Abstract
Chronic stress precipitates or exacerbates the symptoms of functional bowel disorders, including motility dysfunction. The cellular mechanisms of these effects are not understood. We tested the hypothesis that heterotypic chronic stress (HeCS) elevates the release of norepinephrine from the adrenal medulla, which enhances transcription of the gene-regulating expression of Cav1.2 (L-type) channels in colonic circular smooth muscle cells, resulting in enhanced colonic motor function. The experiments were performed in rats using a 9-day heterotypic chronic stress (HeCS) protocol. We found that HeCS, but not acute stress, time dependently enhances the contractile response to ACh in colonic circular smooth muscle strips and in single dissociated smooth muscle cells, the plasma levels of norepinephrine and the mRNA and protein expressions of the α1C subunit of Cav1.2 channels. These effects result in faster colonic transit and increase in defecation rate. The effects of HeCS are blocked by adrenalectomy but not by depletion of norepinephrine in sympathetic neurons. The inhibition of receptors for glucocortocoids, corticotropin-releasing hormone or nicotine also does not block the effects of heterotypic chronic stress. Norepinephrine acts on α- and β3-adrenergic receptors to induce the transcription of α1C subunit. We conclude that HeCS alters colonic motor function by elevating the plasma levels of norepinephrine. Colonic motor dysfunction is associated with enhanced gene transcription of Cav1.2 channels in circular smooth muscle cells. These findings suggest the potential cellular mechanisms by which heterotypic chronic stress may exacerbate motility dysfunction in patients with irritable bowel syndrome.
Keywords: corticotropin-releasing hormone, corticosterone, enteric neurotransmitters, smooth muscle
stress is the adaptive physiological response of living systems in the face of real or perceived life-threatening situations. This response begins with the release of corticotropin-releasing hormone (CRH) in the paraventricular nucleus of the hypothalamus (56), which activates the neuroendocrine axis to release a group of hormones and neurotransmitters systemically and in select organ tissues (9). The release of neurotransmitters elicits an almost immediate response from the target organs, while the more gradual release of hormones from glands may potentiate and sustain the stress response (9, 14, 31, 51). The stress hormones, such as glucocorticoids, may also help in limiting and terminating the stress response (11, 29). Nontranscriptional mechanisms largely mediate the immediate and short-term effects of acute stress. For example, acute stress releases norepinephrine in the amygdala and hypothalamus to sharpen focus and attention (14). It also increases heart rate and blood flow for the “fight or flight” response (51). In the gastrointestinal tract, acute stress almost immediately stimulates colonic contractile activity, resulting in enhanced defecation in rats and mice (33, 34, 47).
When stress is sustained for long periods or when it is applied repeatedly, the same mediators that initially generate the adaptive response by nontranscriptional mechanisms may become maladaptive by inducing transcription of select genes in the target cell types, such as CNS neurons and cardiac muscle cells (16, 18, 39, 55). The altered gene transcription remodels the cellular regulatory mechanisms resulting in organ dysfunction. The induction of gene transcription in the CNS and cardiac myocytes has received attention during the past few years (16, 35, 39, 55). However, we do not know whether the mediators of chronic stress also remodel the gastrointestinal smooth muscle cells to cause motility dysfunction. Generally, it is chronic stress, rather than acute stress, that precipitates or exacerbates the symptoms of two major motility disorders, irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) (3, 15, 26, 27, 36, 58).
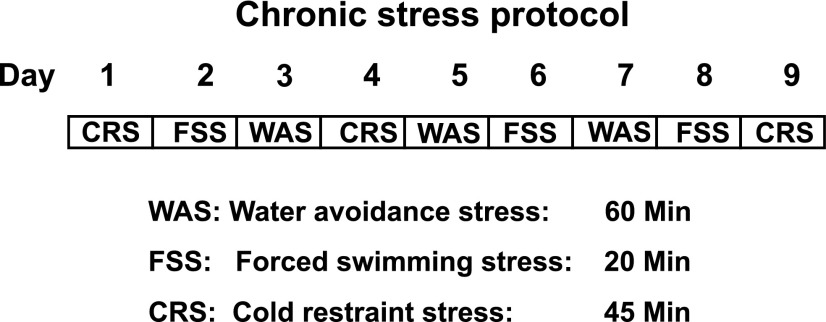
In this study, we tested the hypothesis that heterotypic chronic stress (HeCS), but not acute stress, alters the transcription of a key cell signaling protein of the excitation-contraction coupling, the pore forming α1C subunit of Cav1.2 (L-type) calcium channels, to cause colonic motility dysfunction. We investigated the potential roles of three prominent mediators of heterotypic chronic stress, CRH, norepinephrine, and corticosterone. The experiments were performed on rats employing a 9-day heterotypic stress protocol comprising random distribution of water avoidance stress (WAS), forced swimming stress (FSS), and cold-restraint stress (CRS). A stressor was applied only once per day (Fig. 1).
Fig. 1.
Nine-day heterotypic chronic stress protocol comprising three different randomly arranged stressors.
MATERIALS AND METHODS
Animals.
Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN), and adrenalectomized Sprague-Dawley rats were purchased from Charles River Laboratories (Spencerville, OH). The rats were housed in a controlled environment (22°C and 12:12-h light-dark cycle) and allowed food and water ad libitum. In case of adrenalectomized rats, water was replaced with 0.9% NaCl. The Institutional Animal Care and Use Committee at the University of Texas Medical Branch approved all procedures performed on animals.
Heterotypic chronic stress protocol.
We used a 9-day heterotypic stress protocol comprising three randomly applied stressors: water avoidance stress (WAS) (48), forced swimming stress (FSS) (1), and cold-restraint stress (CRS) (8) (Fig. 1). For acute stress protocol, cold-restraint stress was applied only once. Each stressor was applied between 8 AM and noon. For WAS, the rat was placed for 60 min on a brick (12 cm high × 6 cm wide × 8.5 cm long) in the middle of a plastic container (14 cm high × 36 cm wide and × 54 cm long) filled with water at room temperature (22°C) within 1 cm from the top. For FSS, the rat was forced to swim for 20 min in a plastic container (38 cm high × 24 cm wide × 32 cm long) filled to a depth of 12 cm below the top with water at room temperature (22°C). For CRS, the rat was restrained in a clear plastic container (6 cm diameter × 18 cm long) that prevented it from self-grooming. The container had 2-cm diameter openings at each end for the rat to breathe normally. The restraining container was placed in cold room at 4°C for 45 min. The animals were euthanized by decapitation at various times indicated in the results after the end of HeCS or acute stress protocols for in vitro molecular and muscle bath experiments. Age-matched control rats were brought to the lab and handled without application of stress.
Two rats were housed to a cage during the stress protocols, except during the measurement of moisture content of the pellets. For these measurements, each rat was housed in a single wire bottom cage. The control prestress defecation rate was determined in three consecutive 24-h periods prior to start of the stress protocols. The pellets were collected immediately during the first 2 h, weighed and allowed to dry at 45°C for 24 h to determine percent moisture content.
Measurement of norepinephrine, corticosterone, and corticotropin-releasing hormone in plasma.
Blood samples were collected from the trunk in tubes containing 2.5% sodium citrate and 0.45% citric acid at the time of animal euthanasia by decapitation. In prestress control rats, blood samples were collected from the saphenous vein under 2% isoflurane general anesthesia. Samples were taken on three consecutive days to determine mean value. The tubes were spun in a refrigerated centrifuge; plasma was quickly aliquoted and stored at −80°C for assays. Plasma levels of norepinephrine and corticosterone were measured using the respective radioimmunoassay kits from MP Biomedicals (Solon, OH). Plasma CRH levels were measured using an EIA kit (Penninsula Laboratories, San Carlos, CA). All measurements on samples for each hormone were made using the same standard curve to avoid interassay variation.
Colonic transit.
Colonic transit was measured by the geometric center (GC) method (40). A Silastic catheter (1 mm inside diameter and 2.1 mm outside diameter) was implanted under general anesthesia (2% isoflurane inhalation) into the proximal colon, with its tip resting about 2 cm distal to the cecum. The rats were allowed to recover from surgery for at least 5 days before start of the HeCS protocol. A bolus of 1.5 ml of 1.5% methylcellulose (Fisher Scientific, Fair Lawn, NJ) containing 0.75 mg nonabsorbable phenol red was injected into the colon 6 h and 30 min after the last stressor via the catheter; the catheter was flushed with 0.5 ml saline. Ninety minutes later, the rats were euthanized by CO2 inhalation. The entire colon distal to the tip of the catheter was removed immediately and divided into six segments of equal length. The contents expelled from the anus during this period were collected and referred to as segment 7 for the measurement of geometric center. Each segment, along with its contents, was placed in 25 ml 0.1 N NaOH and homogenized. The homogenate was kept at room temperature for 1 h. One milliliter of the supernatant was added to 0.1 ml of 20% trichloroacetic acid solution to precipitate the protein. After centrifugation at 10,000 g for 30 min, 1 ml of 0.5 N NaOH was added to the supernatant. Phenol red was determined by measuring the absorption at 560 nm using a spectrophotometer (Beckman Instruments, Palo Alto, CA). Colonic transit was calculated as geometric center (GC) of distribution of phenol red: GC = [∑ (OD of phenol red per segment × segment number)]/total OD.
Tissue procurement and protein and total RNA extraction.
The rats were euthanized at various times after the end of HeCS and acute stress protocols. Three- to four-cm-long segments of the distal colon (starting from ∼1 cm oral to the pelvic flexure) were obtained, opened along the mesenteric border, cleaned, and pinned flat in a petri dish with Sylgard base. The mucosal/submucosal layers were separated by microdissection. Adrenal glands were collected. The muscularis externa and adrenal glands were quick frozen in liquid nitrogen and broken into small particles with a chilled pestle for protein and RNA extractions. The tissue particles were homogenized on ice in PBS supplemented with protease inhibitors cocktail (Sigma, St. Louis, MO) for protein extraction. Total RNA was extracted from tissues by using the Qiagen RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse-transcribed using SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) for real-time PCR.
Muscle bath.
Freshly obtained full-thickness colon tissues were stored on ice for no longer than 1 h and then immersed in warm, carbogenated Krebs solution (in mmol/l: 118 NaCl, 4.7 KCl, 2.5 CaC2, 1 NaH2PO4, 1.2 MgCl2, 11 d-glucose, and 25 NaHCO3). The mucosal/submucosal layers were removed by microdissection under a magnifying glass and discarded. Circular muscle strips (4 mm × 10 mm) were mounted in muscle baths (Radnoti Glass, Monrovia, CA) filled with 5 ml carbogenated Krebs solution at 37°C. The contractile activity was recorded with Grass isometric force transducers and amplifiers connected to Biopac data acquisition system (Goleta, CA). Contractile responses to increasing concentrations of ACh were obtained. The bathing solution was replaced every 15 min and 4 min after each concentration of ACh. The strips were left to equilibrate for at least 15 min before adding the next concentration of ACh. The contractile response of circular muscle strips was quantified as increase in the area under contractions during 4 min after addition of ACh to the bath, over the baseline area under contractions during 4 min before the addition of ACh. At least two muscle strips from the distal colon were used from each rat for muscle bath experiments. The mean of the two or more muscle strips at each concentration of ACh was determined for each rat. Statistical analysis was done with n equal to the number of rats.
Cell-dispersion and measurement of cell contraction.
Single smooth muscle cells were isolated by enzymatic digestion with collagenase, as described previously with minor modification (43). The colonic circular muscle sheet was cut into 0.5 × 0.5 cm2 pieces and digested at 31°C with 1.5 mg/ml collagenase (type II, 319 U/mg; Worthington, Freehold, NJ) and 1.0 mg/ml soybean trypsin inhibitor (Sigma, St. Louis, MO) for 40 min. The digested tissue was washed with enzyme-free fresh HEPES medium (Invitrogen, Carlsbad, CA), and the muscle cells were allowed to disperse spontaneously under gentle to-and-fro motion.
Dispersed cells were relaxed at rest, and they responded with cell length shortening in the presence of ACh (10−9–10−5 M). To quantitate muscle cell contraction, an aliquot (0.45 ml) of cells was exposed to 50 μl of ACh or vehicle control for 40 s at 31°C and fixed with 1% acrolein. The lengths of 30 consecutive intact healthy cells were measured.
Western blot analysis.
The proteins in the samples were resolved by standard immunoblotting method. Equal quantities (10–25 μg) of total protein were loaded in each lane. The antibody for α1C subunit of Cav1.2 channels was purchased from Alomone Labs (Jerusalem, Israel; ACC-003) and diluted 1:400. The antibodies for tyrosine hydroxylase and β-actin were purchased from Sigma and diluted 1:1,000 and 1:5,000, respectively.
Real-time PCR.
Real-time PCR assay was used to determine the mRNA expression of α1C subunit, using TaqMan technology on Applied Biosystems 7000 sequence detection system (UTMB Real-Time PCR Core Facility). Applied Biosystems Assays-By-Design containing a 20× assay mix of primers and TaqMan MGB probes (FAMTM dye-labeled) were used for the target gene. Rat 18s RNA served as the endogenous control. The primers spanned exon-exon junctions to avoid amplification of genomic DNA. All primer and probe sequences were searched against the Celera database to confirm specificity. The probe and primer sequences used were for rat Cav1.2 channel α1C intermediate form (CaV1.2b) probe spanning exon 1b and exon 2, CACCAAGGTTCCAACTAT; forward primer, CCATGGTCAATGAGAATACGAGGAT; and reverse primer, GCCGCATTGGCATTCATGTT.
Pharmacological agents.
Bretilium tosylate, hexamethonium, guanethidine, phentolamine, propranolol, and cyanopindolol were purchased from Sigma (St. Louis, MO). All of these agents were dissolved in sterile deionized water and administered intraperitoneally. RU-486 (Sigma) was dissolved in olive oil and administered subcutaneously. Astressin (Sigma) was dissolved in sterile deionized water and administered intravenously. We used the previously established effective doses of pharmacological antagonists.
Statistical analysis.
All data are expressed as means ± SE. Statistical analysis was performed by ANOVA with nonrepeated measures. Post hoc analysis was done by the Fisher's method. A P value of <0.05 was considered statistically significant; n represents the number of rats.
RESULTS
Effect of stress on circular smooth muscle contractile response to ACh, colonic transit, and defection rate.
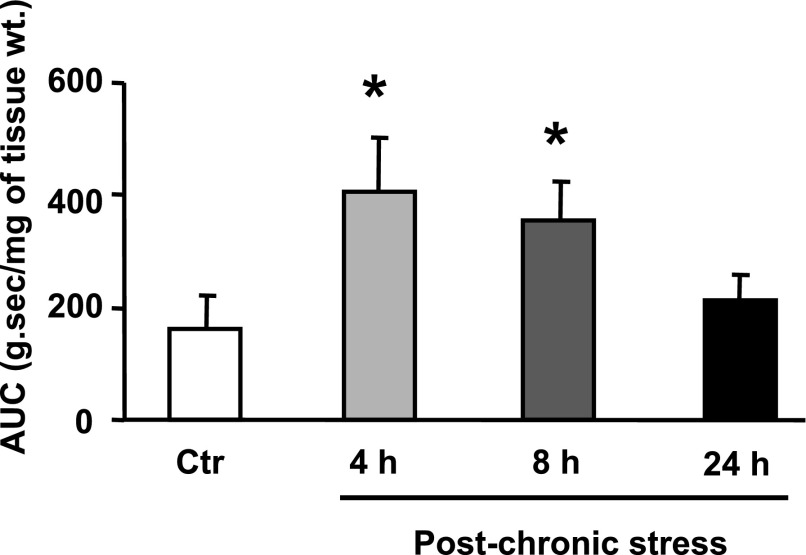
Colonic circular smooth muscle strips were obtained at 1, 4, 8, and 24 h after the last stressor of the 9-day HeCS protocol. The contractile response to ACh increased progressively in muscle strips obtained at these times, reaching significance at 4 and 8 h (Fig. 2A). However, the contractile response to ACh at 24 h after the last stressor was not different from that in age-matched sham-treated controls. By contrast, the contractile responses of the circular muscle strips to ACh at 4, 8, or 24 h after one episode of acute stress were not different from those in age-matched sham-treated controls (Fig. 2B).
Fig. 2.
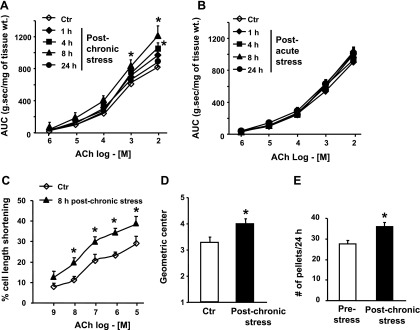
A: contractile response to ACh in colonic circular muscle strips was significantly enhanced at 4 h and 8 h after 9-day heterotypic chronic stress protocol, but it returned to baseline after 24 h (n = 3–5 rats with at least two strips from each rat). B: contractile response to ACh was not affected 1, 4, 8, or 24 h after 1-day acute stress (n = 3–5 with at least two strips from each rat). C: cell-shortening to ACh was significantly enhanced in single dispersed smooth muscle cells obtained 8 h after 9-day heterotypic chronic stress protocol, compared with that in single cells from age-matched sham-treated controls (n = 3 or 4). D: colonic transit (measured as geometric center) was significantly greater 8 h after 9-day heterotypic chronic stress protocol, when compared with sham-treated age-matched controls (n = 4). E: number of fecal pellets/24 h significantly increased after 9-day heterotypic chronic stress protocol (n = 3). *P < 0.05 vs. age-matched sham-treated controls. AUC, area under contractions; Ctr, controls.
The contractile responses to 65 mM KCl in muscle strips obtained at 4 and 8 h after the last stressor of the HeCS were also significantly greater than those obtained in control muscle strips (Fig. 3). However, the contractile responses to KCl in muscle strips obtained 24 h after HeCS did not differ from the controls.
Fig. 3.
The contractile responses to 65 mM KCl in circular muscle strips obtained at 4 h and 8 h after the last stressor of the heterotypic chronic stress protocol were significantly greater than those in strips taken from age-matched and sham-treated controls. The contractile response to KCl at 24 h after heterotypic chronic stress was not different from the control. *P < 0.05 when compared with age-matched sham-treated controls.
We then ascertained that the enhanced contractility of the muscle strips was due to smooth muscle hyperreactivity to ACh by obtaining cell-shortening response in single dispersed circular smooth muscle cells obtained 8 h after the last stressor. The cell-shortening in single dissociated circular smooth muscle cells after 9-day HeCS protocol was significantly greater than that in cells obtained from age-matched sham-treated control rats (Fig. 2C).
We then investigated whether smooth muscle hyperreactivity to ACh after HeCS translates into altered colonic motility function. The GC of a dye bolus injected into the proximal colon 6 h and 30 min after the last stressor of the HeCS protocol was significantly greater than that injected in age-matched sham-treated control rats (Fig. 2D). In addition, the number of pellets defecated in 24 h by the rats subjected to the 9-day HeCS protocol significantly increased when compared with prestressed controls (Fig. 2E). The pellets defecated during application of the last stressor were not included in these numbers. The moisture content of the pellets in chronically stressed rats also was significantly greater from that in pellets prior to the start of the HeCS protocol (57.5 ± 2.5% vs. 52.2 ± 0.9%, n = 6, P < 0.05). By contrast, the number of pellets defecated during 24 h after one episode of WAS was not different from that in sham-treated rats (50.5 ± 3.0 vs. 52.3 ± 1.3, n = 4, P > 0.05). These data suggested that HeCS may remodel the regulatory mechanisms of smooth muscle contractility that persists for several hours after the end of the last stressor. The persistence of the altered contractile response, long after removal and processing of tissues from the chronically stressed animals, suggests a genomic effect mediated by altered gene expression. The stress mediators that may have altered gene expression in intact animals are no longer present in the in vitro environment.
The peripheral mediators of the heterotypic chronic stress response.
Three prominent mediators of stress response are CRH, corticosterone, and norepinephrine (9). We assayed the plasma levels of these mediators to investigate whether one or more of these are candidates to induce persistent colonic circular smooth muscle hyperreactivity to ACh in response to HeCS. The plasma concentrations of corticosterone and norepinephrine significantly increased by about 2.5-fold and 4.5-fold, respectively, 8-h after the last stressor, when compared with those prior to start of the HeCS protocol (Fig. 4A). HeCS had no significant effect on the plasma concentration of CRH (Fig. 4A).
Fig. 4.
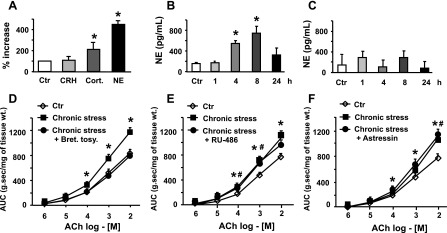
A: plasma levels of norepinephrine (NE) and corticosterone (Cort.), but not those of corticotropin-releasing hormone (CRH), increased significantly 8 h after 9-day heterotypic chronic stress protocol (n = 4). B: plasma levels of norepinephrine increased significantly 4 and 8 h after 9-day heterotypic chronic stress protocol, but they returned to baseline after 24 h (n = 4). C: plasma levels of norepinephrine were not affected by acute stress (n = 4). D: daily intraperitoneal administration of adrenergic receptor antagonist, bretylium tosylate, 30-min before each stress session blocked the induction of smooth muscle hyperreactivity by heterotypic chronic stress (n = 3–5 with at least two strips from each rat). E and F: daily subcutaneous administration of glucocorticoid receptor antagonist, RU-486 or intravenous administration of CRH antagonist, astressin, 30-min before each stress session did not block the induction of hyperreactivity to ACh (n = 3–5). Bret tosy, bretylium tosylate. *#P < 0.05 vs. age-matched sham-treated control.
The data in the previous section indicated that smooth muscle hyperreactivity to ACh increases time dependently after the last stressor (Fig. 2A). Therefore, we examined whether HeCS also increases the plasma concentrations of norepinephrine time dependently. We found that the plasma concentration of norepinephrine at 1 h after the last stressor on day 9 was not different from the control (Fig. 4B). However, the plasma concentrations of norepinephrine at 4 h and 8 h after the last stressor were significantly greater than the control values prior to the start of the stress protocol and at 1 h after end of the last stressor. The plasma concentrations of norepinephrine returned to basal levels 24 h after the last stressor (Fig. 4B). By contrast, the plasma concentrations of norepinephrine at the same time points after 1 day of acute stress did not differ from the basal control value (Fig. 4C).
Which stress mediator induces smooth muscle hyperreactivity to ACh in response to HeCS?
We investigated the role of each of the stress mediators mentioned above in inducing colonic circular smooth muscle hyperreactivity to ACh by blocking their respective receptors, 30 min prior to the start of each daily stress session. In each case, the circular muscle strips were obtained 8 h after the last stressor. The blockade of adrenergic receptors by bretylium tosylate (16 mg/kg ip) (10) prevented the induction of smooth muscle hyperreactivity to ACh HeCS (Fig. 4D). On the other hand, astressin (50 μg/kg iv) (50), the blocker of CRH1 and CRH2 receptors, and RU-486 (mifeprestone) (16 mg/kg sc) (6), the blocker of glucocorticoid receptors, had no significant effect on the induction of smooth muscle hyperreactivity to ACh by HeCS (Fig. 4, E and F).
Source of increase in plasma NE by HeCS.
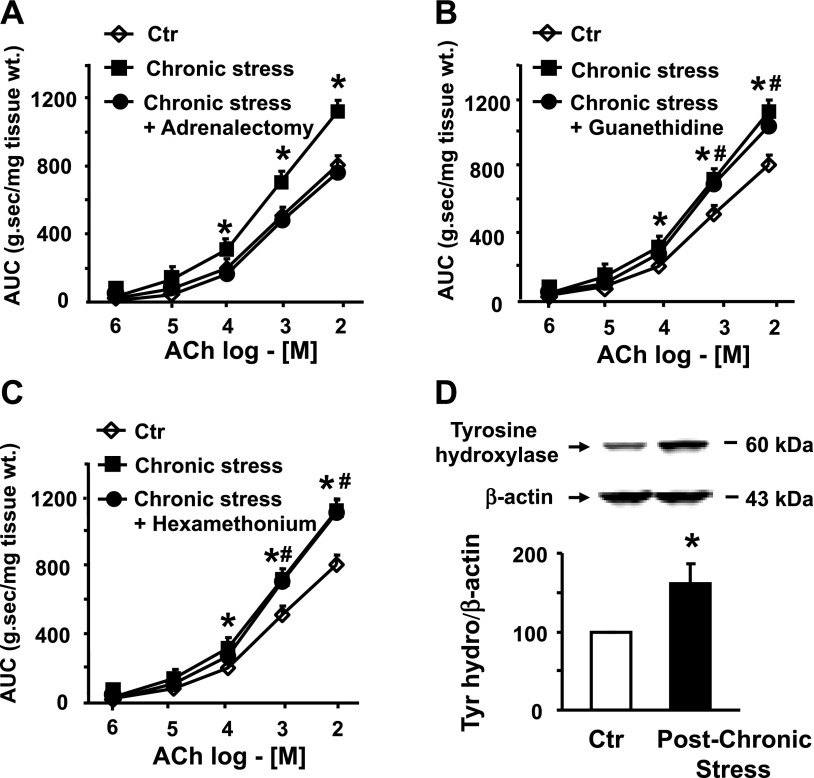
The hypothalamic release of CRH results in stimulation of the sympathetic nervous system to release norepinephrine at the neuroeffector junctions (9). It also results in stimulation of the adrenal medulla to release norepinephrine in peripheral circulation (21). We investigated which of the two sources of norepinephrine induces colonic circular smooth muscle hyperreactivity in response to HeCS. Adrenalectomy completely abolished the induction of smooth muscle hyperreactivity to ACh (Fig. 5A). On the other hand, depletion of norepinephrine in the sympathetic neurons by guanethidine (5 mg/kg ip) (22) and blockade of nicotinic receptors by hexamethonium (10 mg/kg ip) (22) had no significant effect on the induction of smooth muscle hyperreactivity to ACh by HeCS (Fig. 5, B and C). Each substance was administered 30 min prior to application of daily stress.
Fig. 5.
A: adrenalectomy blocked the induction of smooth muscle hyperreactivity to ACh by heterotypic chronic stress (n = 4 or 5 with at least two strips from each rat). B and C: daily intraperitoneal administration of guanethidine or hexamethonium before each stress session had no significant effect on the induction of smooth muscle hyperreactivity to ACh by heterotypic chronic stress (n = 4 or 5 with at least two strips from each rat). D: heterotypic chronic stress significantly increased tyrosine hydroxylase expression in adrenal gland (n = 4). *#P < 0.05 vs. age-matched sham-treated controls.
The generation of norepinephrine is rate limited by tyrosine hydroxylase (9). Therefore, we investigated whether HeCS enhances expression of tyrosine hydroxylase in the adrenal gland, so that it increases the production of norepinephrine, when stimulated by pituitary hormones. Immunoblotting showed that expression of tyrosine hydroxylase significantly increases in the adrenal gland of chronically stressed rats, when compared with that in the glands of age-matched sham-treated controls (Fig. 5D).
In vitro induction of circular smooth muscle hyperreactivity to ACh by norepinephrine?
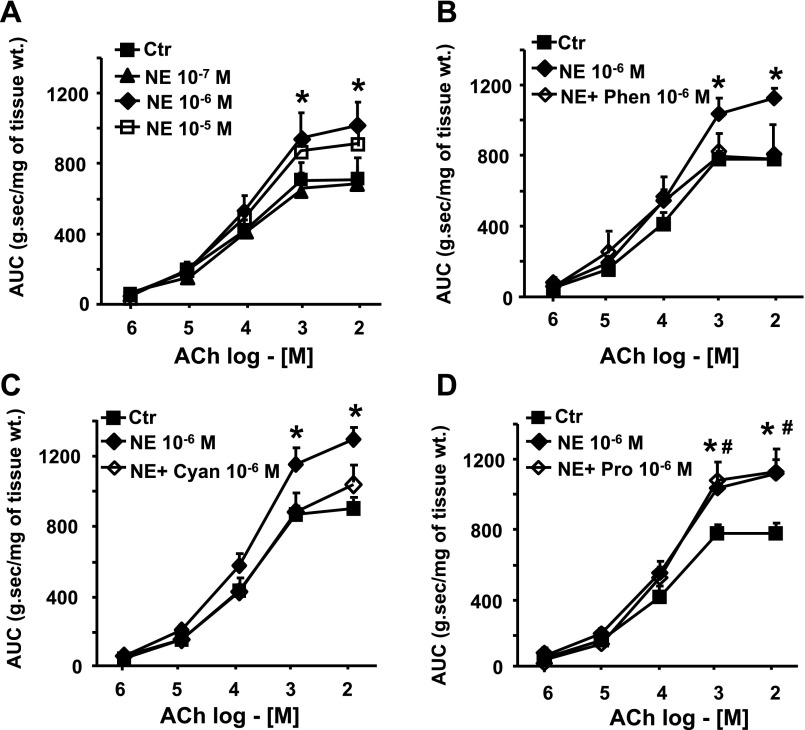
We investigated whether norepinephrine induces colonic circular smooth muscle hyperreactivity to ACh in vitro. The incubation of freshly obtained circular smooth muscle strips from the distal colon of naïve rats for 24 h concentration dependently enhanced their contractile response to ACh (Fig. 6A). We also found that norepinephrine-induced smooth muscle hyperreactivity to ACh was inhibited by the blockade of α-adrenergic receptors with 10−6 M phentolamine (Fig. 6B) (46) and β3-adrenergic receptor antagonist with 10−6 M cyanopindolol (Fig. 6C) (38), but not by 10−6 M β1- and β2-adrenergic receptor antagonist propranolol (Fig. 6D) (46). All antagonists were added to the incubation medium 30 min before the addition of 10−6 M norepinephrine.
Fig. 6.
A: incubation of circular smooth muscle strips from naïve rats with NE concentration dependently induced hyperreactivity to ACh (n = 3 or 4 with at least two strips from each rat). B, C, and D: α-adrenergic receptor antagonist, phentolamine (Phen), and β3-adrenergic receptor antagonist, cyanopindolol (Cyan) blocked the norepinephrine-induced increase in contractility;. β1- and β2-adrenergic receptor antagonist, propranolol (Pro) had no significant effect (n = 3 or 4 with at least two strips from each rat). *#P < 0.05 vs. vehicle-treated control strips.
The effects of heterotypic chronic stress and in vitro treatment with norepinephrine on the expression of α1C subunit of Cav1.2 (L-type) calcium channels in muscularis externa.
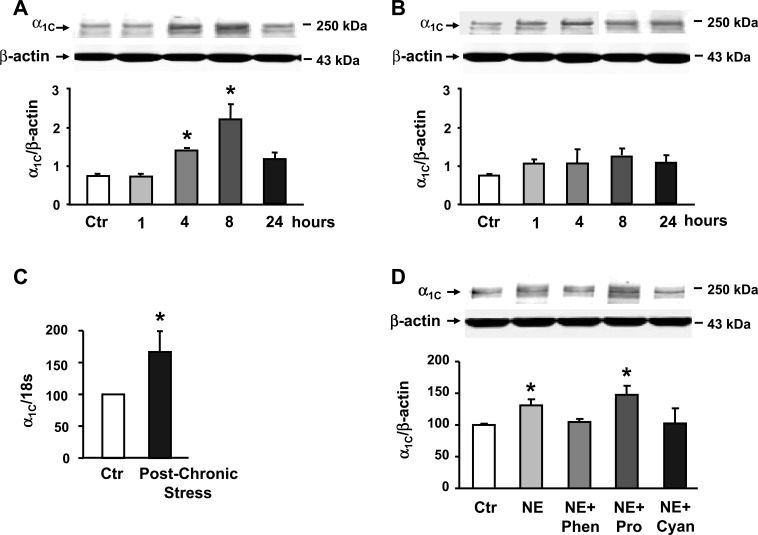
Recent studies show that expression of the α1C subunit of Cav1.2 channels is sensitive to the smooth muscle microenvironment. The exposure of these cells to cytokines suppresses expression of this subunit, resulting in colonic circular smooth muscle hyporeactivity to ACh (43). On the other hand, their exposure to the enteric neurotransmitter VIP enhances expression of this subunit, which, in turn, enhances smooth muscle reactivity to ACh (42). Therefore, we investigated whether smooth muscle hyperreactivity to ACh in response to HeCS is mediated by an increase in expression of the α1C subunit. We found that expression of the α1C subunit in muscularis externa increases time dependently after the last stressor of the HeCS protocol (Fig. 7A). The expression of α1C peaked at about 8 h after the last stressor. However, its expression at 24 h after the last stressor was not different from that in the muscularis externa tissues of age-matched sham-treated controls (Fig. 7A). By contrast, 1-day acute stress had no significant effect on the expression of α1C subunit at any time during the 24-h post-acute-stress period (Fig. 7B). Real-time PCR analysis showed that HeCS also increases mRNA expression of the α1C subunit, indicating a transcriptional effect (Fig. 7C).
Fig. 7.
A: expression of α1C. subunit increased time-dependently after 9-day heterotypic chronic stress protocol (n = 3 or 4). B: 1-day acute stress had no significant effect on the expression of α1C subunit (n = 3). C: percent increase of mRNA in the muscularis externa tissues obtained 8 h after 9-day heterotypic chronic stress protocol, when compared with age-matched sham-treated controls. D: twenty-four-hour incubation of freshly obtained colonic circular smooth muscle strips with NE enhanced the protein expression of α1C subunit. Phentolamine and cyanopindolol blocked the increase in expression of the α1C subunit, while, propranolol had no effect (n = 3). *P < 0.05 vs. age-matched sham-treated controls.
Then we investigated whether norepinephrine enhances expression of the α1C subunit in vitro. The incubation of freshly obtained circular muscle strips from naïve rats for 24 h with 10−6 M norepinephrine enhanced expression of α1C subunit (Fig. 7D). The blockade of α- and β3-adrenergic receptors with 10−6 M phentolamine and 10−6 M cyanopindolol, respectively, prevented an increase in the expression of α1C subunit by norepinephrine (Fig. 7D). However, the β1- and β2-adrenergic receptor antagonist propranolol had no significant effect on the increase in α1C expression by norepinephrine.
DISCUSSION
Previous studies have reported that one episode of acute stress enhances the rate of defecation and myoelectrical/contractile activity in the colon (5, 19, 24). These effects are mediated by the hypothalamic release of CRH, which activates the parasympathetic neurons. The parasympathetic neurons, in turn, stimulate the enteric motor neurons to release ACh at the neuroeffector junction, resulting in the stimulation of colonic motor function (48, 52). Other studies also show that peripheral CRH1 receptors, located on the enteric neurons may mediate the efferent parasympathetic signal to the smooth muscle cells to contract in response to acute stress (23, 32, 49). These effects of acute stress last more or less for the duration of the stressor (19, 47). Our findings also show that the effects of acute stress do not persist after end of the one episode of stress. The effects of chronic stress that precipitates or exacerbates the symptoms of motility dysfunction in IBS and IBD patients, are not known (3, 4, 15, 26, 27, 36, 58). Our findings show that HeCS enhances transcription of gene encoding the α1C subunit of Cav1.2 channels in the rat distal colon. Previous studies found that enhanced expression of this subunit of Cav1.2 channels in colonic circular smooth muscle cells increases Ca2+ influx in response to ACh and KCl, which enhances the contractile response and accelerates colonic transit (42, 44). The increase in expression of the α1C subunit in circular smooth muscle cells of the distal colon in response to heterotypic chronic stress also enhances the contractile response to ACh in integrated circular muscle strips, as well as in single dispersed circular muscle cells, and it accelerates colonic transit. The faster colonic transit is accompanied by an increase in defecation rate and an increase in the moisture content of the pellets due to their shorter duration of contact with the mucosa. These findings suggest that the effects of HeCS on colonic motor function persist for several hours after stress ends due to alterations in the transcription of genes encoding cell-signaling proteins of the excitation-contraction coupling in circular smooth muscle cells. In this study, we focused on expression of α1C subunit because previous studies indicate that its expression is sensitive to environmental factors, and changes in its expression alter colonic motility function. However, we cannot rule out that other cell-signaling proteins may also be affected by HeCS. The net result of transcriptional changes in cell-signaling proteins of the excitation-contraction coupling induced by HeCS, however, is to enhance colonic motility function.
The transcriptional effects of stress depend on the intensity, repetition rate, and the duration of stress (35). In our study, one episode of acute stress had no transcriptional effects on expression of the α1C subunit. However, acute stress may induce the expression of immediate early genes, such as c-fos and c-jun in some cell types, such as epithelial cells and cardiac cells and enteric neurons (30, 53, 54).
Clinical findings show that the intensity of chronic stress correlates with the intensity of gastrointestinal and extra-intestinal symptoms in IBS patients (3, 4). Our heterotypic chronic stress protocol lasted for 9 days, and its genetic effects lasted less than 24 h. The time course of increase of norepinephrine on day 9 of heterotypic chronic stress was similar to that of expression of the α1C subunit and the induction of smooth muscle hyperreactivity to ACh. It is likely that a greater intensity of daily stress or stress sustained for longer periods may produce longer-lasting genetic effects. The transient nature of the genetic effects of chronic heterotypic stress in our study is consistent with clinical observations that the symptoms of IBS improve with the resolution of major life stressors (13, 41, 57). It is noteworthy that the plasma and urine levels of norepinephrine are elevated in IBS, as well as in chronically stressed subjects (2, 17, 28, 37).
Bradesi et al. (7) found that 10-day homotypic chronic stress also develops persistent visceral hyperalgesia that lasts for at least 40 days, but it affects somatic nociception only transiently. These investigators did not identify transcriptional targets for the persistent effects of homotypic chronic stress on visceral hypersensitivity. However, their findings, along with ours, suggest that the duration of transcriptional effects may depend on the targeted genes in different cell types. The transcriptional effects in colonic circular smooth muscle cells last less than 24 h after a 9-day, once daily heterotypic stress protocol.
The hypothalamic release of CRH is an essential initial step for stress response (56). All stress responses are blocked by inhibiting the central CRH1 and CRH2 receptors. The hypothalamic release of CRH stimulates two main neuroendocrine systems that mediate the responses in peripheral organs, the hypothalamic-pituitary-adrenal (HPA) axis, and the autonomic nervous system, including the sympathetic system originating in the locus coeruleus (LC-NE system) and the parasympathetic system in the dorsal motor nucleus of the vagus (9, 52). The stimulation of the HPA axis results in systemic release of corticosterone from the adrenal cortex and norepinephrine from the adrenal medulla. The end neurotransmitters for the sympathetic and parasympathetic systems are norepinephrine and ACh, respectively. Both autonomic neural pathways contain a nicotinic synapse. Our findings show that the parasympathetic neurons, the sympathetic neurons, or the peripheral CRH receptors may not mediate the long-term transcriptional effect of HeCS on the enhancement of colonic motor functions. Systemic administrations of hexamethonium, guanethidine, or astressin did not block the transcriptional effects. In addition, HeCS did not increase the plasma levels of CRH.
On the other hand, adrenalectomy blocked the induction of longer-term colonic circular smooth muscle hyperreactivity to ACh. This suggested that the systemic release of norepinephrine or corticosterone from the adrenal gland might induce colonic circular smooth muscle hyperreactivity to ACh by HeCS. The inhibition of adrenergic receptors by bretylium tosylate blocked the induction of smooth muscle hyperreactivity, but the inhibition of corticosterone by RU-486 had no effect. This suggests that the increase in plasma level of norepinephrine may be the prominent mediator of induction of colonic circular smooth muscle hyperreactivity to ACh in response to heterotypic chronic stress.
Peripheral increases in catecholamine levels are related to the pathophysiology of several stress-related disorders, including post-traumatic stress and major depressive disorders (45, 59). Prolonged or repeated stress activates gene expression for catecholamine biosynthetic enzymes in the adrenal medulla, sympathetic ganglia, and in a number of locations in the brain (35, 39). Prior stressors sensitize the generation of catecholamines in the adrenal medulla (35). We found that the expression of tyrosine hydroxylase, the rate-limiting factor for the synthesis of norepinephrine, increases about 1.6-fold in adrenal tissues after 9 days of HeCS. Consequently, plasma norepinephrine exhibited about a 4.5-fold increase after the last stressor, when compared with no significant increase in response to a 1-day acute stress.
The blood-brain barrier is not absolute; it can be modulated by central release of CRH by acute stress and activated mast cells (12). Blood-brain barrier transports specific peptides from the brain to the periphery (20). Central injection of radioactively labeled CRH accumulates in the spleen and releases β-endorphin (25). However, our findings show that the plasma levels of CRH do not increase in response to HeCS. In addition, peripheral administration of astressin did not block the induction of colonic circular smooth muscle hyperreactivity to ACh. Therefore, in contrast to the potential mediation of stimulation of colonic motor activity by acute stress (23, 32, 49), CRH may not mediate the induction of persistent hyperreactivity to ACh by heterotypic chronic stress.
In vitro studies found that incubation of naïve colonic circular muscle strips with norepinephrine for 24-h concentration dependently increases the contractile response to ACh, as well as protein expression of the α1C subunit of Cav1.2 channels. The increase in expression of the α1C subunit is blocked by α-adrenergic and β3-adrenergic receptor antagonists, but not by β1- and β2-receptor antagonists.
In conclusion, heterotypic chronic stress causes colonic circular smooth muscle hyperreactivity to ACh, which persists for several hours after the end of the last stressor. HeCS upregulates the expression of tyrosine hydroxylase in the adrenal gland, which progressively increases the synthesis and release of norepinephrine in plasma in response to subsequent stressors. Systemic release of norepinephrine from the adrenal medulla mediates the induction of hyperreactivity to ACh. The increase in plasma norepinephrine enhances transcription of the pore-forming α1C subunit of Cav1.2 channels in colonic circular smooth muscle cells by activation of α- and β3-adrenergic receptors. The enhanced expression of the α1C subunit results in smooth muscle hyperreactivity to ACh, accelerated colonic transit, and increase in defecation rate. These effects of heterotypic chronic stress may explain the transient exacerbation of motility dysfunction by chronic stress in IBS patients.
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-079952 and DK-072414 (to S. K. Sarna).
REFERENCES
- 1.Alonso SJ, Castellano MA, Afonso D, Rodriguez M. Sex differences in behavioral despair: relationships between behavioral despair and open field activity. Physiol Behav 49: 69–72, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Aschbacher K, Mills PJ, von Kanel R, Hong S, Mausbach BT, Roepke SK, Dimsdale JE, Patterson TL, Ziegler MG, Ancoli-Israel S, Grant I. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 22: 493–502, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett EJ, Piesse C, Palmer K, Badcock CA, Tennant CC, Kellow JE. Functional gastrointestinal disorders: psychological, social, and somatic features. Gut 42: 414–420, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 43: 256–261, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res 641: 21–28, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bradesi S, Eutamene H, Fioramonti J, Bueno L. Acute restraint stress activates functional NK1 receptor in the colon of female rats: involvement of steroids. Gut 50: 349–354, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol 289: G42–G53, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Brodie DA Ulceration of the stomach produced by restraint in rats. Gastroenterology 43: 107–109, 1962. [PubMed] [Google Scholar]
- 9.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol 463: 235–272, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol Gastrointest Liver Physiol 271: G884–G892, 1996. [DOI] [PubMed] [Google Scholar]
- 11.de Kloet ER Steroids, stability and stress. Front Neuroendocrinol 16: 416–425, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther 303: 1061–1066, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie E, Creed F, Dawson D, Tomenson B. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology 100: 450–457, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am 30: 695–728; vii-viii, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hart A, Kamm MA. Review article: mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment Pharmacol Ther 16: 2017–2028, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Hebert MA, Serova LI, Sabban EL. Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem 95: 484–498, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF, Walker E. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol 91: 906–913, 1996. [PubMed] [Google Scholar]
- 18.Iwaki K, Sukhatme VP, Shubeita HE, Chien KR. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem 265: 13809–13817, 1990. [PubMed] [Google Scholar]
- 19.Jimenez M, Bueno L. Inhibitory effects of neuropeptide Y (NPY) on CRF and stress-induced cecal motor response in rats. Life Sci 47: 205–211, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Kastin AJ, Pan W, Maness LM, Banks WA. Peptides crossing the blood-brain barrier: some unusual observations. Brain Res 848: 96–100, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kwetnansky R Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann NY Acad Sci 1032: 117–129, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol 283: G544–G552, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol 494: 63–74, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maillot C, Million M, Wei JY, Gauthier A, Tache Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology 119: 1569–1579, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Martins JM, Banks WA, Kastin AJ. Transport of CRH from mouse brain directly affects peripheral production of β-endorphin by the spleen. Am J Physiol Endocrinol Metab 273: E1083–E1089, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Maunder R Mediators of stress effects in inflammatory bowel disease: not the usual suspects. J Psychosom Res 48: 569–577, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Mayer EA, Naliboff BD, Chang L, Coutinho SVV. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 280: G519–G524, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Mazur M, Furgala A, Jablonski K, Madroszkiewicz D, Ciecko-Michalska I, Bugajski A, Thor PJ. Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. J Physiol Pharmacol 58 Suppl 3: 131–139, 2007. [PubMed] [Google Scholar]
- 29.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci 18: 49–72, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Miampamba M, Million M, Yuan PQ, Larauche M, Tache Y. Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport 18: 679–682, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller DB, O'Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism 51: 5–10, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Tache Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res 985: 32–42, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Tache Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol 292: R1429–R1438, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu V, Wei JY, Rivier J, Vale W, Mayer EA, Tache Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut 55: 172–181, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nankova BB, Sabban EL. Multiple signalling pathways exist in the stress-triggered regulation of gene expression for catecholamine biosynthetic enzymes and several neuropeptides in the rat adrenal medulla. Acta Physiol Scand 167: 1–9, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Niess JH, Monnikes H, Dignass AU, Klapp BF, Arck PC. Review on the influence of stress on immune mediators, neuropeptides and hormones with relevance for inflammatory bowel disease. Digestion 65: 131–140, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut 53: 1102–1108, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts SJ, Papaioannou M, Evans BA, Summers RJ. Characterization of beta-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for β1-, β2- and β3-adrenoceptors in rat ileum. Brit J Pharmacol 127: 949–961, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci 24: 91–98, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Sallam HS, Oliveira HM, Gan HT, Herndon DN, Chen JD. Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Regul Integr Comp Physiol 292: R253–R257, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Shaw G, Srivastava ED, Sadlier M, Swann P, James JY, Rhodes J. Stress management for irritable bowel syndrome: a controlled trial. Digestion 50: 36–42, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Shi XZ, Choudhury BK, Pasricha PJ, Sarna SK. A novel role of VIP in colonic motility function: induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology 132: 1388–1400, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-κB. Gastroenterology 129: 1518–1532, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Shi XZ, Sarna SK. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: a novel approach to designing promotility and antimotility agents. Am J Physiol Gastrointest Liver Physiol 295: G187–G196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychol 46: 1192–1204, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Stark ME, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol 444: 743–761, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol 280: G173–G177, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann NY Acad Sci 697: 233–243, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil 16 Suppl 1: 137–142, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Taylor MM, Samson WK. Stress hormone secretion is altered by central administration of intermedin/adrenomedullin-2. Brain Res 1045: 199–205, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53: 865–871, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Tsukamoto K, Nakade Y, Mantyh C, Ludwig K, Pappas TN, Takahashi T. Peripherally administered CRF stimulates colonic motility via central CRF receptors and vagal pathways in conscious rats. Am J Physiol Regul Integr Comp Physiol 290: R1537–R1541, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Ueyama T, Saika M, Koreeda C, Senba E. Water immersion-restraint stress induces expression of immediate-early genes in gastrointestinal tract of rats. Am J Physiol Gastrointest Liver Physiol 275: G287–G295, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Ueyama T, Senba E, Kasamatsu K, Hano T, Yamamoto K, Nishio I, Tsuruo Y, Yoshida K. Molecular mechanism of emotional stress-induced and catecholamine-induced heart attack. J Cardiovasc Pharmacol 41 Suppl 1: S115–S118, 2003. [PubMed] [Google Scholar]
- 55.Ueyama T, Yoshida K, Senba E. Emotional stress induces immediate-early gene expression in rat heart via activation of alpha- and beta-adrenoceptors. Am J Physiol Heart Circ Physiol 277: H1553–H1561, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213: 1394–1397, 1981. [DOI] [PubMed] [Google Scholar]
- 57.Whitehead WE Assessing the effects of stress on physical symptoms. Health Psychol 13: 99–102, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 33: 825–830, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yehuda R, Siever LJ, Teicher MH, Levengood RA, Gerber DK, Schmeidler J, Yang RK. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry 44: 56–63, 1998. [DOI] [PubMed] [Google Scholar]