Abstract
The enteric nervous system (ENS) controls gut function. P2X receptors and nicotinic acetylcholine receptors (nAChRs) are ligand-gated cation channels that mediate fast synaptic excitation in the ENS. Close molecular coupling in enteric neuronal membranes contributes to a mutually inhibitory interaction between these receptors; this effect is called cross-inhibition. We studied the molecular mechanisms responsible for cross-inhibition. Whole cell patch-clamp techniques were used to measure P2X- and nAChR-mediated currents in cultured enteric neurons and HEK-293 cells. In cultured myenteric neurons, ACh (3 mM) and ATP (1 mM) coapplication evoked an inward current that was only 57 ± 6% (P < 0.05) of the predicted current that would have occurred if the two populations of channels were activated independently. In HEK-293 cells coexpressing α3β4 nAChR/P2X2 receptors, coapplication of ATP and ACh caused a current that was 58 ± 7% of the predicted current (P < 0.05). To test the importance of P2X subunit COOH-terminal tail length on cross-inhibition, P2X3 and P2X4 subunits, which have shorter COOH-terminal tails, were studied. Cross-inhibition with α3β4 nAChRs and P2X3 or P2X4 subunits was similar to that occurring with P2X2 subunits. P2X receptor or α3β4 nAChR desensitization did not prevent receptor cross-inhibition. These data indicate that the α3β4-P2X receptor interaction is not restricted to P2X2 subunits. In addition, active and desensitized conformations of the P2X receptor inhibit nAChR function. These molecular interactions may modulate the function of synapses that use ATP and ACh as fast synaptic transmitters in the ENS.
Keywords: enteric nervous system, synaptic transmission, ligand-gated ion channels
the enteric nervous system (ENS) is the division of the autonomic nervous system (ANS) that controls gastrointestinal function (8). Although parasympathetic, sympathetic, and sensory nerves supply the intestine, the ENS can control gut function independently from central nervous system control. This is possible because the ENS contains all the neural elements needed for reflex control of gastrointestinal function (10, 12). These elements include motoneurons, interneurons, and sensory neurons that respond to mechanical and chemical stimuli provided by intraluminal content (9).
There are two classes of ligand-gated ion channel receptors that mediate fast synaptic excitation of enteric neurons; cys-loop receptors and P2X receptors. Cys-loop receptors include nicotinic acetylcholine receptors (nAChRs), 5-HT3 receptors, and GABAA receptors. Cys-loop receptors are pentameric, and each subunit has four transmembrane domains and extracellular NH2- and COOH-terminal tails (17, 18). Neuronal nAChRs are composed of two α- and three β-subunits; there are eight neuronal α (α2–α9) and three neuronal β (β2–β4) nAChR subunits (17). In the ENS, the predominant nAChR subtype is composed of α3- and β4-subunits (39), and ACh acting at nAChRs is the primary fast excitatory neurotransmitter in the ENS (10). P2X receptors are trimeric, and each subunit has two transmembrane domains and intracellular NH2- and COOH-terminal tails. P2X receptors are homomeric or heteromeric receptors composed of one or more of seven (P2X1–P2X7) subunits (7, 14, 26, 27, 31, 35). Enteric neurons express P2X2 and P2X3 subunits, which can form homomeric and heteromeric P2X2/P2X3 receptors (6, 29, 38). ATP acting at P2X receptors contributes to fast synaptic excitation in subsets of enteric neurons, and ATP and ACh are cotransmitters at many synapses in the ENS (10, 16, 19, 28, 30).
P2X receptors and nAChRs are structurally different, and it would be expected that they would function independently. However, P2X receptors and nAChRs coexpressed by rat pheochromcytoma-12 (PC-12) cells (23, 25) and superior cervical sympathetic neurons (24) are functionally linked. Simultaneous activation of both channels produces inward currents that are smaller than the additive sum of individual currents (predicted current) that would occur if the channels functioned independently. The functional interaction between nAChRs and P2X receptors was subsequently shown to occur in guinea pig celiac ganglion neurons (32) and cultured myenteric neurons from the guinea pig ileum (38). This latter result is particularly important because ATP and ACh are cotransmitters at functionally identified synapses in the guinea pig ileum myenteric plexus (2, 16, 27) and ATP modulates the function of nAChRs activated by synaptically released ACh (13). The functional interaction between nAChRs and P2X receptors is proportional to receptor density, and the interaction occurs in cell-free patches obtained from cultured myenteric neurons (38). These data suggest that intracellular signaling mechanisms are not required for the functional interaction and that there may be direct molecular interactions between the two types of receptors. A similar functional interaction occurs between P2X receptors and other cys-loop receptors including 5-HT3 receptors in guinea pig submucosal neurons (1) and GABAA receptors in dorsal root ganglion neurons (34). Studies of heterologously expressed P2X2 and 5-HT3 receptors revealed that a portion of the cytoplasmic COOH-terminal tail of the P2X2 receptor and a portion of the second cytoplasmic loop of the 5-HT3A subunit not only are responsible for a functional interaction but also may mediate a physical interaction between these receptors (3). Similarly, the COOH-terminal tail is required for interaction of P2X3 receptors with GABAA receptors expressed in Xenopus oocytes or dorsal root ganglion neurons (34). These studies suggest that the P2X receptor COOH-terminal tail might contribute to interactions with cys-loop receptors, a hypothesis that we test by studying interactions between nAChRs and P2X subunits with varying COOH-terminal tail lengths.
Although a functional interaction between P2X2 receptors and α3β4 nAChRs occurs when these receptors are expressed in Xenopus oocytes (13), the molecular mechanism responsible for the interaction between P2X2 and α3β4 nAChRs has not been studied in detail. The present study was designed to test the hypothesis that the functional interaction between P2X2 receptors and α3β4 nAChRs could be reproduced in a heterologous expression system (HEK-293 cells) and that this system could be used to probe the molecular mechanisms responsible for interactions between these receptors.
MATERIALS AND METHODS
Tissue Culture
Cultured myenteric neurons.
All animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Michigan State University. Neurons were cultured as described previously (34, 35). Two newborn guinea pigs (<36 h old) were killed by severing the major neck blood vessel after halothane anesthesia. The small intestine was removed from the animals and placed in cold (4°C) Krebs solution of the following composition (mM): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. The longitudinal muscle myenteric plexus was stripped free with a cotton swab and cut into 5-mm pieces. Tissues were digested with 1,600 U of trypsin, followed by trituration with a fire-polished Pasteur pipette. After incubation with 2,000 U of crab hepatopancreas collagenase, the tissues were triturated again. The neurons were resuspended in Eagle's minimum essential medium (MEM) containing 10% fetal bovine serum, gentamicin (10 μg/ml), penicillin (100 U/ml), and streptomycin (100 U/ml) and plated onto sterile, poly-l-lysine-coated 35-mm plastic dishes and maintained in an incubator at 37°C in a 5% CO2 atmosphere for up to 3 wk.
Human embryonic kidney (HEK-293) cells.
Cells were obtained from American Type Culture Collection and grown in Dulbecco's modified Eagle's medium (DMEM)-F-12 containing 10% fetal bovine serum, 10% GluMax (Invitrogen, Carlsbad, CA), and 100 U/ml penicillin and streptomycin, except for the rat α3β4 nAChR-stable HEK-293 cell line, which also contained 0.5 mg/ml Geneticin (GIBCO, G418). Cells were maintained at 37°C in a 5% CO2 atmosphere in a humidified incubator. Cells were passaged once every 3 days when they reached 90% confluence. Transient transfection of cells was accomplished by electroporation. A 0.4-cm Gene Pulser Cuvette (Bio-Rad) was used with 2 μg of total plasmid protein along with 0.2 μg of green fluorescent protein (GFP) to help identify transfected cells. The electroporation machine used was the Gene Pulser Xcell Electroporation System (Bio-Rad). This resulted in 80% transfection efficiency. Afterwards, cells were plated on 35-mm coverslips and maintained in the incubator for 24 h before use in electrophysiology experiments. A rat α3β4 nAChR-stable HEK-293 cell line was provided by Dr. Yingxian Xiao from Georgetown University (Washington, DC; Ref. 36). Plasmids containing the coding sequences for rat P2X2, P2X3, and P2X4 receptor subunits were a gift from Dr. Alan North (University of Manchester, Manchester, UK). Plasmids containing the coding sequences for the murine α3 and murine β4 nAChR subunits were provided by Dr. Jerry A. Stitzel (University of Colorado, Denver, CO).
Whole Cell Recording
Whole cell voltage-clamp measurements were obtained at room temperature with standard methods. Coverslips containing cells were placed on a stage of an inverted microscope (Nikon Diaphot, Mager Scientific, Dexter, MI) using phase-contrast optics. For identifying cotransfected cells, a mercury bulb with excitation wavelength of 495 nm was used with a filter for 520-nm emission. The pipette solution contained (mM) 122.5 K-aspartate, 20 KCl, 1 MgCl2, 10 EGTA, 5 HEPES, and 2 ATP; pH was adjusted to 7.3 with KOH. The extracellular solution was a HEPES-based buffer composed of (mM) 155 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 12 glucose; pH was adjusted to 7.4 with NaOH. All recordings were made with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Data were acquired with pCLAMP 9.1 software (Molecular Devices). Whole cell recordings were carried out with patch pipettes with tip resistances of 3–5 MΩ; seal resistances were >1 GΩ. Steady-state current-voltage relationships for agonist-induced whole cell currents were obtained with step depolarization that changed the holding potential from an initial value of −70 mV to 50 mV in 20-mV increments. There was a 2-min interval between all successive agonist applications at each holding potential, −70 mV for HEK-293 cells and −60 mV for myenteric neurons.
Drug Application
Agonists were applied onto individual neurons/cells by gravity flow from linear array quartz tubes placed near the cell. The distance from the mouth of the tubes to the cells was ∼200 μm, with flow controlled manually with a micromanipulator. Computer-controlled solenoid valves (General Valve, Fairfield, NJ) were used to gate solution flow through the tubes. Agonist-induced currents were measured as the peak current amplitude. The difference between the predicted sum of the individual peak currents was compared with the current amplitude caused by coapplication of two agonists.
Statistics
Data are expressed as means ± SE, and n refers to the number of cells from which the data were obtained. The predicted current amplitude that would occur during agonist coapplication was calculated by measuring the peak currents caused by previous individual agonist applications and then summing these values. These data were used for statistical comparisons of actual and predicted currents. Traces illustrating predicted currents were constructed with a simple math function in Origin 7 (OriginLab, Northhampton, MA). Student's t-test or analysis of variance was used to establish significant differences between control and treatment groups. The significance level was P < 0.05. Agonist concentration-response curves were fit to the following logistic function with Origin: y = (Ymin − Ymax)/[1 + (x/EC50)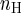 ] + Ymax, where EC50 is the half-maximal effective concentration, nH is the slope (Hill coefficient), and Ymin and Ymax are the minimum and maximum, respectively.
] + Ymax, where EC50 is the half-maximal effective concentration, nH is the slope (Hill coefficient), and Ymin and Ymax are the minimum and maximum, respectively.
RESULTS
Inhibitory Interaction Between nAChRs and P2X Receptors in Cultured Myenteric Neurons
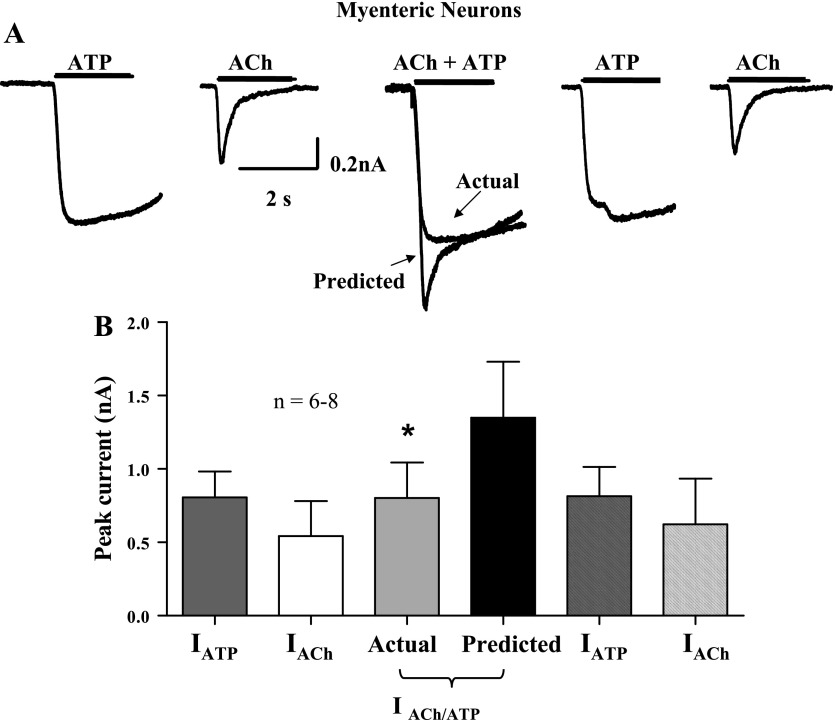
ACh (3 mM) caused an inward current in 98% of the myenteric neurons tested, while ATP (1 mM) caused an inward current in 80% of the neurons tested. The mean current amplitudes caused by individual application of ACh (3 mM) and ATP (1 mM) were not statistically different (Fig. 1). However, when the two agonists were applied simultaneously to the same neurons the peak inward current was only 57 ± 6% of the predicted current that would have occurred had the two channels functioned in an independent manner (n = 8, P < 0.05; Fig. 1); these data confirm previously published work (35). In the remainder of this article, the inhibitory interaction between nAChRs and P2X receptors is referred to as “cross-inhibition.”
Fig. 1.
Cross-inhibition between P2X receptors and nicotinic acetylcholine receptors (nAChRs) in guinea pig ileum cultured myenteric neurons. A: inward currents caused by ATP (1 mM), an agonist for P2X receptors, and ACh (3 mM), an agonist for nAChRs, in the same neuron. Coapplication of ACh and ATP caused an inward current that was smaller than the predicted current that would have occurred if the 2 channels functioned independently (P < 0.05). Individual reapplication of ATP and ACh caused inward currents (I) that were not statistically different from the original responses (P > 0.05). B: pooled data from experiments shown in A; n = no. of neurons. *Significantly different from predicted current.
Properties of P2X2 Receptors and α3β4 nAChRs Expressed in HEK-293 Cells
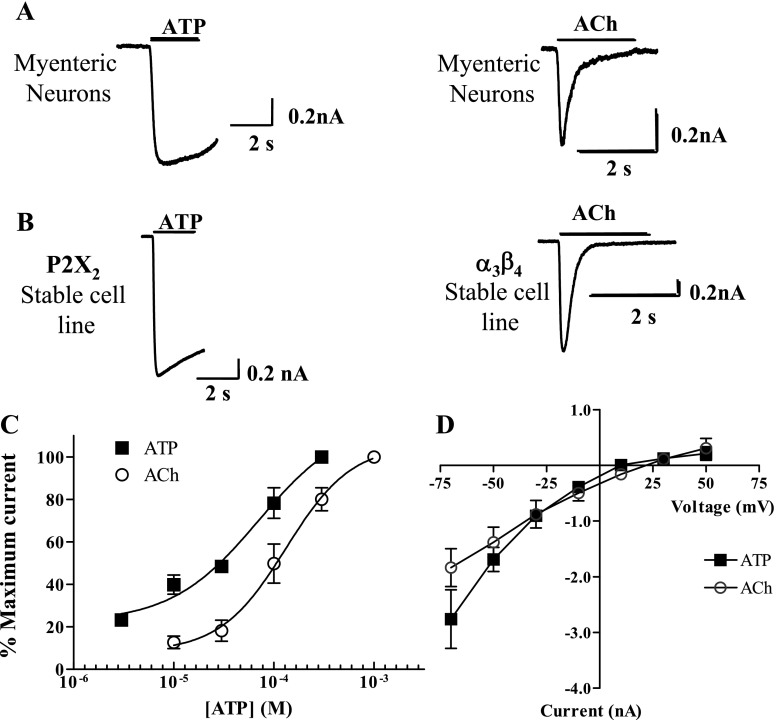
We verified that the pharmacological and functional properties of heterologously expressed P2X2 receptors and α3β4 nAChRs were similar to those seen in cultured myenteric neurons. In HEK-293 cells stably expressing P2X2 receptors, ATP (1 mM) evoked a rapidly developing current that was largely sustained throughout the period of ATP application. This response was similar to that caused by ATP in cultured myenteric neurons (Fig. 2A). ACh (3 mM) evoked a rapidly developing and desensitizing current in HEK-293 cells stably expressing α3β4 nAChRs, and this response was similar to that measured in cultured myenteric neurons (Fig. 2B). Concentration-response curves and current-voltage relationships were determined for ATP in HEK-293 cells stably expressing P2X2 receptors and for ACh in HEK-293 cells stably expressing α3β4 nAChRs. ATP caused a concentration-dependent increase in current amplitude with a EC50 of 20 ± 10 μM; the maximum current occurred at 300 μM (Fig. 2C). The Hill slope was 0.9 ± 0.3 (n = 6). These values were similar to those reported previously for ATP-evoked inward currents in cultured myenteric neurons (38).
Fig. 2.
Comparison of pharmacological and functional properties of P2X2 receptors and α3β4 nAChRs expressed by cultured myenteric neurons and HEK-293 cells. A: inward currents caused by ATP (1 mM; left) and ACh (3 mM; right) in a cultured myenteric neuron. The ATP response desensitized slowly, while the ACh response desensitized completely during ACh application. B: inward currents caused by ATP (1 mM) in an HEK-293 cell line stably expressing P2X2 receptors (left) and by ACh (3 mM) in an HEK-293 cell line stably expressing α3β4 nAChRs (right). Although larger in amplitude, the properties of the currents in cultured myenteric neurons and HEK-293 cells were similar. C: concentration-response curves for ATP and ACh in HEK-293 cells stably expressing P2X2 and α3β4 nAChRs. D: current-voltage relationship for ATP (1 mM)- and ACh (3 mM)-induced currents in HEK-293 cells stably expressing P2X2 receptors and α3β4 nAChRs.
ACh caused a concentration-dependent inward current in HEK-293 cells with an EC50 of 130 ± 20 μM and a Hill slope of 2.0 ± 0.6 (Fig. 2C). These values were similar to those reported previously for ACh-induced activation of nAChRs expressed by cultured myenteric neurons (36). Current-voltage relationships for ACh- or ATP-induced currents were studied in HEK-293 cells stably expressing α3β4 nAChRs or P2X2 receptors, respectively. ACh-induced currents exhibited an inwardly rectifying current voltage relationship with a reversal potential of 8.0 ± 4.5 mV (n = 5, Fig. 2D). The slope conductance at −50 mV was 33 ± 6 nS, while at 50 mV this value was 7 ± 4 nS. ATP-induced currents in P2X2-stable cells also exhibited inward rectification, and the reversal potential was 15 ± 6 mV (n = 4 or 5). At −50 mV the slope conductance was 48 ± 5 nS, while at 50 mV this value was 6 ± 3 nS. These properties are very similar to those reported previously for nAChRs and P2X receptors expressed by cultured myenteric neurons (37–39). All subsequent experiments used saturating concentrations of ATP (1 mM) and ACh (3 mM) unless specified otherwise.
Cross-Inhibition Between P2X2 Receptors and α3β4 nAChRs in HEK-293 Cells
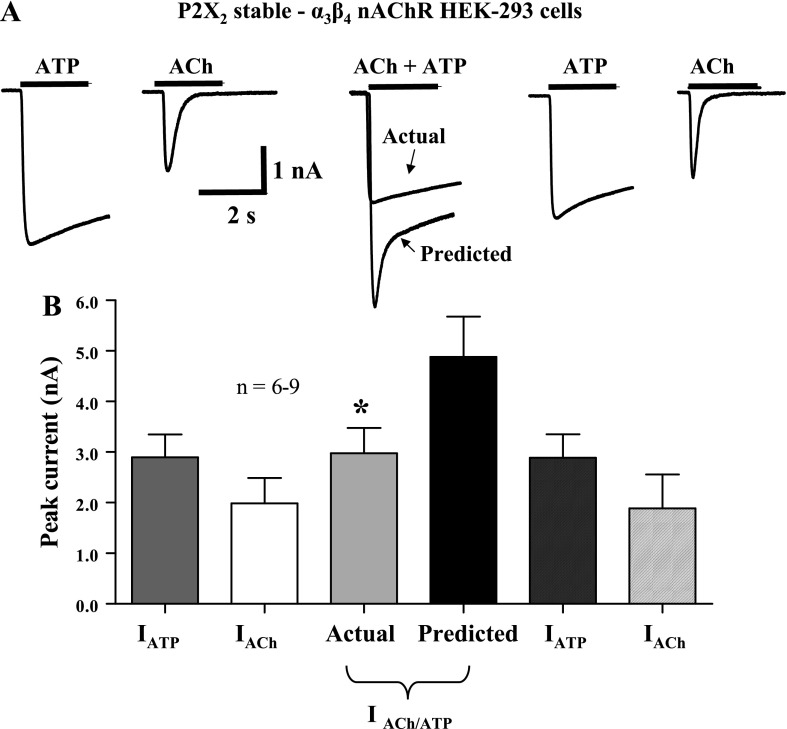
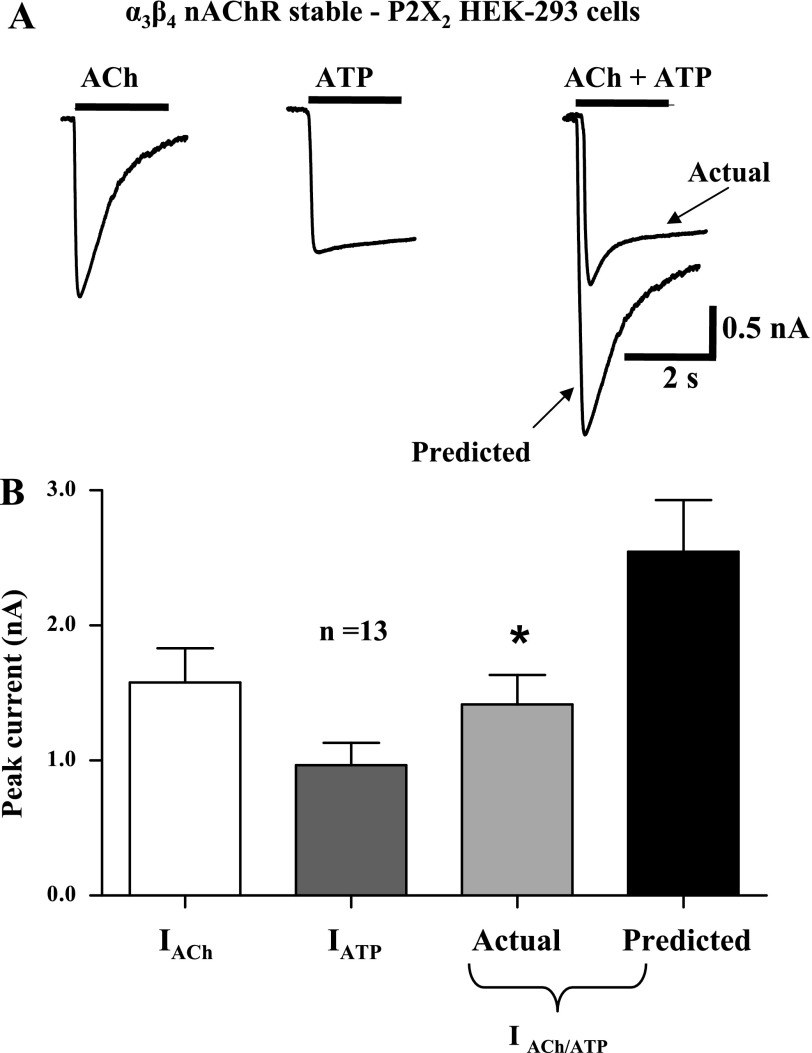
The data described above indicate that the functional and pharmacological properties of heterologously expressed nAChRs and P2X receptors are similar to those of the receptors expressed by cultured myenteric neurons. Therefore, we used HEK-293 cells that stably expressed rat P2X2 receptors and that were also transiently transfected with murine α3 and β4 nAChR subunits to study cross-inhibition between these receptors. Because of receptor overexpression, the mean current amplitudes caused by individual application of ACh and ATP were larger than those recorded from cultured myenteric neurons (Fig. 3). However, as was observed in cultured myenteric neurons, simultaneous application of maximum concentrations of ACh and ATP caused an inward current that was only 61 ± 3% (P < 0.05, n = 9) of the predicted current that would have occurred had the heterologously expressed P2X2 receptors and α3β4 nAChRs functioned independently. Responses caused by individual application of ACh and ATP obtained after agonist coapplication were not statistically different from the original current amplitudes (P > 0.05, n = 9; Fig. 3). Furthermore, when the predicted current value was calculated with individual agonist-evoked currents obtained after agonist coapplication, the actual current was significantly smaller than this predicted value (P < 0.05, n = 6). In the next experiments, we used a cell line that stably expressed α3β4 nAChRs and that was transiently transfected with P2X2 receptors. In these cells, currents caused by simultaneous application of ACh and ATP were only 58 ± 5% of the predicted current (Fig. 4; P < 0.05, n = 13) that would have occurred if the receptors functioned independently. Subsequent individual reapplication of ACh and ATP caused currents that were not statistically different from those elicited before agonist coapplication (P > 0.05, n = 13; data not shown). Furthermore, when the predicted current value was calculated with individual agonist-evoked currents obtained after agonist coapplication, the actual current was significantly smaller than this predicted value (P < 0.05, n = 13).
Fig. 3.
Cross-inhibition between P2X2 receptors and α3β4 nAChRs expressed by HEK-293 cells. A: ATP (1 mM)- and ACh (3 mM)-induced inward currents recorded from a HEK-293 cell stably expressing P2X2 receptors and transiently transfected with α3β4 nAChRs. Currents caused by agonist coapplication exhibited cross-inhibition. Individual reapplication of ATP and ACh caused inward currents that were not statistically different from the original responses (P > 0.05). B: pooled data from experiments shown in A; n = no. of cells. *Significantly different from predicted current.
Fig. 4.
Cross-inhibition between P2X2 receptors and α3β4 nAChRs expressed by HEK-293 cells. A: ATP (1 mM)- and ACh (3 mM)-induced inward currents recorded from a HEK-293 cell stably expressing α3β4 nAChRs and transiently transfected with P2X2 receptors. Currents caused by agonist coapplication exhibited cross-inhibition. B: pooled data from experiments shown in A; n = no. of cells. *Significantly different from predicted sum of currents (P < 0.05).
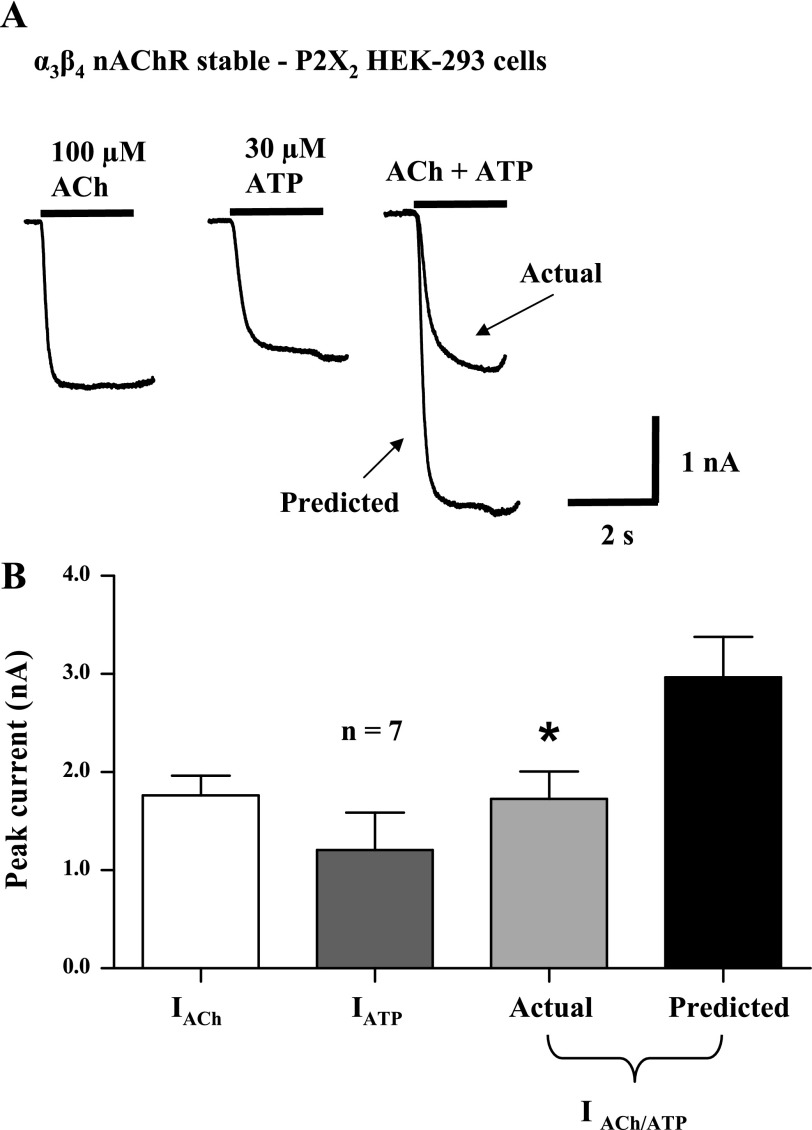
As mentioned above, receptor overexpression resulted in current amplitudes in HEK-293 cells that were larger than those in cultured myenteric neurons. It is possible that nAChR and P2X receptor activation caused by coapplication of maximum agonist concentrations produced local Na+ or Ca2+ depletion, reducing the driving force required for inward current. Another concern would be poor voltage control of large-amplitude currents evoked by coapplication of maximum agonist concentrations. To rule out these possibilities, EC50 concentrations of ACh (100 μM) and ATP (30 μM) were used to activate nAChRs and P2X receptors, respectively. Because submaximal agonist concentrations were used, neither receptor desensitized (Fig. 5). However, the inward current caused by simultaneous activation of α3β4 nAChRs and P2X2 receptors was only 58 ± 7% of predicted response (P < 0.05, n = 7).
Fig. 5.
Cross-inhibition between P2X2 receptors and α3β4 nAChRs expressed by HEK-293 cells is maintained when receptors were activated by agonist half-maximal effective (EC50) concentrations. A: ATP (30 μM)- and ACh (100 μM)-induced inward currents recorded from a HEK-293 cell stably expressing α3β4 nAChRs and transiently transfected with P2X2 receptors. ACh current activated by the EC50 concentration does not desensitize. Currents caused by agonist coapplication exhibited cross-inhibition. B: pooled data from experiments shown in A; n = no. of cells. *Significantly different from predicted current (P < 0.05).
ACh may interact directly with P2X receptors to modulate their function, or ATP may directly interact with nAChRs to modulate their function. Cultured myenteric neurons cannot be used to evaluate this possibility because nearly all neurons express both receptors. Therefore, we used HEK-293 cells expressing only P2X2 or α3β4 nAChRs to test for the direct agonist modulation mechanism of cross-inhibition. ACh (3 mM) did not cause an inward current in HEK-293 cells expressing only P2X2 receptors, and ACh did not alter currents caused by ATP in these cells (P > 0.05, n = 4; Fig. 6A). Likewise, ATP (1 mM) did not cause an inward current in cells expressing only α3β4 nAChRs and ATP did not alter currents caused by ACh in these cells (P > 0.05, n = 7; Fig. 6B).
Fig. 6.
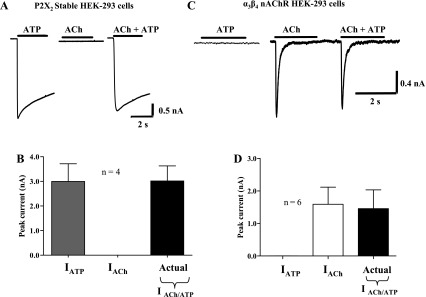
ACh and ATP do not directly modulate P2X2 receptor or α3β4 nAChR function. A: ATP (1 mM) caused an inward current in a HEK-293 cell stably expressing only the P2X2 receptor. ACh (3 mM) did not cause an inward current in these cells. ATP and ACh coapplication caused an inward current that did not differ from the response caused by ATP alone. B: pooled data from experiments shown in A. In cells expressing only the P2X2 receptor, coapplication of ATP and ACh caused an inward current that is not different from the response caused by application of ATP alone (P > 0.05). C: ACh (3 mM) caused an inward current in a HEK-293 cell stably expressing only the α3β4 nAChR. ATP (1 mM) did not cause an inward current in these cells. ATP and ACh coapplication caused an inward current that did not differ from the response caused by ACh alone. D: pooled data from experiments shown in C. In cells expressing only the α3β4 nAChR, coapplication of ATP and ACh caused an inward current that was not different from the response caused by application of ACh alone (P > 0.05).
P2X3 and P2X4 Subunits Interact with nAChRs
The specificity of the P2X receptor and nAChR interaction was determined by transiently expressing P2X3 or P2X4 subunits in HEK-293 cells stably expressing α3β4 nAChRs. P2X3 and P2X4 subunits differ from P2X2 subunits in the length of the intracellular COOH-terminal tail (Fig. 7). In cells coexpressing α3β4 nAChR/P2X3 receptors, coapplication of ACh and ATP caused an inward current that was only 53 ± 5% of the predicted current (Fig. 8, A and B; n = 25, P < 0.05). In cells coexpressing α3β4 nAChR/P2X4 receptors, agonist coapplication caused an inward current that was only 58 ± 5% of the predicted current (P < 0.05, n = 21; Fig. 8, C and D).
Fig. 7.
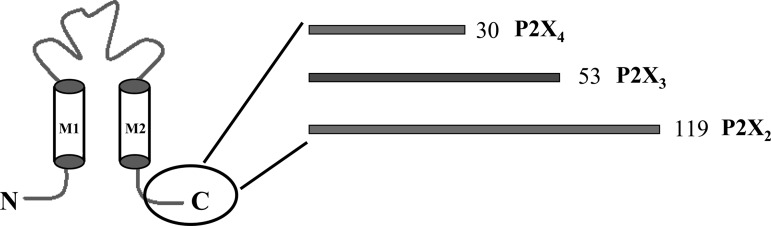
Cartoon illustrating P2X subunit COOH-terminal tail length differences among P2X2, P2X3, and P2X4 subunits. M1 and M2 are the first and second transmembrane domains.
Fig. 8.
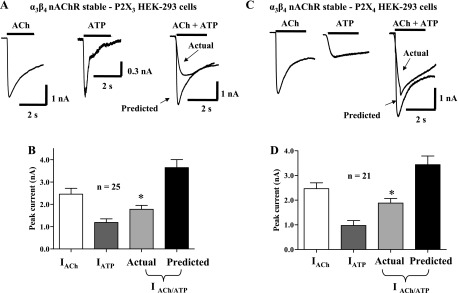
P2X subunit COOH-terminal tail length does not alter cross-inhibition of α3β4 nAChR function. P2X2, P2X3, and P2X4 subunits differ mainly in their COOH-terminal tail amino acid length. P2X4 subunits have the shortest COOH-terminal tail (30 amino acids). A: inward currents caused by ACh (3 mM) and ATP (1 mM) in HEK-293 cells stably expressing α3β4 nAChRs and transiently transfected with P2X3 receptors. Inward currents carried by P2X3 receptors desensitize rapidly. The inward currents caused by coapplication of ACh and ATP were smaller than the predicted current that would occur if the receptors functioned independently. B: pooled data from experiments shown in A. C: inward currents caused by ACh and ATP in HEK-293 cells stably expressing α3β4 nAChRs and transiently transfected with P2X4 receptors. The inward currents caused by coapplication of ACh and ATP were smaller than the predicted current that would occur if the receptors functioned independently. D: pooled data from experiments shown in C. *Significantly different from predicted current (P < 0.05) in both sets of experiments.
Receptor Desensitization
We next investigated whether the desensitized state of nAChRs could maintain cross-inhibition of P2X2 receptors. Individual activation currents were 1.9 ± 0.3 nA and 2.5 ± 0.3 nA for ACh and ATP, respectively. ACh was continuously applied to HEK-293 cells stably expressing α3β4 nAChRs and transiently transfected with P2X2 receptors. This treatment caused nAChR desensitization, and then ATP and ACh were coapplied. Because the nAChR was desensitized, the current should only be due to activation of P2X2 receptors. The ATP-induced current was only 71 ± 7% of the initial current obtained before nAChR desensitization (P < 0.05, n = 7; Fig. 9A). The current recorded during simultaneous agonist application in the presence of nAChR desensitization was 71% of the predicted current; this value is higher than but not statistically different from (58 ± 7%; n = 13, P > 0.05) that obtained during ATP and ACh application when both the P2X2 and α3β4 nAChRs were fully function (i.e., not desensitized). The same experiment was repeated, except this time the P2X2 receptors were desensitized with prolonged ATP application immediately followed by coapplication of ACh and ATP. After P2X2 receptor desensitization, the ACh-induced current was only 72 ± 6% (P < 0.05, n = 11; Fig. 9B) of the initial ACh-induced current. Again, this value was higher than but not statistically different from (P > 0.05, n = 13) that measured during ATP and ACh coapplication when the P2X2 receptors and α3β4 nAChRs were fully functional.
Fig. 9.
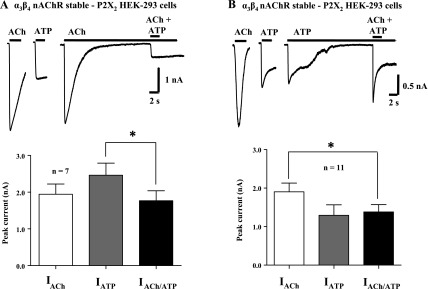
Cross-inhibition between P2X2 receptors and α3β4 nAChRs is maintained during receptor desensitization. A: inward currents caused by ACh (3 mM) and ATP (1 mM) in HEK-293 cells stably transfected with α3β4 nAChRs and transiently transfected with P2X2 receptors. Prolonged ACh application desensitized the α3β4 nAChR, but the inward current caused by ATP in the continued presence of ACh was smaller than the current caused by ATP before nAChR desensization. Histograms show pooled data from experiments shown in traces at top. B: similar results were obtained in the same cells when the P2X2 receptor was first desensitized by prolonged application of ATP. The inward current caused by ACh during P2X2 receptor desensitization was smaller than the ACh current recorded before P2X2 receptor desensitization. Histograms show pooled data from experiments shown in traces at top. *Significantly different from the current recorded before desensitization (P < 0.05) in both sets of experiments.
To determine the specificity of this interaction, experiments similar to those above were performed with the P2X3 and P2X4 subtypes of the receptor. For nAChR/P2X3 cells, ACh-induced currents after P2X3 receptor desensitization were 74 ± 9% of the initial ACh current (n = 9, P < 0.05; Fig. 10A). This value was significantly different from that occurring during simultaneous P2X3 receptor and nAChR activation when these receptors were fully functional (nondesensitized) (see above, 53 ± 5%; P < 0.05). For nAChR/P2X4 cells, ACh-induced currents after P2X4 receptor desensitization were only 75 ± 6% of initial activation. (P < 0.05, n = 9; Fig. 10B). This value was significantly different from that occurring during simultaneous P2X4 receptor and nAChR activation when these receptors were fully functional (see above, 58 ± 5%; P < 0.05).
Fig. 10.
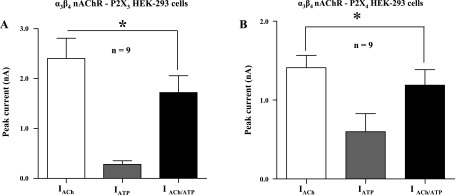
Cross-inhibition between P2X3 or P2X4 receptors and α3β4 nAChRs is maintained during receptor desensitization. A: histograms showing the amplitude of ACh (3 mM) and ATP (1 mM) currents before P2X3 receptor desensitization. ATP was then applied continuously to desensitize the P2X3 receptors, and ACh was reapplied in the continued presence of ATP (see Fig. 8 for protocol). The ACh current was significantly smaller than that recorded before P2X3 receptor desensitization. B: histograms showing results of experiments similar to those in A but using cells coexpressing P2X4 receptors and α3β4 nAChRs. The ACh current recorded during P2X4 receptor desensitization was smaller than that recorded before the desensitization protocol. *Significantly different from current recorded before desensitization (P < 0.05) in both sets of experiments.
DISCUSSION
ACh and ATP are cotransmitters in the ENS (10, 28, 30), and interaction between their target receptors has been shown in guinea pig celiac ganglion neurons (32, 33), rat PC-12 cells (23, 25), superior cervical sympathetic neurons (24), and cultured myenteric neurons (38) and in heterologously expressed receptors in Xenopus oocytes (13). In the present study, we have reproduced this receptor interaction in mammalian cells expressing α3β4 nAChRs and several P2X receptor subunits.
Functional and Pharmacological Properties of P2X2 Receptors and nAChRs Are Retained in HEK-293 Cells
We previously confirmed that P2X2 receptors and nAChRs are functionally linked in guinea pig cultured myenteric neurons (38), and we show that this interaction can be reproduced in a heterologous expression system. P2X2 receptors desensitize slowly, while responses mediated by enteric α3β4 nAChRs desensitize in <2 s; these properties were retained in HEK-293 cells expressing α3β4 nAChRs or P2X2 receptors. Concentration-response curves for ACh- and ATP-induced inward currents were also similar in cultured myenteric neurons and HEK-293 cells expressing α3β4 nAChRs or P2X2 receptors. The current-voltage relationship for currents carried by P2X2 receptors and α3β4 nAChRs exhibits inward rectification (37, 38, 39), and this property was also retained in HEK-293 cells. Therefore, HEK-293 cells are an appropriate model system to study the details of the molecular interactions between P2X and nAChRs.
Cross-Inhibition Between nAChRs and P2X Receptors Expressed in HEK-293 Cells
We used HEK-293 cells that stably expressed P2X2 receptors and transiently expressed α3β4 nAChRs or HEK-293 cells that stably expressed α3β4 nAChRs and transiently expressed P2X2 receptors to study receptor cross-inhibition. Similar data were obtained with both types of receptor combinations. As occurs with cultured myenteric neurons, when P2X2 receptors and nAChRs are coactivated in HEK-293 cells expressing both receptors, the resulting inward current is substantially smaller than the current that would have occurred if the two receptors functioned independently. If P2X receptors and nAChRs function independently, coactivation of receptors would cause fully additive currents. The response amplitude would be predicted by summing the currents evoked by individual agonist application; this is the predicted current. The data obtained in HEK-293 cells indicate that cross-inhibition is not a neuron-specific response and it does not require neuron-specific proteins or other molecules to maintain the receptor interaction. We have also ruled out receptor rundown or desensitization as a mechanism responsible for the proposed cross-inhibition, because we were able to show that the currents caused by individual application of ACh or ATP were stable in amplitude through the time course of our studies.
It is possible that cross-inhibition occurs because P2X receptors have an allosteric inhibitory binding site for ACh, or that nAChRs have a similar binding site for ATP (25, 31). Another possibility is that ACh activates muscarinic receptors or ATP activates P2Y receptors, which are both endogenously expressed by HEK-293 cells (22). These receptors link to protein kinase C activation, which could then alter the function of nAChRs or P2X receptors. These hypotheses would be difficult to test in cultured myenteric neurons, because nearly every neuron coexpresses P2X2 receptors and nAChRs (37, 38) and many myenteric neurons also express muscarinic and P2Y receptors (11, 20). However, by using HEK-293 cells it is possible to control receptor expression. We showed that ACh does not activate P2X2 receptors and, more importantly, ACh does not modify activation of the P2X2 receptor by ATP in the absence of nAChR expression. Similarly, ATP does not modify nAChR function in the absence of P2X2 receptor expression. These data indicate that ACh and ATP do not directly modulate the function of P2X2 receptors or nAChRs and do not indirectly modify their function via activation of muscarinic or P2Y receptor-linked signaling pathways.
We used saturating agonist concentrations in the studies of cross-inhibition. It is possible that cross-inhibition is not due to direct receptor interaction but could be due to local depletion of Na+ or Ca2+ ions near the channel during maximal activation. Inadequate voltage control during maximal receptor activation could also account for nonadditivity of agonist responses. To address these concerns, we studied receptor cross-inhibition with EC50 concentrations of ACh and ATP. We found that even during submaximal receptor activation, agonist coapplication produced responses that were smaller in amplitude than the predicted additive response. These data indicate that nonadditivity between P2X2 and nAChRs is not attributable to local ion depletion or poor voltage clamp of large-amplitude currents.
Cross-Inhibition of α3β4 nAChR Function Is Not Specific for P2X2 Receptors
We next determined whether inhibition of α3β4 nAChR function was specific for P2X2 subunits. P2X receptor subunits differ largely in the length and composition of their COOH-terminal intracellular tail. The P2X2 subunit has a COOH-terminal tail length of 119 amino acids (26). We focused on P2X3 subunits, with a COOH-terminal tail length of 53 amino acids, because these are expressed by myenteric neurons (29) and the P2X4 subunit because it has the shortest COOH-terminal tail at 30 amino acids (26). By narrowing down the region of the COOH-terminal tail that mightf be responsible for this interaction, a better understanding of the sequence regions or motif that mediate this interaction can be elucidated. We detected cross-inhibition between α3β4 nAChRs and both P2X3 and P2X4 receptors, suggesting that α3β4 nAChRs can interact with multiple P2X receptor subunits. Although P2X2 and P2X4 COOH-terminal tails do not have identical sequences (24), the 30-amino acid COOH-terminal tail length of the P2X4 subunit is sufficient to produce the proposed allosteric linking to the α3β4 nAChR. Others have shown that a segment of the COOH-terminal tail of the P2X2 subunit (amino acids 22-119 from the end of the second transmembrane domain) interacts with the cys-loop 5-HT3A and GABAA receptors (3, 4).
Desensitized States of P2X Receptors and nAChRs Can Mediate Cross-Inhibition
Previous work (20) showed that cross-inhibition between neuronal nAChRs and P2X receptors requires the open state of the channels and little cross-inhibition occurred during desensitization of one of the receptors. However, we found cross-inhibition of α3β4 nAChR function when P2X2, P2X3, or P2X4 receptors coexpressed by the same cells were desensitized. Similarly, P2X2 receptor function could be inhibited by desensitized α3β4 nAChRs in cells expressing both receptors. Although cross-inhibition was maintained during receptor desensitization, the amount of cross-inhibition was not equivalent to that occurring when fully active, nondesensitized receptors were studied. This difference was most prominent when nAChRs were coexpressed with P2X3 or P2X4 subunits. This suggests that the desensitized state of one receptor (either P2X receptor or α3β4 nAChR) is less efficient at impairing current flow through the linked receptor. Therefore, differences in our data from those of Nakazawa (24) are based on the magnitude of cross-inhibition caused by desensitized receptors rather than complete inability of the desensitized receptor to cause cross-inhibition. Khakh and coworkers (13) studied the effects of receptor desensitization on interactions between P2X2 receptors and α3β4 nAChRs expressed in Xenopus oocytes. They used a mutant P2X2 receptor (T18A) that desensitizes >10-fold faster than wild-type P2X2 receptors. These studies showed that currents carried by α3β4 nAChRs were inhibited when the T18A P2X2 receptor was fully activated but that nAChR currents partly recovered as the T18A P2X2 receptor desensitized. These data are again consistent with our data showing that the desensitized state of one receptor in the interacting pair (α3β4 nAChR-P2X2) inhibits current flow through the coupled receptor, but the inhibition is reduced compared with that occurring when the channels are in the fully open state.
Conclusions
Cross-inhibition between cys-loop-type receptors and P2X receptors may be a mechanism of receptor modulation in the ENS (1, 38). This interaction does not require intracellular signaling mechanisms and occurs in cell-free patches of cultured neurons, suggesting close coupling of the two receptors in the neuronal membrane (38). Functional interaction between nAChRs and P2X2 receptors also occurs when these receptors are expressed heterologously in HEK-293 cells, suggesting that the receptor interaction does not require nervous system-specific proteins. Conformational spread could be a mechanism for cross-inhibition between α3β4 nAChRs and P2X receptors (13, 14) as may also occur with other receptors and channels (5). Conformational spread would require that nAChRs and P2X receptors are so closely associated in the membrane that opening of one channel impairs opening of the second channel. Fluorescence resonance energy transfer analysis of α4β2 nAChRs (brain-specific receptors) and P2X2 receptors expressed in HEK-293 cells revealed that the receptors were localized within 100 nm of each other in the plasma membrane. This close association would make conformational spread during receptor activation possible, perhaps through heterodimer formation (15). Conformational spread would also be consistent with our desensitization data. P2X receptors and nAChRs adopt different conformations in the closed, open, and desensitized states (12, 21). Conformation spread from the open state readily impairs current flow in the adjacent channel, while conformation spread from the desensitized state of one receptor impairs current flow in the adjacent channel less effectively.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-57039.
Acknowledgments
We are grateful to Dr. Yingxian Xiao for generously donating the α3β4 nAChR-stable HEK-293 cell line and Dr. Alan North for the P2X2, P2X3, and P2X4 plasmids. We also thank Dr. Jerry A. Stitzel for providing the mouse α3 and β4 plasmids.
REFERENCES
- 1.Barajas-López C, Montaño LM, Espinosa-Luna R. Inhibitory interactions between 5-HT3 and P2X channels in submucosal neurons. Am J Physiol Gastrointest Liver Physiol 283: G1238–G1248, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bian X, Bertrand PP, Bornstein JC. Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol 528: 551–560, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boué-Grabot E, Barajas-López C, Chakfe Y, Blais D, Bélanger D, Emerit MB, Séguéla P. Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. J Neurosci 23: 1246–1253, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boué-Grabot E, Toulmé E, Emerit MB, Garret M. Subunit-specific coupling between gamma-aminobutyric acid type A and P2X2 receptor channels. J Biol Chem 279: 52517–52525, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bray D, Duke T. Conformational spread: the propagation of allosteric states in large multiprotein complexes. Annu Rev Biophys Biomol Struct 33: 53–73, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem Cell Biol 117: 415–422, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Edwards FA, Gibb AJ. ATP—a fast neurotransmitter. FEBS Lett 325: 86–89, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Furness JB, Sanger GJ. Intrinsic nerve circuits of the gastrointestinal tract: identification of drug targets. Curr Opin Pharmacol 2: 612–622, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Furness JB The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci 30: 1–5, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gallligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 81: 97–103, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Gwynne RM, Bornstein JC. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol 5: 1–17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He ML, Koshimizu TA, Tomić M, Stojilkovic SS. Purinergic P2X2 receptor desensitization depends on coupling between ectodomain and C-terminal domain. Mol Pharmacol 62: 1187–1197, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature 406: 405–410, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Khakh BS Molecular physiology of P2X receptors and ATP signaling at synapses. Nat Rev Neurosci 2: 165–174, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Khakh BS, Fisher JA, Nashmi R, Bowser DN, Lester HA. An Angstrom scale interaction between plasma membrane ATP-gated P2X2 and alpha4beta2 nicotinic channels measured with fluorescence resonance energy transfer and total internal reflection fluorescence microscopy. J Neurosci 25: 6911–6920, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LePard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 276: G529–G538, 1999. [DOI] [PubMed] [Google Scholar]
- 17.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57: 521–546, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423: 949–955, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol 556: 571–584, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monro RL, Bornstein JC, Bertrand PP. Synaptic transmission from the submucosal plexus to the myenteric plexus in Guinea-pig ileum. Neurogastroenterol Motil 20: 1165–1173, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mourot A, Rodrigo J, Kotzyba-Hibert F, Bertrand S, Bertrand D, Goeldner M. Probing the reorganization of the nicotinic acetylcholine receptor during desensitization by time-resolved covalent labeling using [3H]AC5, a photoactivatable agonist. Mol Pharmacol 69: 452–461, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Mundell SJ, Benovic JL. Selective regulation of endogenous G protein-coupled receptors by arrestins in HEK293 cells. J Biol Chem 275: 12900–12908, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa K, Fujimori K, Takanaka A, Inoue K. Comparison of adenosine triphosphate- and nicotine-activated inward currents in rat phaeochromocytoma cells. J Physiol 434: 647–660, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa K ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci 14:740–750, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakazawa K Reduction of acetylcholine-activated current by low concentrations of extracellular adenosine-triphosphate. Life Sci 57: 351–356, 1995. [DOI] [PubMed] [Google Scholar]
- 26.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40: 563–580, 2000. [DOI] [PubMed] [Google Scholar]
- 27.North RA Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Nurgali K, Furness JB, Stebbing MJ. Analysis of purinergic and cholinergic fast synaptic transmission to identified myenteric neurons. Neuroscience 116: 335–347, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci 101: 39–47, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Bertrand PP. Purinergic receptors and synaptic transmission in enteric neurons. Purinergic Signal 4: 255–266, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson SJ, Ennion SJ, Evans RJ, Edwards FA. Synaptic P2X receptors. Curr Opin Neurobiol 11: 378–386, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Searl TJ, Redman RS, Silinsky EM. Mutual occlusion of P2X ATP receptors and nicotinic receptors on sympathetic neurons of the guinea-pig. J Physiol 510: 783–791, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silinsky EM, Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J Physiol 464: 197–212, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toulmé E, Blais D, Léger C, Landry M, Garret M, Séguéla P, Boué-Grabot E. An intracellular motif of P2X3 receptors is required for functional cross-talk with GABAA receptors in nociceptive DRG neurons. J Neurochem 102: 1357–1368, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Vial C, Roberts JA, Evans RJ. Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci 25: 487–493, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat α3/β4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol 54: 322–333, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Galligan JJ. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J Physiol 496: 719–729, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Galligan JJ. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol 513: 685–697, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Ren J, Brown E, Schneider D, Caraballo-Lopez Y, Galligan JJ. Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. J Pharmacol Exp Ther 302: 889–897, 2002. [DOI] [PubMed] [Google Scholar]