Abstract
The Ussing chamber provides a physiological system to measure the transport of ions, nutrients, and drugs across various epithelial tissues. One of the most studied epithelia is the intestine, which has provided several landmark discoveries regarding the mechanisms of ion transport processes. Adaptation of this method to mouse intestine adds the dimension of investigating genetic loss or gain of function as a means to identify proteins or processes affecting transepithelial transport. In this review, the principles underlying the use of Ussing chambers are outlined including limitations and advantages of the technique. With an emphasis on mouse intestinal preparations, the review covers chamber design, commercial equipment sources, tissue preparation, step-by-step instruction for operation, troubleshooting, and examples of interpretation difficulties. Specialized uses of the Ussing chamber such as the pH stat technique to measure transepithelial bicarbonate secretion and isotopic flux methods to measure net secretion or absorption of substrates are discussed in detail, and examples are given for the adaptation of Ussing chamber principles to other measurement systems. The purpose of the review is to provide a practical guide for investigators who are new to the Ussing chamber method.
Keywords: colon, duodenum, ion transport, nutrient absorption
the ussing chamber provides a valuable, time-proven method for the measurement of electrolyte, nutrient, and drug transport across epithelial tissues. The method was developed over 50 years ago by the Danish biologist Hans H. Ussing as a means to understand the phenomenon of active NaCl transport. Active transport, i.e., the capacity of an epithelium to move ions or nutrients against an electrical and/or concentration gradient, had been previously demonstrated by isotopic tracer experiments. Frog skin was used by Ussing and colleagues as a model system because it had the capacity to move NaCl from the skin surface into the interstitium against more than a 100-fold concentration difference. However, a major difficulty was distinguishing the movement of ions actively transported by epithelial cells from the passive movement of ions through paracellular, i.e., intercellular, pathways. Ussing solved this problem by developing an experimental system whereby dissected frog skin separated two halves of a chamber, each of which superfused the frog skin with identical electrolyte solutions of the same volume. Thus paracellular ion movements driven by the passive forces of transepithelial concentration and osmotic and hydrostatic gradients were eliminated. The passive transepithelial driving force created by the spontaneous electrical potential across the epithelium was eliminated by clamping the potential to zero with an external current passed across the epithelium. This current, known as the short-circuit current (Isc), is equivalent to the algebraic sum of electrogenic ion movement by active transport (i.e., when using conversion with the Faraday constant). Thus, by eliminating transepithelial diffusion forces (osmotic and electrochemical gradients), the movement of ions as measured by isotopic tracers or the Isc in the Ussing chamber resulted from active transport. Observations from this system led to the Koefoed-Johnsen-Ussing two-membrane model for epithelial transport in which the apical membrane is permeable to Na+ and the basolateral membrane is permeable to K+. This paradigm served as the foundation for our present model of transepithelial transport in which primary active transport by Na+-K+ ATPase in the basolateral membrane provides the electrochemical gradient for secondary active transport by Na+ channels (as well as Na+-coupled cotransporters/antiporters) at the apical membrane. For more information regarding the genius and discoveries of Hans Ussing, see the Hans Ussing Memorial issue of the Journal of Membrane Biology, issue 184, 2001. Today, the Ussing chamber method has been applied to virtually every epithelium in the animal body, including the reproductive tract, exocrine/endocrine ducts, intestine, airway, eye, and choroid plexus. Furthermore, the method has been extensively used for studies of cultured epithelial cells (primary cells and cell lines) where tight junction integrity maintains apical and basolateral membrane polarity.
Ussing chamber studies of intestinal mucosa have provided many of the key observations that moved our understanding of transepithelial transport processes toward a molecular basis. The laboratories of Peter Curran, Michael Field, and Stanley Schultz trained a generation of scientists, most notably in studies of rabbit ileum, which precisely defined the transport mechanisms that exist in many epithelial tissues. Together, with observations from model tissues (amphibian epithelia, shark rectal gland), studies of intestinal mucosa have been instrumental in elucidating the well-known processes of electrogenic Cl− secretion, electrogenic Na+-coupled glucose absorption, and electroneutral NaCl absorption. The Isc and transepithelial voltage potential (Vt) measurements in Ussing chamber studies have shown that electrogenic Cl− secretion is not only important to normal digestive physiology but also serves as the target for enterotoxic and inflammatory-mediated secretory diarrhea. On the basis of these studies, it is now known that the process involves the activities of the Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR) at the apical membrane and the Cl− uptake mechanisms of the Na+/K+/2Cl− cotransporter NKCC1 and the Cl−/HCO3− exchanger AE2 at the basolateral membrane (8, 20, 41, 53). Loss of CFTR in the genetic disease cystic fibrosis compromises hydration of the mucus and debris on epithelial surfaces, which leads to the disease manifestations of intestinal obstruction, pancreatic insufficiency, and failure of the pulmonary mucociliary apparatus (57). Bioelectric measurements in Ussing chambers established the process of Na+-coupled glucose transport as the prototype for Na+-coupled nutrient absorptive mechanisms, and the eventual identification of the molecular entity SGLT1 has resulted in the discovery of a family of hexose transporters that support viability of a variety of cell types (for review, see Ref. 59). Electroneutral NaCl transport (also known as coupled NaCl absorption) does not exhibit rheogenic properties, which was disadvantageous for Isc-dependent studies in Ussing chambers and thus was first defined in perfused intestinal preparations (50). However, isotopic flux measurements in Ussing chambers led to a broader understanding of the characteristics and regulation of coupled Na+/H+ and Cl−/HCO3− exchangers (4, 7, 35, 42). Molecular identification of the exchanger families has led to pharmacological and knockout (KO) mouse studies that indicate that electroneutral NaCl absorption principally involves the coupling of the Na+/H+ exchanger NHE3 (5, 12, 39) to the Cl−/HCO3− exchanger DRA (55). The central point to be made from these examples is that Ussing chamber studies revealed the characteristics and relative importance of transport processes in the intestinal epithelium, which were eventually identified at the molecular level. The contemporary investigative techniques of patch-clamp and fluorescence microscopy are used to define the molecular physiology of these proteins through the advantages of isolation and amplification in heterologous expression systems. However, the application of these methods to native intestinal epithelial cells is strongly limited by technical demands. As our understanding of the molecular interactions of transporters is refined, the methodology of the Ussing chamber will continue to provide a “gold standard” in the application of this knowledge to the physiological complexities of healthy and diseased intestinal mucosa.
Genetically manipulated mouse models have provided an important confluence between our molecular descriptions of transporters and their functions in native intestinal epithelium. Transgenic and gene-targeted mice are used to elucidate the activity of a transporter in both overexpression and loss-of-function (KO) experiments. Studies of transporter KO mice reveal previously unrecognized functional or regulatory properties of other transporters. For example, studies of the CFTR KO mouse intestine demonstrated Isc changes indicative of cAMP regulation of Na+-coupled glucose absorption, an effect that is normally obscured by the large Isc response induced by CFTR activation (18). However, serendipitous findings such as these illustrate the need for investigations of genetically manipulated mice to ensure that functional or anatomical compensatory changes are not responsible for observational data before assigning specific roles to the targeted transporter. The CFTR KO mouse was developed in 1992 and was among the first genetically modified models of a major human genetic disease, i.e., cystic fibrosis (48). To this day, the CFTR KO mouse continues to be a valuable reagent for the investigations of cystic fibrosis and a variety of other diseases that invoke alterations in CFTR function. However, when the CFTR KO mouse was developed in 1992, there were less than 20 publications identified by MedLine search describing the normal transport physiology of the murine gastrointestinal tract. Despite the fact that the mouse intestinal tract demonstrates many similarities in function to more traditional models of intestinal physiology such as rat and rabbit, the specificity of molecular manipulations requires more exacting descriptions of comparative intestinal function and protein (e.g., transporter) physiology/pharmacology in the mouse. Since resources for these types of investigations are limited, the information will have to be gleaned from the control studies of various research efforts.
The importance of understanding the normal intestinal physiology of the mouse may gain wider acceptance with increasing efforts to generate “humanized” murine models in which human genes are inserted against the background of a mouse homolog KO (62). Although conceptual and technical difficulties of humanized mice are foreseen, these models provide an alternative approach to understanding physiological function that is not possible with studies in heterologous expression systems. On the basis of these considerations, the intersection of molecular and integrative physiology in these genetically manipulated mouse models will likely require the application of the Ussing chamber method. Although the method is sometimes criticized for its “black box” limitations (27), the negative is far outweighed by the instances where scientific deductions resulting from Ussing chamber studies have provided an accurate paradigm for discoveries of a molecular nature. Thus the Ussing chamber will continue to provide a useful method for integrating our knowledge of transporter molecules into the complex physiological functions of an intestinal epithelium.
Description of the Method
All animal experiments and protocols used to demonstrate the Ussing chamber method were approved by the University of Missouri Animal Care and Use Committee.
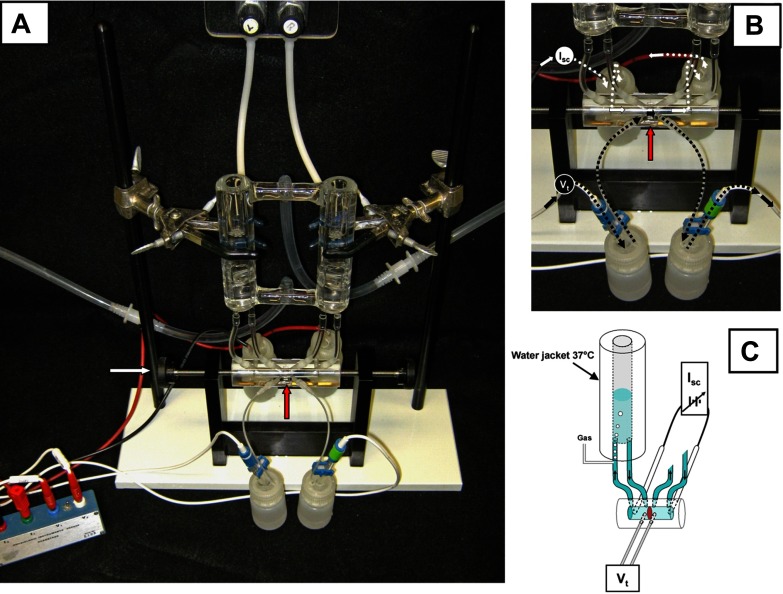
The basic design of a classical Ussing chamber is illustrated in Fig. 1. Although the design is specific for the classic technique, the basic principles described herein also apply to new equipment designs. The intestinal section is opened and oriented as a flat sheet to separate the two halves of the chamber. In Fig. 1, A and B, the intestinal preparation (red arrow) is situated vertically such that the mucosal membrane (also referred to as the apical or luminal-side membrane) is facing one chamber half, whereas the serosal membrane (also referred to as the basolateral, nutrient, or blood-side membrane) is facing the other half-chamber, thus separating the solutions that independently bathe each chamber half. The reservoirs above each chamber are water jacketed to enable warming the superfusate to body temperature for mammalian intestine. Typically, a CO2/HCO3−-buffered Ringer solution like Krebs bicarbonate Ringer (KBR) is used as the superfusate (see Standard protocol and reagents). Oxygen and carbon dioxide tension are maintained in a physiological buffer by injection ports positioned such that they also provide gas lift circulation in the tubing that leads from each chamber half. For mammalian physiological buffers, the gas is a mixture of 95% O2 and 5% CO2 (also known as carbogen), which oxygenates the solutions to a fairly high level of Po2 (>400 mmHg) that is necessary to overcome the lack of hemoglobin delivery by arterial blood supply. Carbogen also provides a Pco2 approximately equivalent to venous blood, which maintains the HCO3− buffer at the physiological pH 7.4. The gas lift circulation is typically set at rates that reduce the unstirred layer at either side of the mucosa without damaging the tissue. A rule of thumb is to set the gas lift so that the individual bubbles of gas are just barely discernible to the naked eye. Needle valves (cat. no. 06393-70, Cole-Parmer) situated in the gas line before entering the chamber port are very helpful in minimizing variability in gas lift rates. In this type of Ussing chamber design, it is important to level the two reservoirs so that the hydrostatic pressure on each side is identical. Ballooning of the mucosa into one chamber during an experiment indicates an imbalance in either solution height or rates of gas lift. Volumes of superfusate that are 5 ml or less are used for mouse intestine because of limitations in the surface area and tissue strength of the intestinal preparation.
Fig. 1.
Classic Ussing chamber design. A: assembled apparatus with water-jacketed reservoirs, Ussing chamber (intestinal preparation is mounted vertically; red arrow) secured by thumb wheel screws (white arrow), and electrodes attached to voltage clamp head stage. B: close-up view of voltage (Vt)-measuring and short-circuit current (Isc)-passing pathways. Calomel half-cell electrodes used for Vt measurements are connected by 3 M KCl salt bridges at each side of intestinal preparation (red arrow). Ag-AgCl electrodes used for Isc passing across the intestinal preparation are connected to the chamber by Krebs bicarbonate Ringer (KBR) salt bridges at each end of the chamber. C: schematic cut-away diagram of Vt and Isc circuits of the Ussing chamber. Short circuiting (Isc) is provided by an automatic voltage clamp (symbol). Note superfusion circulation of KBR is driven by gas lift using 95%O2-5% CO2. Intestinal preparation (red disc) separates the mucosal and serosal baths.
Under the above conditions, the intestinal mucosa or epithelial monolayer will exhibit a spontaneous transepithelial voltage potential (Vt), which for mouse intestine is typically in the range of 1–20 mV. By current convention, the serosal bath serves as ground, and normal murine intestine will typically have a negative Vt (e.g., −2 mV). The Vt is measured by a potentiometer and electrodes (typically calomel half-cells or Ag-AgCl) that are connected by salt bridges to each chamber half. Inside the chamber, the bridge ends are located in close proximity to the mucosa on the mucosal and serosal sides of the intestinal sheet. Salt bridges are used both for convenience and to prevent exposure of the epithelium to the toxic effects of Ag from Ag-AgCl electrodes. The salt bridges for the Vt-sensing electrodes typically consist of 3% agar melted in 3 M KCl solution that is congealed in the tubing. With the exception of protons and hydroxyl ions, K+ and Cl− are the major physiological electrolytes with high and nearly equivalent electric mobilities in aqueous solution, which, in an agar bridge, provides an electrical conduit with low resistance (19, 32). If 3 M KCl salt bridges are used, the diameter of the bridge can be small (e.g., 0.4–1.2 mm), which minimizes KCl contamination of the bathing physiological buffers. As shown in Fig. 1, B and C, a Vt-measuring circuit is established from the potentiometer of the voltage clamp to a calomel electrode (in 3 M KCl) connected to the chamber via a 3 M KCl salt bridge across the mucosa in KBR to a second 3 M KCl salt bridge and calomel electrode connected to the potentiometer. Another circuit is used to pass current (i.e., the Isc) across the epithelium for the purposes of clamping the spontaneous Vt to zero. The Isc-passing electrode can also be used to briefly clamp the intestinal preparation to a defined voltage for the purpose of measuring the transepithelial conductance Gt or its reciprocal, the transepithelial resistance Rt (see below, Standard protocol and reagents). The magnitude of the Isc necessary for voltage clamping is determined from Vt and the series resistance of the circuit plus mucosa, and it is applied continuously during an experiment by an automatic voltage clamp (see Equipment). To minimize circuit resistance, the Isc is typically passed from paired Ag-AgCl electrodes on each side of the mucosa to the chamber by salt bridges of larger diameter (3 mm ID). If larger salt bridges are used, they should be composed of 3% agar melted in the same physiological buffer (e.g., KBR) used for bathing the mucosa to avoid alterations in ionic composition of the superfusate. The Isc-passing electrode salt bridges are situated externally to the Vt-sensing bridges. As shown in Fig. 1, B and C, an Isc-passing circuit is established from the voltage clamp to a Ag-AgCl electrode in KBR connected to the chamber via a KBR salt bridge across the mucosa in KBR to a second KBR salt bridge and Ag-AgCl electrode connected to the automatic voltage clamp. Any complete interruption in these circuits will be immediately obvious, resulting in loss of Vt measurement or the ability to voltage clamp the preparation. However, more subtle effects can result from poor connections in the circuit, which can be due to small bubbles on the salt bridge ends, broken agar in the salt bridges, slippage of the agar within the salt bridges (the gravity-dependent end of the bridge should contain a narrowing to avoid slippage), deteriorating solder between the Ag-AgCl electrode and the conduit wire, and poor connections at the head stage of the voltage clamp.
The application of the Ussing chamber technique to mouse intestine required miniaturization of the chamber dimensions. To reduce circuit resistance, the optimal geometry of the superfusion compartment of the half-chamber is a cone with the apex at the Isc bridge expanding to the tissue aperture. However, a cylindrical bore with a diameter equivalent to the tissue aperture suffices for smaller chambers used with mouse intestine. As shown in Fig. 2, one technique is to affix (or “mount”) the mouse intestine across the aperture of the half-chamber by impaling the edges of the intestinal mucosa on a circle of pins outside the chamber aperture (see preparation of intestinal mucosa below in Standard protocol and reagents). The pins enter opposing blunt end holes when the two half-chambers are assembled and secured by the peripheral force of thumbwheel-driven screws at the end of each half-chamber (see white arrow, Fig. 1). We have found that the optimal pin circle diameter and aperture diameter for small intestinal sections from adult mice to be 8 mm and 5.5 mm, respectively. This arrangement minimizes stretching/tearing at the pins and “edge damage” of the mucosal preparation (see Troubleshooting). We also employ Parafilm that is cut to form gaskets about the aperture to minimize mucosal edge damage in our acrylic chambers. The gaskets are held in place by modest application of stopcock grease. The chambers in our laboratory have a circular aperture, but even greater surface area can be achieved with an elliptical aperture. However, the elliptical aperture requires a longer section of unblemished intestinal mucosa, thereby increasing the possibility that Peyer's patches in small intestinal preparations or inadvertent mucosal damage from the tissue preparation will be included in the exposed surface area. Moreover, a circular aperture reduces edge damage by minimizing the edge length to aperture area ratio.
Fig. 2.
Murine small intestinal preparation (black arrow) mounted on pins of the serosal half of an acrylic Ussing chamber. Note corresponding holes for pins in opposing chamber half and guideposts for assembly of chamber.
Equipment.
Several vendors carry Ussing chamber equipment and accessories. Water-jacketed reservoirs, acrylic chambers, and voltage clamps for classical Ussing chamber applications are available from Physiologic Instruments (San Diego, CA), Warner Instruments, a subsidiary of Harvard Apparatus (Hamden, CT and Holliston, MA), and World Precision Instruments (Sarasota, FL). The acrylic chambers used in our laboratory were custom designed at the University of Missouri Scientific Instrument Shop. Two useful features for the chambers are the use of guideposts for fitting the two chamber halves together and luer fittings to provide leak-free connections for the salt bridges and reservoir perfusion tubing. Custom chamber design and production is also available commercially at PlexiCraft (Iowa City, IA; formerly Jim's Instrument Shop). Calomel electrodes can be purchased from a variety of sources. Our laboratory uses calomel half-cells manufactured by Radiometer America (www.radiometeramerica.com). Tubing for salt bridges can also be obtained from a variety of vendors and can be fitted with plastic luers for easy connections to the Ussing chamber.
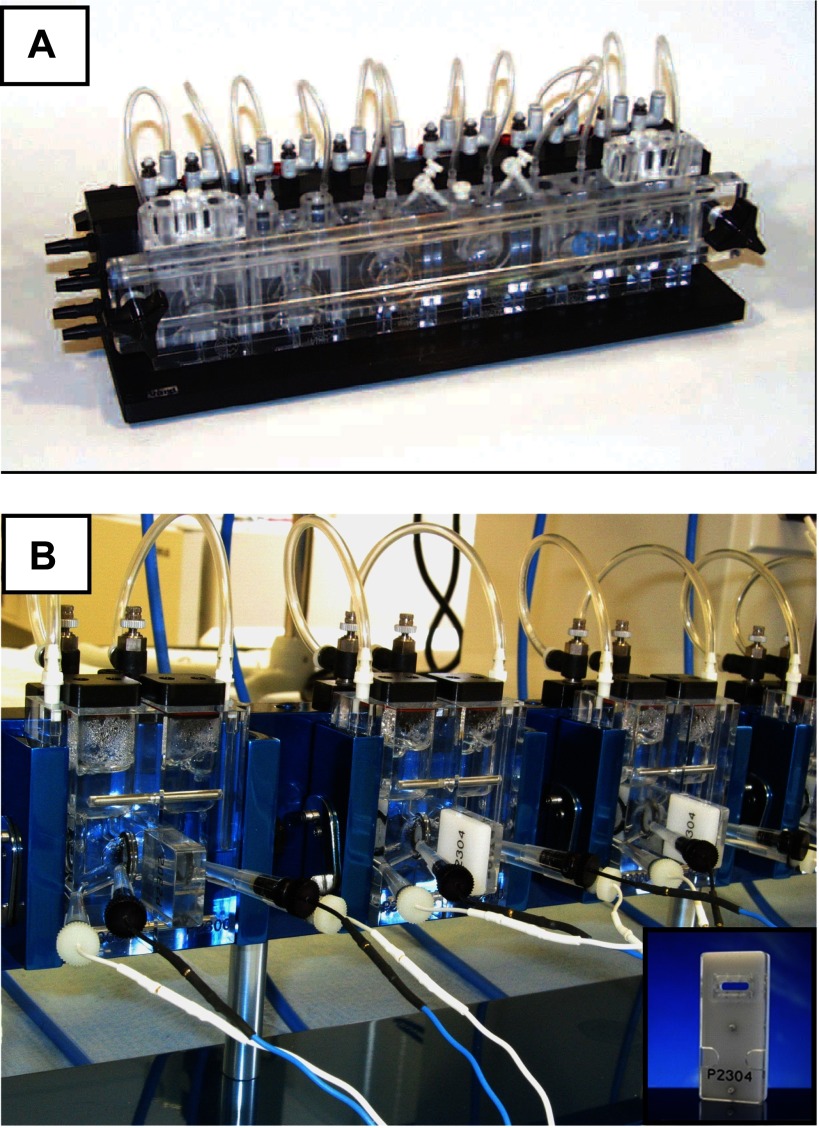
Navicyte (SDR Clinical Technology, Sydney, Australia; distributed by Harvard Apparatus) and Physiologic Instruments also manufacture modular systems in which the reservoir and gas lift are made integral with the tissue chamber within the same acrylic block (see Fig. 3). Heating to body temperature is made possible by conduction from a surrounding metal block into which each chamber is secured. With the Physiologic Instruments system, the intestinal mucosa is mounted on pins in a “slider” that fits into a space between the two halves of the chamber. Multiple slider designs are available with apertures optimized for specific tissues and also for securing various cell culture preparations.
Fig. 3.
Modular Ussing chamber systems. Commercially available systems include acrylic blocks where the chamber is made integral with reservoirs and gas lift. A: modular system available from Navicyte (Harvard Apparatus). B: modular system available from Physiologic Instruments. Inset: “slider” with pins and aperture for mounting murine intestinal preparations.
Standard protocol and reagents.
SUPERFUSATE.
The superfusate that bathes both sides of the intestinal preparation in an Ussing chamber experiment is typically a physiological Ringers-type solution. Although several recipes are available, our laboratory routinely uses KBR, which has the following composition (in mM): 115 NaCl, 25 NaHCO3−, 2.4 K2HPO4, 1.2 CaCl2, 1.2 MgCl2, 0.4 KH2PO4, at pH 7.4 when gassed with 95% O2-5% CO2 at 37°C. In most studies, 10 mM glucose is added to the serosal bath to provide an energy substrate and 10 mM mannitol is added to the mucosal bath to maintain osmotic balance across the mucosa. Identification of transporter activity sometimes requires symmetrical changes in ionic concentration of the KBR, which typically involves substitution with ions that are impermeable to cellular ion transport processes such as N-methyl-d-glucamine+ for Na+ and isethionate− or gluconate− for Cl−. Organic anions used for Cl− substitution are known to reduce free Ca2+ concentration, and gluconate−, in particular, causes more than a 10-fold decrease in free Ca2+ in Ringers solutions (52). Typically, an additional 4–12 mM of a Ca2+ salt (calcium gluconate, CaCl2, CaSO4) is added to superfusate solutions containing gluconate− to maintain physiological levels of free Ca2+. Special care must also be taken when altering the HCO3− concentration of the solution, which is routine for some Ussing chamber studies (see pH stat in Special Uses of the Ussing Chamber). Removing or altering the HCO3− concentration requires a corresponding change in the Pco2 to maintain pH at 7.4. For CO2/HCO3−-buffered media like KBR, these concentrations are calculated using the Henderson-Hasselbalch equation: [HCO3−] = 10pH − pK × [CO2], where [CO2] = Pco2 × CO2 solubility coefficient. The pK and CO2 solubility coefficient must be adjusted for ionic strength, temperature, and pH. For example, standard cell culture solutions containing other buffers (HEPES) and/or lower levels of HCO3− will be acidic (pH ≈ 5.5–6.5) if gassed with 95% O2-5% CO2. Hence oxygenation of these solutions often requires gassing with 100% O2.
ELIMINATION OF ELECTRICAL BIAS.
The standard protocol for Ussing chamber studies involves steps to eliminate bias in the electrical measurements, and this is performed during set up by operating the chamber in the absence of an intestinal preparation. Thus the Ussing chamber apparatus is assembled without mucosa, and the reservoirs and chambers are filled with superfusate. All bubbles near bridge ends or interfering with chamber circulation are removed, and any leaks in the system are secured. After the superfusate warms to 37°C, bias in the electrical measurements is eliminated by “zeroing.” First, the voltage difference between the two Vt-sensing electrodes is nullified by application of an offset voltage, thus ensuring that voltage clamping to 0 mV applies only to the Vt. Second, the resistance of the superfusate must be compensated so that it is not included in the determination of the Gt (or Rt). Most automatic voltage clamps include the feature of “fluid resistance compensation.” The magnitude of compensation will vary with the electrolyte content of the solution, and the distance between the Isc-passing salt bridge ends. Hence the zeroing procedure will need to be repeated if a salt bridge is replaced or dislodged before the experiment. It is advisable to readjust the offset and fluid resistance compensation setting after ∼10–15 min since electrode drift and fluid junction potentials (see Troubleshooting) require time to achieve equilibrium. After zeroing is completed, the voltage clamp circuitry is set to standby mode, and the intestinal preparation is mounted in the chamber with fresh superfusate.
INTESTINAL PREPARATION.
Following euthanasia, the murine intestine is removed by sharp dissection. Care must be taken to cut but not pull the intestine from its mesenteric attachment to avoid damaging the epithelium where the arterial-neural network penetrates the intestinal musculature to the submucosa. The intestinal section is opened longitudinally along the mesenteric attachment remnant so that the antimesenteric mucosa, which is likely to undergo less excision damage, will be situated in the aperture of the chamber. The seromusculature layers (serosa, longitudinal, and circular smooth muscle) are relatively thin in the mouse intestine, so some studies use whole-thickness mouse intestine for Ussing chamber studies to preserve the intramural neuromuscular activity and avoid additional pharmacological treatments that minimize this activity. There are two important considerations when using this approach. First, the seromusculature layers present a significant diffusion barrier to experimental drugs/isotopes and to nutrients/oxygen, which reduces the viability of the intestinal preparation. An excellent investigation of the effect of seromusculature layer on the viability of rat intestine in the Ussing chamber (as determined by isotopic Na+ flux and glucose addition) has been provided by Binder and Rawlins (3). Second, whole-thickness intestinal preparations undergo rhythmical neuromuscular contractions that produce corresponding changes in the Vt and thus Isc by physiological means.
The focus of our laboratory has been to isolate as much as possible the properties and regulation of ion transport by the epithelium. Therefore, before mounting in the Ussing chamber, we prepare the murine intestine by both pharmacological treatment and seromusculature “stripping” to minimize the influence of the intrinsic neuromuscular system. Seromusculature stripping removes the serosa (visceral peritoneum) and the longitudinal/circular muscle layers of the intestinal wall, leaving only the underlying submucosal elements, remnants of muscle, and the epithelium (see seromuscular stripping). The submucosal damage from seromuscular stripping induces phospholipase C or A2 activity, resulting in liberation of arachidonic acid and subsequent eicosanoid generation (46). In particular, prostanoid exposure has variable but well-documented effects on coupled NaCl absorption and CFTR activation attributable to stimulation of intracellular cAMP and Ca2+ mobilization (6). Therefore, it is useful to incubate freshly excised intestinal sections in ice-cold, gassing KBR containing 1 μM indomethacin for 10 min before seromuscular stripping. The incubation allows blockade of tissue cyclooxygenases, which prevents exposure of the mucosa to prostanoids elaborated during seromuscular stripping. If cyclooxygenase activity is desirable during an Ussing chamber experiment, e.g., activation secondary to infectious agents, then prostanoid exposure during muscle stripping and treatment with cyclooxygenase inhibitors can be avoided by washing the stripped mucosal preparation in a series of baths before mounting.
SEROMUSCULAR STRIPPING.
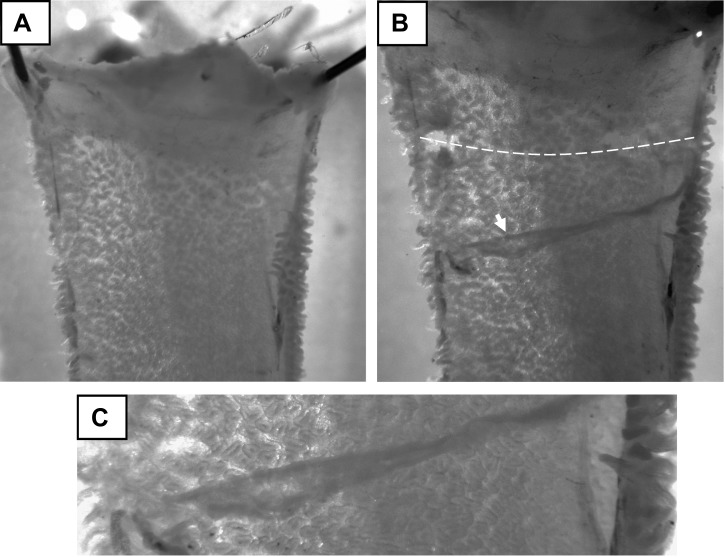
Seromusculature stripping can be achieved by a variety of methods such as scraping the serosal side of the intestine with the edge of a glass slide or by pulling a taut thread longitudinally between the mucosa and muscle layers. However, we have found less damage to the mucosa occurs when the seromuscular layers are bluntly dissected under a dissection stereomicroscope. As shown in Fig. 4A, the intestinal section (typically 1 cm in length) is pinned mucosal side down to a plate containing cured Sylgard (∼0.5 cm thick). The intestinal section is kept cold during dissection either by prechilling the plate or covering the section with ice-cold KBR. Intestinal sections should only be handled at the tissue edges (using fine forceps). Bottom lighting of the stereomicroscope is essential to inspect the intestinal section for damage (or the presence of Peyer's patches in small intestinal sections) and for dissection of the seromuscular layer, especially during the learning stages of the procedure. As shown in Fig. 4, B and C, the seromuscular layer is “scored” or cut with a scalpel blade, and the edge of the layer is reflected along the longitudinal axis of the intestine with the use of fine forceps. After completion of seromusculature stripping, the mucosa is typically mounted on the pins of Ussing half-chamber under the stereomicroscope (Fig. 2). Once the assembled Ussing chamber is secured by the pressure clamps, superfusion is initiated. Typically, we include tetrodotoxin (0.1 μM) in the serosal bath to eliminate residual neural activity. The effect of tetrodotoxin requires ∼20 min, during which the Isc decreases to a steady-state level.
Fig. 4.
Seromuscular stripping method for murine intestine. A: intestinal preparation (duodenum; note pylorus at upper end) is pinned mucosal side down to a cooled Sylgard-cast plate under a dissection stereomicroscope with bottom illumination (magnification = ×7) B: scalpel blade is used to score the seromuscular layer (white dashed line; note occasional full thickness cuts). The seromuscular layer is reflected using fine microforceps by blunt dissection (white arrow). C: higher magnification of reflected seromuscular layer (magnification = ×32).
SHORT-CIRCUIT CURRENT.
The Isc of an intestinal preparation is a summation of all ionic currents across the epithelium: Isc ≈ INa+ + IK+ + ICl− + IHCO3− − IK+ (IK+ is typically an outward current). For murine small intestine, the Isc under these conditions is slightly negative (∼−30 μA/cm2), which reflects the balance of a basal ICl− + IHCO3− secretory current primarily attributable to CFTR activity and a smaller K+ secretory current.
TRANSEPITHELIAL CONDUCTANCE.
Calculation of the Gt using Ohm's law requires measurement of the Isc and Vt of the preparation. Determination of Gt is performed at intervals either by briefly unclamping the mucosal preparation to read the spontaneous Vt (Gt = Isc/ Vt) or by pulsing a small command voltage, e.g., 5 mV, in voltage clamp mode and recording the resulting change in the Isc (Gt = ΔIsc /5 mV). The latter technique is more accurate for preparations with low resistance or displaying low Vt, i.e., the small intestine.
The Gt, or its reciprocal Rt, is a useful measure of the integrity of the intestinal preparation, so it is important to discuss Gt (and Rt) in relation to the paracellular pathway across the intestinal epithelium. In “leaky” epithelium like the intestine, the ionic conductance through the paracellular pathway, in contrast to the transcellular pathway, accounts for >90% of the total transepithelial ionic conductance Gt (14). Hence changes in Gt can signal untoward effects on the tissue integrity. Unlike tight epithelia (26), changes in the transcellular conductance (e.g., activation of a channel) are usually difficult to discern in Ussing chamber experiments of the intestine. Ion conductance through the paracellular pathway of the epithelium is limited by both the tight junctional complex and the relative apposition of the basolateral membranes of adjacent epithelial cells, which determines the volume of the surrounding aqueous column, i.e., the lateral intercellular space (LIS). In addition to Gt, measurements of paracellular flux are often used to estimate intestinal permeability under a variety of conditions. Typically, hydrophilic solutes (mannitol, dextran) that do not undergo transcellular transport are included in both the serosal and luminal baths (equal concentrations), and a trace amount of labeled solute (e.g., [3H]mannitol) is introduced on one side. After an equilibration period of 20–30 min, the labeled solute will reach a steady-state rate of flux into the opposite bath, which can be sampled. Hydrophilic solutes of differing sizes are used to probe the permeability of the paracellular pathway. Although a limit to the size of the paracellular pathway in polarized intestinal cell lines has been estimated at ∼9.0 A, there is evidence from studies of small intestinal preparations indicating a second pathway for much larger probes (e.g., inulin, ∼5,000 molecular wt) but at 100–1,000 times lower permeability than that for mannitol (28). In general, the paracellular flux of a smaller hydrophilic solute probe, e.g., [3H]mannitol, will change in parallel with measured changes in Gt, but one should not assume that this is always the case. Subtle changes in solute permeability can occur without corresponding changes in Gt (for example, see Ref. 31).
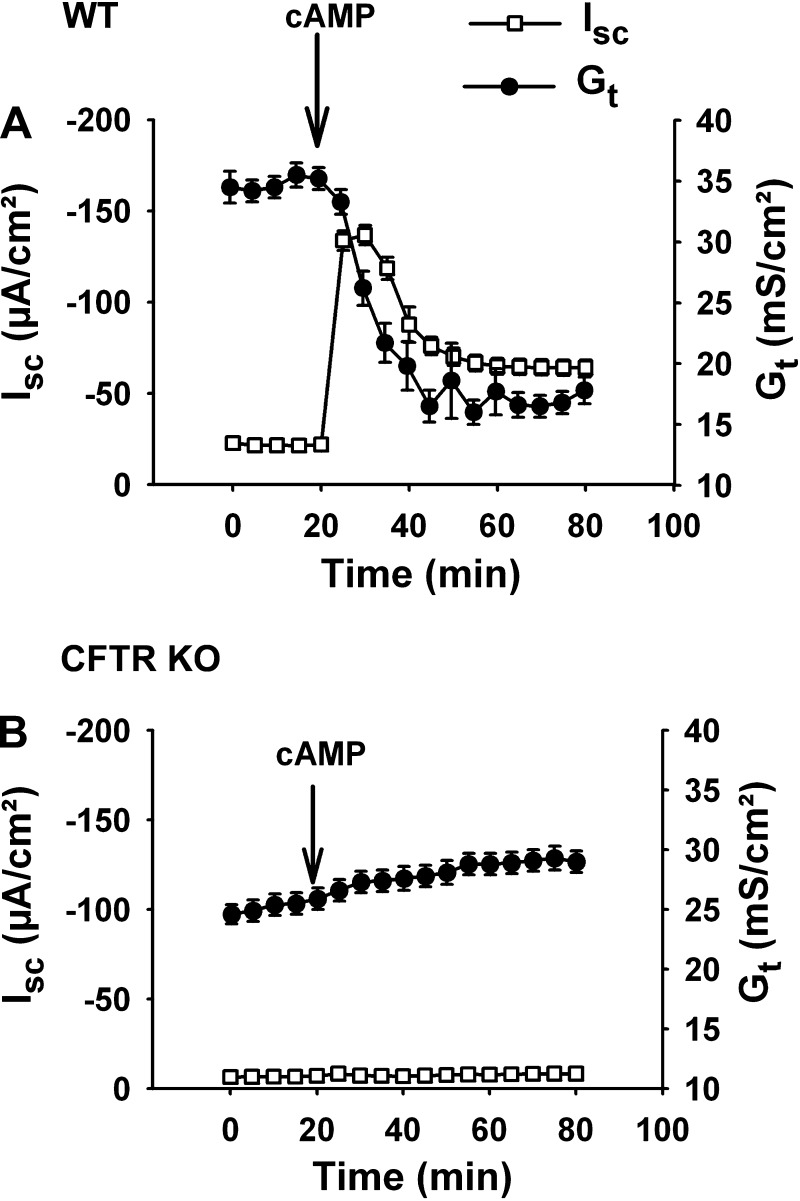
Regulation of the molecular elements of the tight junction is presently an area of avid research interest (for reviews, see Refs. 51, 56, and 61), and it is known that many pathological conditions can induce disruption of the tight junctional complex, which is often accompanied by epithelial shedding. In an Ussing chamber experiment, an insult to tissue integrity can be measured in real time by the Gt. However, under many pharmacological and physiological conditions encountered by the epithelium, rapid changes in Gt can also result from alterations in LIS volume. Significant reductions in NaCl and water absorption cause “collapse” of the LIS, i.e., loss of LIS volume and closer apposition of the lateral cell surfaces, which decreases Gt (or increases Rt) (49). Similarly, as shown in Fig. 5, rapid induction of CFTR-mediated anion secretion in the intestine will collapse the LIS, decrease Gt, and, interestingly, limit the magnitude/duration of the anion secretory response (perhaps because of temporary dehydration of the LIS volume) (15). In contrast, the absence of CFTR prevents acute collapse of the LIS and changes in Gt during cAMP treatment. Although altered Gt can signal disruption of the epithelium, it is important to recognize that Gt also reflects physiological regulation of LIS volume, and, therefore, interpretation of changes in Gt often requires supporting studies (typically histological or ultrastructural evidence).
Fig. 5.
Effect of cystic fibrosis transmembrane conductance regulator (CFTR) activation on transepithelial conductance (Gt). A: time course of short-circuit current (Isc) and transepithelial conductance responses of wild-type (WT) murine jejunum (not seromuscular stripped) to treatment with a cocktail of 10 μM forskolin and 100 μM IBMX (cAMP). Note that the decline in Gt after cAMP treatment slightly precedes the decline in the Isc from its maximal value (n = 6). B: time course of Isc and Gt responses of CFTR knockout (KO) murine jejuna to cAMP treatment (n = 6). [Modified from Gawenis et al. (15).]
Troubleshooting.
EDGE DAMAGE.
Edge damage refers to the extrusion into the chamber compartment of a small portion of the crushed mucosa along the outer diameter of the aperture when the two half-chambers are pressure clamped together. Since the crushed mucosa is a shunt pathway between the chamber halves, the actual Vt is artificially reduced and the Gt is artificially increased (as well as the “apparent” paracellular movement of isotopes/reagents). An excellent examination of this artifact in Ussing chamber experiments was provided by Dobson and Kidder, who showed that the effect on these electrical parameters of the mucosa increased proportionally with a reduction in aperture surface area, i.e., the ratio of edge damage surface area to the exposed surface area increased with smaller apertures (11). Thus the aperture size providing the largest exposed surface area of the intestinal mucosa is optimal.
CONTAMINATION.
One of the more serious but often overlooked problems in Ussing chamber studies is drug or reagent contamination. The Ussing chambers are designed for reuse and are constructed of plastic polymers. Drugs, particularly those dissolved in DMSO vehicle and, to a lesser extent, ethanol, can penetrate plastic surfaces. Although less a problem with the hard plastics, e.g., acrylics like Plexiglas used for the chamber, any soft plastic or latex tubing used to connect the reservoirs to the chamber are particularly troublesome and should be changed after each use. To minimize contamination when drugs using DMSO vehicle are used, the acrylic chambers should be soaked in soapy water (10–15 min), rinsed, and soaked briefly in a 20% DMSO aqueous solution for 5 min before a final rinsing. Acrylics should not be cleaned with alcohols or other organic solvents because damage may result. Glass reservoirs can remain attached to the support stand but should be thoroughly washed, rinsed with 70% ethanol, and rinsed with distilled water. Another source of contamination is the reuse of agar salt bridges, where the drug or isotope can penetrate ∼1 cm along the length of the bridge end during a typical experiment. Thus agar salt bridges are changed after use (the solidified agar is easily ejected from tubing by application of compressed air).
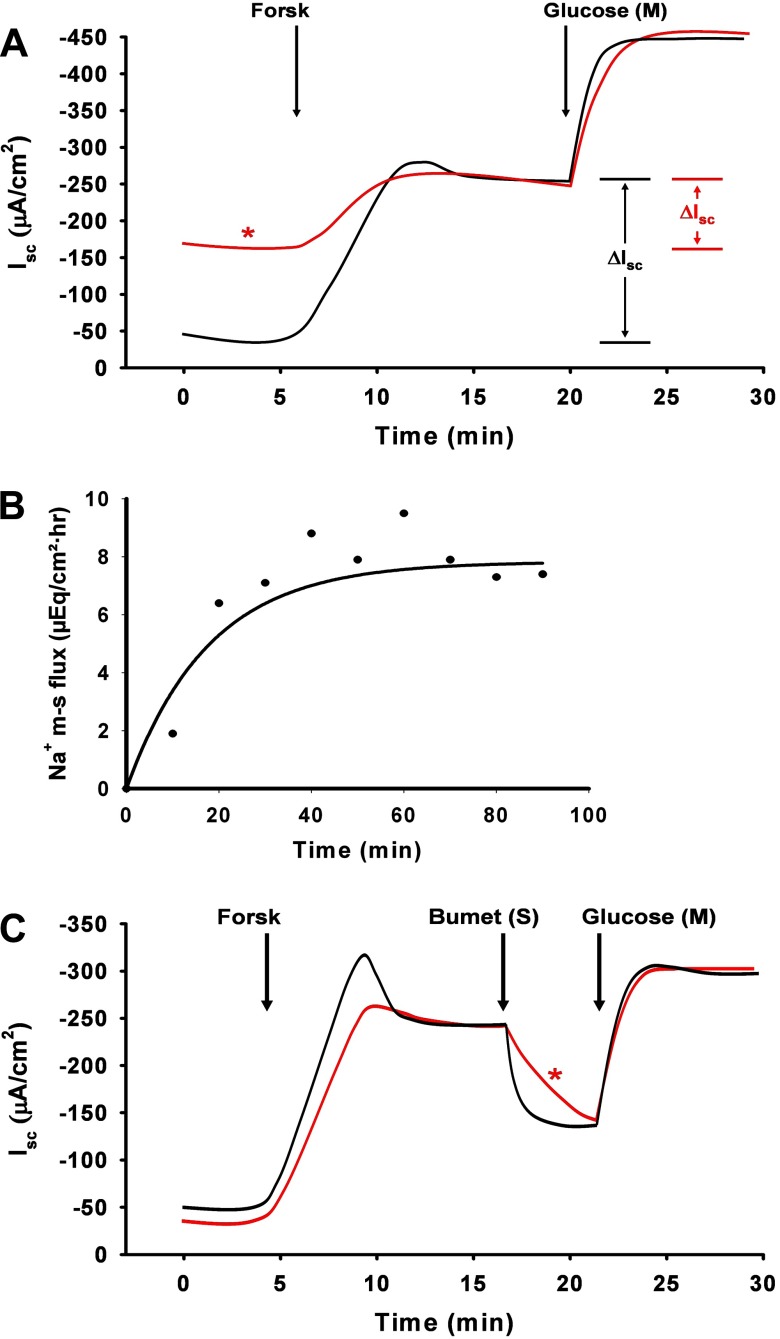
The effects of drug contamination can be insidious and, unfortunately, are often recognized only after a number of experiments yield measurements of basal transport that do not match past studies. Forskolin, ionomycin, and EIPA are among the drugs that we have found to be particularly troublesome with regard to contamination. Figure 6A shows a representation of the changes in the basal and stimulated Isc that occurred in normal mouse intestine during a series of experiments evaluating forskolin effects. In one group (black line), the Ussing chamber system was properly cleaned as above, whereas, in the second group (red line), the soft plastic tubing between the reservoirs and Ussing chambers was not changed (only washed and rinsed between experiments). Note the elevated baseline and reduced response to forskolin treatment in the second group. Another source of contamination is sequestration of reagents in small cracks or “crazing” of the inner chamber surface of the acrylic chambers that occur with repeated use. Routine inspection of the chamber under a dissection microscope enables tracking of changes in the integrity of the chamber surface.
Fig. 6.
Common problems encountered in Ussing chamber studies of murine intestine. A: representation of forskolin (Forsk) contamination in Ussing chamber apparatus. Short-circuit (Isc) measurements during sequential additions of forskolin (10 μM, mucosal and serosal addition) and glucose (10 mM, mucosal addition). Black trace shows normal Isc response to forskolin and glucose additions. *Red trace shows elevated baseline Isc and reduced response (ΔIsc) to forskolin addition attributable to forskolin contamination from previous experiments. Although the system was thoroughly rinsed and washed, soft plastic tubing was not replaced and served as the source of forskolin contamination. B: time period required for 22Na added to the mucosal bath to attain a steady-state flux into the serosal bath. Samples taken from the serosal (sink) bath after addition of 3 μCi addition of 22Na into the mucosal (source) bath attained a steady-state rate after 20–30 min. C: representation showing diffusion barrier to bumetanide (50 μM, serosal addition) attributable to the seromuscular layer. Black trace shows sequential Isc responses to forskolin (10 μM, mucosal and serosal addition), bumetanide (50 μM, serosal addition), and glucose (10 mM, mucosal addition) in murine jejunal preparation stripped of the seromuscular layers. Bumetanide effect (inhibition of electrogenic Cl− secretion) occurs within 5 min. Red trace shows identical experiment using a whole-thickness murine jejunal preparation. *Note that the bumetanide inhibition is only 40% complete at 5 min.
DRUG RESPONSE.
One of the more unanticipated aspects of Ussing chamber studies of native intestine are differences in reagent concentrations that are necessary to achieve the same effect reported for cell expression systems. For example, drug dosages to elicit transport changes in native intestine are sometimes one to three orders of magnitude greater than that reported for responses in expression systems. The reason for this difference is multifactorial. First, the native intestine has many diffusion barriers at the epithelial surfaces. On the apical membrane, both the glycocalyx at the cell surface and goblet cell secretions present a mucous barrier that limits diffusion from the bath, both by enhancing the unstirred layer effect and by binding certain compounds. Accumulation of mucus on the apical surface can often be observed macroscopically during an Ussing chamber experiment. The observable mucus layer can be removed near the end of an equilibration period (i.e., before the experiment) by applying a gentle stream of superfusate to the apical surface to dislodge the mucus followed by refreshing the apical solution. Mucolytic agents such as DL-dithiothreitol (100 μM for 5 min) can also be used, but, as a sulfhydryl reagent, control studies are necessary to ensure relevant protein function is not affected. On the basolateral membrane, the submucosa, composed of collagen and cellular elements such as fibroblasts, also presents a structural diffusion barrier that enhances the unstirred layer in a seromusculature-stripped preparation. Isotopes of univalent ions such as 22Na and 36Cl require almost 30 min to equilibrate across the epithelium before attaining a steady-state rate of flux (Fig. 6B). Thus complex compounds may require a longer period of equilibration, especially if the villous epithelium is targeted. The basolateral diffusion barrier is further limiting when the seromuscular layer is left intact although this effect can be lessened by tetrodotoxin treatment (43). Figure 6C shows a representation of the difference in the Isc responses of seromuscular-stripped and -unstripped mouse intestine to blockade of stimulated Cl− secretion by the diuretic bumetanide. Bumetanide acts by blocking NKCC1, the Na+/K+/2Cl− cotransporter that is located at the basolateral membrane in murine intestinal epithelium. Note that maximal blockade of NKCC1, as denoted by the decrease in the forskolin-stimulated Isc, occurs at 5 min in the stripped preparation but is only 40% of maximal blockade at 5 min in the unstripped preparation.
Second, the ultrastructural and microscopic architecture of the intestine also contributes to the alterations in drug responses by the epithelium. The orderly epithelial cell layer of the native intestine often produces greater responses in transport function (e.g., Isc response to forskolin) compared with polarized cell monolayers simply because of the number of available epithelial cells within the same gross surface area. However, compared with cell monolayers, the microvilli of the brush border are tightly compacted, and the interdigitation of adjacent basolateral membranes is more complex, which slows the diffusion of compounds, especially to the extracellular domains of membrane proteins. The microscopic architecture of the crypt-villus arrangement in native intestine also can alter drug action, which is typically not encountered in studies of polarized cell monolayers. Diffusion of compounds to the apical membrane of crypt epithelial cells from the luminal solution or to the basolateral membrane of the villous epithelium from the basolateral solution is reduced relative to the opposite condition (i.e., diffusion to the basolateral membrane of the crypt epithelial cell from the basolateral solution and to the apical membrane of the villous epithelial cells from the luminal solution). If drug action elicits tachyphylaxis or receptor desensitization, then the magnitude of the response to a given dose may be artificially reduced because of slow diffusion. Stripping of the seromuscular layer lessens the effect of the microscopic architecture, apparently by widening the crypt openings and villous bases. An example of this effect came from preliminary studies evaluating the effects of uroguanylin and Escherichia coli heat-stable toxin (STa) on intestinal Cl− and HCO3− secretion in mice (22). STa acts almost exclusively on apical membrane guanylate cyclase C receptors to stimulate intracellular cGMP levels and activate CFTR-dependent anion secretion, which is principally located in the crypt epithelium. Initial studies using unstripped intestinal preparations yielded inconsistent, moderate Isc responses to luminal STa treatment, whereas rapid, sustained Isc responses to luminal STa were elicited in seromusculature-stripped intestinal preparations (L.L. Clarke, unpublished observations).
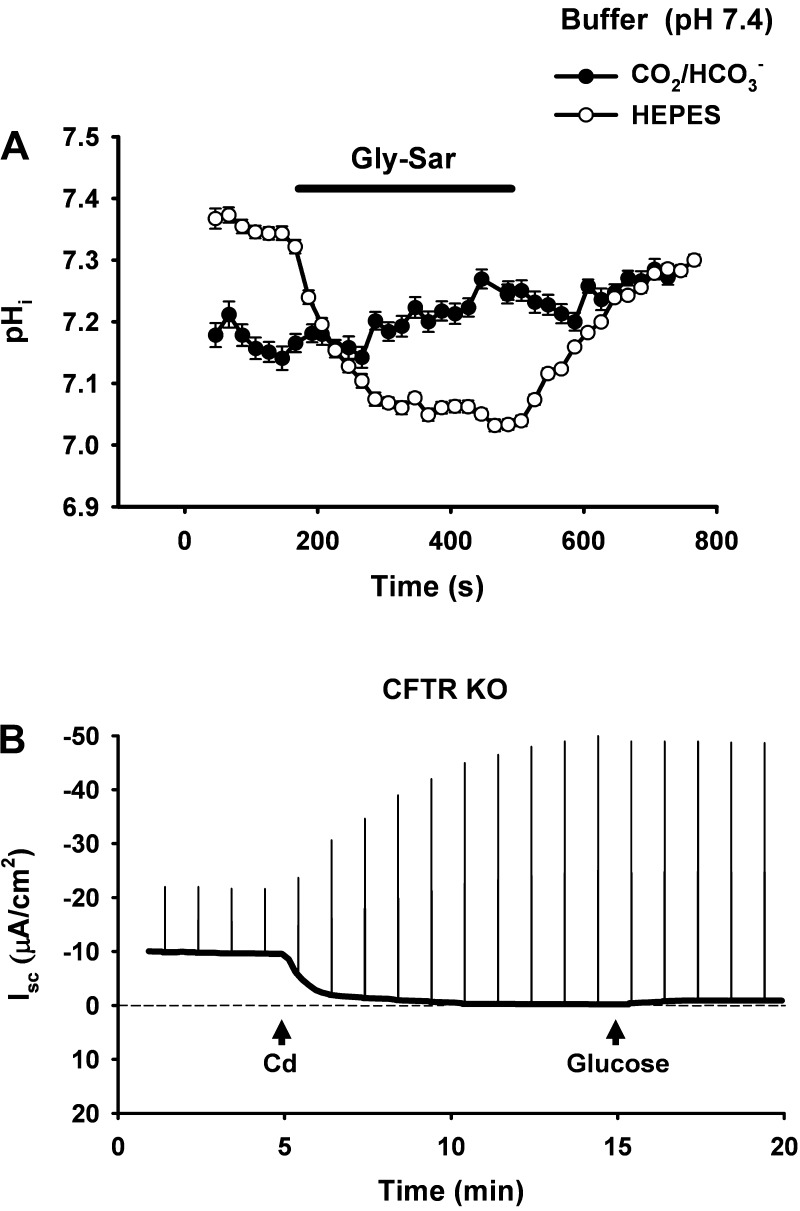
A third factor to be considered in the alteration of drug responses is the composition of physiological buffer solutions that are typically employed in Ussing chamber studies of intestine. The presence of CO2/HCO3− buffering has an important influence on drug action not only through its buffering and chaotropic characteristics but also through its contribution to the total buffering capacity of the epithelial cell (44). Because of the difficulties in sustaining CO2 tension in cell culture preparations (e.g., carbogen gassing), buffers such as HEPES, which do not contribute to the cell's buffering capacity, are used in most studies of cell expression systems. Thus reagents may elicit abrupt nonphysiological changes in cell pH that go undetected when added to superfusate buffered without CO2/HCO3−. The effect of using CO2/HCO3− buffering vs. HEPES buffering on reagent action can be demonstrated using intestinal preparations mounted in a modified Ussing chamber system for intracellular pH (pHi) measurement (see Future Uses below). For example, H+-dipeptide transport via the transporter Pept1 is an electrogenic process that stimulates the Isc in jejunal preparations (58). Figure 7A shows pHi measurements of intact murine villous epithelium during treatment with luminal application of the inert dipeptide gly-sar. At the same extracellular pH, gly-sar treatments elicit rapid acidification of the villous epithelium bathed in HEPES-buffered Ringers solution but has no effect on pHi in villous epithelium bathed in KBR solution containing CO2/HCO3− buffer. The ionic strength of a physiological Ringers solution is also a factor when comparing drug effects on native intestine with those reported for cell systems. For example, previous studies have shown that physiological levels of Na+ significantly alter the dose of EIPA required for blockade of Na+/H+ exchangers in native epithelium (parotid duct) compared with cell studies where EIPA dose-response relationships were performed in solutions with low Na+ concentration (33). Similarly, the effect of the anion transport inhibitor DIDS is more effective at blockade of the Cl−/HCO3− exchanger putative anion transporter-1 when applied in buffers containing low Cl− concentrations (∼5 mM) compared with physiological Ringers solutions with ∼120 mM Cl− concentration (16, 44, 45).
Fig. 7.
Difficulties with interpretation of Ussing chamber experiments performed using murine intestine. A: effect of nonphysiological buffer (HEPES) on changes of intracellular pH (pHi) induced by application of the Pept1 substrate (glycine-sarcosine, gly-sar). Gly-sar (25 mM) induces an Isc response in the Ussing chamber attributable to H+-dipeptide transport via Pept1. Measurement of pHi in the upper villous epithelium was performed on murine duodenum situated in a horizontal Ussing chamber. In the presence of physiological buffer, i.e., CO2/HCO3− in KBR (•), pHi is unaffected by gly-sar transport. In contrast, in the presence of HEPES-buffered Ringers solution (○), pHi becomes markedly acidic during gly-sar exposure. B: effect of sequential additions of cadmium (100 μM, mucosal addition) and glucose (10 mM, mucosal addition) on the Isc and Gt of murine jejunum. Cd2+ treatment was predicted to reduce the Isc as a means to evaluate the contribution of CLC-2 Cl− channels to the residual Isc in CFTR knockout mouse jejunum. Although the experiment could be interpreted positively on the basis of the abrupt decrease in Isc, the large increases in Gt (as indicated by the upward deflections of the Isc in response to repeating 5 mV command pulses) and the lack of response to glucose indicated metabolic insult and loss of tissue viability.
PREPARATION VIABILITY.
Upon removal from the animal, the ex vivo intestinal preparation incubated in Ringers solution has limited viability. One can reasonably expect a strongly viable mouse intestinal preparation for up to 3 h in an Ussing chamber. Viability decreases with longer time periods or during treatment with certain reagents. The magnitude of the Gt (or Rt) is one real-time indicator of viability as tissue integrity deteriorates and can be useful in discriminating between mechanism of action and nonspecific effects of reagents. For example, as shown in Fig. 7B, cadmium (Cd2+, 100 mM), which has been shown to block CLC-2 chloride channels (63), was added to the luminal bath solution to evaluate the role of CLC-2 in the residual Isc exhibited by CFTR KO small intestine (9). If one was monitoring only the Isc (dark line), application of Cd2+ was found to rapidly reduce the Isc, consistent with Cl− channel blockade. However, a repeating command voltage was imposed across the intestine as a means to monitor changes in the Gt. As shown by the increased magnitude of the resulting Isc deflections, Cd2+ also induced a rapid increase in Gt indicating an untoward effect on tissue viability. Both an inability to reverse the Cd2+ effect by washout and the subsequent absence of an Isc response to luminal glucose application (activating Na+-coupled glucose transport) confirmed loss of preparation viability. In addition to experimental time, an important consideration is the age of the mice that are used for the intestinal preparations. Mice at 6 wk of age have achieved ∼90% of their growth potential; therefore, adult mice between 2 and 4 mo of age are used for most of our studies. After 5 mo of age, we have found significant decreases in the magnitude of ion transport processes, which includes cAMP-activated anion secretion, basal NaCl absorption, and gastric HCl secretion. Thus control and treatment mice should be matched not only for sex but also age, and preferably as littermates or siblings.
LIQUID JUNCTION AND DIFFUSION POTENTIALS.
It is sometimes desirable to use solutions of different ionic composition on each side of the mucosa in an Ussing chamber experiment, e.g., to maximize the chemical gradient for an ion channel or to perform pH stat experiments in which the luminal bath is devoid of HCO3− (see pH stat). These experiments should be carefully considered since there are both technical and conceptual difficulties that are encountered that necessitate a number of assumptions. Significant imbalances in the major electrolytes (Na+, Cl−, K+) between the luminal and basolateral bath results in voltage measurement artifact. Under this condition (e.g., replacement of luminal Cl− with an impermeant anion like gluconate−), the measured voltage potential (apparent Vt) is a combination of the true Vt, the bi-ionic diffusion potential created by the imbalanced ion moving through the leaky paracellular pathway (Vp) and the liquid junction potential (Vlp). Vlp is the voltage potential resulting from the different mobilities of ions at the interfaces between different solutions (e.g., the junction of the 3 M KCl salt bridge on the side of ion substitution). Voltage clamping to 0 mV yields an Isc that is not a direct measure of active transport but includes the effect of clamping Vp and Vlp, both of which may change over time as ions contaminate the second solution. Thus the Isc under this condition has little meaning unless efforts are made to differentiate the different voltage components (for example see Ref. 2). Some useful information from the apparent Vt or Isc can be gleaned if acute experiments of agonist or antagonist actions are studied, e.g., activation of CFTR and measurement of the acute change in Isc (ΔIsc) in the absence of luminal Cl−. However, these studies require the assumption that the agonist-antagonist does not alter the permselectivity of the paracellular pathway to the imbalanced ion, or, if the experiment is internally controlled (e.g., by comparing wild-type and KO mouse intestine), that the ionic permselectivity of the paracellular pathway across the intestine of each group is identical.
Recent investigation into the molecular nature of the tight junctional complex has spawned an interest in measurements of the bi-ionic diffusion potential Vp, i.e., where the concentration of either the cation or anion of a salt is the same on each side of the mucosa, but the concentration of the counter ion on one side is different because of substitution (e.g., isethionate− for Cl−). These experiments can be used to estimate changes in permselectivity of the paracellular pathway, which is largely determined by a family of cell adhesion proteins known as claudins (28). Rows of claudins on the surface of each epithelial cell meet to form the strands of the tight junctional barrier. The expression of different members of the claudin family largely determines the size, charge, and conductance properties of the pathway through the tight junctions of epithelia. Furthermore, it is known that naturally occurring mutations in claudins can result in disease, e.g., mutations in claudin-16, which is located in tight junctions of the thick ascending limb of the loop of Henle, result in urinary magnesium loss and hypomagnesia (28). Experiments to determine Vp are best performed when the superfusate contains only one anion and one cation of equal charge (e.g., NaCl). The change in Vt (ΔVt) measured immediately after imposition of a transepithelial concentration gradient can be used to estimate the junctional permeability (P) of the cation relative to the anion, or vice versa. For NaCl, the apparent junctional selectivity (A) for Na+ relative to Cl− (i.e., PNa+:PCl−) is described by the equation ΔVt = A ln (c2/c1), where c is equal to the Na+ concentration before (c1) and after (c2) imposing the Na+ concentration gradient and A is equal to (RT/F)(PNa − PCl)/(PNa + PCl), where R is the gas constant, T is the absolute temperature (°K), and F is the Faraday constant (38). For quantitative measurements of Vp, efforts to minimize or estimate the true Vt may be necessary, which often requires transporter blockade or transporter KO mice. Additionally, correction must be made for liquid junction potentials. The magnitude of Vlp typically falls between 2 and 12 mV for most physiological solutions (32). For Ussing chamber experiments, the Vlp can be estimated by placing a short 3 M KCl salt bridge between the half-chambers at the solution interface (instead of the mucosa). During measurement of Vt, a switch is made from identical solutions on each side to the test solution on one side of the central bridge. The ΔVt is an empirical estimate of the Vlp and can be compensated in subsequent experiments by appropriately altering the offset voltage function of the voltage clamp. Early but elegant examples of experiments measuring bi-ionic diffusion potentials across epithelia are given in Refs. 34 and 37.
PHYSICAL IMBALANCES.
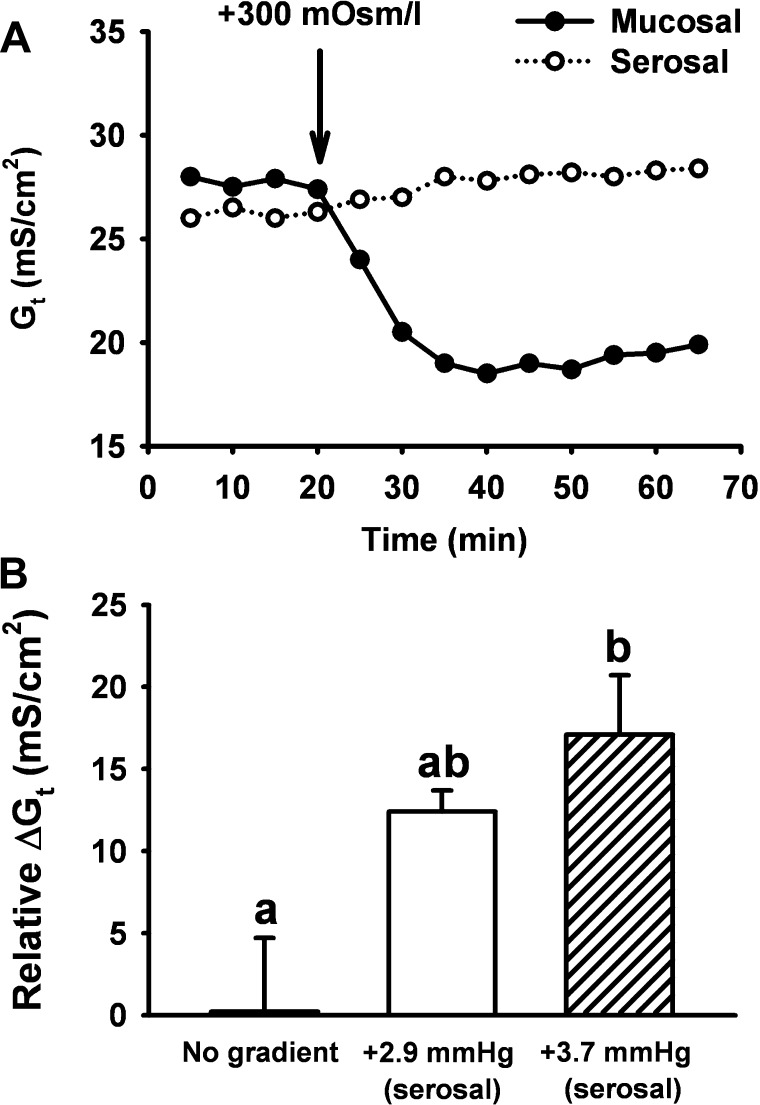
As discussed previously, the Gt of an intestinal preparation is largely a measure of paracellular conductance, which is limited by both the tight junctional complex and the relative volume of the lateral intercellular space LIS. The intestinal epithelial LIS is altered by physical imbalances that demonstrate “sideness” with regard to the effect on Gt (15). Hyperosmotic conditions not only induce cell shrinkage but have different effects on Gt depending on whether the mucosal or serosal side of the epithelial preparation is exposed to a hypertonic solution (47). As shown by the experiment on murine intestine depicted in Fig. 8A, hypertonicity of the luminal bath abruptly collapses the LIS and decreases Gt, whereas hypertonicity of the basolateral bath distends the LIS and moderately increases Gt (electron micrograph of changes in LIS morphology for the latter effect is shown in Ref. 15). Thus a fortunate “valve” effect is created in which extracellular fluid loss via the paracellular pathway is minimized during luminal hypertonicity of the intestine, whereas gain of extracellular fluid can result from basolateral hypertonicity, i.e., dehydration. Small hydrostatic pressure imbalances also affect Gt. As shown in Fig. 8B, a hydrostatic pressure difference of <3 mmHg on the basolateral side of the murine intestinal mucosa increases Gt. This latter effect is consistent with alterations in submucosal Starling forces that accompany conditions resulting in filtration secretion diarrhea. Thus any departure from the balanced physical conditions of an Ussing chamber experiment requires consideration of LIS dynamics in the interpretation of data, especially with regard to studies addressing the barrier function of the intestine.
Fig. 8.
Effects of unbalanced physical forces on the Gt of murine intestine in Ussing chamber experiments. A: effect of hypertonic medium (+300 mOsm/l mannitol) in either the mucosal (•) or serosal (○) bath on the Gt of murine jejunum. Note that mucosal hypertonicity results in an abrupt decrease in Gt attributable to the collapse of the lateral intercellular spaces, whereas serosal hypertonicity moderately increases Gt. B: effect of small increases in serosal hydrostatic pressure on the Gt of murine jejunum. Either 0 (no gradient), +2.9, or +3.7 mmHg was applied by increasing the fluid height of the serosal bath for 10 min on mouse jejunum in the Ussing chamber. Changes in Gt under all conditions were adjusted for the mean decrease in Gt (−9.9 mS/cm2) that normally occurs when no hydrostatic pressure gradient is applied. a,bMeans with different letters are significantly different (P < 0.05). [Modified from Gawenis et al. (15).]
Special Uses of the Ussing Chamber
Isotopic flux measurements.
One of the most useful aspects of the Ussing chamber is the capability for isotopic or fluorescent tracer flux measurements. Flux studies measure the steady-state rate of transfer of an electrolyte or other substrate across the epithelium from the luminal bath to the basolateral bath or vice versa. These measurements provide an undeniable standard that serves as the starting point for investigation into the mechanism of transfer or its alterations by pharmacological or pathological action. Most studies employ isotopic tracers due to the desirable properties of specificity and sensitivity of measurement. However, the safe handling and clean up of radioactive materials adds a laborious aspect to their use. Present interests focused on intestinal permeability in disease have resulted in an increasing number of studies that use fluorescent tracers often of specific size or composition to probe the permeability characteristics of the paracellular pathway (16, 23).
Isotopic flux measurements of murine intestinal mucosa involve the basic set up and principles of the Ussing chamber as described above. Bidirectional flux studies measure isotopic flux from the mucosal bath to the serosal bath (Jms) and vice versa (Jsm), which enables the calculation of net flux using the equation: Jms − Jsm = Jnet. Thus net absorption of an isotope is indicated by +Jnet, whereas net isotope secretion is indicated by −Jnet. Since different isotopes of the same ion or substrate are often not available, bidirectional flux studies involve measuring the unidirectional flux (i.e., Jms or Jsm) on different intestinal preparations from the same mouse. Since the Gt of intestinal preparations is >90% paracellular conductance, significant bias in the determination of Jnet occurs if one of the unidirectional measurements is performed on a preparation with a significantly greater Gt compared with the opposite unidirectional measurement. Thus a critical aspect is that the mucosal preparations must be paired on the basis of similar Gt. For large intestine, 25% difference in Gt as a paired preparation is acceptable, whereas for the leakier small intestine a 15% difference in Gt is used for pairing. However, small intestine studies are advantageous in that a greater number of preparations can be studied from the same mouse (e.g., 3 Jms and 3 Jsm preparations), which increases the likelihood of obtaining at least one pair from each mouse.
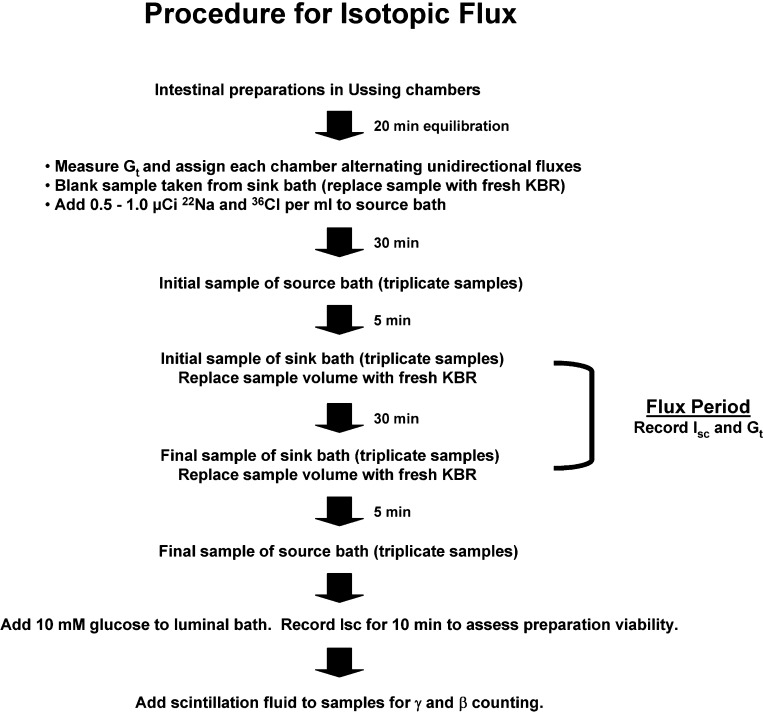
The sequence of a typical isotopic flux experiment in which 22Na and 36Cl tracers are used is shown in Fig. 9. The sequence begins after seromuscular-stripped mucosal preparations are mounted in the Ussing chambers for ∼20 min to allow TTX blockade of neural function (if desired). First, Gt is measured and each preparation is assigned either Jms or Jsm direction in an alternating pattern from the preparation with lowest Gt to the one with highest Gt. Since the Gt of a preparation may change during an experiment (especially if two flux periods with an intervening treatment are performed), assignment of this pattern yields more successful pairs by the end of the experiment. Second, before isotope addition, a sample is taken from the “sink” side (e.g., the serosal bath in a Jms measurement) to serve as a “blank” for background radiation determination in counting and to ensure that that the chamber is not contaminated with isotope from previous use. After the blank sample, isotopes are added to the “source” side (e.g., the mucosal bath in a Jms measurement). Approximately 0.5–1.0 μCi/ml provides a specific activity in the source bath of murine intestinal preparations to ensure that initial samples taken from the sink side will have sufficient counts/min (cpm) to be at least 10× above background radiation. The source bath should contain “cold” ion or substrate to minimize the effects of nonspecific binding to glass or plastic in the apparatus. The source specific activity is determined from the cpm of samples taken at the beginning and end of the experiment (normalized to 1 ml), using the following formula: specific activity [disintegrations/min(dpm)/μeq or dpm/μmol] = sample dpm ÷ μeq ion (or μmol substrate), where sample dpm = sample cpm/counter efficiency. Counter efficiency (expressed as the fraction cpm/dpm) is calculated by dividing the cpm of a known quantity of isotope (in μCi) by the calculated dpm, which is equal to the μCi × 2.22 × 106 dpm/μCi (1 μCi = 2.22 × 106 dpm). The μeq ion (or μmol substrate) is the total content (cold and hot) of the ion or substrate in 1 ml of the source solution.
Fig. 9.
Flow chart for performing 22Na36Cl flux studies of murine intestine.
Third, an equilibration period is necessary to allow a steady-state flux of the isotope across the mucosa into the sink bath. To determine the length of the equilibration period for the segment of interest, preliminary studies are necessary in which timed samples are taken from the sink bath over an ∼1-h period beginning immediately after isotope addition to the source bath (for example, see Fig. 6B). Surprisingly, we have found differences in the rate of unidirectional equilibration for Jms (30 min) vs. Jsm (40 min) for murine intestinal preparations with the seromuscular layer intact, whereas the rate of equilibration is the same (∼30 min) in both Jms and Jsm directions for seromuscular-stripped preparations. Routinely, we provide a 30-min equilibration period before starting a flux period.
Fourth, a flux period is performed by sampling from the sink bath at the beginning and end of a timed period. Longer time periods for flux measurements are more accurate (36), so we typically use a 30-min period. To avoid hydrostatic pressure differences across the mucosa during a flux period, samples taken from the sink side are replaced with identical volumes of bath medium but require that the flux calculation be corrected for dilution of the end sample. Alternatively, samples from both the source and sink sides can be taken and not replaced, provided that sufficient superfusate is maintained and the flux calculation is corrected with each sample. Since flux experiments often require 2–3 h, it is necessary to place a glass or plastic condenser over the reservoir to minimize evaporative water loss. Depending on the treatment and the viability of the preparation, time control experiments are often necessary to ensure accurate accounting of treatment effects.
The final step is calculation of isotopic flux from the sample measurements. The method we use for transepithelial flux calculation was described by Schultz and Zalusky (40). The unidirectional flux across each preparation in μeq/cm2 of intestinal surface area·h is calculated from the equation: Jms (or Jsm) = V × (S2 - S1 × dil)/(specific activity × surface area × time), where V is the volume of sink superfusate (in ml); S1 and S2 are the dpm/ml of samples taken at the beginning and end of the flux period, respectively; dil is the dilution of the sink superfusate resulting from sample fluid replacement; specific activity is the specific activity of the isotope in the source bath (dpm/μeq); surface area is the area of exposed mucosa, i.e., aperture area in cm2; and time is the length of the flux period in hours. During a flux period, Isc and Gt are recorded and each averaged. The mean Gt is used to make the final pairing of the unidirectional fluxes after an experiment where all preparations are from the same mouse. When an electrogenic ion transport process is the subject of study, the magnitude of mean Isc can be compared with the rate of ion flux by converting current to flux units. The movement of an equivalent of univalent ions (eq) is approximately equal to the flow of one faraday of electrons (95,485 electrons). The Isc in amperes (A) can be expressed as coulombs (C)/s, which is divided by F (coulombs/equivalent) to yield the rate of univalent ion flux.
Thus Isc = μA/cm2 = μA (μC/s) × 1/cm2 × 1/F (μC/μeq) × 3,600 s/h = μeq (or μmol)/cm2·h, which simplifies to Isc = μA/cm2 × 0.0373= μeq (or μmol)/cm2·h.
If 22Na and 36Cl tracers are used simultaneously in a flux study, then it is necessary to correct the dpm of β-emitting 36Cl for “22Na spillover” (fNaspill, i.e., fraction of 22Na spillover). The 22Na spillover results from γ-radiation from 22Na that activates the scintillation cocktail of the sample and, therefore, must be subtracted from the β cpm of the sample to achieve an accurate measurement of 36Cl. The scintillation spectrum of 22Na spillover overlaps the scintillation spectrum for β emission by 36Cl. The fNaspill can be minimized by reducing the amount of 22Na used in the study and by focusing the acquisition window settings of the liquid scintillation counter for the 36Cl β emission spectrum. However, the residual fNaspill still introduces a significant error in the 36Cl measurement. Correction requires determination of fNaspill by measuring both the β and γ dpm of a 22Na standard, i.e., a known quantity of 22Na, in liquid scintillation cocktail. The fNaspill is calculated by dividing the β dpm by the γ dpm of the 22Na standard. All flux samples (plus a 36Cl standard for the liquid scintillation counter) are measured for γ and β dpm. The γ dpm of each sample is used not only for the determination of the 22Na activity but is also multiplied by the fNaspill, and this quantity is subtracted from the β dpm to yield actual 36Cl activity. With conditions optimized for our scintillation counter and technique, we find that the fNaspill is ∼0.15. A comparison of this method with other methods for simultaneous measurement of 22Na and 36Cl in aqueous samples has been provided by Rangachari and McWade (36).
pH stat.
The pH stat technique is used to measure the flux of H+ (acid) or HCO3− (base) across the intestinal mucosa in the Ussing chamber. Typically, the practice of this method involves the removal of buffers (HCO3−, K2HPO4, and KH2PO4 in KBR) from either the mucosal or serosal bath, but one should be mindful that this violates one of the basic principles of the Ussing chamber, i.e., balanced solution composition across the mucosa. The reason for using a buffer-free solution (e.g., saline) is that the elaboration of H+ or HCO3− into the bath medium on the side of interest must be accurately measured to achieve continuous titration at a certain pH value, i.e., clamping pH typically at pH 7.0 or 7.4. This deviation from the classical Ussing chamber method requires several considerations that can be illustrated by a description of the pH stat technique used to measure the secretory flux of HCO3− (JsmHCO3−) across murine small intestine. In the measurement of JsmHCO3−, the removal of HCO3− from the mucosal bath establishes a concentration gradient (25 mM) since the serosal bath typically contains standard KBR plus glucose gassed with 95% O2-5% CO2, pH 7.4. Fortunately the permeability of the paracellular pathway to HCO3− is fairly low in the small intestine, and the small paracellular contribution to JsmHCO3− is relatively unchanged by moderate differences in the Gt of duodenal preparations (± 10 mS/cm2) (53). An important factor in these measurements is the requirement to constantly displace CO2 (solubility ∼1.1 g/kg water, 37°C) from the unbuffered solution with a less soluble gas to minimize the pH effects from spontaneous hydration of CO2. Thus the luminal solution is vigorously gassed with 100% O2 (solubility ∼0.03 g/kg water, 37°C) or 80% N2 (solubility ∼0.01 g/kg water, 37°C)-20% O2 mixture (simulating air) to minimize the partial pressure of CO2 in solution. If measurements of HCO3− secretion in the absence of transepithelial gradients of CO2/HCO3− are desired, the reader is referred to an in vitro method developed by Feldman et al. (13).
The flux of acid or base equivalents is estimated by the rate of base or acid addition, respectively. For example, JsmHCO3− into the unbuffered luminal bath is determined by number of acid equivalents of titrant (e.g., 5 mM HCl) necessary to maintain the pH at 7.4 during a timed flux period. Clamping to a predetermined pH value can be accomplished using an automatic titration burette system (e.g., Radiometer America PHM290 pH stat controller/ ABU900 autoburette) or manually with a pipette. An important consideration is that pH measurement of real-time changes in acid or base secretion is delayed by the time necessary to distribute the secreted equivalents or the titrant additions throughout the volume of the chamber. As shown in Fig. 10A, even automated titration results in a series of pH excursions about the target pH attributable to the mixing necessary to distribute secreted HCO3− or added titrant. Hence single time points (e.g., “peak” secretion) may misrepresent the data since pH stat algorithms cannot accurately predict titrant addition during rapid changes in acid-base secretion, and calculation of the flux rates from short time periods further amplifies the magnitude of this error. pH measurement also lags behind the instantaneous measurements of the Isc, which can result in an artificial incongruity between acute Isc and JsmHCO3− responses. An example of incongruity between the time course of the Isc and electrogenic JsmHCO3− during an acute response of murine duodena to cAMP stimulation is shown in Fig. 10B. Therefore, measurement of acid-base flux should be performed during a steady-state flux period. We typically perform 30-min basal flux periods, allowing 15–20 min for agonist-antagonist responses to attain a steady-state before a second 30 min flux period. However, multiple flux periods for pH stat of native intestine are often problematic because of the accumulation of mucus at the mucosal surface that affects the action of some drugs. In these instances, accumulated mucus on the mucosal surface is removed before a flux period, allowing enough time for the rate to return to steady state.
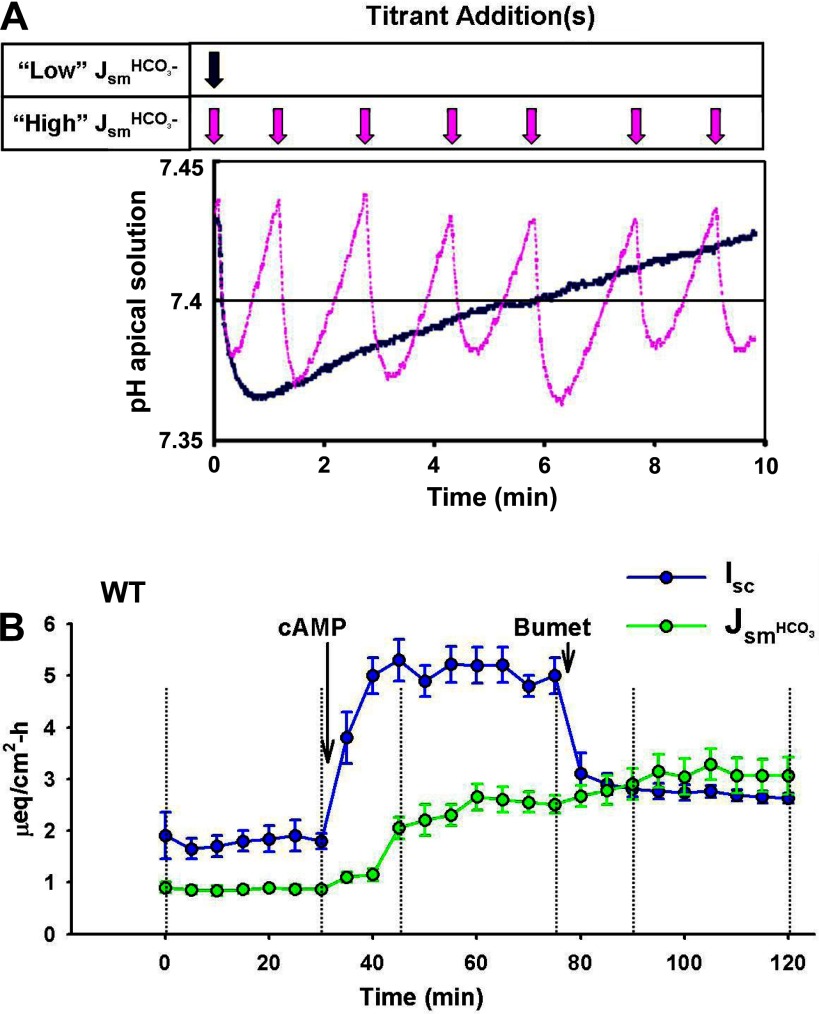
Fig. 10.
A: changes in pH of an unbuffered luminal solution during pH stat measurements of fast and slow rates of HCO3− secretion. Traces showing changes in pH of the luminal solution during pH stat studies. Note the pH excursions about the target pH 7.4 (solid line) for an epithelium with high HCO3− secretory flux (Jsm) (pink trace and arrows) and low HCO3− secretory flux (blue trace and arrow). [Modified from Krouse et al. (24).] B: pH stat measurements of HCO3− secretion (JsmHCO3−) and Isc across WT murine duodenum during sequential treatments with forskolin (cAMP; 10 μM, mucosal and serosal addition) and bumetanide (Bumet; 50 μM, serosal addition). Compare the rapid increase in Isc (blue line and symbols) with the slower changes in JsmHCO3− (green line and symbols) after cAMP treatment. The different time courses for Isc and JsmHCO3− demonstrate the lag in pH measurement attributable to mixing of the superfusate. Bumetanide treatment inhibits Cl− secretion and reduces the Isc without affecting the JsmHCO3−. Vertical dashed lines indicate 3 successive steady-state flux periods (30 min each). n = 5–7 duodenal preparations.
The unbuffered solutions used in pH stat experiments are typically saline or saline-type solutions that are similar in composition to KBR. The replacement of NaHCO3 in these solutions is a troublesome aspect in that the ideal replacement anion should not demonstrate appreciable buffering properties nor alter transport function. Unfortunately, these characteristics are not demonstrated by most physiologically nontoxic Na+ salts. One method typically used is the replacement of NaHCO3 and K2HPO4/KH2PO4 with NaCl and KCl, respectively. Although this establishes a mucosal-serosal Cl− concentration gradient (opposite the HCO3− gradient), the magnitude of the Cl− gradient is unlikely to significantly affect Cl− transport. Another alternative is to replace NaHCO3− with a poorly permeable anion such as isethionate− or gluconate−. However, gluconate has a low pKa (3.86), resulting in significant buffering capacity at pH 7.0 or 7.4. In contrast, Na+ isethionate is an alkali salt, which, at this concentration, yields a pH of ∼8 and therefore exhibits much less buffering capacity at pH 7.4. The use of an essentially unbuffered solution in the pH stat method requires two additional considerations. First, rapid titration to the target pH may result in transient exposure of the epithelium to an adverse pH, which may elicit a physiological response. In pH stat studies of the duodenum, we have found that titration with NaOH to rapidly attain pH 7.4 will result in a prolonged cessation of HCO3− secretion. Since most preparations seem to initially acidify the mucosal bath (likely due to Pco2 from the dissection bath that has penetrated the mucus layer), we allow HCO3− secretion of the preparation sufficient time to alkalize the medium to pH 7.4 before a flux period, which improves reproducibility. Second, the addition of agonists-antagonists, even in small quantities, to the unbuffered mucosal bath may have drastic effects on the bath pH; if possible, the drug solution should be neutralized before application.
Electrical field stimulation.
One of the more novel uses of the Ussing chamber technique has been electrical field stimulation, a technique largely pioneered by Helen J. Cooke and colleagues, which is used to investigate regulation of ion transport by intramural neurons. The method involves the placement of opposing foil electrodes on the serosal half-chamber, which enable the passage of low intensity currents in the plane of the submucosa of the intestinal preparation. Without directly altering the Isc of the preparation, en masse activation of ganglionic cell bodies is possible. Stimulation using 2.5 to 4.0 mA at frequencies in the 5–10 Hz range causes maximal increases in the Isc (primarily attributable to anion secretion), which returns to basal levels after cessation of the stimulus. Studies of acetylcholine release by enteric neurons have shown that stimulation at low frequency (0.1 Hz) induces maximal release from the myenteric plexus, whereas high frequencies (5–10 Hz) induce maximal release from the submucosal plexus (21, 60). The technique of electrical field stimulation combined with specific receptor blockade has been instrumental in elucidating not only cholinergic regulation but also other neurotransmitters (e.g., VIP, tachykinin, histamine) involved in the regulation of intestinal ion transport. A description of the technique and an excellent review of the role that electric field stimulation has played in dissecting the various neurotransmitters controlling ion and water transport in the intestine has been provided by HJ Cooke and RA Reddix (10). Application of this method to intestine from genetically manipulated mouse models (e.g., receptor KO) should provide new routes of discovery in understanding neural regulation of ion transport in the intestine.
Intestinal restitution and barrier function.
The phenomenon of epithelial restitution involves the rapid dedifferentiation of columnar epithelial cells near a site of superficial injury for the purpose of covering the submucosa and reestablishing barrier function. Early work on epithelial restitution was performed using in vivo intestinal preparations, which seemed necessary given the physiological complexity of the process (25). However, the Ussing chamber provides an organ culture system that sustains viability of the intestinal preparation for hours and therefore has been adapted to studies of barrier damage and restitution. Superficial damage to intestinal epithelium has been induced both in vitro in the Ussing chamber (17) and in vivo followed by intestinal resection for Ussing chamber studies (29). There are clear advantages afforded by the Ussing chamber method including the ability to continuously monitor the Gt or its inverse, transepithelial electrical resistance, as barrier integrity is restituted (although caution must be applied to interpretation of this measurement; see transepithelial conductance). Furthermore, the Ussing chamber provides the opportunities to measure changes in transport properties and manipulate the restitution process through various reagent treatments. The application of this technique to the intestine from genetically manipulated mouse models provides a powerful means of elucidating the key molecular constituents in this physiological process (29).
Strengths and Weaknesses of the Method
The principle strength of the Ussing chamber technique is that it provides a short-term organ culture method that enables precise measurement of electrical and transport parameters of intact, polarized intestinal epithelium. Although the technique has been in existence for over 50 years, its utility continues to be relevant in the postgenomic era. The discoveries made possible by amplification of gene expression in heterologous systems require justification in a physiological context. The Ussing chamber method provides this transition whereby newly discovered reagents or genetic manipulations in mice can be evaluated in native intestinal epithelium.
The principle weakness of the Ussing chamber technique lies in the interpretation of a relatively small number of measurements to describe the complex physiological system of the intestinal mucosa. The intestinal mucosa contains many cell types communicating through a variety of systems, e.g., cell-to-cell contact and paracrine humoral agents, which may not be discerned or even considered during Ussing chamber measurements. The native epithelial cell expresses its full complement of ion transporters available to compensate for or otherwise alter the responses of a transporter in an ostensibly focused investigation. For example, the assignment of an ionic current to either the apical or basolateral membrane of the epithelial cell often requires extensive conceptual and experimental considerations. Another concern often raised with the Ussing chamber method relates to the limited viability and optimal function of an ex vivo intestinal preparation.
Despite the number of limitations, progress in Ussing chamber studies is possible through the application of specific inhibitors, transgenic mice, and, as past successes have taught us, deductive reasoning. Many of the technical difficulties with Ussing chamber methodology have been overcome by the introduction of modular systems (for example, Fig. 3) that make the technique more widely accessible. The cautionary note here is that the positive result (e.g., Fig. 7B) or the exciting discovery may be misleading and, on closer inspection, can often be explained by well-documented phenomena. Thus experience and strict adherence to the basic principles of the Ussing chamber method are valuable in the proper application of the technique.
Future Uses
In essence, the Ussing chamber technique has provided life sciences with an important conceptual paradigm. Far beyond Ussing's landmark discovery that the Isc of frog skin is a Na+ absorptive current, our present understanding of how to handle intestinal mucosa, preserve epithelial polarity, and sustain viability in ex vivo studies can largely be traced to discoveries made using the Ussing chamber technique. This knowledge serves as the basis for designing chambers that enable the study of individual epithelial cells within the native intestinal mucosa. Although the measurement of cell membrane potential and resistances using intracellular microelectrode and modified patch-clamp techniques were early cell-based assays, the application of fluorometry and confocal microscopy are furthering the study of transport and other functions of individual epithelial cells in situ. Horizontal Ussing-type chambers allowing superfusion of both mucosal and serosal surfaces of intestinal preparations have been developed to enable imaging of cell function. For example, as illustrated by the method shown in Fig. 11, microfluorimetry of intracellular pH using cell-permeant fluorescent dyes combined with KO mice allows mapping of acid-base transporter function in epithelial cells of the lower and upper regions of individual villi (1, 45, 54). Similar studies, but employing confocal microscopy, have described the pH regions established by acid-base transporters at the surface of the apical and basolateral membranes of colonic surface epithelium (30). The application of fluorescently labeled transporter proteins can provide in situ localization to specific cell types, although limits even in the best of optical systems prevent adequate resolution of important events occurring over short distances such as trafficking of transporters between the cell surface and submembrane vesicles. Nonetheless, together with the advantages of genetically manipulated mouse models, new advances in the imaging of cell functions applied with the Ussing chamber method will further our progress in the quest to understand the integration of cellular components in performing the complex functions of intestinal epithelium.
Fig. 11.
Adaptation of microfluorimetry to measurement of pHi in intact villous epithelium of murine jejunum using a horizontal Ussing chamber system. A: duodenal villi loaded with BCECF, a pH-sensitive ratiometric dye, and observed using a water-immersion objective. B: higher magnification of a single villus immobilized for pHi measurements using a nylon mesh overlay (not seen). C: higher magnification of upper villous epithelium with regions of interest for capture of fluorescence emission. [Reprinted by permission from Simpson et al. (44).]
GRANTS
Our studies mentioned in this review were funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK48816, to L. Clarke) and the Cystic Fibrosis Foundation Therapeutics (CLARKE06XX0).
Acknowledgments
The author acknowledges helpful discussions with Nancy M. Walker (University of Missouri, Dalton Cardiovascular Research Center) and Dr. Lara R. Gawenis (Department of Physiology, University of Utah Medical Center).
REFERENCES
- 1.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acute adaptive cellular base uptake in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 280: G1083–G1092, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Argenzio RA, Whipp SC. Effect of theophylline and heat-stable enterotoxin of Escherichia coli on transcellular and paracellular ion movement across isolated porcine colon. Can J Physiol Pharmacol 61: 1138–1148, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Binder HJ, Rawlins CL. Electrolyte transport across isolated large intestinal mucosa. Am J Physiol 225: 1232–1239, 1973. [DOI] [PubMed] [Google Scholar]
- 4.Binder HJ, Sandle GI. Electrolyte absorption and secretion in the mammalian colon. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1987, pp. 1389–1418.
- 5.Bookstein C, Depaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchanger, NHE-1 and NHE-3, of rat intestine. J Clin Invest 93: 106–113, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukhave K, Rask-Madsen, J. Saturation kinetics applied to in vitro effects of low prostaglandin E2 and F2α concentrations on ion transport across human jejunal mucosa. Gastroenterology 78: 32–42, 1980. [PubMed] [Google Scholar]
- 7.Chang EB, Rao MC. Intestinal water and electrolyte transport: Mechanisms of physiological and adaptive responses. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1994, pp. 2027–2081.
- 8.Clarke LL, Grubb BR, Gabriel SE, Smithies O, Koller BH, Boucher RC. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science 257: 1125–1128, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol Gastrointest Liver Physiol 270: G259–G267, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Cooke HJ, Reddix RA. Neural regulation of intestinal electrolyte transport. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1994, pp. 2083–2132.
- 11.Dobson JG, Kidder GW. Edge damage effect in in vitro frog skin preparations. Am J Physiol 214: 719–724, 1968. [DOI] [PubMed] [Google Scholar]
- 12.Donowitz M, Tse M. Molecular physiology of mammalian epithelial Na+/H+ exchangers NHE2 and NHE3. In: Gastrointestinal Transport: Molecular Physiology, edited by Barrett KE and Donowitz M. San Diego, CA: Academic, 2001, pp. 437–498.
- 13.Feldman GM, Berman SF, Stephenson RL. Bicarbonate secretion in rat distal colon in vitro: a measurement technique. Am J Physiol Cell Physiol 254: C383–C390, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Frizzell RA, Schultz SG. Ionic conductances of extracellular shunt pathway in rabbit ileum: influence of shunt on transmural sodium transport and electrical potential differences. J Gen Physiol 59: 318–337, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gawenis LR, Boyle KT, Palmer BA, Walker NM, Clarke LL. Lateral intercellular space volume as a determinant of CFTR-mediated anion secretion across small intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 286: G1015–G1023, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl−/OH− exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gookin JL, Galanko JA, Blikslager AT, Argenzio RA. PG-mediated closure of paracellular pathway and not restitution is the primary determinant of barrier recovery in acutely injured porcine ileum. Am J Physiol Gastrointest Liver Physiol 285: G967–G979, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grubb BR Ion transport across the jejunum in normal and cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 268: G505–G513, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Hille B Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer, 1984, pp. 1–20.
- 20.Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3− secretion. Am J Physiol Gastrointest Liver Physiol 272: G872–G878, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Javed N, Cooke HJ. Acetylcholine release from colonic submucous neurons associated with chloride secretion in the guinea pig. Am J Physiol Gastrointest Liver Physiol 262: G131–G136, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Joo NS, London RM, Kim HD, Forte LR, Clarke LL. Regulation of intestinal Cl− and HCO3− secretion by uroguanylin. Am J Physiol Gastrointest Liver Physiol 274: G633–G644, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Kovbasnjuk ON, Bungay PM, Spring KR. Diffusion of small solutes in the lateral intercellular spaces of MDCK cell epithelium grown on permeable supports. J Membr Biol 175: 9–16, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Krouse ME, Talbott JF, Lee MM, Joo NS, Wine JJ. Acid and base secretion in the Calu-3 model of human serous cells. Am J Physiol Lung Cell Mol Physiol 287: L1274–L1283, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Lacy ER Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol 10, Suppl 1: S72–S77, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Lindemann B Hans Ussing, experiments and models. J Membr Biol 184: 203–210, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lucas ML Amendments to the theory underlying Ussing chamber data of chloride ion secretion after bacterial enterotoxin exposure. J Theor Biol 234: 21–37, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Ma TY, Anderson JM. Tight junctions and the intestinal barrier. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. London, UK: Academic, 2006, pp. 1559–1594.
- 29.Moeser AJ, Nighot PK, Ryan KA, Simpson JE, Clarke LL, Blikslager AT. Mice lacking the Na+/H+ exchanger 2 have impaired recovery of intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 295: G791–G797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montrose MH, Chu S. Transepithelial SCFA gradients regulate polarized Na/H exchangers and pH microdomains in colonic epithelia. Comp Biochem Physiol A 118: 389–393, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest 110: 1739–1747, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neher E Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Park K, Olschowsk JA, Richardson LA, Bookstein C, Chang EB, Melvin JE. Expression of multiple Na+/H+ exchanger isoforms in rat parotid acinar and ductal cells. Am J Physiol Gastrointest Liver Physiol 276: G470–G478, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Powell DW Intestinal conductance and permselectivity changes with theophylline and choleragen. Am J Physiol 227: 1436–1443, 1974. [DOI] [PubMed] [Google Scholar]
- 35.Powell DW Intestinal water and electrolyte transport. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1987, pp. 1267–1306.
- 36.Rangachari PK, McWade D. Simultaneous measurement of 22Na and 36Cl in aqueous samples: a comparison of three different methods. Am J Physiol Gastrointest Liver Physiol 252: G436–G438, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Reuss L, Finn AL. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol 25: 115–139, 1975. [DOI] [PubMed] [Google Scholar]
- 38.Reuss L, Simon B, Cotton CU. Pseudo-streaming potentials in Necturus gallbladder epithelium. J Gen Physiol 99: 317–338, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultheis P, Clarke LL, Meneton P, Miller M, Soleimani M, Gawenis LR, Riddle T, Duffy J, Doetschman T, Wang T, Giebisch G, Aronson P, Lorenz J, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Schultz SG, Zalusky R. Ion transport in isolated rabbit ileum. I. Short-circuit current and Na fluxes. J Gen Physiol 47: 567–584, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-,cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 505: 411–423, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellin JH Intestinal electrolyte absorption and secretion. In: Gastrointestinal Disease, edited by Sleisenger MH and Fordtran JS. Philadelphia, PA: W. B. Saunders, 1998, pp. 954–976.
- 43.Sheldon RJ, Malarchik ME, Fox DA, Burks TF, Porreca F. Pharmacological characterization of neural mechanisms regulating mucosal ion transport in mouse jejunum. J Pharmacol Exp Ther 249: 572–582, 1988. [PubMed] [Google Scholar]
- 44.Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Smith C, Marks AD, Lieberman M. Metabolism of the eicosanoids. In: Basic Medical Biochemistry. Philadelphia, PA: Lippincott, Williams and Wilkins, 2005, pp. 654–667.
- 47.Smulders AP, Tormey JM, Wright EM. The effect of osmotically induced water flows on the permeability and ultrastructure of the rabbit gallbladder. J Membr Biol 7: 164–197, 1972. [DOI] [PubMed] [Google Scholar]
- 48.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Spring KR Epithelial fluid transport—a century of investigation. News Physiol Sci 14: 92–100, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Turnberg LA, Bieberdorf FA, Morawski SG, Fordtran JS. Interrelationship of chloride, bicarbonate, sodium and hydrogen transport in human ileum. J Clin Invest 49: 557–567, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc 1: 38–41, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Vanoye CG, Altenberg GA, Reuss L. Inhibition of P-glycoprotein-mediated transport by a hydrophobic contaminant in commercial gluconate salts. Am J Physiol Cell Physiol 276: C1439–C1442, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Walker NM, Flagella M, Gawenis LR, Shull GE, Clarke LL. An alternate pathway of cAMP-stimulated Cl− secretion across the NKCC1-null murine duodenum. Gastroenterology 123: 531–541, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135: 1645–1653, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut 56: 6–8, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welsh MJ, Tsui LC, Boat TF, Beaudet AL. Cystic fibrosis. In: Metabolic and Molecular Basis of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Valle D, and Fredrickson DS. New York: McGraw Hill, 1995, pp. 3799–3863.
- 58.Winckler C, Breves G, Boll M, Daniel H. Characteristics of dipeptide transport in pig jejunum in vitro. J Comp Physiol [B] 169: 495–500, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 261: 32–43, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Yau W, Dorsett J, Youther M. Modulation of submucosal cholinergic neurons by 5-hydroxytryptamine and neuropeptides. Am J Physiol Gastrointest Liver Physiol 259: G1019–G1024, 1990. [DOI] [PubMed] [Google Scholar]
- 61.Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta 1778: 709–716, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Dey CR, Wert SE, Duvall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266: 1705–1708, 1994. [DOI] [PubMed] [Google Scholar]
- 63.Zuniga L, Niemeyer MI, Varela D, Catalan M, Cid LP, Sepulveda FV. The voltage-dependent ClC-2 chloride channel has a dual gating mechanism. J Physiol 555: 671–682, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]