Abstract
Background:
Methamphetamine use is a common problem among women of childbearing age, leading to an increasing number of children with prenatal methamphetamine exposure. Whether microstructural brain changes associated with prenatal methamphetamine exposure can be detected with diffusion tensor imaging (DTI) is unknown.
Method:
Twelve-direction DTI was performed in 29 methamphetamine-exposed and 37 unexposed children ages 3–4 years on a 3-T MRI scanner. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) were determined in the corpus callosum (genu and splenium) and bilaterally in the frontal and parietal white matter (WM), basal ganglia (caudate, putamen, globus pallidus), and thalamus.
Results:
Children with prenatal methamphetamine exposure had lower ADC in the frontal (right: −2.1%, p = 0.04; left: −2.0%, p = 0.09) and parietal WM (right: −3.9%, p = 0.002; left: −3.3%, p = 0.02) compared to unexposed children. The methamphetamine-exposed children also showed a trend for higher FA in the left frontal WM (+4.9%, p = 0.06) compared to the unexposed children.
Conclusion:
Since less myelination and higher dendritic or spine density have been reported in animals exposed to methamphetamine, lower diffusion in our children may reflect more compact axons or greater dendritic or spine density associated with prenatal methamphetamine exposure. These findings suggest alterations in white matter maturation in these children exposed to methamphetamine in utero.
GLOSSARY
- ADC
= apparent diffusion coefficient;
- ANOVA
= analysis of variance;
- ASI
= Addiction Severity Index;
- CES-D
= Center for Epidemiologic Studies–Depression scale;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- DTI
= diffusion tensor imaging;
- EPI
= echoplanar imaging;
- FA
= fractional anisotropy;
- ISP
= Index of Social Position;
- NART-R
= National Adult Reading Test;
- ROI
= region of interest;
- SASSI
= Substance Abuse Subtle Screening Inventory;
- TE
= echo time;
- TI
= inversion time;
- TR
= repetition time;
- VIQ
= verbal intelligence;
- WM
= white matter.
Methamphetamine use is an increasing problem among women of childbearing age. Prenatal methamphetamine exposure has been associated with smaller subcortical brain volumes1 and elevated striatal creatine in children ages 3–16 years.2 Methamphetamine increases synaptic dopamine, which may stimulate dopamine receptors that are present in the human brain as early as the first trimester.3 Since D1 receptors can regulate the cell cycle during corticogenesis,4 methamphetamine has the potential to alter cortical development. Methamphetamine also stimulates several catecholamine systems that are present prenatally and play diverse roles in brain development.5
Diffusion tensor imaging (DTI) allows the evaluation of brain microstructure.6 The apparent diffusion coefficient (ADC) is a measure of the water molecules’ average freedom to diffuse in a given area. In contrast, anisotropy measures the degree of restricted diffusion along certain directions and provides information about structural orientation. Normal brain development shows the greatest changes in FA and diffusivity during the first 5 years of life.7,8 DTI may also differentiate between stages of brain maturation.9 In adult methamphetamine users, DTI demonstrated lower FA in the frontal white matter (WM).10 Likewise, children with prenatal exposure to cocaine, another psychostimulant, showed higher diffusion in frontal WM.11 Since the effects of prenatal methamphetamine exposure on the developing brain remain unknown, we performed a study to determine whether children with prenatal methamphetamine exposure would demonstrate higher diffusion and lower FA in the subcortical and frontal WM regions.
METHODS
Subjects.
Twenty-nine methamphetamine-exposed and 37 unexposed children, ages 3 or 4 years, were recruited from local hospitals, drug rehabilitation centers, and the local community. Cumulative gestational methamphetamine exposure was determined by detailed interviews of the mothers regarding quantity of methamphetamine used/day, days used/week, duration of use, and lifetime usage patterns as assessed by the Addiction Severity Index (ASI) and Substance Abuse Subtle Screening Inventory (SASSI) questionnaire. These children had careful physical and neurologic evaluations and were screened to ensure they had no contraindications for the MR studies. To evaluate possible environmental influences on brain development, the children’s primary care providers (typically the biologic mothers but also some legal guardians) were evaluated for their estimated verbal intelligence (VIQ) using the National Adult Reading Test (NART-R), mood with the Center for Epidemiologic Studies–Depression scale (CES-D), and socioeconomic status using the Hollingshead Two-Factor Index of Social Position (ISP).12
Methamphetamine-exposed children meeting the following criteria were included: 1) ages 36–59 months and 2) any reported maternal methamphetamine use during pregnancy or positive toxicology at birth. Children were excluded from the study if their legal guardian was unable to give informed consent (non-English speaking, low cognitive functioning with VIQ <80) or the mother had 1) HIV-1 during the pregnancy, 2) a history of comorbid psychiatric illness that may confound study analysis (e.g., schizophrenia, bipolar disorder with psychosis), 3) a medical condition during pregnancy that might alter the child’s brain development (e.g., severe preeclampsia, sickle cell anemia, cardiac, renal, or liver failure, syphilis), or 4) a history of DSM-IV dependence on alcohol or other drugs except nicotine during pregnancy. In addition, potential participants were excluded from the study if they 1) had a congenital, genetic, or infectious neurologic disorder (e.g., fetal alcohol syndrome, Down syndrome, fragile X), 2) were preterm (<36 weeks), 3) had a history of failure to thrive within the first year of life, 4) had an overt TORCH infection [Toxoplasmosis, Other (Treponema pallidum, varicella zoster virus, parvovirus), Rubella virus, Cytomegalovirus, and Herpes simplex virus] at birth, 5) had a major neurologic disorder since birth (e.g., bacterial meningitis, epilepsy), or 6) had other contraindications for MR studies, such as metallic or electronic implants in the body or severe claustrophobia.
Comparison subjects fulfilled the same inclusion and exclusion criteria as the methamphetamine-exposed children, but had no history of methamphetamine exposure. Legal guardians signed an informed consent approved by the University of Hawaii, Committee on Human Studies.
Imaging acquisition and processing.
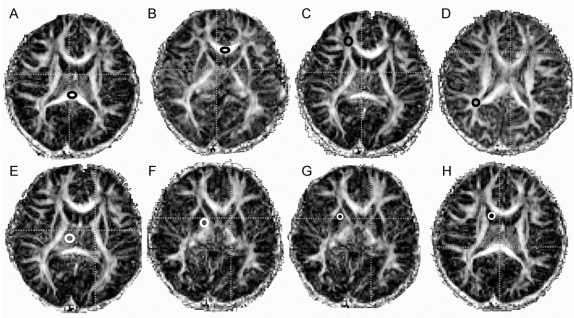
MR scans were performed on unsedated children in a 3 T Siemens Trio scanner (Siemens Medical Solutions, Erlangen, Germany), using an eight-channel phase-array RF coil. The children were typically scanned during a nap, after a meal and acclimation to the scanner, or while watching a favorite movie. One to five sessions were required to obtain good quality scans in each child. The following structural sequences were acquired: 1) three-plane localizer, 2) sagittal high-resolution three-dimensional magnetization-prepared rapid gradient echo (repetition time [TR]/echo time [TE]/inversion time [TI] = 2,200/4.91/1,000 msec, 208 × 256 × 144 matrix, 1 mm isotropic resolution), and 3) transversal fluid-attenuated inversion recovery (TR/TE/TI = 10,000/79/2,500 msec; 1 excitation; 205 × 320 matrix, 28 slices; voxel size = 0.9 × 0.7 × 4.0 mm3). Next, two axial spin-echo echoplanar imaging (EPI) scans with diffusion weighting and full-brain coverage were acquired consecutively, with 1) 3 diffusion directions (28 slices, 4 mm slice, 1 mm gap, TR/TE = 3,400/80, 128 × 128 matrix, b = [0,1000] s/mm2) and 2) 12 diffusion directions (28 slices, 4.6 mm slice, 0.46 mm gap, TR/TE = 3,700/88 msec, 128 × 128, b factor = [0,1000]s/mm2). All images were reviewed for structural abnormalities and movement or other artifacts. FA and ADC values for each voxel were calculated using DTIStudio version 2.0313 using the 12-axis DTI data. Regions of interest (ROIs) were manually drawn, using fixed size ROIs, on FA and ADC maps and cross-referenced with other available images for accurate placement. ROIs included the genu and splenium of the corpus callosum (4 × 3 mm) and bilateral frontal and parietal WM (4 × 4 mm), caudate (3 × 3 mm), putamen (4 × 4 mm), globus pallidus (3 × 3 mm), and thalamus (6 × 6 mm). One investigator (L.F.), who was blinded to subjects’ exposure status, completed all drawings. ROIs for each area were drawn on the axial slices in which the respective structure appeared largest and most delineated (figure 1).
Figure 1 Representative region of interest placements on fractional anisotropy maps for the seven regions studied
Thalamus (E), globus pallidus (F), putamen (G), caudate (H), and white matter (WM) regions [frontal WM (C) and parietal WM (D)] were drawn bilaterally, while corpus callosum was drawn as indicated in the splenium (A) and genu (B).
To calculate intraoperator reliabilities, the investigator redrew the same ROIs from 10 sets of scans, and remeasured the FA and ADC values for all ROIs. Intraclass correlation coefficients for the ADC values of all regions were r > 0.8 and p ≤ 0.0001, while FA values of all regions were r > 0.6 and p ≤ 0.03.
Statistical analysis.
Statistical analyses were performed using StatView 5.0.1 and SAS Enterprise Guide 4.1.0.471 (SAS Institute Inc., Cary, NC). Repeated measures analyses of variance (ANOVA) of FA and ADC were performed to determine group differences between methamphetamine-exposed and control subjects for all regions (with the regions as within-subject repeated measures for global changes in ADC or FA). Similarly, repeated measures ANOVAs were then performed to test for changes in the WM regions (frontal, parietal, and corpus callosum), the striatal regions (caudate, putamen, and globus pallidus), and within individual regions (within subject genu and splenium for the corpus callosum and left and right hemisphere for other regions). t Tests were performed for those measures with significant findings or trends on the ANOVAs (p ≤ 0.1). Mean ± SEM are reported for all measures. To test for possible relationships between methamphetamine exposure and diffusion measures, linear regression analyses were performed using imaging measures as dependent variables, and drug use measures as independent variables. To reduce the number of comparisons, only imaging measures with significant group differences (methamphetamine vs control) were tested with the regression analyses. To test for possible confounds from other drug exposure, regions with significant methamphetamine effects on overall ANOVA were reexamined with repeated measure analyses of covariance using other drug exposures as covariates. A type I error probability ≤0.05 was used to determine significance.
RESULTS
Subject characteristics.
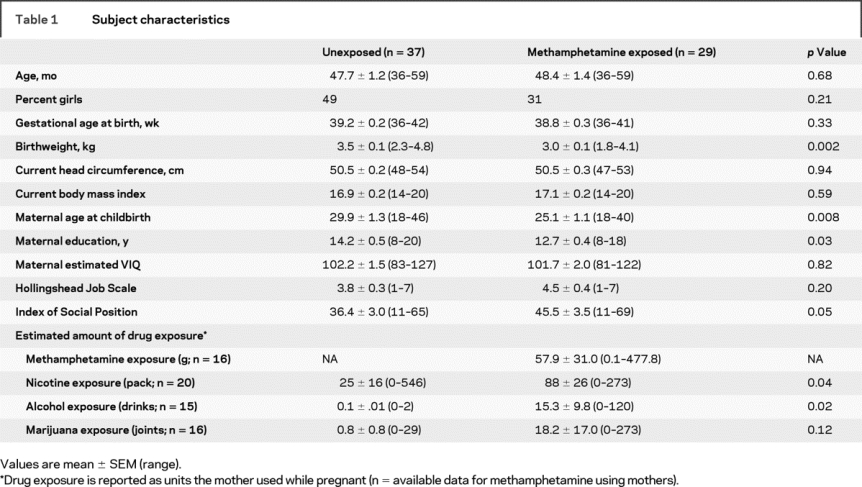
The two subject groups had similar age and parental estimated verbal intelligence, but the mothers or primary caretakers of the methamphetamine-exposed group had lower education and Hollingshead ISP (table 1). Because some children were not raised by their biologic mothers, detailed drug exposure histories were not available for all children. The children with prenatal methamphetamine exposure were exposed to methamphetamine for an average of 2.5 ± 0.2 trimesters. Of the 17 children who had records of urine or meconium toxicology, performed only in those with a suspicion of maternal methamphetamine use, 65% tested positive for methamphetamine at birth. During pregnancy, mothers had a broad range of methamphetamine usage (average 58 ± 31 g). Although none of the mothers met DSM-IV criteria for any other drug dependence except for methamphetamine or nicotine while pregnant, the mothers who used methamphetamine smoked more cigarettes, drank more alcohol, and showed a trend for more marijuana use (table 1).
Table 1 Subject characteristics
DTI findings.
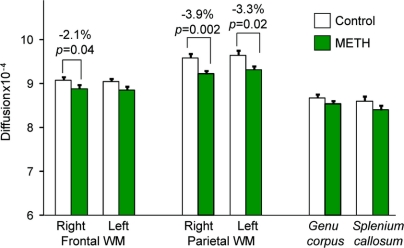
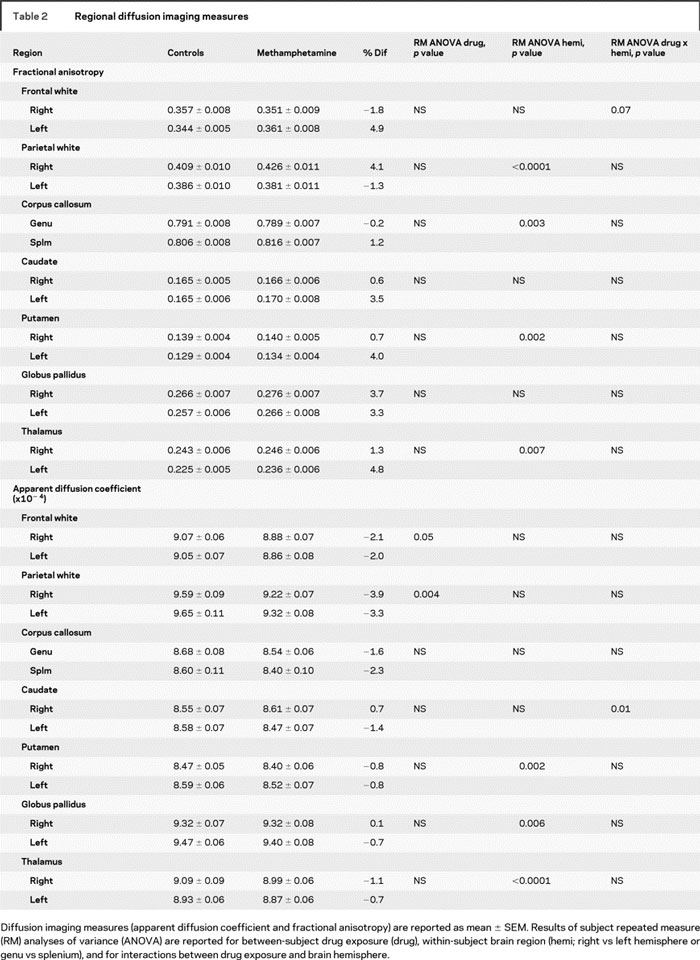
Despite normal-appearing MRIs, children with prenatal methamphetamine exposure had lower diffusivity across all regions measured (p = 0.05), with some regions having greater reductions than others (group by region interaction [p = 0.04]). There was also a trend for higher FA across all regions (p = 0.10). Post hoc analyses showed methamphetamine-exposed children had lower diffusivity across all WM regions (p = 0.007), specifically in the right frontal (−2.1%, p = 0.04), right parietal (−3.9%, p = 0.002), and left parietal (−3.3%, p = 0.02) WM (figure 2), as well as a trend in the left frontal WM (−2.0%, p = 0.09). Conversely, there were no group differences in FA, except for a trend for higher FA in the left frontal WM (+4.9%, p = 0.06) of methamphetamine-exposed children. No group differences were observed in the basal ganglia or thalamus.
Figure 2 Lower white matter diffusion in children with prenatal methamphetamine exposure (repeated measures analysis of variance for all white matter regions p = 0.007)
Means and SEM are shown with percent change for significant between-group post hoc t tests for each region. METH = methamphetamine; WM = white matter.
Potential covariates.
Lower frontal and parietal WM diffusivity in the methamphetamine-exposed children retained significance or a trend for significance when covaried for the number of trimesters exposed to nicotine (frontal p = 0.05; parietal p = 0.002), marijuana (frontal p = 0.01; parietal p = 0.008), or alcohol (frontal p = 0.03; parietal p = 0.02), as well as when covaried for the number of cigarettes (frontal p = 0.09; parietal p = 0.004), joints of marijuana (frontal p = 0.02; parietal p = 0.02), or drinks of alcohol (frontal p = 0.05; parietal p = 0.04) used while pregnant, although fewer subjects were available for analyses.
Correlations between DTI measures and participant characteristics.
Consistent with prior reports,6 as the children become older, their parietal WM ADC became lower (r = −0.2, p = 0.02) across all participants. Children whose mothers had higher lifetime maternal drug use disorder probability (SASSI FVOD Drug scale) had lower ADC in the parietal (r = −0.18, p = 0.04) and frontal (r = −0.19, p = 0.03) WM. However, there were no correlations between WM ADC and gestational drug exposure (number of trimesters used, or quantity used), including methamphetamine, nicotine, marijuana, and alcohol.
Regional and hemispheric differences in DTI.
As expected and similar to prior reports,14 diffusion measures varied by region (FA, p < 0.001; ADC, p < 0.001) (table 2). FA was highest in the corpus callosum and lowest in the putamen, while ADC was highest in the parietal WM and also lowest in the putamen. Across all subjects, left hemisphere showed lower FA than the right hemisphere in the parietal WM, putamen, and thalamus (table 2). In contrast, across all subjects, higher ADC was observed in the left vs the right hemisphere for the putamen and globus pallidus, but right greater than left in the thalamus. The splenium of the corpus callosum had higher FA values than the genu, with no difference in ADC. Only the caudate ADC showed an interaction between hemisphere and drug exposure, due to a nonsignificantly lower ADC in the left caudate (−1.4%) and a nonsignificantly higher ADC (+0.7%) in the right caudate of the methamphetamine-exposed children.
Table 2 Regional diffusion imaging measures
DISCUSSION
Children with prenatal methamphetamine exposure showed lower brain diffusivity than unexposed subjects, particularly in the frontal and parietal WM. Additionally, there was a trend for higher FA in the frontal WM in the methamphetamine-exposed children relative to the unexposed children. Although the mothers who used methamphetamine also used more nicotine, alcohol, and marijuana than the mothers of the unexposed children, including these variables in the comparisons did not alter the finding of lower diffusion in these brain regions of the methamphetamine-exposed children.
These findings are opposite from our initial hypothesis, which was based on findings in adult methamphetamine users and preadolescents with prenatal cocaine exposure. A study of normally developing but older children (ages 6–17 years) found age-related decreases in diffusion along with age-related increases in FA in the inferior longitudinal fasciculus and the inferior frontal occipital fasciculus,9 which are fiber tracts that traverse the frontal and parietal WM regions in the current study. Such normal age-related decreases in diffusion and increases in anisotropy in children were reported by others as well.15 Our prenatal methamphetamine-exposed children showed lower diffusion than the unexposed children, which would correspond to the lower diffusion typically observed with older age in normally developing children. However, the prenatal methamphetamine children did not show the normal concomitant increases in FA in these WM regions. We also observed greater FA in right than left hemisphere in most brain regions in both subject groups, which is opposite from those reported previously in similar brain regions (i.e., the inferior-frontal-occipital fasciculus) of normally developing older children. However, in the frontal WM, the methamphetamine-exposed children showed no hemispheric difference, unlike the trend for higher right hemisphere FA in our age-matched unexposed children, which again suggests a more similar pattern to the older children.9 Furthermore, fewer girls were included in the prenatal methamphetamine-exposed group (31%) compared to the unexposed group (49%), which might have led to an apparently higher diffusion (i.e., masking the even lower diffusion) in the methamphetamine-exposed group since girls were found to have lower transverse and mean diffusion in these brain regions than boys.9 Taken together, our findings suggest prenatal methamphetamine exposure accelerates brain development in an abnormal pattern. Such abnormal brain development may account for the slower maturation of behavioral measures observed in neonates with prenatal methamphetamine exposure.16,17
While the exact mechanism of how prenatal methamphetamine exposure may lead to lower brain diffusivity is unknown, lower WM diffusivity typically reflects more compact axonal fibers. Consistent with this interpretation, children with prenatal methamphetamine exposure showed smaller subcortical structures.1 Animal models of prenatal methamphetamine exposure also demonstrated greater cellularity and compactness of cells. Specifically, in utero exposure of rat pups to methamphetamine caused a reduction of the optic nerve diameter, due to thinner myelination but not fewer axons.18 If prenatal methamphetamine exposure has similar effects on the development of axonal fiber tracts within the frontal and parietal lobes, this might lead to more densely packed axons within the WM and lower diffusion values without a measurable effect on the FA. These findings are also consistent with prior reports of greater dendritic branching19 and spine densities in the dorsolateral striatal regions after methamphetamine administration.20 Such increased complexity in the neuronal structures would hinder diffusion of water molecules and hence lower mean diffusion in the brains of children with prenatal methamphetamine exposure.
Lower diffusivity and higher FA have been observed in several WM regions of young (1–3 years old) autistic children, and were thought to be related to decreased synaptic pruning.8 Less pruning, if present in our children with prenatal methamphetamine exposure, also could lead to higher cell density and restriction of water movement, and lower ADC, without specifically affecting directionality or FA.
Two other studies have evaluated prenatal stimulant drug exposure using DTI. One study of children with prenatal nicotine exposure found higher FA in frontal WM and the genu, but diffusion was not evaluated.21 While some women in our study smoked cigarettes, adjusting for nicotine or marijuana usage did not alter the methamphetamine effects. Contrary to our findings, another study found higher than normal diffusivity in the frontal WM of 10- to 12-year-old children with prenatal exposure to cocaine, another stimulant that causes dopamine release.11 The discrepancy between these studies may reflect differences in maternal drug usage, since post hoc analyses found that the prenatally cocaine exposed children with additional alcohol or marijuana exposure had even higher diffusion.11 The different age ranges may be another factor since WM volumes and anisotropy increase into adolescence,22,23 and the preadolescent cocaine-exposed children would have been subjected to many more environmental influences compared to the young preschoolers in our study.
Like age-related changes in diffusion and FA, brain lateralization is well established and likely begins early in cortical development.24 We observed regional differences in laterality in our children; specifically, FA was greater in the right than left parietal WM, putamen, and thalamus. These findings are consistent with those of a study of 2- to 8-year-old children that found right greater than left FA in the inferior longitudinal fasciculus,25 but contrary to another study of older (6–17 years) children that showed trends for left greater than right FA in the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus.9 We also found greater diffusivity in the left than right putamen and globus pallidus, but greater diffusivity in the right than left thalamus. It is unclear whether these asymmetries in diffusivity are normal since diffusion in these brain regions was not evaluated in the prior studies of 2- to 8-year-old or 6- to 12-year-old children. Asymmetric organization of the brain also includes monoamine asymmetries,26 with right greater than left D2 and D3 receptor availability in the human adult caudate. Therefore, dopamine system disruption by methamphetamine could alter normal lateralization, particularly in the dopamine dense caudate, where we observed methamphetamine exposure by hemisphere interaction on diffusion. Longitudinal follow-up is necessary to determine if the hemispheric asymmetries in diffusion or FA in these brain regions remain with continued development, and if asymmetries in dopamine-rich regions will be associated with altered psychomotor skill development.
The findings of this study are confounded by incomplete drug histories on some subjects that may minimize or exacerbate the effects of methamphetamine. In addition, genetic and environmental influences cannot be ruled out as a source for the lower WM diffusion, since the children whose mothers with greater lifetime substance use disorder probability had lower diffusivity, while estimated amount of prenatal drug exposure did not appear to influence brain diffusivity. However, we attempted to minimize the effect of many potential confounders, for instance, by narrowing the age range to minimize variability on DTI measures associated with age, recruiting children at a young age to minimize environmental influences, and excluding children who were considerably preterm, since that has been shown to affect DTI measures and brain maturation,27 or had other prenatal infections. We also had rigorous quality assurance, and scans were repeated until imaging quality was acceptable. Longitudinal studies, which are ongoing, will determine if the lower WM diffusion observed in these young children will remain low, normalize, or change to higher diffusion with age.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by C.C. Cloak and L. Fujii with consultation from C. Jiang, MS, the UH Neuroscience and MRI Research group statistician.
ACKNOWLEDGMENT
The authors thank the research participants, as well as K. Abe for subject evaluation, K. Jaremko for data entry, R. Yakupov and K. Yue for MR data collection and processing, and C. Jiang for statistical consultation.
Supplementary Material
Address correspondence and reprint requests to Dr. Christine Cloak, Department of Medicine, John A. Burns School of Medicine, Queen’s University Tower, 1356 Lusitana Street, Honolulu, HI 96813 cloak@hawaii.edu
Editorial, page 2062
e-Pub ahead of print on April 15, 2009, at www.neurology.org.
Supported by funds from the National Institute on Drug Abuse (R01-DA21016-LC, K24-DA16170-LC; K02-DA16991-TE; K01-DA021203-CC) and core resources from the National Center for Research Resources (G12 RR003061-21, 1P01 RR011091-11), the National Institute of Neurological Disorders and Stroke (U54 NS56883), and the Office of National Drug Control Policy.
Disclosure: The authors report no disclosures.
Medical Devices: Siemens Trio scanner (Siemens Medical Solutions, Erlangen, Germany).
Received July 8, 2008. Accepted in final form December 22, 2008.
REFERENCES
- 1.Chang L, Smith LM, LoPresti C, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res 2004;132:95–106. [DOI] [PubMed] [Google Scholar]
- 2.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology 2001;57:255–260. [DOI] [PubMed] [Google Scholar]
- 3.Unis AS, Roberson MD, Robinette R, Ha J, Dorsa DM. Ontogeny of human brain dopamine receptors: I: differential expression of [3H]-SCH23390 and [3H]-YM09151-2 specific binding. Brain Res Dev Brain Res 1998;106:109–117. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Lidow MS. D1 dopamine receptor regulation of cell cycle in FGF- and EGF-supported primary cultures of embryonic cerebral cortical precursor cells. Int J Dev Neurosci 2002;20:593–606. [DOI] [PubMed] [Google Scholar]
- 5.Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol 2004;190 suppl 1:S8–21. [DOI] [PubMed] [Google Scholar]
- 6.Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: application to the study of the developing brain. J Am Acad Child Adolesc Psychiatry 2007;46:213–223. [DOI] [PubMed] [Google Scholar]
- 7.Ben Bashat D, Ben Sira L, Graif M, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging 2005;21:503–511. [DOI] [PubMed] [Google Scholar]
- 8.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage 2007;37:40–47. [DOI] [PubMed] [Google Scholar]
- 9.Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 2007;17:2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung A, Lyoo IK, Kim SJ, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol 2007;10:765–775. [DOI] [PubMed] [Google Scholar]
- 11.Warner TD, Behnke M, Eyler FD, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics 2006;118:2014–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 13.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006;81:106–116. [DOI] [PubMed] [Google Scholar]
- 14.Mamata H, Jolesz FA, Maier SE. Characterization of central nervous system structures by magnetic resonance diffusion anisotropy. Neurochem Int 2004;45:553–560. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin North Am 2006;16:19–43. [DOI] [PubMed] [Google Scholar]
- 16.Smith LM, Lagasse LL, Derauf C, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol 2008;30:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:82–88. [DOI] [PubMed] [Google Scholar]
- 18.Melo P, Moreno VZ, Vazquez SP, Pinazo-Duran MD, Tavares MA. Myelination changes in the rat optic nerve after prenatal exposure to methamphetamine. Brain Res 2006;1106:21–29. [DOI] [PubMed] [Google Scholar]
- 19.Blaesing B, Nossoll M, Teuchert-Noodt G, Dawirs RR. Postnatal maturation of prefrontal pyramidal neurones is sensitive to a single early dose of methamphetamine in gerbils (Meriones unguiculatus). J Neural Transm 2001;108:101–113. [DOI] [PubMed] [Google Scholar]
- 20.Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci 2007;25:847–853. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen LK, Picciotto MR, Heath CJ, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci 2007;27:13491–13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorgio A, Watkins KE, Douaud G, et al. Changes in white matter microstructure during adolescence. Neuroimage 2008;39:52–61. [DOI] [PubMed] [Google Scholar]
- 23.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- 24.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci 2003;4:37–48. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram SK, Sivaswamy L, Makki MI, Behen ME, Chugani HT. Absence of arcuate fasciculus in children with global developmental delay of unknown etiology: a diffusion tensor imaging study. J Pediatr 2008;152:250–255. [DOI] [PubMed] [Google Scholar]
- 26.Tucker DM, Williamson PA. Asymmetric neural control systems in human self-regulation. Psychol Rev 1984;91:185–215. [PubMed] [Google Scholar]
- 27.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics 2008;121:306–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.