Abstract
Exposure of maternal mice to inorganic arsenic through the drinking water induces liver tumors and aberrant gene expression in offspring when they reached adulthood. To help define if these are direct fetal effects of arsenic, fetal liver cells were isolated from untreated mice at gestation day 13.5 by mechanical dissection and centrifugation. Two hrs after seeding the cells on collagen1-coated plates in William E media containing 10% fetal bovine serum, 1x ITS (insulin, transferrin and selenium) and antibiotics, inorganic arsenite (0, 0.1, 0.3, and 1.0 µM) was added to the fresh media for 72 hr. Cell morphology and viability were not significantly altered by these arsenic concentrations. At the end of arsenic exposure, cells were harvested into Trizol, and total RNA was extracted, purified, and subjected to real-time RT-PCR analysis. Arsenite exposure produced a concentration-dependent induction of heme oxygenase-1 (up to 8-fold) and metallothionein-1 (up to 5-fold), indicative of stress response to adapt to arsenic insult. Expression of genes related to steroid metabolism, such as 17β-hydroxysteroid dehydrogenase-7 (HSD17β7) and Cyp2α4, were increased approximately 2-fold, together with increases in estrogen receptor-α (ER-α) and ER-α-linked genes such as anterior gradient-2, keratin 1–19, and trefoil factor-3. Arsenic in vitro induced a 3-fold increase in the expression of α-fetoprotein, a biomarker associated with transplacental arsenic-induced mouse liver tumors. Thus, exposure of mouse fetal liver cells to arsenic induces adaptive responses and aberrant gene expression, which could alter genetic programming at the very early life stage, potentially contributing to tumor formation much later in life.
Keywords: Arsenic, Fetal mouse liver cells, Adaptive response, Aberrant gene expression
Introduction
Inorganic arsenic is a human carcinogen, inducing tumors of the skin, lung, urinary bladder, liver, prostate, kidney, and possibly other sites. 1–4 We have shown that short-term exposure of mice to inorganic arsenic in utero produces a variety of internal tumors in offspring when they reach adulthood.5–9 Gestation is a period of high sensitivity to chemical carcinogenesis in rodents and probably in humans. 10 Inorganic arsenic can readily cross the rodent and human placenta and enter the fetus.1–3, 11–12 After in utero exposure to inorganic arsenic at carcinogenic doses, significant amounts of inorganic arsenic and its methylated metabolites (DMA and MMA) are detected in fetal blood and tissues in mice. 12
The liver is a major target organ of arsenic toxicity in humans, 13–14 and a potential target of arsenic carcinogenesis. 3–4 In accord with human data, transplacental exposure of mice to inorganic arsenic induces a marked, dose-related increase in liver tumors, including hepatocellular carcinoma, in adult offspring. 5–8 An array of aberrantly expressed genes in arsenic-induced liver tumors and/or the normal tumor-surrounding tissues, including genes critical to the carcinogenic process and aberrant estrogen signaling, also occur in adult mice after in utero exposure to arsenic. 15–18 The hypothesis that arsenic might somehow act through aberrant estrogen signaling is further supported by synergistic increases in tumor incidence, including increases in liver tumors, in CD1 mice when in utero arsenic exposure is combined with postnatal exposure to the synthetic estrogen, diethylstilbestrol (DES), on postpartum day 1 to 5. 7–8
To define early molecular events associated with transplacental arsenic carcinogenesis, gene expression profiling was performed at early time points in fetal mouse livers,19 newborn mouse livers, 20 and fetal mouse lungs 21 after in utero exposure to a carcinogenic level of inorganic arsenic from gestation day 8 to 18. Significant alterations in gene expression occurred in these fetal or newborn tissues, including genes related to arsenic adaptation, steroid metabolism and estrogen signaling, and genes related to insulin growth factor signaling and a cancer biomarker, α-fetoprotein (AFP).19–21
Clearly, maternal/in utero exposure to arsenic causes marked changes in the expression of various genes in the fetal liver. However, the pregnant animal represents a complex system for potential gene expression control emanating from various sites including maternal and fetal non-liver sites. Thus, to help define the potential direct effects of inorganic arsenic on mouse livers, fetal liver cells were isolated from CD1 mice at gestation day 13.5.22 Fetal liver cells were then seeded onto collagen-coated plates and cultured in William E medium containing 10% fetal bovine serum, 1 × ITS (insulin, transferrin and selenium), antibiotics, and various concentrations of inorganic arsenite for 72 hr. Total RNA was then isolated for real-time RT-PCR analysis. The results clearly showed that exposure of fetal liver cells to inorganic arsenic at tolerable concentrations directly induced an adaptive response and aberrant gene expression, including the expression of stress-related genes and genes involved in estrogen signaling. Perhaps most interestingly, the expression of AFP, a tumor biomarker associated with transplacental arsenic-induced liver tumors, was greatly increased. Thus, arsenic directly acts on fetal liver cells to alter gene expressions, which could be an important mechanism in transplacental arsenic carcinogenesis.
Materials and Methods
Chemicals
Sodium arsenite (NaAsO2) was obtained from Sigma Chemical Co. (St. Louis, MO). The primers for real-time RT-PCR analysis were synthesized by Sigma-GenoSys (The Woodlands, TX). All other chemicals were of reagent grade.
Fetal liver cell isolation
Timed pregnant CD1 mice were used at gestation day 13.5 for fetal liver cell isolation. Animal care was provided in accordance with the US Public Health Policy on the Care and Use of Animals, and the NIEHS Institutional Animal Care and Use Committee approved the study proposal. Mice were anesthetized with pentobarbital (50 mg/kg, ip), and fetuses removed. The very small (3–6 mg) fetal livers (n = 6–9 fetus) were pooled from each dam. Fetal livers were mechanically dissected with flat forceps in culture medium containing 3 × antibiotics and passed through 70 G filter to remove big clots and non-dissociated cells. The cell suspensions were then centrifuged (500 × g, 3 min) to remove debris. The liver cells obtained were washed twice with culture media and counted. Cells were seeded into collagen-1-coated 6-well plates (Beckson Dickson, Bedford, MA) at 2 ×/105 cells/well. Two hours after seeding, cultures were changed to fresh William E media containing 10% fetal bovine serum, 1 × ITS (insulin, transferrin and selenium), 1x antibiotics, and various concentrations of inorganic arsenite (0.1, 0.3 and 1.0 µM) for 72 hr.
Cell morphology
Cultured fetal liver cells were monitored daily under the Olympus CK40 microscope equipped with Polaroid MicroCam (Paramus, NJ). In the pilot study, high concentrations of arsenic (3 and 10 µM) produced cytotoxicity and morphology changes (data not shown). Cultures tolerable to lower concentrations of arsenic (0.1, 0.3, and 1.0 µM) were used for gene expression analysis.
Real-time RT-PCR Analysis
The levels of expression of the selected genes were quantified using real-time RT-PCR analysis. The forward and reverse primers for selected genes were designed using ABI Primer Express software (Applied Biosystems, Foster City, CA) and listed in Table 1. Total RNA was reverse transcribed with MuLV reverse transcriptase and oligo-dT primers, and subjected to real-time PCR analysis using SYBR green PCR master mix (Applied Biosystems, Cheshire, UK). The cycle time (Ct) values of genes of interest were first normalized with β-actin from the same sample, and then the relative differences between control and treatment groups were calculated and expressed as percentage of controls.
Table 1.
Primer sequences for real-time RT-PCR analysis
| Gene | GenBank Number |
Forward | Reverse |
|---|---|---|---|
| AFP | V00743 | AGCTCAGCGAGGAGAAATGGT | GTTCACAGGGCTTGCTTCATTC |
| Agr2 | NM_011783 | CCTTGCGGCTCACACAAAG | ATGGCCACAAGAAGCAGGAT |
| β-actin | M12481 | GGCCAACCGTGAAAAGATGA | CAGCCTGGATGGCTACGTACA |
| Cyp2a4 | J03549 | GGAAGACGAACGGTGCTTTC | CCCGAAGACGATTGAGCTAATG |
| ER-α | M38651 | TCTCTGGAAGAGAAGGACCACATC | TGCAGAGTCAGGCCAGCTTT |
| HO-1 | M33203 | CCTCACTGGCAGGAAATCATC | CCTCGTGGAGACGCTTTACATA |
| HSD17β7 | NM_010476 | AGCTGATGGAGGCGTTCCT | TGTGTATCACCCAGCGAGATG |
| Krt1-19 | NM_008471 | GAGGACTTGCGCGACAAGA | GGCGAGCATTGTCAATCTGTAG |
| MT-1 | BC027262 | AATGTGCCCAGGGCTGTGT | GCTGGGTTGGTCCGATACTATT |
| Tff3 | NM_011575 | CTCTGTCACATCGGAGCAGTGT | TGAAGCACCAGGGCACATT |
Statistics
For real-time RT-PCR analysis, means and SEM of separate fetal liver isolations (n = 4 to 6 dams) were calculated. For comparisons of gene expression between two groups, Students' t test was performed. For comparisons among three or more groups, data were analyzed using a one-way analysis of variance, followed by Duncan’s multiple range test. The significance was set at p < 0.05 in all cases.
Results
Morphology of cultured fetal liver cells
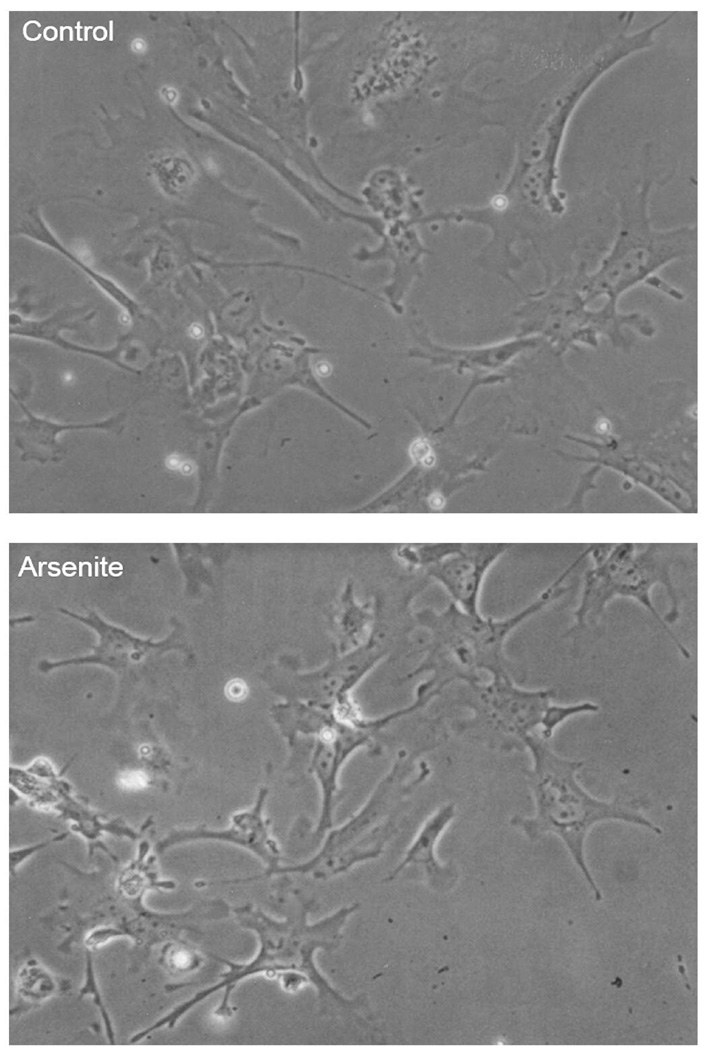
The morphology of cultured fetal liver cells was quite different from adult hepatocytes 23 in that: they are much smaller in size; they proliferate and expand from initially attached cells; and their shape is irregular. The morphology of control fetal liver cells after 72-hr culture (Fig. 1 top) was not significantly different from cultures treated with 1.0 µM arsenite (Fig. 1 bottom).
Figure 1.
Representative morphology of fetal liver cells at 72 hr cultures. Fetal liver cells were isolated from CD1 mice at GD13.5. Top: untreated controls; bottom: cells were continuously exposed to inorganic arsenite (1.0 µM for 72 hr).
Real-time RT-PCR analysis of aberrantly expressed genes
Both heme oxygenase-1 (HO-1) and metallothionein-1 (MT-1) are well known markers of acute arsenic-induced oxidative stress. 1–3 Figure 2 illustrates the concentration-dependent induction of HO-1 (up to 8-fold) and MT-1 (up to 5-fold) in fetal liver cells cultured with 0 to 1.0 µM arsenite for 72 hrs, indicative of an adaptive response occurring to arsenic on these fetal liver cell primary cultures in response to inorganic arsenic insult.
Figure 2.
Expression of oxidative stress-related genes heme oxygenase-1 (HO-1) and metallothionein-1 (MT-1) in response to arsenic insult. Fetal CD1 mouse liver primary cultures were treated with various concentrations of arsenite for 72 hrs, and the gene expression was quantified with real-time RT-PCR. Data are mean and SEM of separate cell isolations (n= 4 – 6). *Significantly different from untreated controls, p < 0.05
The expression of genes related to steroid metabolism and estrogen signaling is altered by in utero arsenic exposure at carcinogenic doses in fetal livers and lungs. 19,21 Thus, the expression of genes involved in these processes were examined in fetal cultured liver cells. Table 2 shows that the expression of hydroxysteroid 17-β dehydrogenase-7 (HSD17β7, involved in estradiol production) and expression of Cyp2a4 (steroid 15α-hydroxylase, involved in testosterone metabolism) were both directly increased by arsenic approximately 2-fold. The expression of estrogen receptor-α (ER-α, 2-fold) and ER-α-regulated genes such as anterior gradient 2 (Agr2, 2-fold), cytokeratin 1–19 complex (Krt1-19, 2.5-fold), trefoil factor 3 (Tff3, 2-fold), and small proline-rich protein 2a (Sprr2a, 2-fold) were also increased following arsenic treatment.
Table 2.
Effects of arsenite on gene expression in fetal mouse liver cell primary cultures
| Genes | Control | As-0.1 µM | As-0.3 µM | As-1.0 µM |
|---|---|---|---|---|
| Steroid metabolism | ||||
| HSD17β7 | 100 ± 12 | 112 ± 36 | 170 ± 26 | 208 ± 10* |
| Cyp2a4 | 100 ± 15 | 103 ± 21 | 110 ± 10 | 195 ± 24* |
| Estrogen signaling and related | ||||
| ER-α | 100 ± 5 | 101 ± 15 | 181 ± 56 | 230 ± 45* |
| Agr2 | 100 ± 9 | 80 ± 21 | 161 ± 45 | 201 ± 24* |
| Krt1-19 | 100 ± 18 | 95 ± 5 | 237 ± 30* | 270 ± 43* |
| Tff3 | 100 ± 10 | 126 ± 43 | 165 ± 25 | 225 ± 45* |
| Sprr2a | 100 ± 25 | 140 ± 60 | 215 ± 70 | 240 ± 60* |
| Cancer related | ||||
| AFP | 100 ± 17 | 165 ± 50 | 215 ± 60 | 290 ± 65* |
Data are mean ±SEM (n= 4 – 6)
Significantly different from controls, p < 0.05.
Transplacental arsenic exposure also alters the expression of a key cancer-related gene, namely AFP. 19–21 AFP has long been considered a superlative tumor cell marker and is increased in liver tumors formed in adults after transplacental arsenic exposure16–18 The expression of AFP was increased in a concentration-related fashion to a maximum of 3-fold (Table 2).
Discussion
The present study clearly demonstrates that inorganic arsenic can directly act on fetal liver cells to alter gene expression at concentrations not overtly cytotoxic. In our transplacental arsenic carcinogenesis studies, the doses of arsenic given to the pregnant mice are also very well tolerated by both dam and fetus, and the resultant inorganic arsenic concentrations in fetal blood and tissues are comparable to levels in human blood from areas where arsenicosis is endemic. 10 Thus, the use of concentrations that are not overtly toxic mimic our transplacental arsenic carcinogenesis model. In general, arsenic-induced aberrant gene expression occurred in a concentration-dependent manner for most genes. The altered gene expressions include stress-related genes (HO-1 and MT-1), genes related to steroid metabolism (HSD17β7 and Cyp2a4), and estrogen signaling (ER-α, Agr2, Krt1-19, and Tff3). The increased expression of AFP, which is clearly a cancer-related gene, is perhaps very important. Thus, exposure of fetal liver cells to arsenic directly alters molecular response, and the data obtained from this in vitro study helps provide insights into the potential contributing factors in the mechanism of arsenic transplacental carcinogenesis.
Gestation is a critical period in development, and is highly sensitive to chemical carcinogenesis, including sensitive to carcinogenesis induced by inorganics such as cisplatin, 24 lead 25 and nickel, 26 due to factors like organogenesis-related cell differentiation, rapid and global proliferative tissue growth, and genetic programming and imprinting.10,27 Inorganic arsenic can readily cross the placenta and enter the fetus in humans 1–3,11 and in rodents. 11 In C3H mice, in utero arsenic exposure at carcinogenic doses produces an array of aberrant gene expression changes in fetal livers19 and fetal lungs, 19–21 as well as in newborn livers.20 The use of CD1 mouse liver cell primary cultures in the present study was based on several considerations: (1) the observed aberrant gene expressions in fetal tissues of C3H mice would be better verified using another mouse strain, e.g., CD1 mice; (2) the aberrant estrogen signaling pathway has been proposed as a mechanism of transplacental arsenic carcinogenesis,15 and when in utero arsenic exposure alone in CD1 mice induces liver tumors, and this effects are synergistically increased in combination with postnatal DES treatment. 7,8 The present study further demonstrates that this effect is due, at least in part, to direct effect of arsenic on fetal liver cells.
HO-1 and MT-1 are well-known biomarkers for arsenic exposure.1–3,28 A concentration-dependent induction of HO-1 and MT-1 by arsenic suggests that the metalloid can directly stimulate fetal liver cells to elicit a stress response, and the increased HO-1 and MT-1 protein would provide a survival advantage to these fetal cells through adaptation to arsenic insult. These adaptive responses are good for initial cell survival, but would be at the expense of the future toxicity, as some damaged cells could escape from apoptosis and inherit potentially genetic lesions for carcinogenesis.29
The alterations of genes related to steroid metabolism could potentially impact estrogen signaling.19 In the present study, the expression of HSD17β7, a gene encoding for an enzyme involved with estradiol biosynthesis, was increased ~2- fold. Similarly, a female dominant gene Cyp2a4 (steroid 15a-hydroxylase) was induced, and its expression also involves an imprinting growth hormone-related factor in mouse liver.30 The increases in these steroid metabolism-related genes by arsenic fortify our hypothesis that arsenic might somehow act as an endocrine disruptor in fetal mouse liver cells.15,19 Arsenic-increased expressions of ER-α and ER-α-linked Agr2, Krt1-19, Tff3 and Sprr2a, were observed in the cultured fetal mouse liver cells. These findings add supporting evidence that aberrant estrogen signaling at the early stage of development could be a mechanism for arsenic hepatocarcinogenesis, 15 as aberrant estrogen signaling has been proposed to be a mechanism for chemical carcinogenesis. 31 These results are also consistent with our recent findings in fetal tissues of C3H mice exposed to arsenic in utero 19,21 in that aberrant estrogen signaling could have important implications in early life programming of genes potentially leading to tumors in adulthood. 15,32
Perhaps the most interesting finding is the aberrant expression of a gene related to cancer, namely AFP. AFP is known as a growth-promoting oncoprotein often overexpressed in embryonic tumors. AFP plays a well-known role in hepatocarcinogenesis. In liver tumors in adult mice induced by transplacental arsenic exposure, AFP overexpression is a prominent feature. 15–18 The present data indicates that arsenic directly impacts expression of this cancer-related genes in a target cell population.
In summary, the present study clearly demonstrates that in utero arsenic exposure resulted in dramatic alterations in gene expression in primary fetal mouse liver cell cultures, including stress responses, a complex interplay between steroid metabolism and estrogen signaling pathways, and genes related to cancer. Arsenic-induced gene expression changes may very well play an important role in early genetic reprogramming, leading to the formation of tumors later in life. 9 As a corollary in humans, increased mortality occurs from lung cancers in young adults following in utero exposure to arsenic in the drinking water.33 Thus, the developing human and mouse fetus appear to be very sensitive to transplacental arsenic carcinogenesis.
Acknowledgements
The authors thank Drs. Jean-Francois Coppin, Wei Qu, and Larry Keefer for their critical review of this manuscript. Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services.
References
- 1.NRC (National Research Council) Arsenic in the drinking water (update) Washington, DC: NRC, National Academy; 1999. [Google Scholar]
- 2.NTP. Arsenic compounds, inorganic, National Toxicology Program Report on Carcinogens. 11th edition. Research Triangle Park, NC: U.S. Department of Health and Human Services; 2004. [Google Scholar]
- 3.IARC (Intermational Agency for Research on Cancer) Some Drinking Water Disinfectants and Contaminants, including Arsenic. France: Lyon; 2004. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 84: Arsenic in drinking water. [PMC free article] [PubMed] [Google Scholar]
- 4.Centeno JA, et al. Pathology related to chronic arsenic exposure. Environ. Health Perspect. 2002;110(Suppl 5):883–886. doi: 10.1289/ehp.02110s5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waalkes MP, et al. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol. Appl. Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 6.Waalkes MP, et al. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- 7.Waalkes MP, et al. Urogenitial system cancers in female CD1 mice induced by in utero arsenic exposure are exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006;66:1337–1445. doi: 10.1158/0008-5472.CAN-05-3530. [DOI] [PubMed] [Google Scholar]
- 8.Waalkes MP, et al. Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicol. Appl. Pharmacol. 2006;215:295–305. doi: 10.1016/j.taap.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Waalkes MP, et al. Transplacental arsenic carcinogenesis in mice. Toxicol. Appl. Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson LM, et al. Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ. Health Perspect. 2000;108(suppl 3):573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concha G, et al. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 12.Devesa V, et al. Speciation of arsenic in the maternal and fetal mouse tissues following gestational exposure to arsenite. Toxicology. 2006;224:147–155. doi: 10.1016/j.tox.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu T, et al. Application of cDNA microarray to the study of arsenic-induced liver diseases in the population of Guizhou, China. Toxicol. Sci. 2001;59:185–192. doi: 10.1093/toxsci/59.1.185. [DOI] [PubMed] [Google Scholar]
- 14.Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 2005;206:169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Waalkes MP, et al. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J. Natl. Cancer Inst. 2004;96:466–474. doi: 10.1093/jnci/djh070. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, et al. Toxicogenomic analysis of aberrant gene expression in liver tumors and nontumorous livers of adult mice exposed in utero to inorganic arsenic. Toxicol. Sci. 2004;77:249–257. doi: 10.1093/toxsci/kfh055. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, et al. Global gene expression associated with hepatocarcinogenesis in adult male mice induced by in utero arsenic exposure. Environ. Health perspect. 2006;114:404–411. doi: 10.1289/ehp.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, et al. Transplacental arsenic plus postnatal 12-O-teradecanoyl phorbol-13-acetate exposures induced similar aberrant gene expressions in male and female mouse liver tumors. Toxicol. Appl. Pharmacol. 2006;213:216–223. doi: 10.1016/j.taap.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, et al. Transplacental exposure to inorganic arsenic at a hepatocarcinogenic dose induces fetal gene expression changes in mice indicative of aberrant estrogen signaling and disrupted steroid metabolism. Toxicol. Appl. Pharmacol. 2007;220:284–291. doi: 10.1016/j.taap.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, et al. Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology. 2007;236:7–15. doi: 10.1016/j.tox.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen J, et al. Fetal onset of aberrant gene expression relevant to pulmonary carcinogenesis in lung adenocarcinoma development induced by in utero arsenic exposure. Toxicol. Sci. 2007;95:313–320. doi: 10.1093/toxsci/kfl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 23.Klaassen CD, et al. Induction of metallothionein in primary rat hepatocyte cultures. Methods Enzymol. 1991;205:567–574. doi: 10.1016/0076-6879(91)05140-q. [DOI] [PubMed] [Google Scholar]
- 24.Diwan BA, et al. Transplacental carcinogenicity of cisplatin: initiation of skin tumors and induction of other preneoplastic and neoplastic lesions in SENCAR mice. Cancer Res. 1993;53:3874–3876. [PubMed] [Google Scholar]
- 25.Waalkes MP, et al. Renal tubular tumors and atypical hyperplasias in B6C3F1 mice exposed to lead acetate during gestation and lactation occur with minimal chronic nephropathy. Cancer Res. 1995;55:5265–5271. [PubMed] [Google Scholar]
- 26.Diwan BA, et al. Transplacental carcinogenic effects of nickel(II) acetate in the renal cortex, renal pelvis and adenohypophysis in F344/NCr rats. Carcinogenesis. 1992;13:1351–1357. doi: 10.1093/carcin/13.8.1351. [DOI] [PubMed] [Google Scholar]
- 27.Birnbaum LS, et al. Cancer and developmental exposure to endocrine disruptors. Environ. Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, et al. Stress-related gene expression in mice treated with inorganic arsenicals. Toxicol. Sci. 2001;61:314–320. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- 29.Waalkes MP, et al. Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol Sci. 2000;56:255–261. doi: 10.1093/toxsci/56.2.255. [DOI] [PubMed] [Google Scholar]
- 30.Jarukamjorn K, et al. Modified expression of cytochrome P450 mRNAs by growth hormone in mouse liver. Toxicology. 2006;219:97–105. doi: 10.1016/j.tox.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Dickson RB, et al. Estrogen receptor-mediated processes in normal and cancer cells. J. Natl. Cancer Inst. Monogr. 2000;27:135–145. doi: 10.1093/oxfordjournals.jncimonographs.a024237. [DOI] [PubMed] [Google Scholar]
- 32.Cook JD, et al. Interaction between genetic susceptibility and early life environmental exposure determines tumor suppressor gene penetrance. Proc. Natl. Acad. Sci. USA. 2005;102:8644–8649. doi: 10.1073/pnas.0503218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AH, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ. Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]