Abstract
Endogenous insulin-like growth factor-I (IGF-I) regulates intestinal smooth muscle growth by concomitantly stimulating proliferation and inhibiting apoptosis. IGF-I-stimulated growth is augmented by the αvβ3 integrin ligands vitronectin and fibronectin. IGF-I expression in smooth muscle is increased in both TNBS-induced colitis and Crohn's disease. We hypothesized that intestinal inflammation increased vitronectin and fibronectin expression by smooth muscle and, along with IGF-I upregulation, increased intestinal muscle growth. Intestinal smooth muscle cells were examined 7 days following the induction of TNBS-induced colitis. Although αvβ3 integrin expression was not altered by TNBS-induced colitis, vitronectin and fibronectin levels were increased by 80 ± 10% and 90 ± 15%, above control levels, respectively. Basal IGF-I receptor phosphorylation in inflamed muscle from TNBS-treated rats was increased by 86 ± 8% over vehicle-treated controls. Basal ERK1/2, p70S6 kinase, and GSK-3β phosphorylation in muscle cells of TNBS-treated rats were also increased by 140–180%. TNBS treatment increased basal muscle cell proliferation by 130 ± 15% and decreased apoptosis by 20 ± 2% compared with that in vehicle-treated controls. The changes in proliferation and apoptosis were reversed by an IGF-I receptor tyrosine kinase inhibitor or an αvβ3 integrin antagonist. The results suggest that smooth muscle hyperplasia in TNBS-induced colitis partly results from the upregulation of endogenous IGF-I and ligands of αvβ3 integrin that mediate increased smooth muscle cell proliferation and decreased apoptosis. This paper has identified one mechanism regulating smooth muscle hyperplasia, a feature of stricture formation that occurs in the chronically inflamed intestine of TNBS-induced colitis and potentially Crohn's disease.
Keywords: Crohn's disease, insulin-like growth factor
trinitrobenzene sulfonic acid (TNBS) induces chronic inflammation in the rat colon that involves all portions of the intestinal wall including the muscularis propria. TNBS-induced colitis is a model of Crohn's disease and similarly is associated with an initial Th1 patterned immunological response within the mucosa, submucosa, and muscularis propria (4, 8). Chronic inflammation of intestinal muscle, whether in TNBS-induced colitis or Crohn's disease, results in hyperplasia and hypertrophy of smooth muscle cells of the muscularis propria and increased production of extracellular matrix proteins, including collagen-I, by the smooth muscle cells and myofibroblasts of the muscularis propria (35, 37). In Crohn's disease, expression of insulin-like growth factor-I (IGF-I) is increased in all layers of the intestine including the muscularis propria (37). Similar events occur in animal models of chronic intestinal inflammation: TNBS-induced and dextran sulfate-induced colitis (28, 33) and peptidoglycan-polysaccharide-induced ileocolitis (38). IGF-I is an endogenous growth factor that plays a central role in the growth and development of visceral and vascular smooth muscle. In the human intestine IGF-I regulates muscle cell growth in normal smooth muscle, by simultaneously stimulating proliferation and inhibiting apoptosis, and in chronically inflamed muscle where it also stimulates collagen-I production (9, 10). The increased levels of smooth muscle cell-derived IGF-I in Crohn's disease may contribute to hyperplasia of the muscularis propria and stricture formation in the ∼30% of patients developing this complication, and in animal models of inflammatory bowel disease (20).
The effects of IGF-I on intestinal smooth muscle cells are mediated by the cognate IGF-I receptor tyrosine kinase, which is coupled to activation of ERK1/2 and p70S6 kinase, which jointly stimulate proliferation (14). In rat colonic smooth muscle cells, IGF-I also regulates collagen I gene expression through the ERK1/2 pathway (30). In human intestinal smooth muscle, where it has been examined, IGF-I regulates PI3-kinase-dependent glycogen synthase kinase-3β activity and inhibits apoptosis (10, 11). We have recently shown that αvβ3 integrin (the cognate vitronectin receptor) and its ligands vitronectin and fibronectin are expressed by intestinal smooth muscle cells in humans. Occupancy of αvβ3 integrin by these ligands increases the intensity and duration of IGF-I stimulated IGF-I receptor tyrosine kinase activation and smooth muscle growth (12). The participation of IGF-I and αvβ3 integrin and their interaction in regulating intestinal smooth muscle cell growth in chronic intestinal inflammation, induced by TNBS, has not been examined. Although increased expression of IGF-I is thought to play a role in the hyperplasia of smooth muscle in the chronic inflammation of TNBS colitis, this has not been directly demonstrated. This article provides evidence that smooth muscle hyperplasia in TNBS-induced colitis partly results from the upregulation of endogenous IGF-I and ligands of αvβ3 integrin that mediate increased smooth muscle cell proliferation and decreased apoptosis.
MATERIALS AND METHODS
Induction of chronic intestinal inflammation with TNBS in the rat colon.
Muscle cells were isolated from the circular muscle layer of the colon of 250-g Sprague-Dawley rats following induction of colitis with TNBS. All animal treatments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. The induction of chronic intestinal inflammation with TNBS was performed according to well-established methods (7, 25, 26, 32, 34, 35). Briefly, rats were deeply anaesthetized with diethyl ether. TNBS at a dose of 90 mg/kg [1.5 ml/kg of 60 mg/ml solution in 50% ethanol (vol/vol)] or 50% ethanol (vol/vol) vehicle alone (control animals) was instilled intrarectally 6 cm from the anal verge. At 7 days following treatment with TNBS, at a time when chronic intestinal muscle inflammation is present, the rats were euthanized by CO2 asphyxiation, and tissues were collected.
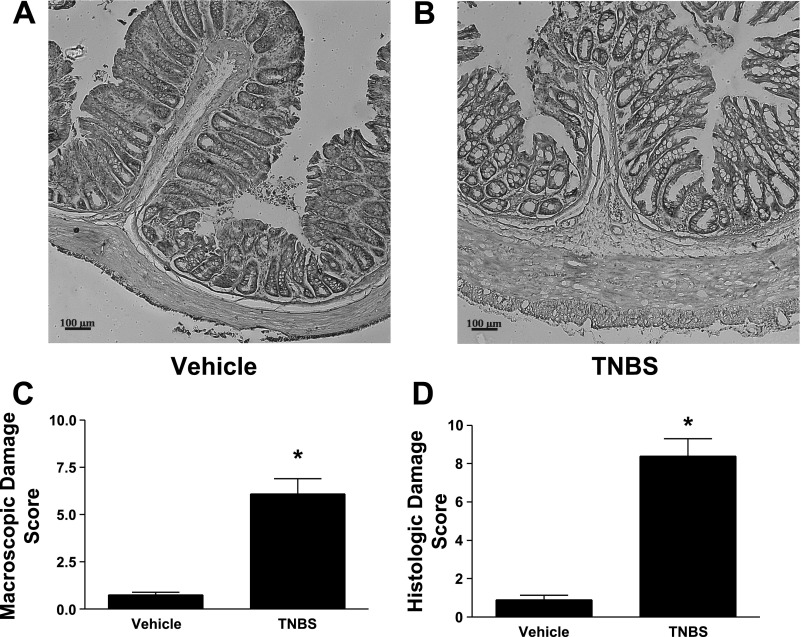
At the time of tissue collection, the severity of inflammation was assessed in two ways according to validated protocols (17, 18): scoring of macroscopic damage and a histological evaluation. Macroscopic scoring was based on wall thickness and the presence and extent of adhesions, ulcerations, hyperemia, and diarrhea. Histological scoring was based on: the extent of destruction of the normal mucosal architecture, cellular infiltration, muscle thickening, crypt abscesses, and goblet cell depletion. All animals treated with TNBS exhibited a significant increase in both macroscopic scores (Fig. 1A) and histological scores (Fig. 1B) compared with vehicle-treated animals.
Fig. 1.
Inflammatory damage increases during the chronic inflammatory phase of TNBS-induced colitis. Representative examples of hematoxylin and eosin (H&E) staining from vehicle control-treated rat (A) and TNBS-treated rat (B). Gross damage scores (C) and histopathology damage scores (D) were measured 7 days following intrarectal administration of TNBS. Gross damage and histopathological damage were significantly increased in the colons of TNBS-treated animals compared with vehicle-treated animals. Results were expressed by validated scoring methods (18). Values represent means ± SE of 6 experiments. *P < 0.05 vs. vehicle-treated animals.
Isolation of colonic smooth muscle cells.
Muscle cells were isolated from the circular muscle layer of rat colon according to previously reported techniques (9, 13, 14). Briefly, segments of colon were obtained from rats after the induction of inflammation with TNBS instillation or from rats after instillation of ethanol vehicle. The mucosa was dissected away and the circular layer separated before being cut into 2×2 cm strips. Isolated muscle cells were prepared by enzymatic digestion at 37°C in 20 ml of Dulbecco's modification of Eagle's medium plus 10% fetal bovine serum (DMEM-10) containing penicillin 200 U/ml, streptomycin 200 μg/ml, gentamicin 100 μg/ml and amphotericin B 2 μg/ml to which was added 0.0375% collagenase (type II), and 0.1% soybean trypsin inhibitor. Muscle cells dispersed from the circular layer were harvested by filtration through 500 μm Nitex mesh and centrifugation at 150 g for 5 min. Cells were resuspended and washed twice by centrifugation at 150 g for 5 min. After resuspension in DMEM-10 containing the same antibiotics, the cells were used for preparation of whole cell lysates to measure protein levels or were placed into culture at a concentration of 5 × 105 cells per ml as determined by counting in a hemocytometer. Cultures were incubated in a 10% CO2 environment at 37°C. DMEM-10 medium was replaced every 3 days until the cells reached confluence.
Primary cultures of muscle cells were passaged upon reaching confluence and used in first passage. We have previously shown cultures of smooth muscle cells isolated in this manner are homogeneous and possess a phenotype characteristic of intestinal smooth muscle as determined by immunostaining for smooth muscle markers and expression of γ-enteric actin (29). Other cell types including epithelial cells, endothelial cells, neurons, and interstitial cells of Cajal are not detected in these cultures.
Immunoprecipitation of IGF-I receptor.
IGF-I receptors were immunoprecipitated as previously described (15). Briefly, cell lysates were prepared in an immunoprecipitation buffer consisting of (in mM) 50 Tris·HCl (pH 7.5), 150 NaCl, 50 NaF, 1 Na orthovanadate, 1 dithiothreitol, 1 phenylmethylsulfonyl fluoride, and 0.5% Nonidet P-40, to which was added 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1 μg/ml aprotinin. The resulting cell lysates were clarified by centrifugation at 14,000 g for 10 min at 4°C. The lysates were precleared by incubation with protein A agarose beads for 1 h at 4°C. Samples containing equal amounts of protein (1 mg) were incubated for 2 h at 4°C with 2 μg of rabbit anti-IGF-I receptor (anti-IGF-IR) β-subunit. The incubation continued overnight at 4°C after the addition of 10 μl of protein A agarose beads. The immune complex-agarose beads were washed three times with ice-cold immunoprecipitation buffer and twice with ice-cold kinase assay buffer. After being washed, the immune complex-agarose beads were resuspended in 25 μl of sample buffer, boiled for 5 min, and then loaded onto a 7.5% polyacrylamide gel and the proteins were separated by SDS-PAGE. Tyrosine-phosphorylated IGF-IR was identified by Western blot analysis as described below.
Western blot analysis.
Analysis of proteins and phosphorylated proteins was performed by Western blot analysis using lysates prepared from freshly isolated smooth muscle cells by standard methods as described above (10, 11, 14, 15). After addition of Laemmli sample buffer, the lysates were boiled for 5 min and equal amounts of total cell protein were separated with SDS-PAGE under denaturing conditions. After the proteins were electrotransferred to nitrocellulose, the membranes were incubated overnight with specific antibodies recognizing the proteins of interest: αv, β3, fibronectin, vitronectin, ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), p70S6 kinase, phospho-p70S6 kinase (Ser389), GSK-3β, or phospho-GSK-3β (Ser9). When appropriate, nitrocellulose membranes were stripped and reblotted to determine levels of total (phosphorylated plus nonphosphorylated) protein. Bands of interest were visualized with enhanced chemiluminescence by using a FluoChem 8800 (Alpha Innotech, San Leandro, CA), and the resulting digital images quantified by use of AlphaEaseFC version 3.1.2 software.
[3H]thymidine incorporation assay.
Proliferation of smooth muscle cells in culture was measured by the incorporation of [3H]thymidine as described previously (9, 11). Briefly, smooth muscle cells growing in 24-well plates were rendered quiescent by incubation in serum-free DMEM for 24 h. The cells were incubated for an additional 24 h in the presence of either the αvβ3 selective disintegrin, echistatin (100 nM), the IGF-I receptor tyrosine kinase inhibitor AG1024 (10 μM), or vehicle alone. In other experiments the cells were incubated for an additional 24 h in the presence of either an ERK1/2 inhibitor (1 μM U1026), a PI3-kinase inhibitor (1 μM LY294002), or a p70S6 kinase inhibitor (1 nM rapamycin). During the final 4 h of this incubation period, 1 μCi/ml [3H]thymidine was added to the medium. [3H]thymidine incorporation into the perchloric acid extractable pool was used as a measure of DNA synthesis.
Nucleosome apoptosis assay.
Apoptosis of smooth muscle cells in culture was quantitated as described previously by measurement of cytoplasmic histone-associated DNA fragments (nucleosomes) using a quantitative sandwich enzyme immunoassay ELISA (Roche Applied Science, Indianapolis, IN) according to the manufacturer's direction (10). Briefly, smooth muscle cells growing in 48-well culture plates were washed free of serum and incubated for 48 h in serum-free DMEM. During the final 24 h the cells were incubated in the presence of either the αvβ3 selective disintegrin echistatin (100 nM), the IGF-I receptor tyrosine kinase inhibitor AG1024 (10 μM), or vehicle alone. The medium was removed and replaced with 100 μl of lysis buffer. The cells were incubated at room temperature for 20 min and centrifuged for 10 min at 200 g. Aliquots of supernatant (20 μl) containing cytoplasmic DNA fragments was transferred to each well of a 98-well ELISA plate precoated with streptavidin. To each well was added 80 μl of a mixture containing mouse monoclonal antibody to histone-biotin (clone H11-4) and mouse monoclonal antibody to anti-DNA-POD (clone MCA-33). The microtiter plates were incubated at room temperature on a microplate shaker at 250 rpm for 2 h. The unbound components were removed by washing each well three times with 250 μl. After substrate 2,2′ azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) solution was added to each well, the microtiter plates were incubated with shaking (200 rpm) for 10 min. Absorbance at 405 nm (A405) was measured by use of a Victor2 1420 multichannel counter (Perkin Elmer, Boston, MA). All samples were run in duplicate and corrected for substrate blanks. Results were reported as the percent change in A405.
Measurement of protein content.
The protein content of cell lysates was measured by using the Bio-Rad DC protein assay kit according to manufacturer's direction. Samples were adjusted to provide aliquots of equal protein content prior to in vitro kinase assay or Western blot analysis.
Statistical analysis.
Values given represent means ± SE of n experiments where n represents the number of experiments on cells derived from separate intestinal specimens or primary cultures. Statistical significance was tested by Student's t-test for either paired or unpaired data as appropriate. Densitometric values for protein bands of phosphorylated signaling intermediates were reported in arbitrary units above background values after normalization to total protein levels. Bands of interest were visualized with enhanced chemiluminescence by using a FluoChem 8800 (Alpha Innotech, San Leandro, CA), and the resulting digital images quantified by using AlphaEaseFC version 3.1.2 software.
Materials.
Collagenase and soybean trypsin inhibitor were obtained from Worthington Biochemical (Freehold, NJ); DMEM and HBSS were obtained from Mediatech (Herndon, VA); fetal bovine serum was obtained from Summit Biotechnologies (Fort Collins, CO); rabbit polyclonal antibodies to p70S6 kinase, GSK-3β, and pGSK-3β (Ser9), mouse monoclonal antibodies to phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, and phosphotyrosine (p-Tyr-100), anti-rabbit IgG-HRP, and anti-mouse-HRP were obtained from Cell Signaling Technology (Beverly, MA); rabbit polyclonal antibody to phospho-p70S6 kinase (Ser389) was obtained from Upstate Biotechnology (Lake Placid, NY); rabbit polyclonal antibody to β3 was obtained from Biosource International (Camarillo, CA); rabbit polyclonal antibody to αv was obtained from Becton-Dickinson (Franklin Lakes, NJ); rabbit polyclonal antibody to IGF-IRβ and mouse monoclonal antibody to αvβ3 (23C6) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); U1026, LY294002, and rapamycin were obtained from Calbiochem (San Diego, CA); Western blotting materials and DC protein assay kit were obtained from Bio-Rad Laboratories (Hercules, CA); Nucleosome Apoptosis ELISA was obtained from Roche Applied Science (Indianapolis, IN); [3H]thymidine (6 Ci/mmol)was obtained from NEN (Boston, MA); and plastic cultureware was obtained from Corning (Corning, NY). Echistatin, 2,4,6-TNBS, and all other chemicals were obtained from Sigma (St. Louis, MO).
RESULTS
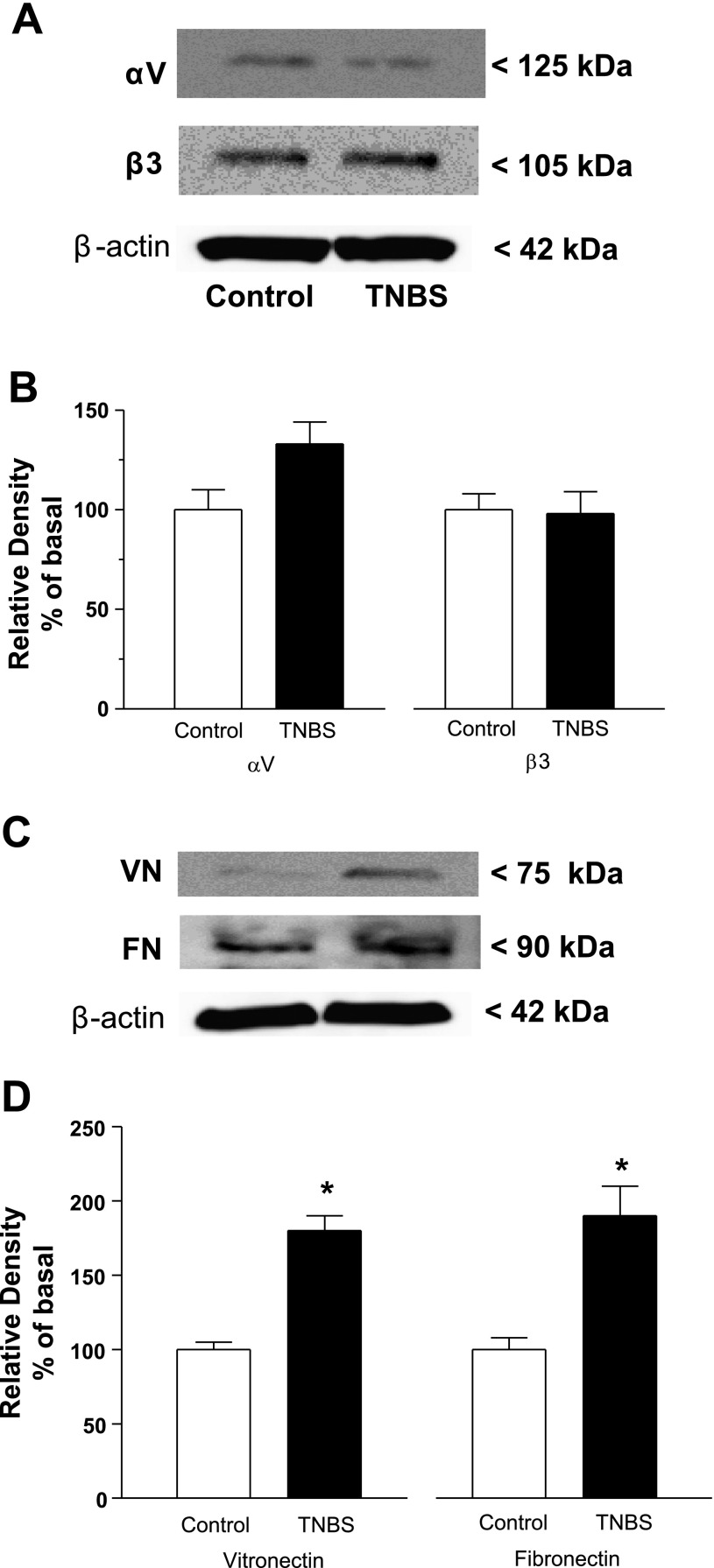
TNBS-induced inflammation of intestinal muscle increases levels of the αvβ3 integrin ligands vitronectin and fibronectin.
The levels of vitronectin and fibronectin produced by smooth muscle cells were assessed by immunoblot analysis in whole cell lysates. TNBS-induced inflammation increased levels of vitronectin by 80 ± 10% and fibronectin by 90 ± 15% above levels in vehicle-treated rats (Fig. 2).
Fig. 2.
Levels of the αvβ3 integrin ligands vitronectin (VN) and fibronectin (FN) are increased in the muscularis propria during chronic intestinal inflammation. Representative immunoblots of αv and β3 integrin subunit expression (A) and vitronectin and fibronectin expression (C) after chronic muscle inflammation was induced by TNBS. The levels of αv and β3 integrin (B) are not increased in the colonic muscle of TNBS-treated animals compared with control. The levels of vitronectin and fibronectin (D) are increased in the colonic muscle of TNBS-treated animals. Integrin levels were measured by immunoblot analysis in lysates prepared from smooth muscle cells isolated from the colon of rats 7 days following rectal administration of TNBS or vehicle alone. Results are expressed in relative densitometric units in samples containing equal amounts of total protein. Values represent means ± SE of 4 experiments on separate animals. *P < 0.05 vs. control.
The levels of protein expression of both subunits of the αvβ3 integrin were also assessed by immunoblot analysis in whole cell lysates. The levels of the αv and β3 integrin subunits were not altered in the presence of TNBS-induced inflammation (Fig. 2).
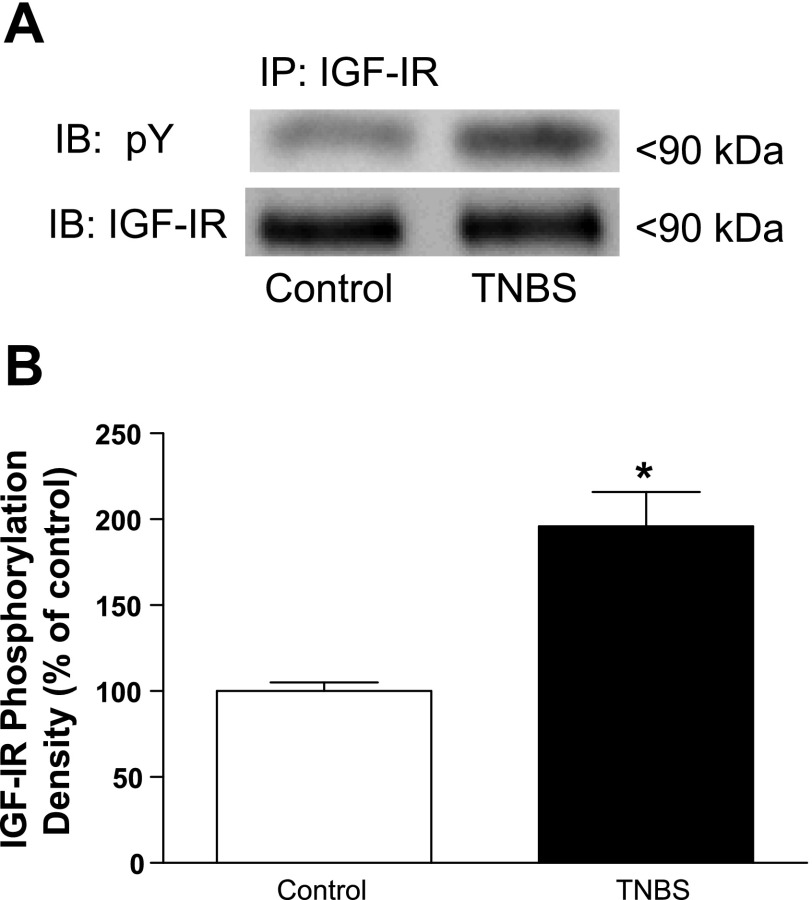
TNBS-induced inflammation of intestinal muscle increases levels of basal IGF-I receptor tyrosine kinase phosphorylation.
In the present study we utilized tyrosine phosphorylation of the immunoprecipitated IGF-I receptor as a measure of IGF-I receptor tyrosine kinase activity. It is important to note that IGF-I is an autocrine growth factor for intestinal smooth muscle. Thus under basal conditions phosphorylation of the IGF-I receptor tyrosine kinase is expected (Fig. 3). The levels of basal IGF-I receptor tyrosine kinase phosphorylation, however, were increased by 80 ± 8% in smooth muscle from TNBS-treated rats compared with control vehicle-treated animals (Fig. 3).
Fig. 3.
Basal levels of IGF-I receptor phosphorylation are increased during chronic intestinal inflammation. A: representative immunoblots (IB) of basal IGF-I receptor (IGF-IR) phosphorylation in colonic smooth muscle from control and TNBS-treated rats. B: densitometric analysis of basal IGF-I receptor phosphorylation in colonic smooth muscle from control and TNBS-treated rats. Basal IGF-I receptor phosphorylation is significantly increased in smooth muscle cells isolated from the chronically inflamed colons of rats treated with TNBS compared with control (vehicle)-treated rats. IGF-I receptor phosphorylation was measured in colonic smooth muscle cells by phosphotyrosine immunoblotting following immunoprecipitation (IP) of IGF-IR in lysates prepared from smooth muscle cells isolated from rat colon 7 days following rectal administration of TNBS or vehicle. Results are expressed in relative densitometric units in samples containing equal amounts of total protein. Values represent means ± SE of 4 experiments on separate animals. *P < 0.05 vs. control.
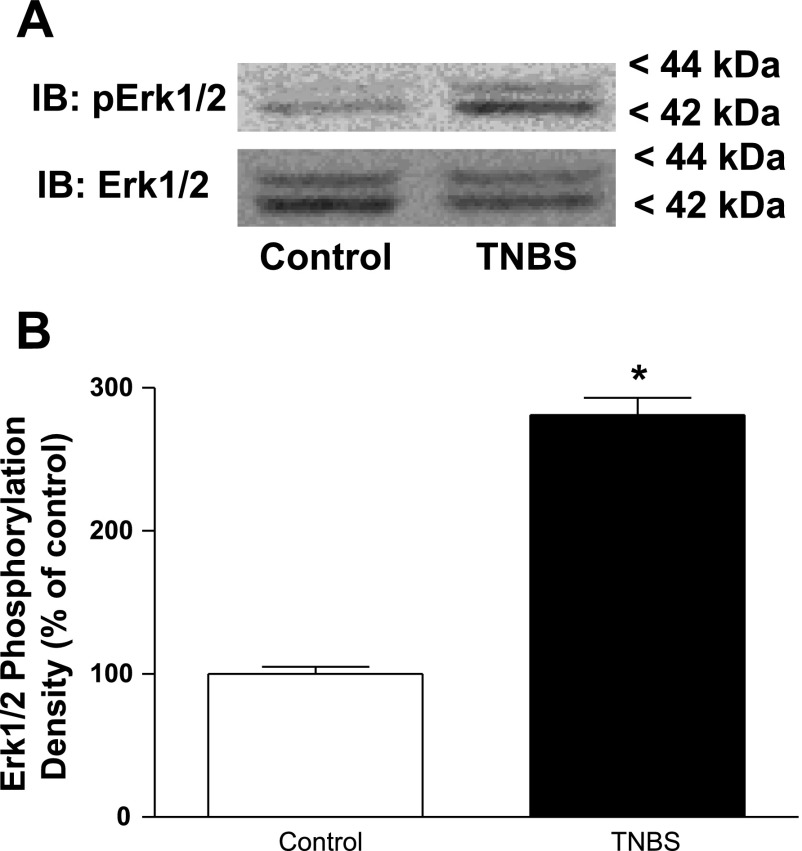
TNBS-induced inflammation of intestinal muscle increases levels of basal ERK1/2 and p70S6 kinase phosphorylation.
IGF-I regulates intestinal smooth muscle proliferation by jointly activating the ERK1/2 signaling pathway and the p70S6 kinase (via PI3-kinase) signaling pathway. In the present study, we used phosphorylation of ERK1/2 (Thr202/Tyr204) as a measure of ERK1/2 enzyme activity and phosphorylation of p70S6 kinase (Ser389) as a measure of p70S6 kinase activity. The levels of basally phosphorylated ERK1/2 were increased by 180 ± 12% in the inflamed muscle of TNBS-treated rats compared with the levels in control vehicle-treated rats (Fig. 4).
Fig. 4.
Basal levels of ERK1/2 phosphorylation are increased during chronic intestinal inflammation. A: representative immunoblots of basal ERK1/2 (Thr202/Tyr204) phosphorylation (pERK1/2) in colonic smooth muscle from control and TNBS-treated rats. B: densitometric analysis of basal ERK1/2 (Thr202/Tyr204) phosphorylation in colonic smooth muscle from control and TNBS-treated rats. Basal ERK1/2 phosphorylation is significantly increased in smooth muscle cells isolated from the chronically inflamed colons of rats treated with TNBS compared with control (vehicle)-treated rats. ERK1/2 (Thr202/Tyr204) phosphorylation was measured in colonic smooth muscle cells isolated from rat colon 7 days following rectal administration of TNBS or vehicle. Results are expressed in relative densitometric units in samples containing equal amounts of total protein. Values represent means ± SE of 4 experiments on separate animals. *P < 0.05 vs. control.
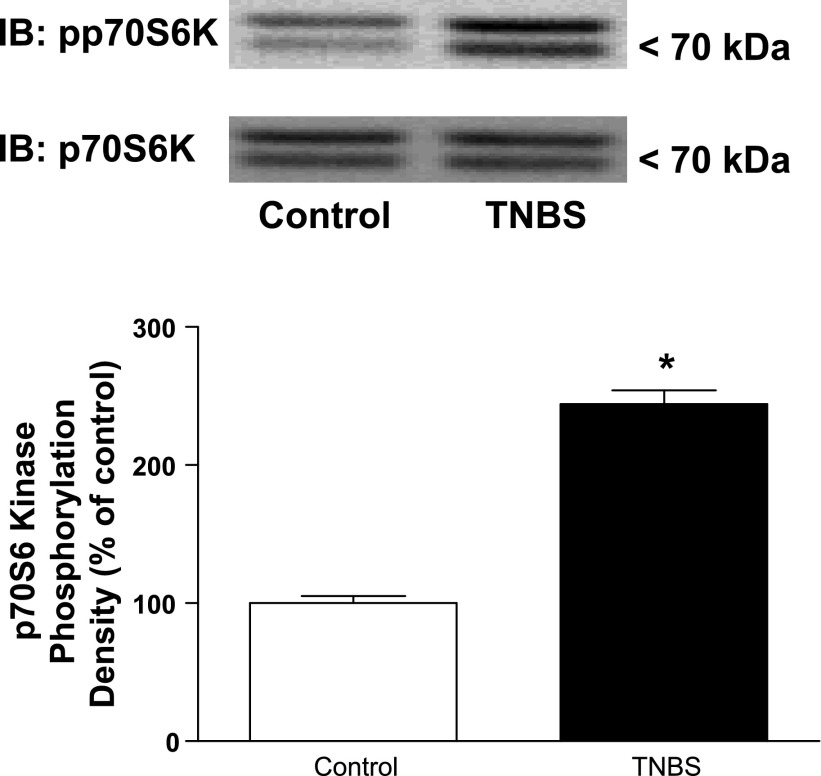
Similarly, the levels of basally phosphorylated p70S6 kinase (Ser389) were increased by 144 ± 4% above basal levels in the inflamed muscle of TNBS-treated rats compared with the levels in control vehicle-treated rats (Fig. 5).
Fig. 5.
Basal levels of p70S6 kinase phosphorylation are increased during chronic intestinal inflammation. A: representative immunoblots of basal p70S6 kinase (Thr389) phosphorylation (pp7056k) in colonic smooth muscle from control and TNBS-treated rats. B: densitometric analysis of basal p70S6 kinase (Thr389) phosphorylation in colonic smooth muscle from control and TNBS-treated rats. Basal p70S6 kinase phosphorylation is significantly increased in smooth muscle cells isolated from the chronically inflamed colons of rats treated with TNBS compared with control (vehicle)-treated rats. The p70S6 kinase (Thr389) phosphorylation was measured by immunoblot analysis in colonic smooth muscle cells isolated from rat colon 7 days following rectal administration of TNBS or vehicle. Results are expressed in relative densitometric units in samples containing equal amounts of total protein. Values represent means ± SE of 4 experiments on separate animals. *P < 0.05 vs. control.
TNBS-induced inflammation of intestinal muscle increases levels of basal GSK-3β phosphorylation.
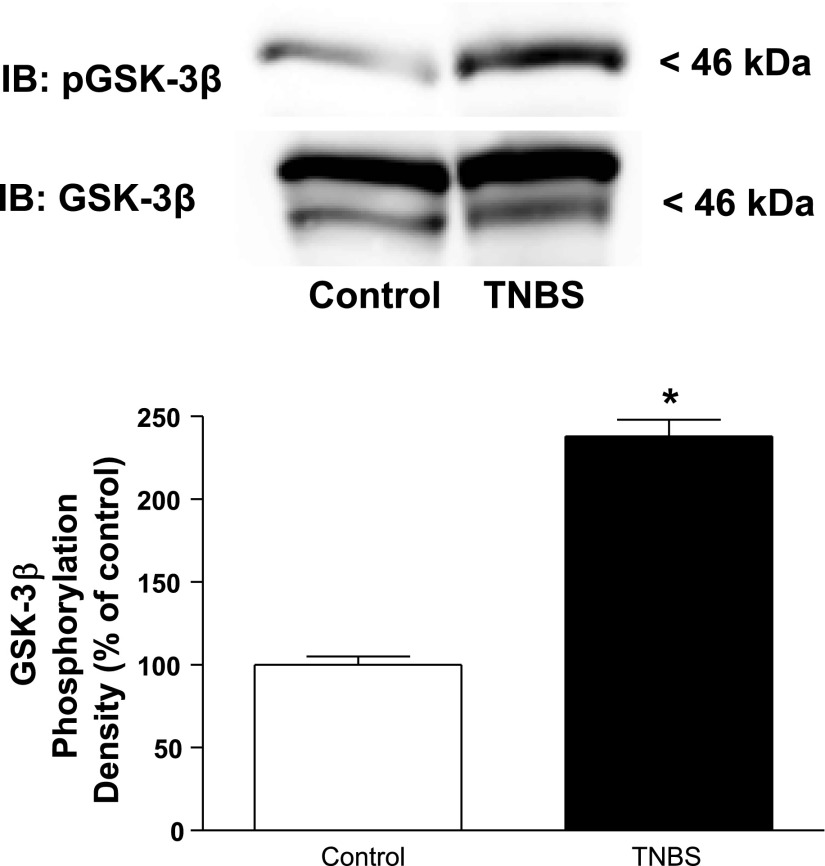
The effects of chronic intestinal inflammation on the levels of GSK-3β (Ser9) phosphorylation were measured as an indicator of apoptosis. We hypothesized that in the setting of TNBS-induced inflammation the levels of GSK-3β (Ser9) phosphorylation would be increased and could contribute to the net increase in muscle growth.
Basal levels of GSK-3β (Ser9) phosphorylation were increased by 140 ± 10% in the inflamed colonic muscle of TNBS-treated rats compared with control vehicle-treated rats (Fig. 6).
Fig. 6.
Basal levels of GSK-3β phosphorylation are increased during chronic intestinal inflammation. A: representative immunoblots of basal GSK-3β (Ser9) phosphorylation in colonic smooth muscle from control and TNBS-treated rats. B: densitometric analysis of basal GSK-3β (Ser9) phosphorylation in colonic smooth muscle from control and TNBS-treated rats. Basal GSK-3β phosphorylation is significantly increased in smooth muscle cells isolated from the chronically inflamed colons of rats treated with TNBS compared with control (vehicle)-treated rats. GSK-3β (Ser9) phosphorylation was measured by immunoblot analysis in colonic smooth muscle cells isolated from rat colon 7 days following rectal administration of TNBS or vehicle. Results are expressed in relative densitometric units in samples containing equal amounts of total protein. Values represent means ± SE of 4 experiments on separate animals. *P < 0.05 vs. control.
TNBS-induced inflammation of intestinal muscle increases basal rates of muscle cell proliferation via αvβ3-dependent and IGF-I-dependent mechanisms.
Basal rates of proliferation were measured by [3H]thymidine incorporation in untreated control smooth muscle cells derived from both vehicle-treated and TNBS-treated rats. Muscle cells were treated with the αvβ3 integrin antagonist echistatin, a neutralizing antibody to the αvβ3 integrin, and with an IGF-I receptor tyrosine kinase inhibitor AG1024.
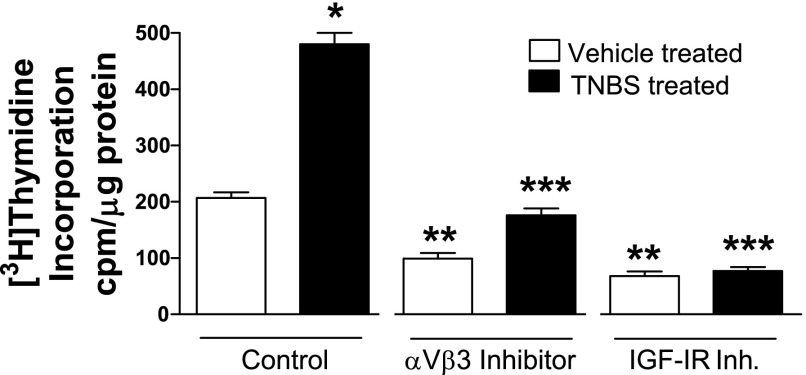
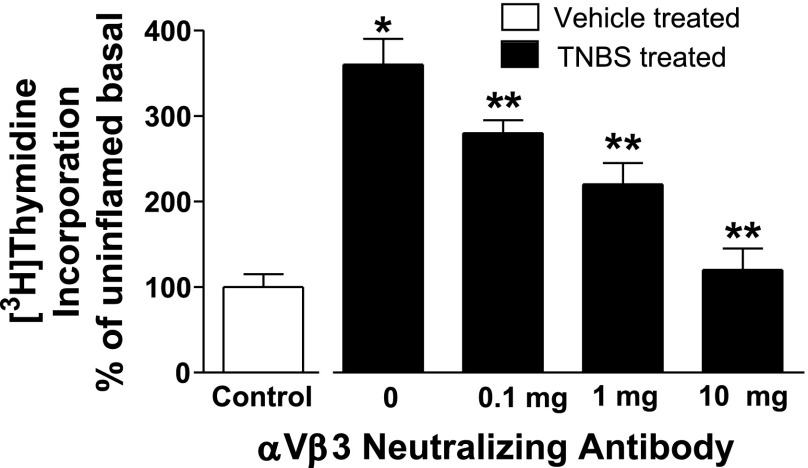
Basal rates of [3H]thymidine incorporation were increased by 130 ± 15% in smooth muscle cells from TNBS-treated rats compared with muscle cells from control, vehicle-treated rats. The increased rates of basal [3H]thymidine incorporation in muscle cells from TNBS-treated rats were inhibited by 63 ± 5% in the presence of echistatin and by 84 ± 7% in the presence of AG1024 (Fig. 7). A neutralizing antibody to αvβ3 integrin also inhibited [3H]thymidine incorporation in smooth muscle cells from TNBS-treated rats in a concentration-dependent manner (Fig. 8).
Fig. 7.
Increased basal rates of smooth muscle cells proliferation in the chronically inflamed intestine are dependent on IGF-I and αvβ3 integrin. Basal rates of smooth muscle cell [3H]thymidine incorporation are increased in the TNBS-treated, chronically inflamed rat colonic smooth muscle (solid bars) compared with vehicle-treated uninflamed colonic muscle (open bars). In the presence of either the αvβ3 integrin inhibitor (Inh.) echistatin (100 nM) or the IGF-I receptor tyrosine kinase inhibitor AG1024 (10 μM), basal rates of [3H]thymidine incorporation were inhibited, implying that activation of αvβ3 integrin and the IGF-I receptor by endogenous ligands regulates proliferation in both the uninflamed and chronically inflamed intestine. Incorporation of [3H]thymidine into the perchloric acid extractable pool was used to measure DNA synthesis. *P < 0.05 vs. proliferation in vehicle-treated colonic muscle cells, **P < 0.05 vs. proliferation in colonic muscle cells isolated from control vehicle-treated rats, ***P < 0.05 vs. proliferation in colonic muscle cells isolated from control TNBS-treated rats.
Fig. 8.
Increased proliferation in chronically inflamed colonic muscle in inhibited by an αvβ3 neutralizing antibody. The increased basal rates of [3H]thymidine incorporation in chronically inflamed colonic smooth muscle are inhibited in concentration-dependent fashion by an αvβ3-neutralizing antibody. [3H]thymidine incorporation was measured in the presence of increasing concentrations of the αvβ3 neutralizing antibody, 23C6. Results were expressed as a percent of response in vehicle-treated control cells. *P < 0.05 vs. [3H]thymidine incorporation in smooth muscle cells isolated from control vehicle-treated rats. **P < 0.05 vs. [3H]thymidine incorporation in smooth muscle isolated from TNBS-treated rats in the absence of αvβ3 neutralizing antibody.
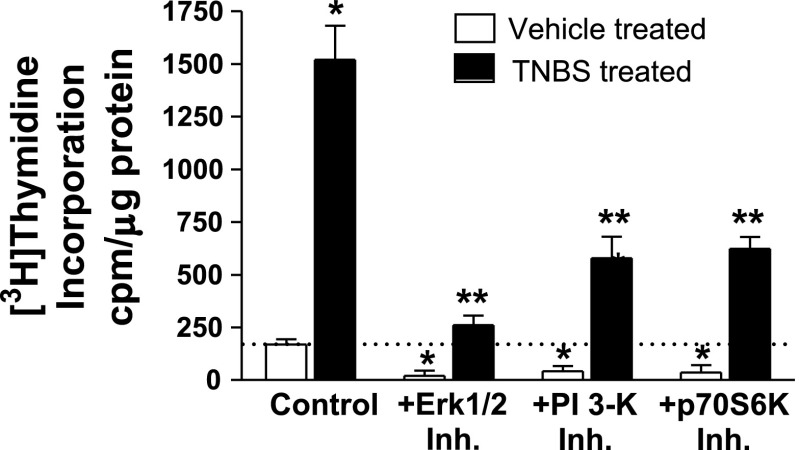
The results obtained with the IGF-I receptor tyrosine kinase inhibitor implied that the higher basal rates of smooth muscle cell proliferation in the chronically inflamed intestine are regulated by endogenous IGF-I and its signaling pathways: ERK1/2, PI3-kinase, and p70S6 kinase (11, 14, 36) (Fig. 9). The increased rate of basal [3H]thymidine incorporation in muscle cells from TNBS-treated rats over that in muscle cells from vehicle-treated rats was inhibited 93 ± 3% by the ERK1/2 inhibitor (1 μM U1026), 70 ± 12% by the PI3-kinase inhibitor (1 μM LY294002), and 61 ± 5% by the p70S6 kinase inhibitor (1 nM rapamycin) (Fig. 9). Taken together, these results show that, in TNBS-treated rats, excess muscle cell proliferation is regulated by endogenous IGF-I and ligands of αvβ3.
Fig. 9.
Increased proliferation of smooth muscle during chronic inflammation is dependent on ERK1/2 and PI-3 kinase activation. The increased basal rates of [3H]thymidine incorporation in chronically inflamed colonic smooth muscle are inhibited by an ERK1/2 inhibitor (Inh.) (1 μM U1026), a PI3-kinase inhibitor (1 μM LY294002), and a p70S6 kinase inhibitor (1 nM rapamycin). *P < 0.05 vs. [3H]thymidine incorporation in smooth muscle cells isolated from control vehicle-treated rats. **P < 0.05 vs. [3H]thymidine incorporation in smooth muscle isolated from TNBS-treated rats in the absence inhibitors.
TNBS-induced inflammation of intestinal muscle inhibits apoptosis via αvβ3-dependent and IGF-I-dependent mechanisms.
Smooth muscle cells derived from vehicle-treated and from TNBS-treated rats were deprived of serum for 24 h, after which the basal rates of apoptosis were measured from the accumulation of nucleosomes. The participation of αvβ3 integrin and IGF-I in this process was examined in muscle cells treated with either echistatin or AG1024.
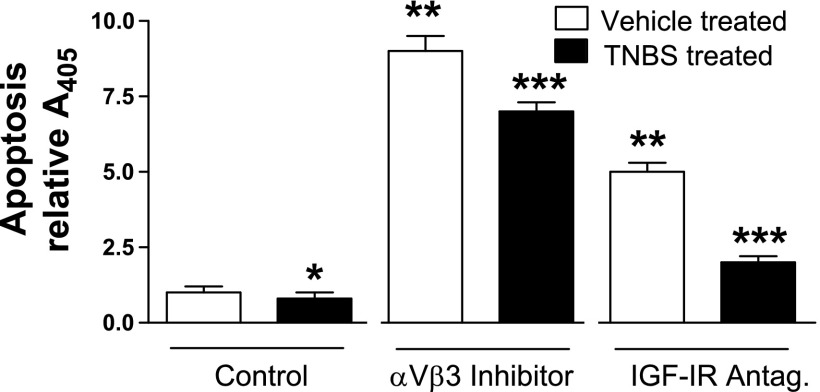
In smooth muscle cells from TNBS-treated rats, basal levels of nucleosome formation were inhibited by 20 ± 2% compared with smooth muscle cells from vehicle-treated rats. In smooth muscle cells from both control untreated and inflamed TNBS-treated rats either echistatin or AG1024 increased nucleosome formation. In smooth muscle cells from vehicle-treated rats nucleosome formation was augmented 9.0 ± 0.5-fold in the presence of echistatin and was augmented by 5.0 ± 0.4-fold in the presence of AG1024 (Fig. 10). In smooth muscle cells from TNBS-treated rats nucleosome formation was augmented 8.8 ± 0.4-fold in the presence of echistatin and was augmented 2.5 ± 0.3-fold in the presence of AG1024 (Fig. 10).
Fig. 10.
Decreased basal rates of smooth muscle cell apoptosis in the chronically inflamed intestine are dependent on IGF-I and αvβ3 integrin. Basal rates of smooth muscle cell apoptosis are decreased in the TNBS-treated, chronically inflamed rat colonic smooth muscle (solid bars) compared with vehicle-treated uninflamed colonic muscle (open bars). In the presence of either the αvβ3 integrin inhibitor echistatin (100 nM) or the IGF-I receptor tyrosine kinase inhibitor AG1024 (10 μM), basal rates of apoptosis were increased, implying that activation of αvβ3 integrin and the IGF-I receptor by endogenous ligands regulate apoptosis in both the uninflamed and chronically inflamed intestine. The levels of nucleosomes were quantified by ELISA and were used as a measure of apoptosis. Results are expressed as relative A405. *P < 0.05 vs. apoptosis in vehicle-treated colonic muscle cells; **P < 0.05 vs. apoptosis in colonic muscle cells isolated from control vehicle-treated rats. ***P < 0.05 vs. apoptosis in colonic muscle cells isolated from control TNBS-treated rats.
The results show that basal rates of apoptosis in smooth muscle cells are lower in TNBS-treated rats and imply that, whether from TNBS-treated or vehicle-treated rats, apoptosis is regulated by endogenous IGF-I and ligands of αvβ3.
DISCUSSION
This paper shows that in the chronically inflamed colon of TNBS-treated rats, growth of intestinal smooth muscle is increased compared with vehicle-treated animals. This growth has been associated with increased expression of IGF-I in the muscle layer (37), but the mechanisms mediating the increased muscle growth that are regulated by the IGF-I system have not been examined. We now show that the increased IGF-I production that occurs in the chronically inflamed smooth muscle in TNBS-induced colitis regulates increased muscle cell proliferation and decreased muscle cells apoptosis. There is also a concomitant increase in the production of the αvβ3 ligands fibronectin and vitronectin. Our previous work provides insight on how these events are linked and can contribute to increased muscle growth in the inflamed intestine. Occupation of αvβ3 integrin by its endogenous ligands in smooth muscle increases the intensity and duration of IGF-I stimulated IGF-I receptor tyrosine kinase activity, signaling, and growth (12). This interplay has relevance in the TNBS-induced model of colitis, in which there is increased production of the αvβ3 integrin ligands vitronectin and fibronectin as well as increased IGF-I expression that results in increased muscle cell growth and hyperplasia of the muscularis propria in the inflamed intestine.
The evidence supporting the participation of αvβ3 integrin and IGF-I in the increased smooth muscle growth in TNBS-induced inflammation can be summarized as follows: 1) αvβ3 integrin is expressed by colonic smooth muscle cells and TNBS-induced inflammation increased the expression of both vitronectin and fibronectin, ligands of αvβ3 integrin; 2) basal IGF-I receptor tyrosine kinase phosphorylation is increased in the inflamed colonic muscle; 3) basal phosphorylation of ERK1/2 and p70S6 kinase, which are coupled to stimulation of proliferation, is increased in the inflamed colonic muscle; 4) basal phosphorylation of GSK-3β, which is coupled to inhibition of apoptosis, is increased in the inflamed colonic muscle; 5) basal levels of smooth muscle cell proliferation in the inflamed colonic muscle are increased and are dependent on IGF-I and αvβ3 integrin since they were decreased by antagonists of IGF-I and αvβ3 integrin; 6) the increased basal levels of smooth muscle cell proliferation in the inflamed colonic muscle are dependent on ERK1/2, PI3-kinase, and p70S6 kinase; and 7) apoptosis in the inflamed colonic muscle are decreased and are increased by antagonists of IGF-I and αvβ3 integrin.
Other events that occur during TNBS-induced colitis contribute to altered smooth muscle function and muscle hyperplasia. Chronic inflammation causes alterations in the enteric nervous system that lead to relative loss of intrinsic cholinergic neural input to the intestinal muscle layer resulting in muscle hyperplasia in vitro (2, 21). During the course of TNBS-induced colitis, smooth muscle changes from a solely contractile phenotype to a myofibroblast phenotype with increased synthetic potential including for cytoskeletal proteins such as α-actin and vimentin and decreased production of desmin (21). The regulation of extracellular matrix homeostasis is disordered following TNBS-induced colitis. Collagen I expression is increased in response to chronic inflammation whereas collagenase activity is decreased (35). As shown in this paper, production of the extracellular matrix proteins fibronectin and vitronectin is increased in the chronically inflamed intestine.
Release of inflammatory cytokines and mediators from macrophages, mast cells, and lymphocytes resident in the muscularis propria or subjacent lamina propria or those recruited to the muscle layer contribute to altered smooth muscle growth. Studies in mast cell-deficient mice suggest that tissue mast cells do not mediate the response of murine intestinal muscle to TNBS-induced inflammation (5). Although tissue mast cell numbers are decreased during the inflammatory response to TNBS and in Crohn's disease generally (19, 22, 27), an increase in mast cells in the submucosa and both circular and longitudinal smooth muscle layers is seen during the chronic, fibrotic response to TNBS and in the strictures of human Crohn's disease patients suggesting mast cells play a role in tissue fibrosis (6, 23, 31). During the course of chronic inflammation induced by TNBS in the mouse and in other models of colitis, a sequential pattern of immunological response is observed (1, 3, 4). Within the first 7 days (inflammatory phase) a Th1 patterned response characterized by IL-12 and IFN-γ is seen, followed at 3 wk by a Th17 patterned response characterized by IL-23 and IL-17. After 5 wk (fibrotic stage) a Th2 patterned response is seen characterized by IL-4 and TGF-β1. Throughout all these periods TNF-α levels remain elevated. In human Crohn's disease these responses are simultaneous with the combined Th1/Th17 response (reviewed in Ref. 3). The utility of animal models of colitis lie in the ability to examine alterations of smooth muscle growth and function that occur in the setting of a more limited immunological profile at a given point in time.
Upregulation of IGF-I expression occurs concomitantly with upregulation in the production of αvβ3 integrin ligands by the chronically inflamed intestinal smooth muscle in TNBS-induced colitis. Upregulation of IGF-I expression is a common feature of chronically inflamed intestine whether in animal models of colitis or in Crohn's disease (16, 24, 37). Within the muscle layer, IGF-I plays an important role in the regulation of smooth muscle cell growth: it jointly stimulates growth and inhibits apoptosis (10, 11). IGF-I stimulated growth is enhanced by the activation of the αvβ3 integrin by its ligands. When αvβ3 integrin is activated by fibronectin or vitronectin, IGF-I-stimulated IGF-I receptor tyrosine kinase activity, the activity of its signaling intermediates, and the resultant growth are increased. In summary, this paper has identified a mechanism that may play a role in the muscle hyperplasia component of stricture formation that occurs in the chronically inflamed intestine in TNBS-induced colitis and potentially Crohn's disease.
GRANTS
This work was supported by Grants DK49691 (J. F. Kuemmerle), DK34153 (J. R. Grider), and DK077917 (L.-Y. Qiao) from the National Institutes of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology 128: 654–666, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Blennerhassett MG, Bovell FM, Lourenssen S, McHugh KM. Characteristics of inflammation-induced hypertrophy of rat intestinal smooth muscle cell. Dig Dis Sci 44: 1265–1272, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3: 521–533, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A, Strober W. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol 178: 5859–5870, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Fukumoto Y, Kasai H, Takahashi H, Sugiyama H, Hase N, Kaneko H, Hamamura I, Aoki Y, Ota M, Kobayashi T, Katsuura Y, Kamimura T, Komoriya K. The role of mast cells in the development of 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in rats. Scand J Gastroenterol 37: 555–560, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Gelbmann CM, Mestermann S, Gross V, Kollinger M, Scholmerich J, Falk W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut 45: 210–217, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseini JM, Goldhill JM, Bossone C, Pineiro-Carrero V, Shea-Donohue T. Progressive alterations in circular smooth muscle contractility in TNBS-induced colitis in rats. Neurogastroenterol Motil 11: 347–356, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita K, Hori M, Fujisawa M, Sato K, Ohama T, Momotani E, Ozaki H. Role of TNF-alpha in muscularis inflammation and motility disorder in a TNBS-induced colitis model: clues from TNF-alpha-deficient mice. Neurogastroenterol Motil 18: 578–588, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Kuemmerle JF Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology 113: 817–824, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Kuemmerle JF Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3 beta activity. Am J Physiol Gastrointest Liver Physiol 288: G101–G110, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Kuemmerle JF IGF-I elicits growth of human intestinal smooth muscle cells by activation of PI3K, PDK-1, and p70S6 kinase. Am J Physiol Gastrointest Liver Physiol 284: G411–G422, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kuemmerle JF Occupation of αvβ3-integrin by endogenous ligands modulates IGF-I receptor activation and proliferation of human intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 290: G1194–G1202, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Kuemmerle JF Synergistic regulation of NOS II expression by IL-1β and TNF-α in cultured rat colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 274: G178–G185, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Kuemmerle JF, Bushman TL. IGF-I stimulates intestinal muscle cell growth by activating distinct PI 3-kinase and MAP kinase pathways. Am J Physiol Gastrointest Liver Physiol 275: G151–G158, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle: Gbetagamma-dependent mitogen-activated protein kinase activation and growth. J Biol Chem 276: 7187–7194, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis 7: 16–26, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 285: G207–G216, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil 17: 565–574, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd G, Green FH, Fox H, Mani V, Turnberg LA. Mast cells and immunoglobulin E in inflammatory bowel disease. Gut 16: 861–865, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut 49: 777–782, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lourenssen S, Wells RW, Blennerhassett MG. Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp Neurol 195: 497–507, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Menozzi A, Pozzoli C, Poli E, Lazzaretti M, Grandi D, Coruzzi G. Long-term study of TNBS-induced colitis in rats: focus on mast cells. Inflamm Res 55: 416–422, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96: 795–803, 1989. [PubMed] [Google Scholar]
- 24.Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. IGF-I and procollagen α1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol 279: G1307–G1322, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol 204: 667–679, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil 20: 928–938, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson IR, Leung KB, Pearce FL, Walker-Smith JA. Lamina propria mast cells in biopsies from children with Crohn's disease. J Clin Pathol 39: 279–283, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savendahl L, Underwood LE, Haldeman KM, Ulshen MH, Lund PK. Fasting prevents experimental murine colitis produced by dextran sulfate sodium and decreases interleukin-1 beta and insulin-like growth factor I messenger ribonucleic acid. Endocrinology 138: 734–740, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 275: G342–G351, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Xin X, Hou YT, Li L, Schmiedlin-Ren P, Christman GM, Cheng HL, Bitar KN, Zimmermann EM. IGF-I increases IGFBP-5 and collagen α1(I) mRNAs by the MAPK pathway in rat intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 286: G777–G783, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Weksler-Zangen S, Pikarsky A, Pappo O, Wengrower D, Bischoff SC, Pines M, Rivkind A, Goldin E, Levi-Schaffer F. Mast cells involvement in the inflammation and fibrosis development of the TNBS-induced rat model of colitis. Scand J Gastroenterol 37: 330–337, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology 102: 1524–1534, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Zeeh JM, Hoffmann P, Sottili M, Eysselein VE, McRoberts JA. Up-regulation of insulinlike growth factor I binding sites in experimental colitis in rats. Gastroenterology 108: 644–652, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Zeeh JM, Mohapatra N, Lund PK, Eysselein VE, McRoberts JA. Differential expression and localization of IGF-I and IGF binding proteins in inflamed rat colon. J Recept Signal Transduct Res 18: 265–280, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Zeeh JM, Riley NE, Hoffmann P, Reinshagen M, Goebell H, Gerken G. Expression of insulin-like growth factor binding proteins and collagen in experimental colitis in rats. Eur J Gastroenterol Hepatol 13: 851–858, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann EM, Li L, Hou YT, Cannon M, Christman GM, Bitar KN. IGF-I induces collagen and IGFBP-5 mRNA in rat intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 273: G875–G882, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol 280: G1022–G1029, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann EM, Sartor RB, McCall RD, Pardo M, Bender D, Lund PK. Insulinlike growth factor I and interleukin 1 beta messenger RNA in a rat model of granulomatous enterocolitis and hepatitis. Gastroenterology 105: 399–409, 1993. [DOI] [PubMed] [Google Scholar]