense of completely emptying their bowels.
Compliance with study medication.
Nurses administered the first dose of the loading phase of the study medication at the clinic on day 1 and the first dose of the treatment phase of the study medication on day 8. The patients were given medication to be taken at home on days 2 through 7 and days 9 through 15. Instructions were given to take the medication in the morning between 7:00 and 9:00 AM and to record this in their diary. Hence, compliance was checked by diary and pill count.
Study end points.
The primary end point, on which the sample size and statistical power were based, was the effect of pexacerfont on colonic transit at 24 h. Secondary end points were other transit data, time to first bowel movement after first drug intake, and stool frequency, stool consistency, ease of passage, sensation of complete evacuation, and visceral symptoms during the treatment period relative to a predrug baseline period.
Statistical analysis.
An intent-to-treat analysis using all patients who were randomized was used. An analysis of covariance (ANCOVA) was used to compare treatment groups, incorporating the corresponding baseline value (e.g., baseline GC at 24 h) when measured, age, or body mass index (BMI) as covariates. The analysis of the daily diary data first computed the mean scores per period (baseline and, separately, the last 7 days of treatment) in each subject. The treatment period mean scores were then compared by using a similar ANCOVA model with the corresponding baseline mean diary score as covariate. There were no dropouts in the study. Fisher's exact test was used to compare the proportion of patients reporting adverse effects in the two treatment groups.
Sample size considerations.
Sample sizes planned for the study were based on mean and coefficient of variation (CV) data for the primary response measures from prior studies performed in our laboratory in patients with functional diarrhea or D-IBS using the same methods. The results have been published in the literature (49). The mean colonic GC 24 was 4.05 with a CV of 23%. The effect size (i.e., the difference in group means as a percentage of the overall mean for each response) detectable with 80% power based on a two-sample t-test at a two-sided α level of 0.05 and assuming 13 subjects per group was 26% for the GC 24.
RESULTS
Participants, study conduct, and completion.
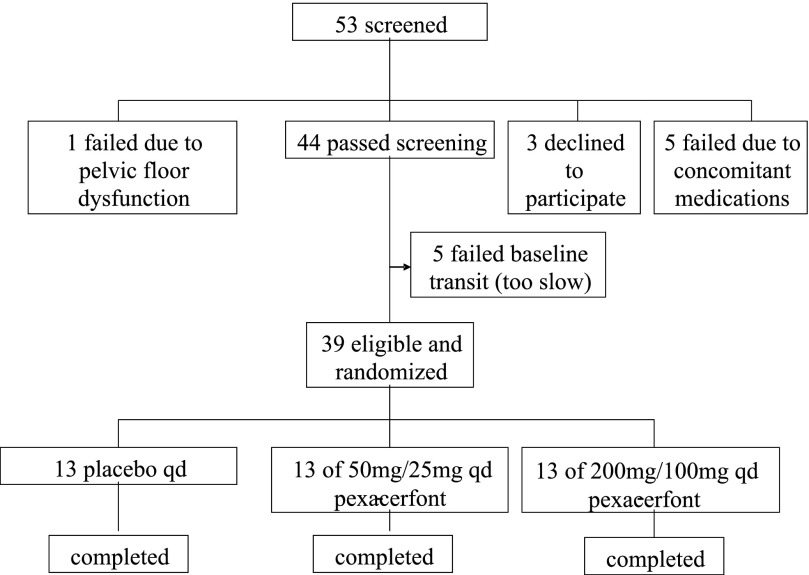
Fifty-three patients were recruited for the study, as shown in the study flow sheet in Fig. 2; 39 of 53 patients fulfilled eligibility criteria. Six were ineligible because of concomitant medication or evidence of a rectal evacuation disorder; three declined to participate; five had colonic transit at baseline that was too slow, relative to the preset criteria. Demographic data, anxiety and depression scores, and baseline colonic transit data of all randomized patients are shown in Table 1. The three groups were comparable at baseline (age, BMI, colonic GC 24 h). All 39 randomized female patients completed the study and received all doses of study medication. Three (one in each group) did not fill out anxiety and depression questionnaires for the treatment period. No safety issues were identified.
Fig. 2.
Study flow chart. A total of 53 individuals were screened and 14 were excluded for the indicated reasons. All 39 individuals who were eligible and randomized completed the study.
Table 1.
Demographics, somatic symptom, anxiety and depression scores, colonic transit, and bowel function at baseline
| Parameter | Placebo (n = 13) | 25 mg BMS-562086 (n = 13) | 100 mg BMS-562086 (n = 13) | P value |
|---|---|---|---|---|
| Age, yr | 44±4.2 | 49±3.5 | 38±2.7 | 0.13 |
| BMI, kg/m2 | 25.7±1.3 | 28.7±2.0 | 29.6±2.3 | 0.45 |
| Anxiety score | 7.8±1.1 | 6.7±1.4 | 5.1±1.0 | 0.24 |
| Depression score | 3.1±0.6 | 3.8±1.5 | 1.6±0.4 | 0.14 |
| GC 24 h, pretreatment | 3.60±0.25 | 3.85±0.20 | 3.14±0.24 | 0.15 |
| No. of stools/day, pretreatment | 2.9±0.3 | 2.9±0.4 | 2.1±0.3 | 0.18 |
| Stool form (BSFS 1-7), pretreatment | 4.6±0.2 | 4.1±0.3 | 4.7±0.2 | 0.47 |
Values are means ± SE. BMI, body mass index; GC, geometric center; BSFS, Bristol stool form scale.
Effect of pexacerfont on gastrointestinal and colonic transit.
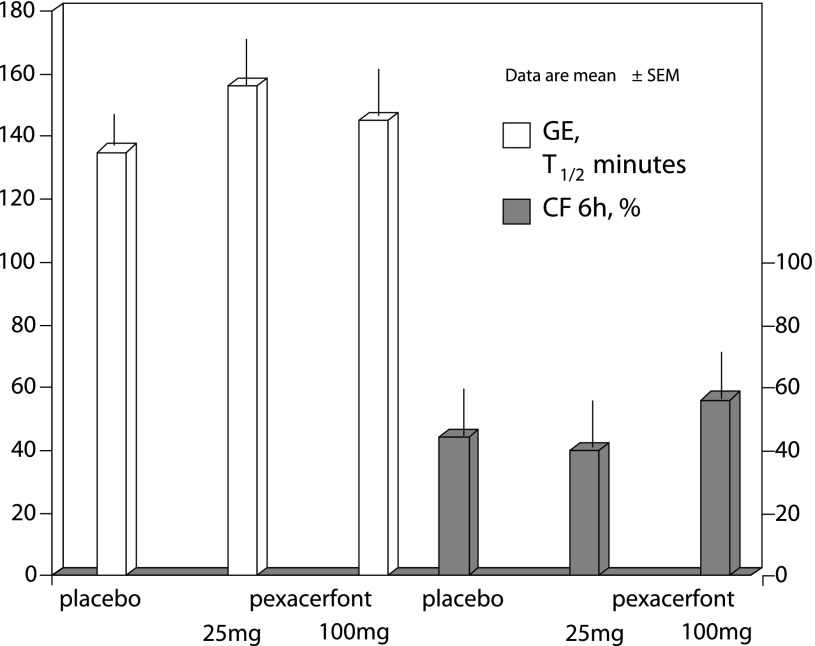
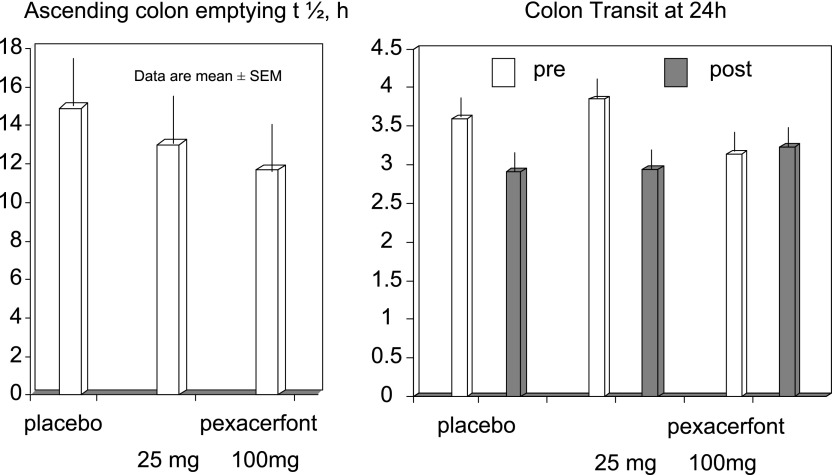
Significant effects of pexacerfont relative to placebo on overall colonic transit at 24 h (primary end point) were not detected (P = 0.53). No other meaningful effects were noted on gastric emptying, orocecal transit, and ascending colon emptying t1/2 (Table 1, Figs. 3 and 4). Data on colonic transit at 32 and 48 h were also not significantly different (data not shown).
Fig. 3.
Gastric emptying (GE) half-time (T1/2) and orocecal transit [colonic filling (CF)] were secondary end points, and there were no statistically significant differences in the 3 treatment groups in regards to the effect of pexacerfont on gastric emptying and colonic filling.
Fig. 4.
There was no significant effect of pexacerfont on overall and ascending colonic transit relative to placebo (P = 0.53); note that the numerical difference in ascending colon emptying half-time (t1/2) between placebo and 100 mg pexacerfont was not significant.
Effect of pexacerfont on bowel function and symptoms.
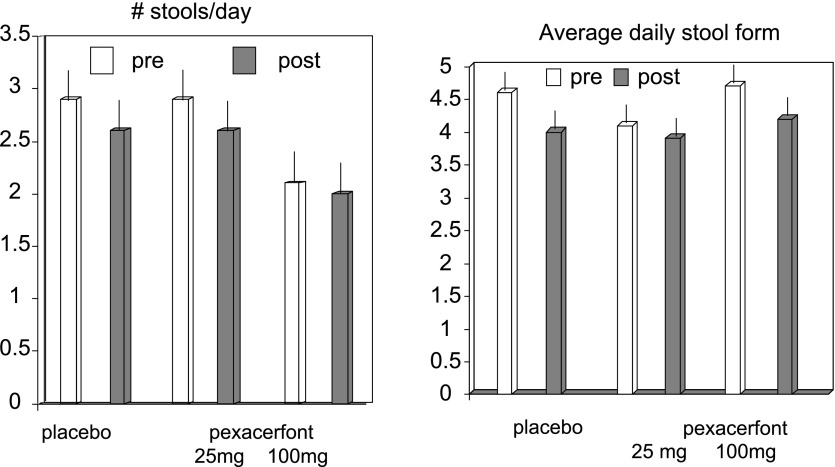
There were no significant treatment effects detected of pexacerfont on the number of stools per day (P = 0.998, Fig. 5), ease of passage scores (P = 0.137), decrease of stool consistency (P = 0.954, Fig. 5), or decrease in subjective symptoms of bloating (P = 0.39), gas (P = 0.59), urgency (P = 0.85), or abdominal pain (P = 0.5), as shown in Table 2.
Fig. 5.
Two of the secondary end points included stool frequency and form, and there were no statistically significant effects noted in regards to these end points.
Table 2.
On treatment average symptoms
| Placebo (N =13) |
BMS-562086 25 mg* (N =13) | BMS-562086 100 mg* (N =13) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Load | Treat | Baseline | Load (50 mg) | Treat (25 mg) | Baseline | Load (200 mg) | Treat (100 mg) | |
| Bloating | 32±6.7 | 32±5.6 | 32.5±6.2 | 32.8±6.9 | 27.7±7.6 | 26±7.3 | 27.9±6.1 | 21.1±4.9 | 20.5±5.5 |
| Gas | 37.3±5.1 | 37±5.7 | 30.7±5.6 | 37.4±6.2 | 31.7±5.4 | 29.8±6.2 | 33±6.1 | 31.5±6.4 | 28±6.5 |
| Urgency | 30.7±6.6 | 26.5±5.6 | 26.8±6.1 | 39.2±5.8 | 34±6.7 | 30±7.4 | 34.1±5.6 | 27.9±5.4 | 25.9±6.1 |
| Pain | 25.7±6.1 | 25.8±6.0 | 23.3±5.5 | 29.2±6.0 | 21.8±6.2 | 26±7.6 | 35±5.1 | 25.6±5.4 | 23.8±5.9 |
| Aggregate | 125.6±21 | 121.1±20.9 | 118.3±21.3 | 138.6±23.1 | 115.2±24 | 111.8±27.7 | 129.3±20.5 | 104.4±21.1 | 97.7±23 |
Values are means ± SE.
Dose refers to treatment phase; patients received double dose during preceding week (“loading” phase).
Anxiety and depression scores.
The average baseline anxiety rating for the entire cohort of 39 patients in the present study was 6.5 ± 0.7; the average depression rating at baseline was 2.8 ± 0.6. Seven of the 39 participants had anxiety ratings of 8 or above, which is associated with case identification of significant anxiety.
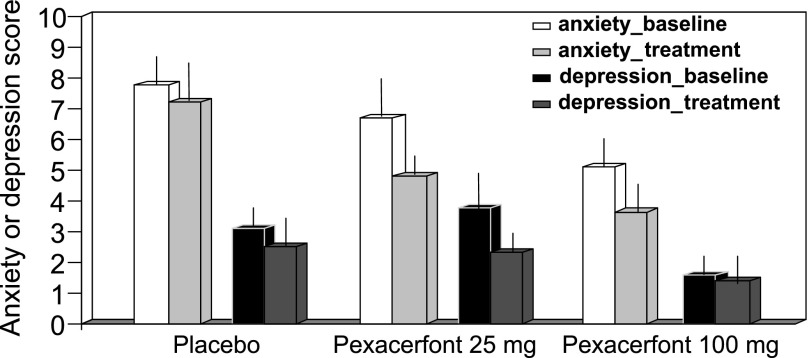
The three groups' anxiety and depression scores were not different at baseline, and no treatment effects were detected (P > 0.5, ANCOVA with age and baseline as covariates, Fig. 6), though the posttreatment scores were associated with baseline scores (P < 0.01).
Fig. 6.
Effect of pexacerfont on anxiety and depression scores; there were no statistically significant differences in the 3 treatment groups.
Pharmacokinetics.
During the maintenance phase (2nd week of dosing), all patients receiving 100 mg pexacerfont had plasma concentrations >500 nm at each time point assessed over each 24-h period. Only 69% of subjects receiving 25 mg of pexacerfont had concentrations >500 nM from 3–24 h over each 24-h period.
Adverse events.
There were no serious adverse events, and no patient had to stop treatment due to an adverse event (AE). Recorded AEs occurring in 10% of participants in any group are shown in Table 3. AEs were generally classified as mild, possibly related to study drug, or resolved without intervention or treatment. There were no significant differences by treatment group.
Table 3.
Adverse effects recorded in >10% of participants
| Placebo | BMS-562086 25 mg | BMS-562086 100 mg | P value | |
|---|---|---|---|---|
| N in group | 13 | 13 | 13 | |
| Total no. (%) with adverse event | 7 (53.8%) | 7 (53.8%) | 9 (69.2%) | 0.77 |
| Gastrointestinal | 2 (15.4%) | 3 (23.1%) | 2 (15.4%) | 1.0 |
| Diarrhea | 1 (7.7%) | 1 (7.7%) | 1 (7.7%) | 1.0 |
| Flatulence | 1 (7.7%) | 2 (15.4%) | 0 | 0.76 |
| Abdominal distension | 0 | 0 | 1 (7.7%) | 1.0 |
| Nervous system disorders: headache | 3 (23.1%) | 2 (15.4%) | 5 (38.5%) | 0.54 |
P values were determined by Fisher's Exact test.
DISCUSSION
This phase IIa study shows that, in women with D-IBS, 25 or 100 mg once daily pexacerfont does not significantly alter colonic transit or induce clinically relevant or statistically significant effects on other regional transit or bowel function. The results were unexpected, since prior studies in several mammalian species including rodents, dogs, and humans have shown that peripheral administration of CRF alters gut motility (22, 32–34, 40, 42). The three groups had similar bowel symptoms (frequency and form), colonic transit, anxiety, and depression scores at baseline. We chose to study the effect of the CRF1 antagonist in patients with IBS rather than healthy volunteers to avoid the criticism that the agent may not be effective because of the absence of stress or anxiety, which are associated with upregulation of CRF mechanisms. Indeed, the average anxiety score of participants was 6.7 (cases defined by scores of 8 or above), in contrast to the average scores of 2.9 in healthy volunteers in concurrent studies in our laboratory (11). However, a subgroup of IBS patients reports a close association between acute stressors and diarrhea. Furthermore, studies indicate that acute stress stimulates transit through the bowel, and CRF may play a role in this response (38). This study did not specifically attempt to differentiate acute stress-associated symptoms vs. chronic stress-related symptoms.
The strengths of the study include the randomized, double-blind, placebo-controlled, dose-response design; the validated method used to assess gut transit; robust statistical power; and the track record of the method in establishing potential efficacy of drugs in development for IBS, especially medications that influence bowel function (6, 12, 16, 41, 49). The pharmacokinetic measurements also demonstrated that for all participants receiving 100-mg dose, and for 69% of those receiving 25 mg pexacerfont had concentrations >500 nM from 3–24 h over each 24-h period. The target plasma concentration of 500 nM was estimated to be associated with inhibition of the effects of stress on colonic transit in experimental animals.
On the other hand, CRF1 receptors also mediate visceral hyperalgesia induced by repeated psychological stress in rats (27); thus effects of pexacerfont on visceral sensation in humans would be of great interest. Nevertheless, it is important to note that inhibition of CRF1 receptors with CP-154,526 (administered acutely or chronically) significantly reduced EMG responses to pressure-based colorectal distension in rats exposed to chronic water avoidance stress but had no effect on the visceromotor response in rats receiving sham stress (27). These observations raise the question whether the CRF-mediated changes in colonic motor or sensory functions are pivotal in the absence of significant forms of stress, which might be continually experienced by patients with D-IBS. Although anxiety levels are higher in patients with IBS (21), it is unclear whether this level of anxiety in humans results in the degree of stimulation of CRF that would make CRF receptors a viable target for CRF antagonists. In fact, there are data that suggest that the anxiety levels may differ in IBS subgroups. Thus a Swedish study recently reported that the D-IBS group showed less body awareness, less psychological symptoms, and a more normal sense of coherence and psychosocial rating; constipation-predominant IBS patients had a higher degree of body dysfunction and psychological symptoms, as well as the lowest sense of coherence compared with controls and D-IBS (20). Our experience that the vast majority of 122 patients with different subtypes of IBS (11) had anxiety scores on a Hospital Anxiety Depression Scale of 4.8 ± 0.3, lower than the score of 8 that is associated with caseness of anxiety disorders in somatic, psychiatric, and primary care patients and in the general population (5). This argues against chronic high level anxiety in many patients with D-IBS in our secondary and tertiary care practice. However, the average baseline anxiety rating for the entire cohort of 39 patients in the present study was 6.5 ± 0.7; 7 of the 39 participants in the study had anxiety ratings of 8 or above, which is associated with case identification of significant anxiety. These data suggest that the IBS participants in the present study would be representative of patients with IBS and that the anxiety levels were, on average, high enough to detect an effect of pexacerfont if comorbidity with mild to moderate anxiety was a factor predisposing to response to this CRF-1 receptor antagonist. Effect of pexacerfont may be different in patients with severe stress or chronic anxiety disorder or in response to acute stressors, and further studies in such patients would be of significant interest. Pexacerfont's effects on anxiety and depression scores were not significantly different from those of placebo, and Fig. 6 generally shows placebo effect of regression to the mean. However, it is important to note that the study size did not provide sufficient power to detect a significant effect on anxiety or depression scores.
The apparent lack of efficacy of pexacerfont on colonic transit or bowel function in this study may have occurred for several reasons, including insufficient dose or route of administration for biological effect, inadequate study duration, variable CRF receptor subtype localization, or a Type 2 error.
The first consideration is whether a sufficient dose of pexacerfont was administered to observe the desired biological effect. Sagami et al. (42) performed a study in IBS patients using a CRF antagonist that was administered intravenously (iv) and found decreased colonic motility. This supported a previous report that iv CRF stimulates colonic motility and that blockage of peripheral CRF1 receptors inhibits accelerated colonic motility (32). In our study, pexacerfont was administered orally, as opposed to iv; however, preclinical and phase I human studies have demonstrated that oral administration of pexacerfont reliably produces plasma drug concentrations substantially greater than those associated with drug activity in preclinical models. Because good plasma drug exposure in this study was confirmed by measurement of trough drug concentrations, it is uncertain whether a sufficient dose was administered to elicit the desired biological effect or whether the preclinical models are not predictive of effects in patients with D-IBS. It is also unclear whether sufficient drug was achieved at the central or peripheral sites of action of the drug. Although preclinical studies show that pexacerfont crosses the blood brain barrier, there are presently no human data that demonstrate the degree at which pexacerfont antagonizes central vs. peripheral CRF1 receptors, and this information is important given that both central and peripheral CRF pathways modulate colonic function.
Another factor that may have contributed to the lack of observed effect of pexacerfont is inadequate study duration to observe the desired biological effects of prolongation of colonic transit and improved bowel function. In animal models, there is evidence that CRF modulates gastrointestinal motility on the myenteric neuronal level without a latency period (4). However, it is conceivable that, in humans, efficacy of CRF1 antagonist may require neuroplasticity changes in either the enteric or central nervous system to induce a measurable biological effect.
In the only prior study employing a CRF antagonist in humans, the agent utilized was nonselective, in contrast to the CRF1 selectivity of pexacerfont. The literature does not provide a consistent understanding on CRF receptor subtype localization. Some data suggest that CRF1 receptors are predominantly expressed in the brain and CRF2 receptors in peripheral sites (26, 31). In other studies, CRF1 receptors are mainly located on myenteric neurons (4, 35). Furthermore, preclinical studies show that pexacerfont crosses the blood-brain barrier, although experiments to demonstrate this in humans have not been undertaken. Therefore, the degree at which pexacerfont antagonizes central vs. peripheral CRF1 receptors is unknown, and this information is important given that both central and peripheral CRF pathways modulate colonic function. Variability of CRF receptor subtype localization among mammalian species and pexacerfont's variable ability to cross the human blood-brain barrier may explain the lack of efficacy of a potent and selective CRF1 antagonist in this study in humans. For example, it is conceivable that, in the study by Sagami et al. (42), the nonselective CRF antagonist affected colonic motility by antagonism of peripheral CRF2 receptors. Therefore, the CRF1 peripheral antagonism targeted by pexacerfont may not be playing a significant role in modulating gastrointestinal motility in humans.
Lastly, we considered whether a Type 2 error may have led to failure to demonstrate efficacy of pexacerfont. We are confident that a false-negative result in this study is unlikely, given that the 80% power based on a two-sample t-test at a two-sided α of 0.05 was used for sample size calculations, based on data in women with D-IBS from the study by Viramontes et al. (49). Moreover, a post hoc analysis of the observed baseline colonic GC at 24 h and its coefficient of variation were similar to those on which the sample size was estimated. On the other hand, Fig. 4 shows numerical differences in ascending colon emptying t1/2. The largest difference between 100 mg pexacerfont and placebo was, on average, 3 h, or ∼22% difference from the observation with placebo. The sample size in this study was sufficient to detect a >60% difference in ascending colon transit between placebo and drug. The observed magnitude of difference (<25%) in ascending colon t1/2 observed is not associated with a change in stool consistency or frequency in other studies with experimental agents; typically a 50% change in ascending colon emptying t1/2 is associated with significant alteration in stool consistency (2, 12). However, the study may have missed a clinically meaningful change in bowel symptoms, pain, or anxiety, given that it was not powered to identify these as primary end points.
In conclusion, our observations showed that, with the selective CRF1 antagonist pexacerfont, there is no significant reduction in colonic transit or bowel symptoms in women with D-IBS. These observations in patients with D-IBS differ from the effects of CRF1 inhibition in stressed animals. Further studies are needed to determine whether CRF1 or CRF2 antagonists are important in the treatment of D-IBS, to assess the potential efficacy of this class of compounds in clinical studies, and to evaluate the role of the hypothalamic-pituitary-adrenal axis and stimulation of CRF mechanisms in the bowel dysfunction in patients with D-IBS.
DISCLOSURES
R. Croop, G. Tong, R. Dockens, and L. Castenada are employees of Bristol-Myers Squibb.
This is a Phase IIa trial. The statistical analysis of the entire data sets pertaining to efficacy (specifically primary and major secondary efficacy end points) and safety (specifically, serious adverse events as defined in federal guidelines) has been independently confirmed by a biostatistician (A. R. Zinsmeister) who is not employed by Bristol-Myers Squibb. The corresponding author, M. Camilleri, had full access to all of the data and takes full responsibility for the veracity of the data and analysis.
GRANTS
M. Camilleri was supported by grant K24-DK02638 from National Institutes of Health. This study was funded by a single center research grant from Bristol-Myers Squibb.
Supplementary Material
Acknowledgments
The excellent secretarial support of Cindy Stanislav is gratefully acknowledged.
This study is registered as NCT00399438 with ClinicalTrials.gov.
Footnotes
Supplemental data for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website.
REFERENCES
- 1.Andresen V, Camilleri M. Irritable bowel syndrome: recent and novel therapeutic approaches. Drugs 66: 1073–1088, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Andresen V, Camilleri M, Busciglio I, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, Currie MG, Zinsmeister AR. Effects of linaclotide, a novel guanylate cyclase-C agonist, on gastrointestinal transit and bowel function in patients with constipation-predominant irritable bowel syndrome. Gastroenterology 133: 761–768, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bao AM, Swaab DF. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology 85: 27–36, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bisschops R, Vanden Berghe P, Sarnelli G, Janssens J, Tack J. CRF-induced calcium signaling in guinea pig small intestine myenteric neurons involves CRF-1 receptors and activation of voltage-sensitive calcium channels. Am J Physiol Gastrointest Liver Physiol 290: G1252–G1260, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: 69–77, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology 120: 354–360, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bristol-Myers Squibb Pharmaceutical Research Institute. BMS-562086 Investigator Brochure. Data on file.
- 8.Bristol-Myers Squibb Pharmaceutical Research Institute. Effects of BMS-562086 on CRF-induced colonic motility and diarrhea. Data on file.
- 9.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med 38: 1807–1810, 1997. [PubMed] [Google Scholar]
- 10.Camilleri M Mechanisms in IBS: something old, something new, something borrowed. Neurogastroenterol Motil 17: 311–316, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, McKinzie S, Buscigilio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychological and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 772–781, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M, McKinzie S, Fox J, Foxx-Orenstein A, Burton D, Thomforde G, Baxter K, Zinsmeister AR. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2: 895–904, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology 103: 36–42, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil 21: 149–159, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary PD, Mechanic D, Greenley JR. Sex differences in medical care utilization: an empirical investigation. J Health Soc Behav 23: 106–119, 1982. [PubMed] [Google Scholar]
- 16.Coulie B, Szarka LA, Camilleri M, Burton DD, Mckinzie S, Stambler N, Cedarbaum JM. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 119: 41–50, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther 16: 1781–1790, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130: 304–311, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson EM, Andren KI, Eriksson HT, Kurlberg GK. Irritable bowel syndrome subtypes differ in body awareness, psychological symptoms and biochemical stress markers. World J Gastroenterol 14: 4889–4896, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esler MD, Goulston KJ. Levels of anxiety in colonic disorders. N Engl J Med 288: 16–20, 1973. [DOI] [PubMed] [Google Scholar]
- 22.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 42: 845–849, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonenne J, Camilleri M, Ferber I, Burton D, Baxter K, Keyashian K, Foss J, Wallin B, Du W, Zinsmeister AR. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol 3: 784–791, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil 17: 415–422, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut 33: 818–824, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, Chrousos GP, Bornstein SR. Expression of corticotropin releasing hormone receptors type 1 and type 11 mRNA in suicide victims and controls. Mol Psychiatry 6: 540–546, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Larauche M, Bradesi S, Million M, McLean P, Taché Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol 294: G1033–G1040, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lembo A, Camilleri M. Chronic constipation. N Engl J Med 349: 1360–1368, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32: 920–924, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 130: 1480–1491, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 136: 4139–4142, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Maillot C, Million M, Wei JY, Gauthier A, Tache Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology 119: 1569–1579, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Martinez V, Wang L, Rivier JE, Vale W, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther 301: 611–617, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Mayer EA, Sytnik B, Reddy NS, Van Deventer G, Tache Y. Corticotropin releasing factor (CRF) increases post-prandial duodenal motor activity in humans. J Gastrointest Motil 4: 53–60, 1992. [Google Scholar]
- 35.Miampamba M, Maillot C, Million M, Tache Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF1 receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol 282: G857–G865, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Tache Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res 985: 32–42, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Monnikes H, Schmidt BG, Tache Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology 104: 716–723, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Narducci F, Snape WJ Jr, Battle WM, London RL, Cohen S. Increased colonic motility during exposure to a stressful situation. Dig Dis Sci 30: 40–44, 1985. [DOI] [PubMed] [Google Scholar]
- 39.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 43: 425–473, 1991. [PubMed] [Google Scholar]
- 40.Pappas T, Debas H, Tache Y. Corticotropin-releasing factor inhibits gastric emptying in dogs. Regul Pept 11: 193–199, 1985. [DOI] [PubMed] [Google Scholar]
- 41.Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology 118: 463–468, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut 53: 958–964, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol 97: 1910–1915, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Stivland T, Camilleri M, Vassallo M, Proano M, Rath D, Brown M, Thomforde G, Pemberton J, Phillips S. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology 101: 107–115, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann NY Acad Sci 697: 233–243, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Tache Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil 16, Suppl 1: 137–142, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ III. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 65: 1456–1479, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut 45: II43–II47, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde G. Gender-related differences in slowing colonic transit by 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 96: 2671–2676, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med 329: 1073–1078, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 33: 825–830, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.