Abstract
The human hyaluronic acid (HA) receptor for endocytosis (HARE/stabilin-2) is the primary clearance receptor for systemic HA, chondroitin sulfates, and heparin, but not for heparan sulfate or keratan sulfate (Harris EN, Weigel JA, Weigel PH. J Biol Chem 283: 17341–17350, 2008). HARE is expressed in the sinusoidal endothelial cells (SECs) of liver and lymph nodes where it acts as a scavenger for uptake and degradation of glycosaminoglycans, both as free chains and proteoglycan fragments. Unfractionated heparin (UFH; ∼14 kDa) and low-molecular-weight heparin (LMWH; ∼4 kDa) are commonly used in treatments for thrombosis and cancer and in surgical and dialysis procedures. The reported half-lives of UFH and LMWH in the blood are ∼1 h and 2–6 h, respectively. In this study, we demonstrate that anti-HARE antibodies specifically block the uptake of LMWH and UFH by isolated rat liver SECs and by human 293 cells expressing recombinant human HARE (hHARE). hHARE has a significant affinity (Kd = 10 μM) for LMWH, and higher affinity (Kd = 0.06 μM) for the larger UFH. Rat liver SECs or cells expressing the recombinant 190-kDa HARE isoform internalized both UFH and LMWH, and both heparins cross-compete with each other, suggesting that they share the same binding sites. These cellular results were confirmed in ELISA-like assays using purified soluble 190-hHARE ectodomain. We conclude that both UFH and LMWH are cleared by HARE/Stab2 and that the differences in the affinities of HARE binding to LMWH and UFH likely explain the longer in vivo circulating half-life of LMWH compared with UFH.
Keywords: Lovenox (enoxaparin), endocytosis, scavenger receptor, biotinylated heparin, coated pit mediated, liver sinusoidal endothelial cells
unfractionated (UFH) and low-molecular-weight (LMWH) heparins are commonly used as treatments for deep vein thrombosis and other thrombotic and cardiovascular disorders. Commercial medical grade heparin is obtained from porcine intestinal mucosa and purified as a sodium salt. UFH, which is defined as a heparin preparation ranging in mass from 3,000 to 30,000 Da, is effective in blocking coagulation factors IIa, IX, Xa, XI, and XII. In contrast, LMWH, which is obtained by chemical breakdown of UFH, is defined as a heparin preparation with a distribution of chain sizes about one-third that of UFH (∼2,000 to 8,000 Da) that binds to and blocks coagulant factors IIa and Xa (8). Over the past decade, the use of LMWH for surgeries, joint replacements, and the treatment of blood disorders has steadily increased (eight indications are currently approved by FDA), primarily because of its longer half-life in the blood and thus effectiveness against the targeted disorder.
Currently in the United States, three preparations of LMWH are FDA approved for use in medical treatments: tinzaparin, dalteparin (e.g., Fragmin), and enoxaparin (e.g., Lovenox). All three preparations have different pharmacological, physiochemical, and functional properties owing to the methods by which they were derived from UFH. For example, tinzaparin and enoxaparin are obtained by β-eliminative cleavage of heparin using heparinase or alkaline treatment, respectively, and dalteparin is obtained by deamination with nitrous acid (10).
The sulfate groups on heparin maintain strong polyanionic charge, which makes this molecule “sticky.” Many of the proteins in blood, in addition to the targeted members in the coagulation cascade, bind heparin and thus act as a saturable system, absorbing a large amount of drug and inducing some resistance to heparin therapy (49, 51). Clearance of heparin is achieved by the reticuloendothelial system, although until recently the mechanism for clearance remained unknown (36). The liver primarily clears larger polymers of heparin, whereas the kidneys filter out smaller fragments (5). Other reports found that macrophages and sinusoidal endothelial cells are responsible for the bulk of the clearance, although a specific heparin receptor was not identified (7, 13, 30, 35, 44). Scavenger receptors in the liver that bind to a large number of ligands [e.g., acetylated low-density lipoprotein (AcLDL), heparan sulfate, chondroitin sulfate] were assumed to also mediate the clearance of heparin (for review see Ref. 50).
Recently, our group identified the human hyaluronic acid receptor for endocytosis (hHARE/stabilin-2) as the primary scavenger for heparin (18, 19). We used UFH to demonstrate that hHARE binds specifically to heparin with high affinity, independently of the hyaluronan-binding site, and rapidly internalizes heparin by coated pit-mediated endocytosis. This efficient HARE-mediated uptake explains the high clearance rate of circulating heparin observed in patients. HARE is highly expressed in the sinusoidal endothelial cells (SECs) of liver, lymph node, and spleen and also mediates the clearance of hyaluronan (HA), chondroitin sulfates (CS), and AcLDL (17, 41, 43). The human type I membrane receptor is present in two forms; a full-length 315-kDa isoform (315-hHARE) and a 190-kDa isoform (190-hHARE) that is identical to the COOH-terminal 1136 aa of the 315-hHARE. The 190-hHARE and 315-hHARE isoforms demonstrate identical binding activities for HA, multiple CS types, Ac-LDL, and heparin (16). These results were based on studies using purified secreted ectodomains (s190- and s315-hHARE) of the receptor for in vitro binding assays and using cultured stable human Flp-In 293 cell lines expressing hHARE cDNA for cell binding/endocytosis assays.
In this report, we show that HARE in freshly isolated rat liver SECs or recombinant human cells also binds specifically to LMWH enoxaparin, but with lower affinity than to UFH, and is internalized more slowly. The results show that HARE mediates clearance of both heparin preparations and explain why LMWH has a longer half-life in the blood than UFH.
MATERIALS AND METHODS
Materials and solutions.
LMWH, under the trade name of enoxaparin (Lovenox), was from Sanofi-Adventis. UFH was from Sigma (St. Louis) and Celsus (Cincinnati, OH). Biotin-LC-hydrazide and streptavidin (SA) were from Pierce (Rockford, IL). Biotin-UFH (b-UFH) and b-LMWH, prepared and characterized as noted in Harris et al. (16), contained 1.5–2 biotins per chain. Na125I and Ni-Sepharpose 6 Fast Flow were from GE/Amersham. Collagenase was from Roche Molecular Biochemicals. Human fibronectin and SA-alkaline phosphatase conjugate were from Sigma. Flp-In cell lines stably expressing 190-hHARE and s190-hHARE were constructed as described (16, 17). Cell culture medium and reagents were from Invitrogen/GIBCO (Carlsbad, CA). Heparin-binding plates (EpranEx) were from Plasso (now BD Biosciences). ELISA-like assays were performed using F8 PolySorp strips from Nunc (Roskilde, DK). AcLDL was from Kalen Biomedical (Montgomery Village, MD). Purified monoclonal antibodies (MAbs) against rat HARE (rHARE) or polyclonal antibody (PAb) against hHARE were prepared as noted previously (18, 54). Hanks' balanced salt solution (HBSS) contains 5 mM KCl, 0.4 mM KH2PO4, 0.8 mM MgSO4, 137 mM NaCl, 0.3 mM Na2HPO4, 5.5 mM glucose, 1.26 mM CaCl2, 0.5 mM MgCl2, and 28 μM phenol red; at the time of use, 3.5 g/100 ml of NaHCO3 was added and the pH was adjusted to 7.2 with HCl. TBS contains 20 mM Tris·HCl, pH 7.0, and 150 mM NaCl. TBST is TBS with 0.1% (vol/vol) Tween-20. TBST-BSA is TBST with 1.0% (wt/vol) BSA. Blocking solution is TBST with 2% BSA. Coating solution for ELISA-like assays contains 15 mM Na2CO3, 36 mM NaHCO3, pH 9.5. Standard assay buffer (SAB) contains 100 mM NaCl, 50 mM sodium acetate, 0.2% (vol/vol) Tween-20, pH 7.2. Endocytosis medium 1 is DMEM containing 0.05% BSA (without serum), and endocytosis medium 2 is RPMI containing 0.15% BSA (without serum). The perfusion buffers are buffer 1 containing 142 mM NaCl, 6.7 mM KCl, 10.0 mM HEPES, pH 7.4; buffer 2 containing 67.0 mM NaCl, 6.7 mM KCl, 4.8 mM CaCl2·2H2O, 101 mM HEPES, pH 7.2; and buffer 3 contains 137.0 mM NaCl, 4.7 mM KCl, 1 mM MgSO4, 1.2 mM CaCl2·2H2O, 10.0 mM HEPES, pH 7.4. BSA if present is at 15 g/l.
Size exclusion chromatography and MALLS analysis.
Weight-average molar mass values for the heparin preparations used were determined by size exclusion chromatography coupled to multiangle laser light scattering (MALLS) as described previously (2). Analyses of 0.2 ml samples (at ∼2.0 mg/ml heparin in PBS) were performed with PL Aquagel-OH60 and Aquagel-OH30 columns in series at a flow rate of 0.4–0.5 ml/min in 50 mM NaPO4, pH 7.0, 150 mM NaCl, 0.05% NaN3 at 22°C. MALLS analysis was performed continuously on the eluate by use of a DAWN DSP laser photometer in series with an OPTILAB DSP interferometric refractometer (Wyatt Technologies).
Isolation of SECs from perfused rat liver.
Animal procedures were performed under Institutional Animal Care and Use Committee protocol 08-073 approved by the University of Oklahoma Health Sciences Center and are within the guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care. SECs were prepared by the liver collagenase perfusion technique of Seglen (40) with minor modifications (6, 32) and purified by using discontinuous Percoll gradients (42). Briefly, Sprague-Dawley rats (200–400 g, Charles River Laboratories) were anesthetized with 11 ml of 25% isoflurane in polyethylene glycol in a glass chamber, placed on a tray face up with a nose cone containing 25% isoflurane and stimulated with 70% ethanol on the abdomen to confirm deep anesthesia. The entire abdominal cavity was exposed and the portal vein was cannulated with an Insyte Autoguard catheter (18 GA, 1.3 × 30 mm, Becton, Dickinson Infusion Therapy Systems) and secured with two loops of surgical silk string. As soon as the catheter was immobilized, other major blood vessels were severed and TBS was flushed (50 ml/min) through the liver for 10 min to remove blood (blanching), while the liver was excised and placed on a plastic net over a funnel that allows fluids to be collected and recirculated. Freshly dissolved collagenase (100 mg/kg weight) in buffer 2-BSA is filtered (0.45 μm), added to 10 volumes of prewarmed buffer 2-BSA and perfused, with recirculation, through the liver 10–12 min. The liver is transferred to a glass dish and the membranes covering the lobes are peeled off and the liver is gently shaken in ∼30 ml of buffer 1-BSA to release the cells. The cells are first filtered through a 100-μm mesh and then twice through a 37-μm mesh. Hepatocytes are pelleted by centrifugation at 150 g for 3 min. The pellets are pooled into two 50-ml tubes and the pellets are washed once with buffer 1-BSA at 22°C, centrifuged at 150 g, and washed twice with buffer 3-BSA under the same conditions. The supernatants from each of these washes (containing SECs, Kupffer cells, and some small hepatocytes) are pooled and then centrifuged at 200 g for 10 min at 4°C. To remove remaining hepatocytes, the cell pellets, resuspended in 5 ml of RPMI-BSA, are pooled and centrifuged at 100 g for 3 min, and then all but the bottom ∼10 ml of the supernatant is removed and saved. The cell pellet is resuspended, the procedure is repeated, and the final pooled supernatants are then centrifuged at 200 g for 10 min to pellet the SECs. The pellets are resuspended in 30 ml RPMI-BSA and 10 ml is layered onto each of three Percoll step gradients (20 ml of 25% over 15 ml of 50% Percoll). The gradients are centrifuged (4°C for 20 min at 900 g) and SECs on the 25/50% interface are collected, resuspended in RPMI, and centrifuged at 350 g for 10 min to remove Percoll. The cells are resuspended in RPMI and incubated on sterile glass petri dishes for 10 min to remove Kupffer cells, which settle out and adhere to the glass, whereas the SECs remain in suspension. For endocytosis experiments, the final SECs, ∼95% pure (32, 42), were allowed to settle and spread on human fibronectin-coated 24-well tissue culture plates at 37°C for 2 h, washed, and used immediately.
Endocytosis of 125I-SA·b-heparin.
Stably transfected cells (clone 9 unless noted otherwise) (17) expressing 190-hHARE were plated in 12-well dishes and grown in DMEM supplemented with 8% fetal calf serum FCS and 100 μg/ml Hygromycin B for at least 2 days prior to experiments. Before the experiment, the medium was replaced with endocytosis medium 1 and incubated at 37°C for 1 h to allow HARE-mediated internalization of any bound serum glycosaminoglycans. For purified SECs, internalization experiments in endocytosis medium 2 started immediately after the 2-h adhesion and recovery period following plating on fibronectin-coated dishes. Endocytosis assays with either cell type were performed at 37°C in the appropriate endocytosis medium containing preformed complexes of 125I-SA·b-heparin (50–100 nM b-UFH or b-LMWH) (18) with or without unlabeled UFH or LMWH as competitor. Specific endocytosis was assessed in two ways. First, cells were incubated in parallel with only 125I-SA·biotin to determine background counts per minute (cpm) values at each time point. Second, cells were incubated with 125I-SA·b-heparin and an excess of unlabeled heparin at each time point. Either the background or heparin competition values, which were approximately equal, were subtracted from all data points to determine specific 125I-SA·b-heparin internalization. At the indicated times, cells were washed three times with ice-cold HBSS and lysed in 0.3 N NaOH, and radioactivity and protein content (17) were determined and expressed as cpm per microgram of protein unless noted otherwise.
Purification of the 190-hHARE ectodomain (s190).
cDNA constructs, generation of stable Flp-In cell lines secreting the soluble ectodomain of the human 190-hHARE, and purification of s190-hHARE protein were described earlier (16). Briefly, conditioned media from s190-hHARE cell cultures were pooled, centrifuged to clear cellular debris, and supplemented to final concentrations of 250 mM NaCl, 10% vol/vol glycerol, and 10 mM imidazole. Ni-saturated Sepharose 6 Fastflow resin (1% vol) was added and, after incubation overnight at 22°C, the resin was collected and washed with 250 mM NaCl, 20 mM NaH2PO4, 10 mM imidazole, pH 7.4, and the protein was eluted with 250 mM NaCl, 20 mM NaH2PO4, 400 mM imidazole, pH 7.4. The eluate contained s190-hHARE as well as several other serum-derived proteins that also adhered to the column. Final purification was achieved by concentrating the eluate, separating the proteins by 5% SDS-PAGE (25), and cutting the s190 band from the copper-stained gel with a clean razor blade. The s190-hHARE protein was electroeluted in 25 mM Tris, 185 mM glycine, and 2.5 mM SDS. Final dialysis and concentration of s190-hHARE (>97% pure) was performed in PBS with the use of Amicon concentrators (30,000 MWCO).
ELISA-like assay for heparin binding and competition to s190-hHARE.
s190-hHARE (1 μg/ml) in 0.2 ml of coating buffer was plated in each well of a F8 Polysorp module, sealed with plastic tape, and incubated overnight at 22°C. All subsequent incubations were performed at 37°C, reagents were always added in blocking solution, and all washes were with TBST. Nonspecific binding sites were blocked by incubation with blocking solution for 1 h. Biotinylated UFH and LMWH (16) and unlabeled heparin (UFH or LMWH) were mixed together in blocking solution, added to each well, and incubated for 2 h. The wells were washed four times, followed by the addition of 0.1 μg/ml SA-alkaline phosphatase and incubation for 15 min. The wells were then washed four times and bound SA-alkaline phosphatase was detected by addition of p-nitrophenolphosphate and color development. A SpectraMax 340 plate reader (Molecular Devices) was used to evaluate changes in A405 at appropriate times.
s190-hHARE binding assay using immobilized heparin.
Assays to assess s190-hHARE binding to heparin adsorbed to EpranEx plates (a reverse situation compared with the ELISA-like assay) were performed as outlined by the manufacturer. Briefly, 25 μg/ml heparin or LMWH solution in PBS was plated in the wells of a heparin-binding plate at 22°C overnight. All subsequent incubations were at 37°C and all washes were repeated three times with SAB. The plates were washed with SAB, incubated with 0.2% (wt/vol) porcine gelatin in SAB for 1 h, and s190-hHARE (1 μg/ml in SAB) was added. The plate was incubated for 2 h, washed, and incubated for 1 h with 0.01 μg/ml anti-V5 PAb (Bethyl Laboratories, Montgomery, TX) in 0.2% (wt/vol) gelatin in SAB. After washing, wells were incubated for 1 h with anti-goat-alkaline phosphatase secondary antibody (1:10,000 dilution; Sigma, cat. no. A-4062) in 0.2% (wt/vol) gelatin in SAB. The wells were washed and incubated with p-nitrophenolphosphate, and A405 was monitored as noted above.
RESULTS
Analysis of mass and concentration by MALLS.
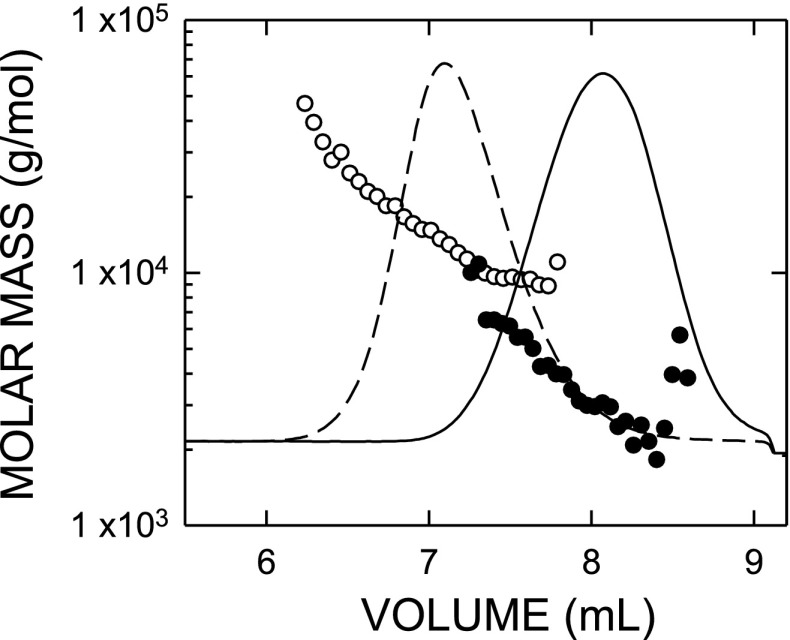
UFH and LMWH (enoxaparin) were analyzed by size exclusion chromatography and MALLS to determine the weight-average molar masses and concentrations of these unmodified heparin preparations (Fig. 1). This is the most accurate method to measure simultaneously the average molecular mass, size distribution, and concentration of purified polymers in solution (3). The weight-average masses of the LMWH and UFH used here were clearly different; the UFH eluted from the column first with a weight-average molar mass (in g/mol) corresponding to 13,500 Da, whereas LMWH eluted later with a weight-average molecular mass of 3,500 Da. Biotinylation of either heparin preparation did not cause degradation (not shown). Peak area is directly related to the amount of the heparin preparation injected onto the column and is used to calculate concentration. Even though the weight-average masses of both heparins are very distinct, there is an overlapping region shared by both preparations because some heparin polymers in each preparation are of similar or the same size and peak broadening also occurs as the polymers fractionate and diffuse through the gel-filtration resin.
Fig. 1.
Polymer mass distributions of unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are different according to multiangle laser light scattering (MALLS). Unmodified UFH and LMWH were individually subjected to mass and concentration analyses using size exclusion chromatography coupled with MALLS. The refractive index concentration traces (lines) and the molar masses (circles) calculated at various elution volumes for UFH (dashed line, ○), or LMWH (solid line, •) are shown. The overall weight-average molar mass for each sample was determined from the concentrations of each molar mass at all points by use of ASTRA 4.73 software (Wyatt Technologies). The weight-average molar mass for UFH and LMWH used in these experiments was 13,500 and 3,500 Da, respectively.
190-hHARE ectodomain binds directly with unmodified or biotinylated heparins.
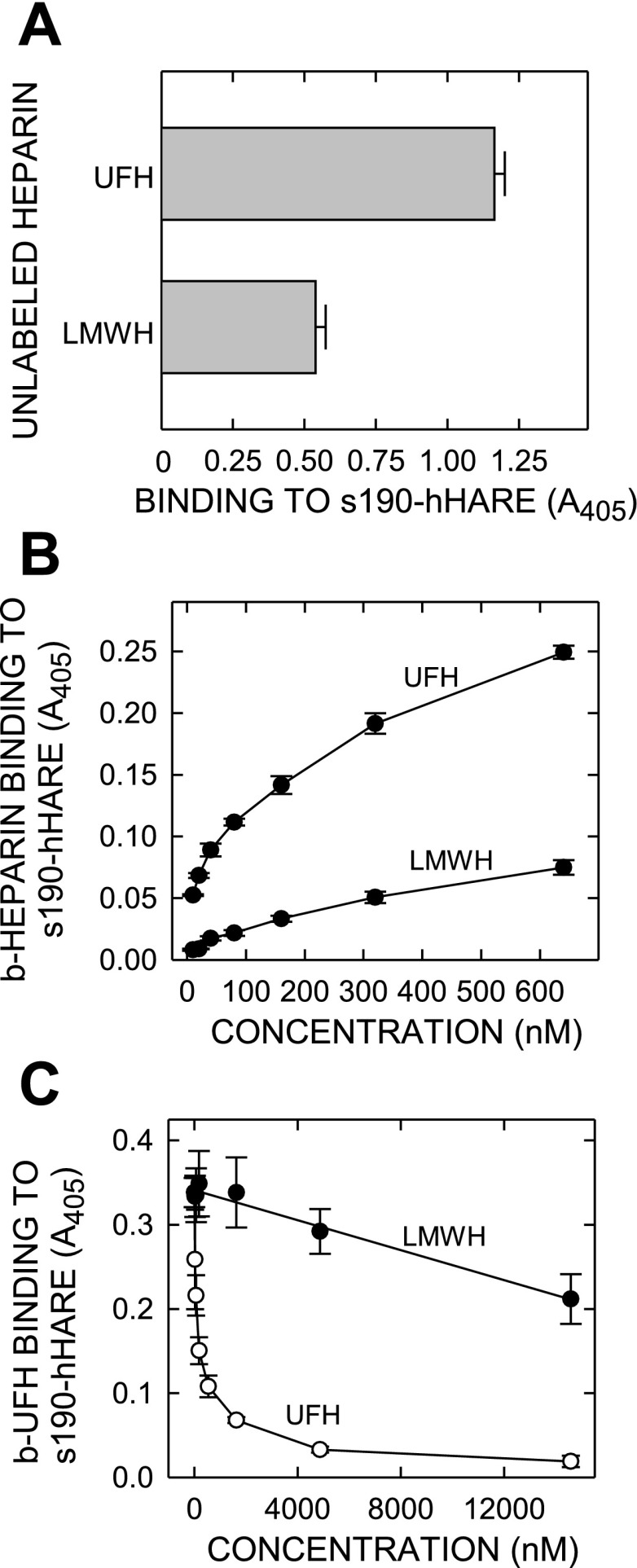
Unlabeled UFH and LMWH were tested for their ability to bind to purified soluble 190-hHARE ectodomain in an ELISA-like assay. Each type of heparin was adsorbed in a saturating amount on heparin-binding EpranEx plates, which are designed to bind unmodified polyanionic GAGs (e.g., heparin or heparan sulfate), and s190-hHARE binding was assessed at 37°C (Fig. 2A). The results show that HARE binds to LMWH. After subtraction of the background antibody binding controls (<5% of total signal), the amount of s190-hHARE binding to immobilized LMWH was 46% of the amount bound to immobilized UFH. We expect the same activity for 315-hHARE, since the s190-hHARE ectodomain has the same ligand-binding activities as the full-length 315 kDa human receptor (19). To test binding and endocytosis, we biotinylated LMWH using the same chemistry as for UFH (1.5–2 biotins per b-LMWH chain; similar to b-UFH).
Fig. 2.
Purified 190-human hyaluronic acid receptor for endocytosis (hHARE) ectodomain binds specifically and directly with both UFH and LMWH. A: unlabeled UFH or LMWH were adsorbed onto wells of EpranEx (heparin-binding) plates, and nonspecific binding sites were blocked with gelatin. Purified s190-hHARE ectodomain (1 μg/ml) was incubated with the adsorbed heparins for 1 h at 37°C and washed, and bound protein was detected with anti-V5 polyclonal antibody (PAb) and then anti-goat IgG-AP as described in materials and methods. Results are presented as the mean ± SE (n = 4) color development at 405 nm. B: to assess direct binding, increasing concentrations biotin (b)-UFH or b-LMWH, as indicated, were incubated with s190-hHARE, adsorbed on PolySorp plastic strips for 1 h at 37°C, and washed, and bound heparin was detected with streptavidin (SA)-AP and quantified at 405 nm as described in materials and methods. C: to assess competition by LMWH or UFH, purified adsorbed s190-hHARE was incubated for 1 h at 37°C with 100 nM b-UFH premixed with the indicated increasing concentrations of unlabeled UFH (○) or LMWH (•). After washing, bound b-UFH was quantified with SA-alkaline phosphatase conjugate, by absorbance (presented as mean ± SE, n = 4) determined at 405 nm.
To confirm directly that LMWH binds to HARE, we incubated increasing concentrations of b-LMWH or b-UFH with purified recombinant s190-hHARE protein adsorbed to plastic wells (Fig. 2B). The dose-response binding curves indicate that b-LMWH binds to s190-hHARE, but at apparently lower affinity and to a lesser extent (∼30%) than b-UFH. In a separate ELISA-like assay, which is the reverse of the above heparin-immobilization assay, we incubated adsorbed purified s190-hHARE, with b-UFH and increasing concentrations of either unlabeled UFH or LMWH. LMWH clearly showed significant dose-dependent inhibition of b-UFH binding (• in Fig. 2C), although as expected the competition by increasing concentrations of UFH was greater (○, Fig. 2C). Unlabeled UFH at 4 μM essentially competed for all (∼100%) b-UFH binding. At this same concentration, ∼24% of the b-UFH binding was competed by LMWH. At the highest concentration tested (15 μM), LMWH competed ∼37% of the b-UFH binding. Based on the observed and extrapolated IC50 values for UFH or LMWH competition for b-UFH binding to purified HARE, we estimate the apparent affinity of UFH to be 114-fold greater than the LMWH affinity for s190-HARE.
LMWH is endocytosed directly or competes for b-UFH endocytosis mediated by 190-hHARE.
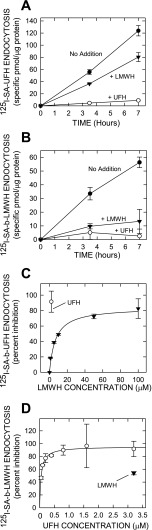
Stable Flp-In 293 cells expressing recombinant membrane-bound 190-hHARE endocytose b-UFH much more efficiently than cells with empty vector (18, 19). Here we compared the inhibitory effects of unlabeled LMWH and UFH on the direct HARE-mediated endocytosis of 125I-SA·b-UFH or 125I-SA·b-LMWH complexes. Cells were incubated for up to 7 h with 125I-SA·b-UFH (Fig. 3A) or 125I-SA·b-LMWH (Fig. 3B) alone or with a 20-fold excess of unlabeled UFH or LMWH as competitor. Regression analysis showed that accumulation of the labeled UFH or LMWH in the presence of either unlabeled heparin preparation was linear over this time. Unlabeled LMWH and UFH decreased the rate of 125I-SA·b-UFH endocytosis by 35 and 93%, respectively. In contrast, for 125I-SA·b-LMWH, the rate of endocytosis in the presence of unlabeled LMWH and UFH decreased by 76 and 95%, respectively.
Fig. 3.
Endocytosis of b-UFH or b-LMWH by 190-hHARE cells is competed by UFH and LMWH. Stably transfected Flp-In 293 cells expressing 190-hHARE were plated and grown in 12-well plates and allowed to internalize 125I-SA·b-UFH (A and C) or 125I-SA·b-LMWH (B and D) at 37°C, as described in materials and methods, either with no addition (•), or with excess unlabeled LMWH (▾) or UFH (○). Cells were washed 3 times with HBSS and lysed in 0.3 N NaOH, and the radioactivity and protein content were determined. Nonspecific binding, assessed in parallel control wells incubated with 125I-SA-biotin, was subtracted, and values are presented as the mean ± SE (n = 3) specific pmol/μg protein (A and B) or percent inhibition (C and D). Lines were calculated by linear or best-fit regression analysis (r > 0.98 in all cases) by using SigmaPlot v10. Cells in medium containing either 125I-SA·b-UFH (A) or 125I-SA·b-LMWH (B) were incubated for the indicated times without competitor, or with a 40-fold excess of unlabeled LMWH or UFH. C: cells were incubated for 4 h with 125I-SA·b-UFH alone or mixed with up to 100 μM LMWH, as indicated, or with 2 μM UFH. D: cells were incubated for 4 h in the presence of 125I-SA·b-LMWH alone or mixed with up to 3.2 μM UFH, as indicated, or with 3.2 μM LMWH.
To assess the dose response of LMWH on 125I-SA·b-UFH endocytosis (Fig. 3C), we incubated cells for 4 h with 100 nM 125I-SA·b-UFH alone or with 2 μM unlabeled UFH (○) or 1–100 μM LMWH (▾). Inhibition of 125I-SA·b-UFH internalization by 2 μM LMWH was ∼19%, whereas 2 μM UFH inhibited 125I-SA·b-UFH endocytosis by 91%. Similarly (Fig. 3D), cells were incubated for 4 h with 100 nM 125I-SA·b-LMWH alone or with 3.2 μM unlabeled LMWH (▾) or 0.05–3.2 μM UFH (○). In this case, inhibition of 125I-SA·b-LMWH internalization by ≥1 μM UFH was ∼95%, whereas inhibition by 3.2 μM LMWH was 54%. The above results indicate that UFH has higher affinity for HARE and is a much better competitor than LMWH for the heparin-binding site(s).
We previously estimated a Kd of ∼0.02 μM for UFH binding to purified s190-hHARE (18). Based on these above results, the estimated concentration of LMWH for a 50% decrease (IC50) in the rate of b-UFH endocytosis was ∼10 μM, which is an approximation of the Kd for HARE binding to LMWH in live cells at 37°C. In contrast, the estimated IC50 for unlabeled UFH in competition with 125I-SA·b-LMWH was ∼0.06 μM (in agreement with a 0.02 μM Kd for binding of purified s190-hHARE to UFH). Thus in live cells the apparent affinity of HARE for UFH is ∼166-fold higher (0.06 μM vs. 10 μM) than for LMWH, which is in close agreement with our data in Fig. 2 and our previous report (18).
HARE binding and endocytosis of heparin is specific.
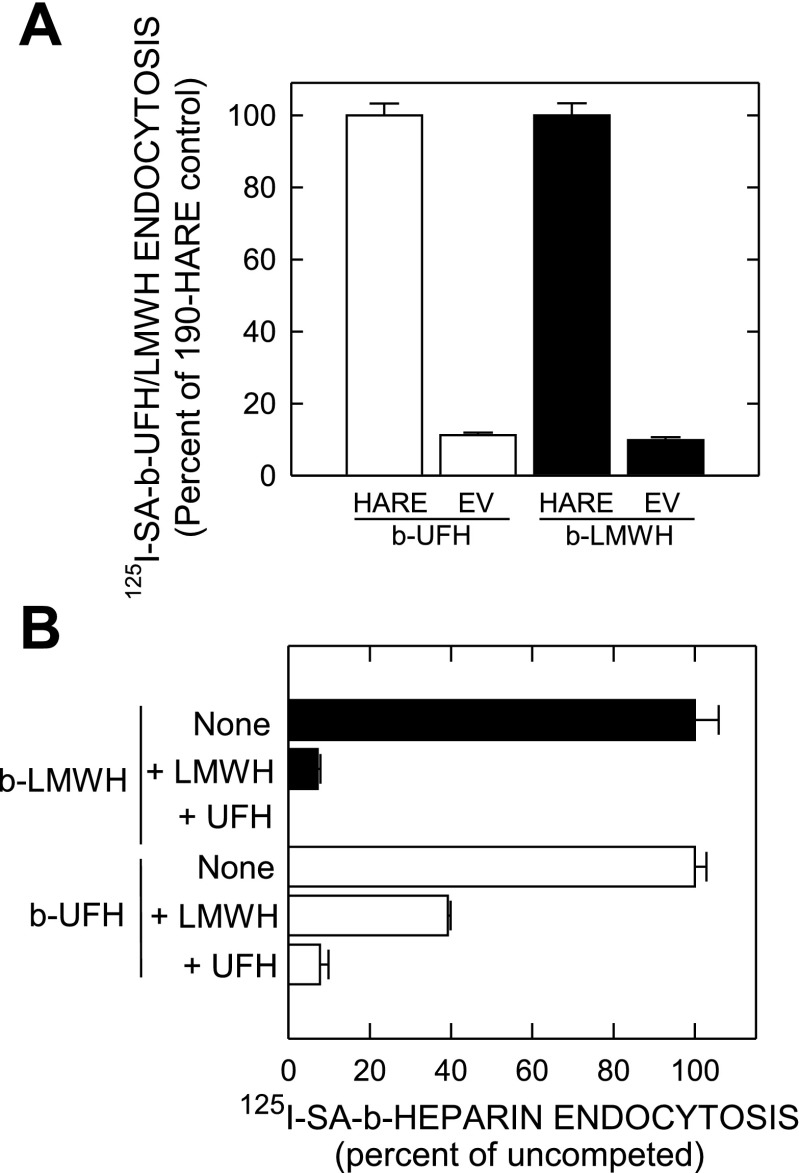
Since heparin is highly charged and binds nonspecifically to other cell components, we measured the rates of 125I-SA·b-UFH and 125I-SA·b-LMWH endocytosis by empty vector (EV) and 190-hHARE cell lines, derived from the parent Flp-In 293 cell line. EV and 190-HARE cells showed the same relative nonspecific binding (∼10%) of 125I-SA·b-LMWH (Fig. 4A, solid bars) compared with n 125I-SA·b-UFH (Fig. 4A, open bars); ∼60% of total LMWH uptake by 190-hHARE cells was specific (mediated by HARE). As noted previously (18, 19), ∼95% of internalized 125I-SA·b-UFH in 190-hHARE cells was HARE-specific, compared with EV cells. Cross-competition of both labeled heparins occurred in 190-hHARE cells; endocytosis of 125I-SA·b-LMWH (Fig. 4B, solid bars) and 125I-SA·b-UFH (Fig. 4B, open bars) were both competed with unlabeled LMWH or UFH. For cells incubated with 125I-SA·b-LMWH, UFH was a slightly better competitor than LMWH at saturation (∼100% vs. 93%). 125I-SA·b-UFH uptake was competed more by UFH than LMWH (92% vs. 69%) at saturation.
Fig. 4.
Cells expressing 190-hHARE mediate specific endocytosis of both UFH and LMWH. Cells were washed and lysed, and the amounts of radioactivity and protein were determined as described in materials and methods. Specific endocytosis for each experimental sample was calculated by subtracting the counts per minute (cpm)/μg protein values for cells incubated in parallel with 125I-SA with free biotin alone (i.e., no b-heparin). A: empty vector (EV) and 5 individual 190-hHARE Flp-In 293 cell lines (9-5, 9-8, 9-9, 9-17, 14), plated in quadruplicate in 24-well dishes, were allowed to internalize 125I-SA·b-LMWH (solid bars) or 125I-SA·b-UFH (open bars) for 3 h. Data from 2 separate experiments were calculated for EV (n = 8) or 190-HARE (n = 40) cells as the mean ± SE specific cpm/μg protein and are shown as a percent of the 190-hHARE samples (as 100%). B: 190-hHARE cells (clone 9-9) were incubated for 4 h in the presence of either 0.05 μM 125I-SA·b-UFH (open bars) or 125I-SA·b-LMWH (solid bars) without (None) or with 2.0 μM LMWH or UFH as indicated. Data were calculated as the mean ± SE (n = 3) specific cpm/μg protein and are shown as a percent of the no-addition (None) samples (as 100%).
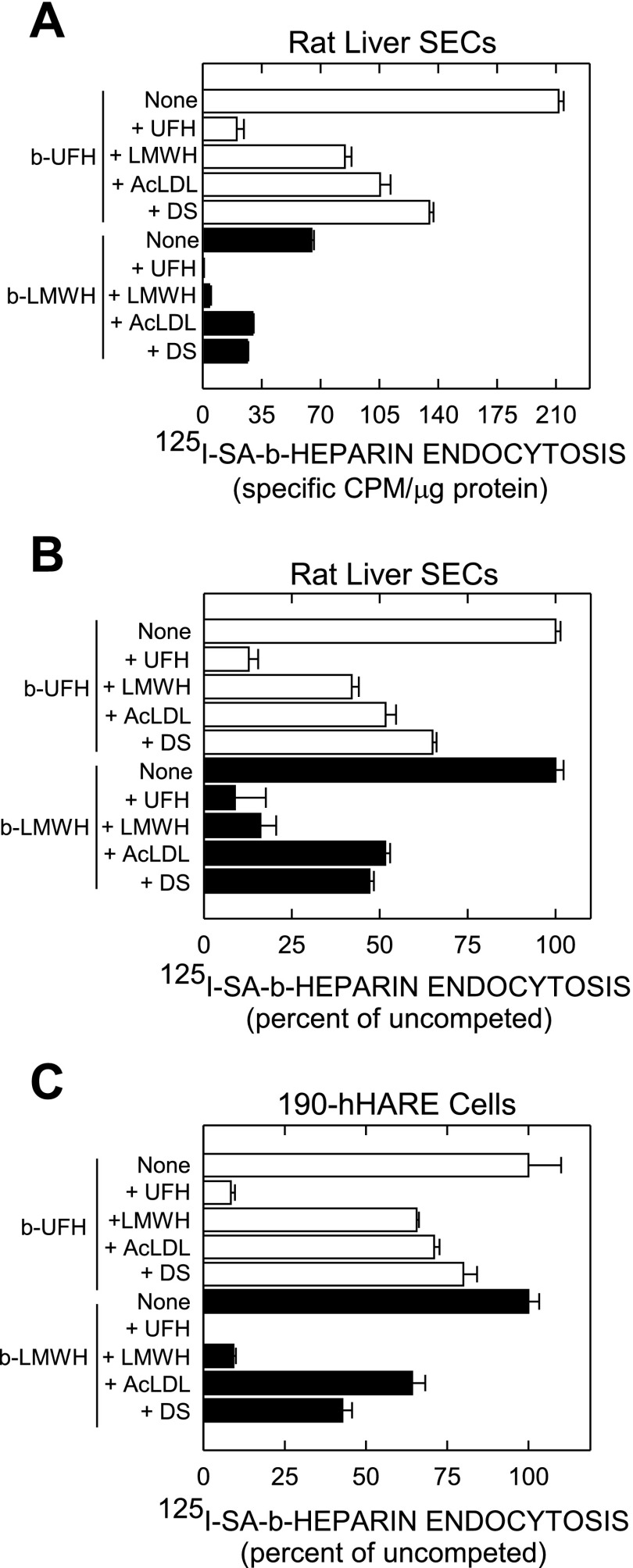
HARE-mediated binding/endocytosis of LMWH by rat liver SECs or 190-hHARE cells is competed by AcLDL and DS.
We previously found that UFH binding and endocytosis by 190-hHARE cells is competed by AcLDL and other polyanions such as dermatan sulfate (DS) and chondroitin sulfate-E (19), both common human ligands. To determine whether binding of both heparins to primary rat liver SECs is similarly competed by these other ligands, cells were incubated with 125I-SA·b-UFH (Fig. 5, A and B, open bars) or 125I-SA·b-LMWH (Fig. 5, A and B, solid bars) with no additions or a 40-fold excess of unlabeled UFH, LMWH, AcLDL, or DS. UFH or LMWH both competed better than AcLDL or DS. Although the amount of each heparin taken up differed (Fig. 5A), both latter ligands blocked uptake of either heparin by ∼50% (Fig. 5B). Thus the results in rat SECs and human cells were virtually the same; LMWH uptake was <50% that of UFH. As a control, the same pattern of competition for 125I-SA·b-LMWH uptake by AcLDL and DS was also observed in 190-hHARE cells (Fig. 5C), as found earlier with UFH (19).
Fig. 5.
Specific endocytosis of UFH and LMWH by rat liver sinusoidal endothelial cells (SECs) and 190-hHARE cells show cross-competition with heparins, acetylated low-density lipoprotein (Ac-LDL), and dermatan sulfate. Isolated rat liver SECs, prepared by a collagenase perfusion technique, were purified, cultured on fibronectin coated 24-well plates for 2 h in serum-free medium, washed, and used as described in materials and methods. A: SECs were incubated with 0.05 μM 125I-SA·b-UFH (open bars) or 125I-SA·b-LMWH (solid bars) in the absence (None) or presence of a 40-fold excess concentration of unlabeled UFH, LMWH, dermatan sulfate (DS), or Ac-LDL at 30 μg/ml. After 3 h at 37°C, cells were washed 3 times with HBSS, and radioactivity and protein were determined. Nonspecific binding, assessed in parallel control wells incubated with 125I-SA·biotin, were subtracted and values are presented as the mean ± SE (n = 3) specific cpm/μg protein. B: the same data from A are presented as a percent of the uncompeted (None) samples for b-UFH and b-LMWH (each as 100%). C: the same experiment as in A was performed with 190-hHARE cells and the data were normalized as in B.
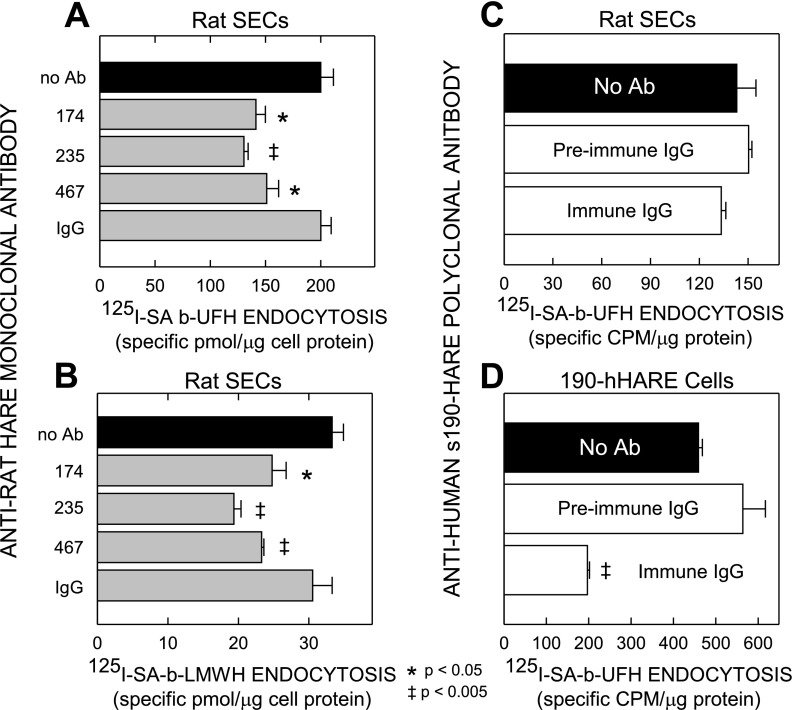
HARE-specific antibodies partially inhibit endocytosis of UFH and LMWH by rat SECs.
To assess whether HARE mediates heparin binding and uptake by rat liver SECs, we tested eight MAbs raised against rat HARE (53, 54) for their ability to block endocytosis of 125I-SA·b-UFH (Fig. 6A) or 125I-SA·b-LMWH (Fig. 6B). We also tested the ability of a rabbit PAb against s190-hHARE to block endocytosis of 125I-SA·b-UFH by rat SECs (Fig. 6C) or 190-hHARE cells (Fig. 6D). Five of the eight MAbs had no effect (not shown), like the mouse IgG control (Fig. 6, A and B). However, three MAbs (nos. 174, 235, and 467) significantly (P < 0.05 or 0.005) blocked endocytosis of b-UFH (Fig. 6A) or b-LMWH (Fig. 6B) by 25–30%; MAb-235 consistently showed the greatest inhibition.
Fig. 6.
Antibodies to rat or human HARE partially block heparin endocytosis by rat SECs and 190-hHARE cells. Rat liver SECs, isolated and cultured without serum as described in materials and methods and Fig. 5, were preincubated for 15 min at 37°C with medium containing no antibody (Ab) or with 60 μg/ml of purified anti-rat MAb 174, 235, or 467, or mouse IgG (as control). An equal volume of medium containing 0.1 μM 125I-SA·b-UFH (A) or 125I-SA·b-LMWH (B) was then added, diluting the Ab concentrations to 30 μg/ml for the duration of the experiment. Cells were incubated for 3 h at 37°C and then washed, and radioactivity and protein content were determined. Values are presented as the mean ± SE (n = 3) specific pmol/μg protein. Similar endocytosis experiments using 125I-SA·b-UFH and rat SECs (C) or 190-hHARE cells (D) were performed to assess inhibition by total preimmune IgG or immune IgG against purified s190-hHARE. Nonspecific binding was assessed in parallel control wells incubated with 125I-SA·biotin and subtracted from total cell associated values, presented as the mean ± SE (n = 3) specific cpm/μg protein. Symbols in A and B indicate significant differences between no Ab and test samples, as assessed by the Student's unpaired t-test; P < 0.05 (*) or P < 0.005 (‡).
Synergy or additivity of inhibition was not observed with any combinations of the three MAbs. The partial inhibition caused by each MAb reflects a real plateau, since these responses were dose dependent and increasing the MAb concentrations gave no greater inhibition beyond the plateau values. The pattern and degree of inhibition by each of the three MAbs for both UFH and LMWH was similar, suggesting that both types of heparin bind to the same HARE site(s). Anti-s190-hHARE PAb inhibited 125I-SA·b-UFH endocytosis by 190-hHARE cells by ∼60% (Fig. 6D) as expected (19) but had no significant effect on 125I-SA·b-UFH endocytosis by rat SECs (Fig. 6C). Preimmune IgG did not inhibit 125I-SA·b-UFH endocytosis by either cell type. These Ab inhibition results indicate that HARE is a bona fide heparin clearance receptor in SECs.
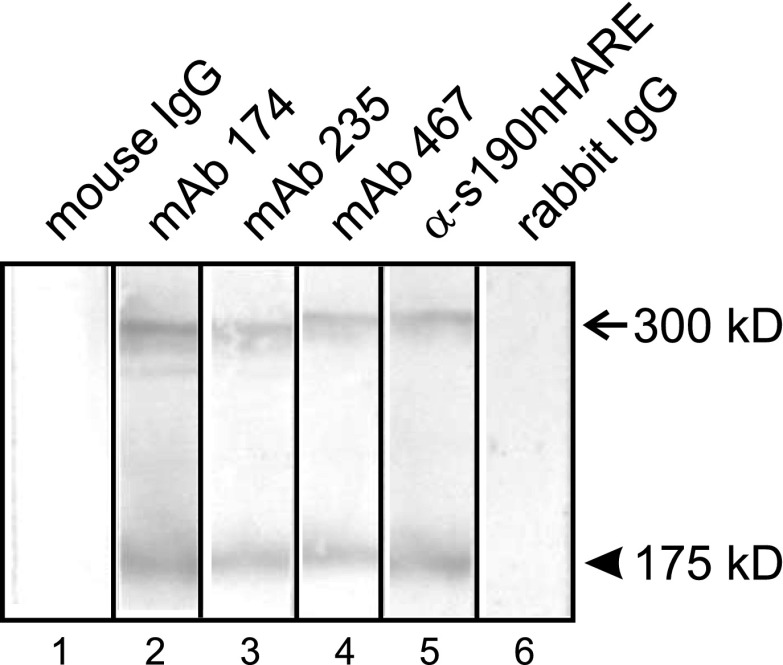
Although anti-human HARE PAb did not block UFH binding to HARE on SECs, we verified that it did cross-react in Western analysis; it readily detected both rat HARE isoforms in whole SEC lysates (Fig. 7, lane 5). The three MAbs that partially inhibited heparin endocytosis in SECs also detected both rat HARE isoforms (Fig. 7, lanes 2-4).
Fig. 7.
Antibody to human HARE does not block heparin uptake by rat liver SECs, but it cross-reacts with both rat HARE isoforms. Rat HARE was immunopurified from isolated rat liver SECs, subjected to SDS-PAGE and electrotransfer. Nitrocellulose strips were incubated for 2 h at 22°C in Tris-buffered saline with Tween-20 (TBST) with 1.0 μg/ml of the indicated purified MAbs, anti-s190-hHARE IgG, nonimmune mouse, or preimmune rabbit IgG. The strips were then washed and probed with appropriate second antibody-AP conjugates as described in materials and methods. The 300-kDa (arrow) and 175-kDa (arrowhead) rat HARE isoforms are both smaller than the corresponding 190- and 315-kDa human HARE isoforms, because of fewer N-glycans (52).
DISCUSSION
This is the first report identifying a specific clearance receptor for LMWH. Most epidemiological studies point out that LMWH is safe to use in prophylactic regimens for prevention of deep vein thrombosis and pulmonary embolism prior to many types of surgeries (11, 26, 33). The primary advantage for using LMWH over UFH is that LMWH has a much longer half-life in the blood. Therefore, it is easier to regulate and reproduce therapeutic dosages of LMWH in patients, and it might be less expensive. However, there are conflicting reports on the cost effectiveness of using LMWH. For example, compared with patients receiving UFH, patients receiving LMWH tend to have a higher incidence of bleeding, which requires higher costs to manage in the long term despite short-term savings (29). All medical-grade heparin is purified from pig small intestine, and most of the US supply comes from foreign sources such as China. When these supplies are disrupted or contaminated, such as occurred during the 2007–08 incident in which >80 US patients died from contaminated heparin from China (14, 23), drug shortages occur and life-saving treatments and surgeries must be deferred. The identification of HARE/Stab2 as a heparin clearance receptor should enhance future efforts to characterize and understand the pharmacokinetics of heparin and to achieve better quality control of heparin preparations.
It has been known since the 1970s that the liver internalizes, and thus removes, heparin from the blood (1, 9, 21, 28), and it is clear now that liver SECs endocytose much more circulating heparin than parenchymal or Kupffer cells (20, 34, 38). Until 2008 (18), no group had conclusively identified the SEC heparin internalization receptor nor shown that SECs deliver the internalized heparin to lysosomes and then digest the heparin polymers down to mono- or oligosaccharides that are then released into the cytoplasm and back into the blood. It was believed that the reticuloendothelial system absorbs heparin nonspecifically and that heparinases break the polymers down to small oligosaccharides (4). However, since heparin is widely used for many applications, the failure to identify the “heparin clearance receptor” and to understand how the body specifically clears heparin has been a huge gap in our physiological knowledge and an obstacle to the development of further therapeutics targeted to this specific clearance function.
On the basis of the now-accepted role of liver SECs in the clearance of HA from blood (12, 27, 47) and our discovery of the high-affinity binding of UFH heparin to hHARE/Stab2, we recently identified HARE as a major clearance receptor for heparin, heparin-like polymers, and probably heparin-protein complexes in the lymphatic and circulatory systems (18). In fact, the HA clearance function mediated by HARE in liver SECs is used to diagnose and assess the prognosis of liver transplant patients and patients with liver damage, by monitoring serum HA levels (15, 37, 39, 48).
The results reported here show that HARE also binds the smaller LMWH preparations, such as enoxaparin, although at lower affinity. Based on in vitro binding assays with purified s190-hHARE and endocytosis assays with cells expressing membrane-bound 190-hHARE, the apparent Kd for hHARE binding to enoxaparin is ∼10 μM, which is 160–500 times higher than the Kd (20–60 nM) for HARE binding to UFH. We compared the abilities of the LMWH enoxaparin vs. standard UFH to interact with the heparin clearance receptor HARE in recombinant 190-hHARE 293 cells and primary rat liver SECs. Our results from cell culture and direct binding assays with the purified s190-hHARE protein reflect the clinical observations related to the half-lives of UFH and LMWH clearance, mainly that LMWH has a lower binding affinity for HARE than UFH. In ELISA-like assays, s190-hHARE had a higher binding affinity for UFH than LMWH. These same results were also reflected in the cell-based assays, which showed that the IC50 for LMWH inhibition of b-UFH internalization is ∼6–10 μM (compared to an IC50 of ∼0.06 μM for UFH). The final proof that HARE is the major liver clearance receptor for heparin will require animal studies to assess the effects of specific anti-HARE heparin-blocking antibodies, which are not yet available, on heparin clearance from the circulation.
We previously developed a panel of eight MAbs against the partially purified rat SEC receptor that enabled us to purify, characterize, and molecularly clone rat HARE for the first time (53–55). MAb-174 was particularly important, since it almost completely blocks HA binding to rat HARE in ligand blots, isolated SECs (46, 53, 55), and perfused liver (45). Three of the eight anti-rat MAbs (nos. 30, 154, and 159) cross-react with human, which allowed us also to purify, characterize, and molecularly clone hHARE from spleen (16, 17, 45, 52). MAb-174 did not block HA binding to hHARE, and none of the 8 MAbs blocked UFH binding to hHARE (unpublished results). In the present study we found that three of the eight MAbs (nos. 174, 235, and 467) partially block heparin binding (both UFH and LMWH) to rHARE but not hHARE. The heparin-blocking MAb-174 and MAb-235 also block HA binding to rHARE, indicating that these two MAbs recognize epitopes that are either shared by or between the independent HA-binding and heparin-binding sites of HARE (19).
The partial and nonadditive inhibition of heparin binding to rHARE by the three different HARE-specific MAbs could be explained in several ways. For example, the different conformational or linear epitopes recognized by each MAb could be part of a subdomain that contributes to, but is not absolutely required for, the overall binding of heparin polymer, (i.e., multiple contact points between HARE and heparin contribute to binding). If the MAbs bind to different subdomains, then we would expect additive inhibition; if the MAbs bind to the same or different epitopes within the same subdomain, then their inhibitory effects would likely not be additive. In agreement with this possible explanation, unpublished preliminary results indicate that MAbs 235 and 467 each block binding (>90%) of the other biotinylated MAb to rHARE; thus their epitopes within HARE are in close proximity.
Definitive animal experiments must await development of specific anti-HARE heparin blocking antibodies, but all our results are consistent with and explain the slower clearance of LMWH from the blood stream. The half-life of circulating UFH in the blood is 30–60 min (22), whereas the half-lives of the smaller LMWH preparations are ∼2–3.5 h, depending on the preparation (10). Smaller heparin chains are more readily cleared by renal filtration than UFH, so the more efficient rapid clearance of UFH is due to HARE-mediated endocytosis by SECs in liver. The present finding that HARE also binds to LMWH, but with lower affinity, now provides a molecular basis to understand why LMWH preparations such as enoxaparin remain in the circulation three to six times longer than UFH. LMWH heparin chains are cleared by two pathways: a renal mechanism in kidney and a HARE-mediated mechanism in liver (and probably lymph node) SECs. The relative contribution of each pathway to LMWH clearance is not yet known.
Previous studies by others showed that rat liver SECs clear UFH from blood in vivo (20, 34, 38). Oie et al. (34) found “HA as the most potent inhibitor (23%)” of UFH uptake but concluded that HARE/Stab2 was not the heparin scavenger receptor, at least in rats, since HA did not block heparin completely, and anti-Stab2 PAb that partially blocks HA binding (31) did not block heparin endocytosis. However, our two essentially concurrent reports using either well-defined cell lines expressing one or both hHARE/Stab2 isoforms (18, 24) or purified glycosaminoglycans and ectodomains of both hHARE isoforms in direct binding studies (19) provide a clear, though surprising, explanation. The HA- and UFH/LMWH-binding sites of hHARE are independent and nonoverlapping; the clearest evidence for this is that 190-hHARE lacking the HA-binding link domain cannot bind HA, but still avidly binds heparin (18, 19, 24). The finding (34) that anti-HARE/Stab2 HA-blocking Ab does not block heparin uptake confirms our results. The above, and our earlier, results show conclusively that heparin uptake is mediated by HARE/Stab2 in rat liver SECs or recombinant cells.
Our results and conclusions regarding clearance of UFH and LMWH by the 190-hHARE also apply to the 315-hHARE, since the heparin-binding site(s) is present within the 190-hHARE isoform (i.e., the COOH-terminal ∼half of the 315 kDa protein). Both isoforms bind heparin, HA, and other glycosaminoglycan ligands in the same fashion (18, 19). The results support the conclusions that the two human and rat HARE isoforms bind to UFH and LMWH similarly, that longer (larger mass) heparin polymers bind more effectively than shorter (smaller mass) heparins, and that HARE/Stab2 is the liver SEC heparin clearance receptor.
GRANTS
This research was supported by National Institute of General Medical Sciences Grant GM69961 from the National Institutes of Health.
DISCLOSURES
The authors have no financial or other interests with Sanofi-Adventis or any other companies mentioned in this report.
Acknowledgments
We thank Jennifer Washburn for help with cell culture and purification of s190-hHARE protein and Amy Padgett-McCue, Janet A. Weigel, and Long Nguyen for general technical assistance.
REFERENCES
- 1.Alant O, Varga L, Antoni F, Karacsonyi S, Faller J. Metabolism and anticoagulant effect of 51Cr-labelled heparin. Acta Physiol Acad Sci Hung 43: 261–267, 1973. [PubMed] [Google Scholar]
- 2.Baggenstoss BA, Weigel PH. Some problems associated with the use of GPC-MALLS to analyze hyaluronan size distributions made by hyaluronan synthases. In: Hyaluronan Structure, Metabolism, Biological Activities, Therapeutic Applications, edited by Balazs EA, Hascall VC. Edgewater, NJ: Matrix Biology Institute, 2005, p. 143–146.
- 3.Baggenstoss BA, Weigel PH. Size exclusion chromatography-multiangle laser light scattering analysis of hyaluronan size distributions made by membrane-bound hyaluronan synthase. Anal Biochem 352: 243–251, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bame KJ Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology 11: 91R–98R, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bunting RW, Wilkinson RA, Callahan RJ, Strauss HW, Fischman AJ. Indium-111 DTPA-heparin: radiolabeling, pharmacokinetics, and biodistribution following intravenous administration in rat and rabbit. Thromb Res 89: 23–30, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Clarke BL, Oka JA, Weigel PH. Degradation of asialoglycoproteins mediated by the galactose receptor system in isolated rat hepatocytes. Evidence for two parallel pathways. J Biol Chem 262: 17384–17392, 1987. [PubMed] [Google Scholar]
- 7.Dawes J, Pepper DS. Human vascular endothelial cells catabolise exogenous glycosaminoglycans by a novel route. Thromb Haemost 67: 468–472, 1992. [PubMed] [Google Scholar]
- 8.Dinwoodey DL, Ansell JE. Heparins, low-molecular-weight heparins, and pentasaccharides. Clin Geriatr Med 22: 1–15, vii, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Estes JW, Poulin PF. Pharmacokinetics of heparin. Distribution and elimination. Thromb Diath Haemorrh 33: 26–37, 1975. [PubMed] [Google Scholar]
- 10.Fareed J, Ma Q, Florian M, Maddineni J, Iqbal O, Hoppensteadt DA, Bick RL. Differentiation of low-molecular-weight heparins: impact on the future of the management of thrombosis. Semin Thromb Hemost 30, Suppl 1: 89–104, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Findik S, Erkan ML, Selcuk MB, Albayrak S, Atici AG, Doru F. Low-molecular-weight heparin versus unfractionated heparin in the treatment of patients with acute pulmonary thromboembolism. Respiration 69: 440–444, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242: 27–33, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Glimelius B, Busch C, Hook M. Binding of heparin on the surface of cultured human endothelial cells. Thromb Res 12: 773–782, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol 26: 669–675, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson S, Bjorkman T. Circulating hyaluronan, chondroitin sulphate and dextran sulphate bind to a liver receptor that does not recognize heparin. Glycoconj J 14: 561–568, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Harris EN, Kyosseva SV, Weigel JA, Weigel PH. Expression, processing and glycosaminoglycan binding activity of the recombinant human 315-kDa HA receptor for endocytosis (HARE). J Biol Chem 282: 2785–2797, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Harris EN, Weigel JA, Weigel PH. Endocytic function, glycosaminoglycan specificity, and antibody sensitivity of the recombinant human 190 kDa HA receptor for endocytosis (HARE). J Biol Chem 279: 36201–36209, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Harris EN, Weigel JA, Weigel PH. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J Biol Chem 283: 17341–17350, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris EN, Weigel PH. The ligand-binding profile of HARE: hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein, dermatan sulfate, and CS-E. Glycobiology 18: 638–648, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiebert L The uptake of heparin by liver sinusoidal cells in normal and atherosclerotic rabbits. Thromb Res 21: 383–390, 1981. [DOI] [PubMed] [Google Scholar]
- 21.Hiebert LM, Jaques LB. The observation of heparin on endothelium after injection. Thromb Res 8: 195–204, 1976. [DOI] [PubMed] [Google Scholar]
- 22.Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126: 188S–203S, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Galcheva-Gargova Z, Al Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med 358: 2457–2467, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyosseva SV, Harris EN, Weigel PH. The hyaluronan receptor for endocytosis (HARE) mediates hyaluronan-dependent signal transduction via extracellular signal-regulated kinases (ERK). J Biol Chem 283: 15047–15055, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 26.Lastoria S, Rollo HA, Yoshida WB, Giannini M, Moura R, Maffei FH. Prophylaxis of deep-vein thrombosis after lower extremity amputation: comparison of low molecular weight heparin with unfractionated heparin. Acta Cir Bras 21: 184–186, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Laurent TC, Fraser JRE. Hyaluronan. FASEB J 6: 2397–2404, 1992. [PubMed] [Google Scholar]
- 28.Losito R, Barlow G, Lemieux E. 3H-heparin and antithrombin III in the isolated liver perfusion. Thromb Res 10: 83–93, 1977. [DOI] [PubMed] [Google Scholar]
- 29.Lynd LD, Goeree R, Crowther MA, O'Brien BJ. A probabilistic cost-effectiveness analysis of enoxaparin versus unfractionated heparin for the prophylaxis of deep-vein thrombosis following major trauma. Can J Clin Pharmacol 14: e215–e226, 2007. [PubMed] [Google Scholar]
- 30.Mahadoo J, Heibert L, Jaques LB. Vascular sequestration of heparin. Thromb Res 12: 79–90, 1978. [DOI] [PubMed] [Google Scholar]
- 31.McCourt PAG, Smedsrod BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology 30: 1276–1286, 1999. [DOI] [PubMed] [Google Scholar]
- 32.McGary CT, Yannariello-Brown J, Kim DW, Stinson TC, Weigel PH. Degradation and intracellular accumulation of a residualizing hyaluronan derivative by liver endothelial cells. Hepatology 18: 1465–1476, 1993. [PubMed] [Google Scholar]
- 33.Mismetti P, Quenet S, Levine M, Merli G, Decousus H, Derobert E, Laporte S. Enoxaparin in the treatment of deep vein thrombosis with or without pulmonary embolism: an individual patient data meta-analysis. Chest 128: 2203–2210, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Oie CI, Olsen R, Smedsrod B, Hansen JB. Liver sinusoidal endothelial cells are the principal site for elimination of unfractionated heparin from the circulation. Am J Physiol Gastrointest Liver Physiol 294: G520–G528, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Oie CI, Olsen R, Smedsrod B, Hansen JB. Liver sinusoidal endothelial cells are the principal site for elimination of unfractionated heparin from the circulation. Am J Physiol Gastrointest Liver Physiol 294: G520–G528, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Palm M, Mattsson C. Pharmacokinetics of heparin and low molecular weight heparin fragment (Fragmin) in rabbits with impaired renal or metabolic clearance. Thromb Haemost 58: 932–935, 1987. [PubMed] [Google Scholar]
- 37.Patel K, Lajoie A, Heaton S, Pianko S, Behling CA, Bylund D, Pockros PJ, Blatt LM, Conrad A, McHutchison JG. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol 18: 253–257, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Praaning-van Dalen DP, Brouwer A, Knook DL. Clearance capacity of rat liver Kupffer, endothelial, and parenchymal cells. Gastroenterology 81: 1036–1044, 1981. [PubMed] [Google Scholar]
- 39.Santos VN, Leite-Mor MM, Kondo M, Martins JR, Nader H, Lanzoni VP, Parise ER. Serum laminin, type IV collagen and hyaluronan as fibrosis markers in non-alcoholic fatty liver disease. Braz J Med Biol Res 38: 747–753, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Seglen PO Preparation of isolated rat liver cells. In: Materials and Methods in Cell Biology, edited by Prescott DM. New York: Academic, 1976, p. 29–78. [DOI] [PubMed]
- 41.Smedsrod B, Malmgren M, Ericsson J, Laurent TC. Morphological studies on endocytosis of chondroitin sulphate proteoglycan by rat liver endothelial cells. Cell Tissue Res 253: 39–45, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Smedsrod B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol 38: 213–230, 1985. [DOI] [PubMed] [Google Scholar]
- 43.Smedsrod B, Pertoft H, Eriksson S, Fraser JRE, Laurent TC. Studies in vitro on the uptake and degradation of sodium hyaluronate in rat liver endothelial cells. Biochem J 223: 617–626, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stehle G, Wunder A, Sinn H, Schrenk HH, Friedrich EA, Dempfle CE, Maier-Borst W, Heene DL. Complexes of a modified low-molecular-weight heparin with protamine are predominantly cleared by a macrophage scavenger receptor-mediated process in rats. J Surg Res 58: 197–204, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Weigel JA, Raymond RC, McGary CT, Singh A, Weigel PH. A blocking antibody to the hyaluronan receptor for endocytosis (HARE) inhibits HA clearance by perfused liver. J Biol Chem 278: 9808–9812, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Weigel PH, McGary CT, Zhou B, Weigel JA. Purification and characterization of the hyaluronan receptor for endocytosis (HARE). In: Hyaluronan: Proceedings of an international meeting, September 2000, North East Wales Institute, UK, edited by Kennedy JF, Philips GO, and Williams PA. Abington, UK: Woodhead, 2002, p. 401–410.
- 47.Weigel PH, Yik JHN. Glycans as endocytosis signals: the cases of the asialoglycoprotein and hyaluronan/chondroitin sulfate receptors. Biochim Biophys Acta 1572: 341–363, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Williams AM, Langley PG, Osei-Hwediah J, Wendon JA, Hughes RD. Hyaluronic acid and endothelial damage due to paracetamol-induced hepatotoxicity. Liver 23: 110–115, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Young E, Prins M, Levine MN, Hirsh J. Heparin binding to plasma proteins, an important mechanism for heparin resistance. Thromb Haemost 67: 639–643, 1992. [PubMed] [Google Scholar]
- 50.Yuasa H, Watanabe J. Are novel scavenger-like receptors involved in the hepatic uptake of heparin? Drug Metab Pharmacokinet 18: 273–286, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Zammit A, Pepper DS, Dawes J. Interaction of immobilised unfractionated and LMW heparins with proteins in whole human plasma. Thromb Haemost 70: 951–958, 1993. [PubMed] [Google Scholar]
- 52.Zhou B, McGary CT, Weigel JA, Saxena A, Weigel PH. Purification and molecular identification of the human hyaluronan receptor for endocytosis. Glycobiology 13: 339–349, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Zhou B, Oka JA, Singh A, Weigel PH. Purification and subunit characterization of the rat liver endocytic hyaluronan receptor. J Biol Chem 274: 33831–33834, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Zhou B, Weigel JA, Fauss LA, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem 275: 37733–37741, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Zhou B, Weigel JA, Saxena A, Weigel PH. Molecular cloning and functional expression of the rat 175-kDa hyaluronan receptor for endocytosis. Mol Biol Cell 13: 2853–2868, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]