Abstract
Objective:
To determine whether the presence of depression predicts higher rate of progression to Alzheimer disease (AD) in patients with amnestic mild cognitive impairment (aMCI) and whether donepezil treatment beneficially affect this relationship.
Methods:
The study sample was composed of 756 participants with aMCI from the 3-year, double-blind, placebo-controlled Alzheimer's Disease Cooperative Study drug trial of donepezil and vitamin E. Beck Depression Inventory (BDI) was used to assess depressive symptoms at baseline and participants were followed either to the end of study or to the primary endpoint of progression to probable or possible AD.
Results:
Cox proportional hazards regression, adjusted for age at baseline, gender, apolipoprotein genotype, and NYU paragraph delayed recall score, showed that higher BDI scores were associated with progression to AD (p = 0.03). The sample was stratified into depressed (BDI score ≥10; n = 208) and nondepressed (BDI <10; n = 548) groups. Kaplan-Meier analysis showed that among the depressed subjects, the proportion progressing to AD was lower for the donepezil group than the combined vitamin E and placebo groups at 1.7 years (p = 0.023), at 2.2 years (p = 0.025), and remained marginally lower at 2.7 years (p = 0.070). The survival curves among the three treatment groups did not differ within the nondepressed participants.
Conclusions:
Results suggest that depression is predictive of progression from amnestic mild cognitive impairment (aMCI) to Alzheimer disease (AD) and treatment with donepezil delayed progression to AD among depressed subjects with aMCI. Donepezil appears to modulate the increased risk of AD conferred by the presence of depressive symptoms.
GLOSSARY
- AD
= Alzheimer disease;
- ADCS
= Alzheimer's Disease Cooperative Study;
- aMCI
= amnestic mild cognitive impairment;
- BDI
= Beck Depression Inventory;
- CDR
= Clinical Dementia Rating;
- ChEI
= cholinesterase inhibitors;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- HAM-D
= Hamilton Depression Rating Scale;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination;
- NINCDS-ADRDA
= National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association.
For many patients, mild cognitive impairment (MCI)1 represents a transitional state between normal aging and dementia. Clinically, MCI was originally defined by subjective concerns and clinical determination of memory deficits in the absence of significant global cognitive and functional impairment.1 Annual progression rates from MCI to dementia range from 5% to 32%1–3 depending on the specific study populations examined and the particular diagnostic criteria applied. Clinical outcomes for MCI can vary greatly, including progression to Alzheimer disease (AD), progression to non-AD dementias, stable cognitive impairment, or reversion to normal cognition. Such variability highlights the heterogeneity of this condition.
Much of the current research in MCI focuses on identifying risk factors that may be predictive of progression to dementia. Early therapeutic intervention in high-risk MCI subjects may potentially delay or even prevent the onset of dementia. Approaches incorporating neuropsychological testing,4,5 structural and functional neuroimaging,6–8 and CSF biomarkers9 have demonstrated varying degrees of success in predicting which subjects with MCI will subsequently progress to AD.
Examination of neuropsychiatric symptoms has also yielded promising results in identifying patients with MCI at high risk of progressing to AD. Cross-sectional studies indicate that behavioral disturbances are frequently reported in MCI, and the overall pattern of psychopathology is similar to that seen in mild AD, with depression, apathy, anxiety, and irritability among the most commonly exhibited symptoms.10–12 Several longitudinal studies of MCI and normal elderly populations suggest that depression and apathy, in particular, may represent prodromal symptoms of AD that are indicative of subsequent dementia,12–15 although not all investigators have confirmed these findings.16,17
Symptoms of depression were assessed as a secondary outcome measure as part of the Alzheimer's Disease Cooperative Study (ADCS) trial of donepezil (Aricept; Pfizer, New York, NY, and Eisai, Tokyo, Japan), vitamin E, and placebo in patients with MCI.4 The longitudinal aspect of the 3-year treatment study represents a unique resource for confirming the utility of depressive symptoms for predicting progression from amnestic MCI (aMCI) to AD. In addition, some studies have suggested a favorable impact of cholinesterase inhibitors (ChEI) on behavioral symptoms in patients with AD18,19; thus we also investigated whether donepezil treatment modified the relationship between depression and time to progression to AD in patients with aMCI.
METHODS
Clinical trial registry information.
This trial is registered at the ClinicalTrials.gov Web site with the following identifier: NCT00000173.
Participants.
The study rationale, design, and participant characteristics for the parent study have been previously described in detail.3 Briefly, 769 participants were recruited from 69 ADCS sites in the United States and Canada. All subjects were between 55 and 91 years of age (inclusive) and met criteria for aMCI of a presumably degenerative nature (insidious onset, gradual progression), defined as 1) subjective memory complaint corroborated by an informant, 2) insufficient global cognitive and functional impairment to meet National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD,20 3) Logical Memory (one paragraph) delayed recall score21 1.5 SD below education-adjusted normative means, 4) Clinical Dementia Rating (CDR)22 score of 0.5, and 5) Mini-Mental State Examination (MMSE)23 score ≥24.
During the screening visit, participants were excluded if they obtained a score of greater than 12 on the Hamilton Depression Rating Scale (HAM-D)24 or a modified Hachinski score of >4. Subjects meeting the entry criteria proceeded to a comprehensive evaluation that included a detailed medical history from the subject and a collateral source and they were excluded if they had been diagnosed with major depression or another psychiatric disorder as described in DSM-IV within the past 2 years. Additional exclusion criteria include history of significant cerebrovascular disease; CNS infarct, infection, or focal lesions of clinical significance on CT or MRI; medical or psychiatric conditions that could interfere with study participation; pregnancy, lactation, or childbearing potential; or taking vitamins or other supplements. Subjects may take stable doses (at least 1 month prior to screening) of antidepressants lacking significant anticholinergic side effects. The evaluation also determined whether the aMCI was of a presumably degenerative nature as characterized by insidious onset and gradual progression.
The study design was a randomized, double-blind, parallel-group comparison of vitamin E (2,000 IU) or donepezil (10 mg) with placebo. The study was conducted according to Good Clinical Practice guidelines, the Declaration of Helsinki, and the US Code of Federal Regulations Title 21 Part 50 (Protection of Human Subjects) and Title 21 Part 56 (Institutional Review Boards). Written informed consent was obtained from all participants and study partners. A data safety monitoring board reviewed the blinded safety data every 3 months during the trial.
Outcome measures.
The primary endpoint was the time to diagnosis of possible or probable AD according to NINCDS-ADRDA criteria.20 When the clinical diagnosis of AD was made, all cognitive and functional data were sent to the ADCS Coordinating Center and forwarded to a five-member central review committee for a consensus diagnosis.
Clinical variables.
Subjects were administered the Beck Depression Inventory (BDI)25 at baseline and every 6 months thereafter. The BDI is a 21-item questionnaire of depressive symptoms presented in Likert scale multiple-choice format. The BDI was modified so that the study partners reported on the subjects' symptoms (i.e., simple word changes from “I” to “The subject”). The maximum possible score is 63, with higher scores indicating greater severity of depression. A score of ≥10 is often considered elevated and indicates the possibility of clinically significant depression.
Statistical analyses.
Differences in the means of continuous variables were tested by the Kruskal-Wallis rank sum test. Differences in proportions were tested by Fisher exact test. Cox proportional hazard regression was used to test the ability of baseline BDI score to predict time to progression to dementia after controlling for age at enrollment, APOE genotype (presence or absence of an ɛ4 allele), gender, treatment group, NYU paragraph delayed recall score, and MMSE score. In the Cox model, subjects were censored at time of early withdrawal (n = 231) or completion of the 36-month follow-up interval without progression to dementia (n = 324). The remaining 201 participants were observed to progress to dementia during the trial. Covariates that did not meet proportional hazards assumptions based on a p value of less than 0.05 on the Schoenfeld residuals test of nonproportionality were controlled for in the analysis by stratification. Analysis of the rate of early study withdrawal revealed that the dropout rate was independent of depression for the overall sample (χ2 contingency table p = 0.48) and within each treatment group (χ2 contingency table p > 0.18).
Kaplan-Meier curves summarizing by treatment arm the probability of survival as a function of years into the trial were produced for various subsets of the study sample. We did not use the Cox model to test for treatment effects because proportional hazards assumptions did not hold.3 Instead, pointwise z-tests (the difference in proportions divided by the standard error of the difference) were used to compare estimated survival (to diagnosis of possible or probable AD) rates between the treatment arms at specific time points on the Kaplan-Meier curves (at 0.7, 1.2, 1.7, 2.2, and 2.7 years).3 All analyses were performed in the statistical programming language R.
RESULTS
Baseline characteristics.
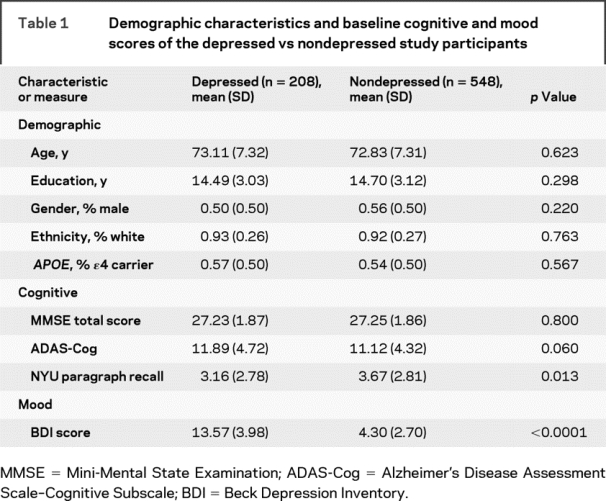
Of the 769 aMCI participants, 756 completed the BDI at baseline and were included in the present analysis. At the onset of the trial, 208 participants met criteria for depression (BDI score of ≥10); the remaining 548 scored within the normal range (BDI <10) and were classified as nondepressed. Baseline demographic characteristics of the study sample are summarized in table 1. The depressed and nondepressed groups had similar mean age, years of education, and sex, ethnicity, and APOE distributions. Baseline MMSE and ADAS-Cog scores were also similar between the two groups. Depressed subjects had significantly lower NYU paragraph recall scores; therefore, this score was included in the subsequent Cox model.
Table 1 Demographic characteristics and baseline cognitive and mood scores of the depressed vs nondepressed study participants
Effects of depression on progression to AD.
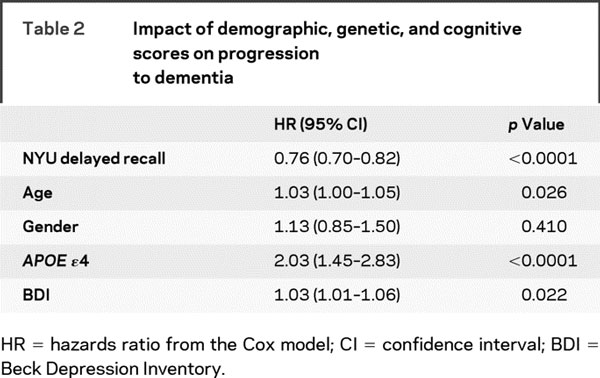
The relationship between BDI scores at baseline and progression to dementia was investigated with Cox proportional hazards regression controlling for age at baseline, gender, APOE genotype, NYU paragraph delayed recall score, MMSE score, and treatment group. MMSE score and treatment group did not meet proportional hazards assumptions (p < 0.05 using the Schoenfeld residuals test for nonproportional hazards). Therefore, we stratified the analysis on MMSE score and treatment group. After stratifying on these two variables, diagnostics for the remaining variables were unremarkable and consistent with the Cox proportional hazards assumptions.
In the final model, gender was not associated with progression to dementia, while age, APOE genotype, and BDI score were significantly associated with progression from aMCI to AD (table 2). Each year increase in age was associated with a 3% higher hazard for dementia, the presence of an APOE ɛ4 allele was associated with a twofold higher hazard, and each point higher on the baseline BDI was associated with a 3% higher hazard of progression to AD. Adding age by BDI interaction term or APOE by BDI interaction term did not improve the model fit (p for interaction >0.18).
Table 2 Impact of demographic, genetic, and cognitive scores on progression to dementia
Effect of donepezil treatment on progression to AD in depressed group.
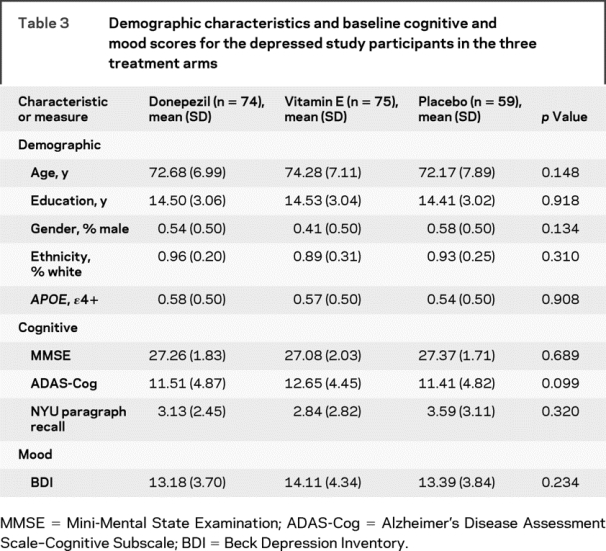
Within the depressed group, demographic and baseline global cognitive performance within the depressed group did not significantly differ among the three treatment arms (table 3).
Table 3 Demographic characteristics and baseline cognitive and mood scores for the depressed study participants in the three treatment arms
The survival curves of the three treatment groups closely resembled each other during the first year of study (figure); the donepezil group began to separate slightly from the vitamin E and placebo groups after 1 year and quite dramatically after 1.5 years of study. Because the survival curves did not differ between the vitamin E and placebo groups in either the larger sample3 or this depressed subsample, these groups were combined to increase the power of the analysis. Pointwise z-tests show that among those with depression, the proportion progressing to AD was lower in the donepezil group than the pooled vitamin E and placebo groups at 1.7 years (p = 0.023) and at 2.2 years (p = 0.025), and marginally lower at 2.7 years (p = 0.070). At 1.7 years, 11% (8 of 74) of the subjects in the donepezil group and 25% (34 of 134) of the subjects in the combined vitamin E/placebo group had progressed to AD. At 2.2 years, 14% of the subjects in the donepezil group progressed to dementia compared to 29% in the combined vitamin E/placebo group. At 2.7 years, the percentage of participants who progressed to AD was 18% for the donepezil group and 32% in the combined vitamin E/placebo group.
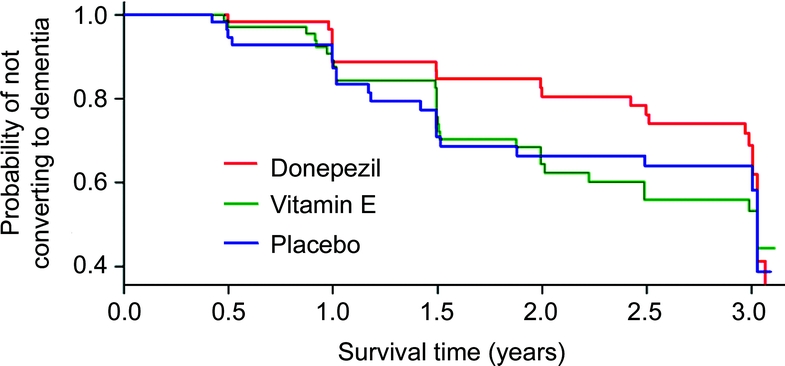
Figure Kaplan-Meier estimates of the rate of progression from amnestic mild cognitive impairment (aMCI) to Alzheimer disease in depressed subjects with aMCI stratified by the three treatment groups
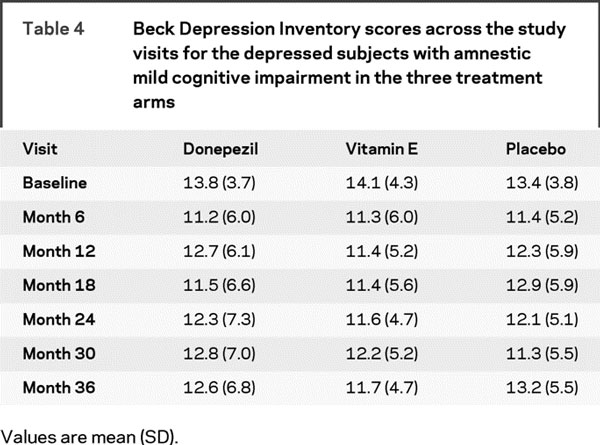
Table 4 provides the means and standard deviations of the BDI scores of the three treatment groups across the study visits, indicating that the BDI scores remained relatively stable across the study visits and were not significantly different among the three treatment arms. Random effects modeling did not yield any treatment group by visit interaction on BDI scores (p = 0.87).
Table 4 Beck Depression Inventory scores across the study visits for the depressed subjects with amnestic mild cognitive impairment in the three treatment arms
Effect of donepezil treatment in the nondepressed group.
Contrary to the pattern observed in the depressed group, the Kaplan-Meier survival (to diagnosis of possible or probable AD) curves for the nondepressed participants showed that patients who received donepezil treatment initially exhibited a slight advantage with regards to slower rate of conversion compared to the vitamin E and placebo groups, but after 1.5 years, the curves for the three groups began to merge and by 2 years, the three curves are virtually indistinguishable from each other.
DISCUSSION
The results from this 3-year, longitudinal clinical trial study reveal that depressive symptoms are predictive of progression from aMCI to clinical diagnosis of AD. Each point increase in the BDI was associated with a 3% higher risk of progression after controlling for other major clinical risk factors for AD (i.e., age, APOE status, and baseline memory function as measured by the NYU delayed paragraph recall test). The present study further demonstrates that donepezil treatment delayed the progression to AD among depressed subjects, and the difference in the proportion surviving without AD between donepezil group and the pooled vitamin E and placebo groups was significant at 1.7 and 2.2 years and marginally significant at 2.7 years. This treatment effect was specific to the depressed group and not observed in the nondepressed participants. The present findings show that the increased risk for progression to AD conferred by the presence of depressive symptomatology in aMCI subjects can be modified by donepezil treatment. These results are analogous to the finding of reduced risk of progression to AD among APOE ɛ4 carriers treated with donepezil.3 Therefore, while APOE ɛ4 allele serves as a genetic marker related to the onset of AD and favorable response to donepezil therapy, the presence of depression may represent a phenotypic marker contributing a similar predictive function and response to donepezil.
The increased incidence of AD associated with neuropsychiatric symptoms, particularly depression and apathy, has been demonstrated in several prior studies of elderly subjects with normal cognition or community-residing and memory disorder clinic MCI subjects.12–15 However, the mechanism underlying this relationship remains incompletely understood. In AD, the development of depression may be the consequence of the underlying CNS pathology, namely the selective loss of noradrenergic cells in the locus ceruleus and possibly the serotonergic raphe nuclei.26–28 Functional and structural neuroimaging studies in subjects with AD have correlated the presence of depression and apathy with atrophy, hypoperfusion, and hypometabolism in the frontal cortex and anterior cingulate.29–31 It is possible that depressed patients with aMCI constitute a distinct subpopulation that more often represents early stage AD and already exhibits similar pathophysiologic changes associated with AD, including those contributing to the depressive syndrome.
The predictive value of neuropsychiatric symptoms for progression to AD raises the possibility that interventions aimed at reducing depressive symptomatology may affect the subsequent outcome of aMCI. Unfortunately, there is no clear evidence favoring cognitive benefits in treating behavioral abnormalities in MCI. Two small studies addressing this question failed to detect improved neuropsychological testing performance following treatment of depressive symptoms.32,33 Moreover, depression in patients with MCI, particularly those who subsequently progressed to dementia, was more persistent and possibly refractory to antidepressant therapy.13,32 The current analysis suggests that donepezil may delay progression to AD in patients with aMCI without impacting symptoms of depression.
Even though some studies have suggested a favorable impact of ChEI on behavioral symptoms of patients with AD,18,19 others have failed to achieve a similar finding.34,35 A recent study on rivastigmine treatment in patients with MCI did not show any significant benefit across a broad range of neuropsychiatric outcomes.36 The results from the present study indicate that donepezil reduced the risk of progression to dementia in a subgroup of aMCI depressed patients. However, this effect was not mediated by the reduction of depressive symptomatology as there was no indication of differences in BDI scores across treatment groups throughout the study period. Neuropathologic studies have demonstrated that subjects with MCI show little or no cholinergic deficits,37,38 which may explain the modest to minimal effects of ChEI in the MCI trials.3,36 The presence of depression, which has also been associated with cholinergic hypofunction,39 may indicate more severe cholinergic deficit resulting in the increased response to ChEI treatment. Alternatively, depression may be a symptom of cholinergic hypofunction and an indicator that the prodromal AD process has advanced to the stage that cholinergic pathways are affected by the disease and are responsive to ChEI treatment.
The strengths of this study include the prospective design with multiple direct assessments of depressive symptomatology, a large treatment-based cohort, and uniform operational definition of aMCI. Several important study limitations should also be acknowledged. Patients with clinically significant depressive symptoms were specifically excluded from the study. These symptoms are now recognized to be prevalent in patients with MCI and have predictive value in identifying individuals at higher risk of progressing to AD. Inclusion of depressed patients might yield even more robust treatment effects. The use of a surrogate reporter might also challenge our results but was required to accommodate for the memory deficit of patients with aMCI.
With regards to additional study limitations, all clinical trials have a propensity toward selection biases in general level of health and education and may not be representative of most community populations. Also, the predominantly Caucasian sample limits the generalizability of the results to other ethnic groups, and the p values for the z-tests comparing survival rates between treatment arms were not adjusted for multiple comparisons. It is interesting to note that despite efforts to screen out patients with clinically significant depression, BDI scores still ranged up to 34, which is indicative of severe depression. The dissociation between HAM-D, which relied on subjects' self-report, and BDI scores, as rated by the study informant, is not a unique observation. The HAM-D scores from the screening visit and baseline BDI scores were weakly correlated (Spearman rho = 0.25, p < 0.001) with minimal shared variance (6%), and discrepancies between self and observer ratings of depression have been well documented.40 A recent study also reported the presence of behavioral symptoms in an MCI sample, as detected by the Neuropsychiatric Inventory, after similar screening procedures and subsequent exclusion of clinically depressed individuals.36 Interestingly, in that study the presence of apathy at baseline was significantly associated with progression to dementia. While it is possible that the “depression” exhibited by the current sample may represent apathy rather than dysphoric mood, the BDI used to document depression contains only two items pertaining to apathy; therefore, it is unlikely to be a major contributor to the overall score.
AUTHOR CONTRIBUTIONS
Statistical analyses for this study were conducted by S.D.E.
DISCLOSURE
Dr. Petersen has served as a paid consultant to GE Healthcare and served on safety monitoring committees for Elan Pharmaceuticals and Wyeth; Dr. Cummings has served as a paid consultant to Abbott, Acadia, Accera, ADAMAS, Astellas, Avanir, Bristol-Myers Squibb, CoMentis, Eisai, EnVivo, Forest, Janssen, Lilly, Lundbeck, Medivation, Merck, Merz, Myriad, Neuren, Novartis, Pfizer, Prana, Schering Plough, Sonexa, Takeda, Toyama, and Wyeth.
APPENDIX
The Alzheimer's Disease Cooperative Study Group consists of the following: Affiliated Research Institute: C.H. Merideth, T.A. Milbrand, S. Mende; Arizona Health Sciences Center: G. Ahern, C. Kells, K. Burton; Barrow Neurology Clinic: A. Schwartz, C. Echols, M. Zomok, L. Dawson; Baumel-Eisner Boca Raton: B. Baumel, J. Crasto, R. Radzivill; Baumel-Eisner Fort Lauderdale: L. Eisner, J. Riveros, A. Johnson; Baumel-Eisner Miami Beach: B. Baumel, J. Crasto, D. Alonso, A. Torres; Baylor College of Medicine: R. Smith Doody, J. Sims, N. Robinson; Brown University: B. Ott, M. Clemens, J. Grace; Burke Medical Research Institute, White Plains: J. Blass, R. Cirio; Cedars-Sinai Medical Center: A. Schneider; Clinical Insights: L. Adler; Clinical Research Systems: R. Margolin, D. Kent; ClinSearch: M. Roffman, I. Marritt; Cognitive Neurology, St. Joseph's Health Center: A. Kertesz, D. Morlog; Columbia University: M. Sano, E. Dominguez, A. Raganuth, R. Santiago, C. Weber; Cornell Medical Center: B. Meyers; Duke University Medical Center: J. Burke, S. Vann Wyne, M. McCart; E. Bruyere Memory Disorder Research: D.A. Guzman, C. Gravelle, I. Bedirian; Emory University: A. Levey, J. Cellar, N. Gauchman, S. Valia; Fletcher Allen Health Care: P.A. Newhouse, E. Gay; Georgetown University Medical Center: P. Aisen, M.A. Cechola, K. Johnson, B. Reynolds; Geriatric and Adult Psychiatry: A. Siegal; Geriatric Medical Research Group: S. Darvesh, J. Cross, G. Sherwood; Glenrose Rehabilitation Hospital: P. McCracken, S. Aloisio, S. Duban, C. McKelvey; Indiana University: M. Farlow, P. Nurnberger, K. Fleming, N. Jessup, J. Pearson, E. Riley; Jewish Hospital Memory Clinic: H. Chertkow, C. Hosein; Johns Hopkins University: J. Brandt, C. Munro, S. Kilada, S. O'Donnell; Kansas University, Kansas City: G.J. Lopez, P. Switzer; Maimonides Medical Center: A. Miller, T. La Rocca, S. Freimark; Massachusetts General Hospital: J. Growdon, M. Tennis; Mayo Clinic, Jacksonville: N. Graff-Radford, F. Parfitt, L.M. Makarov; Mayo Clinic, Rochester: D.S. Knopman, B. Boeve, N. Haukom, M. Mandarino, D. Mullinax, R. Petersen; McGill Centre for Studies in Aging: S. Gauthier, D. Amyot; MCP Hahnemann University: C. Lippa, A.M. Wilson, R. Petrucci; Medical University of South Carolina: D. Bagwell, J.E. Mintzer, M. Stuckey; Memorial Veterans Hospital, Boston University: R.C. Green; Memory Disorders Institute: J. Shua-Haim, V. Shua-Haim, S. Wall, A. Hovick; Mt. Sinai School of Medicine: K. Davis, R.C. Mohs, K. Swedish, M. Casadiego, L. Negroni, K. Ware, B. Knox; Nathan Kline Institute for Psychiatric Research: N. Pomara, C. de la Pena; Neurobehavioral Research: R. Brenner; New York University Medical Center: S. Ferris, M. Vlassopoulos, J. Kastelan, J. Lam; Northwestern University: M.M. Mesulam, L. Herzog; Oregon Health Sciences University: J. Kaye, J. Lear, S. Berman, K. Wild; Pacific Research Network: S. Thein, Jr.; Palm Beach Neurological: D. Cipriani, C. Sadowsky, Y. Ramirez-Rojas; Princeton Biomedical Research: A.A. Sugerman, J.P. Cole-Kady, K. Alvarez, R. Soika; Quantum Labs: J. DeLaGandara; Rush–Presbyterian–St. Luke's Medical Center: N. Aggarwal, D. Bennett, R.M. Ferraro, C. Aldridge, M. Li, R.M. Nance; Southern Illinois University: S. Vicari, F. Schaefer; Southwestern Vermont Medical Center: P. Solomon, B.J. Hathaway, L. Crowe, M. Robinson; Saint Louis University: G. Grossberg; Stanford–Veterans Affairs Aging Clinical Research Center: J.A. Yesavage; Staten Island University Hospital: M. Levy; Sun Health: M. Sabbagh, K. Hatton; Sunnybrook Health Sciences: S. Black, J. Lawrence, M. Evans; SUNY Stony Brook: L. Krupp, D.M. Madigan; Sutter Institute for Medical Research: W.J. Au, D.N. Poff, M. Mulligan, I. Orengo; U.B.C. Clinic for Alzheimer's Disease: H. Feldman, V. O'Neill, K. Gilchrist; University of Calgary Cognitive Assessment Clinic: D. Hogan, P. Mueller; University Hospitals of Cleveland: D. Geldmacher, C. Santillan, P. Talea, M. Sanders; University of California, Davis: C. DeCarli, J. Coleman; University of California, Irvine: C. Cotman, R. Mulnard, C. McAdams-Ortiz, H. Kim; University of California, Los Angeles: J. Cummings, D.L. Masterman, M.F. Carter, N. Bennett, L. Berndt; University of California, San Diego: M. Grundman, J.M. Olichney, S.M. Johnson, C.W. Jenkins; University of California, San Francisco: K. Yaffe, R. Gearhart, V. Smith; University of Kentucky, Lexington: F. Schmitt, J. Cox, S. Anderson, C. Sowards Dearth; University of Miami, Gulf Coast Education and Research: J. Rivero, R. Ownby, J. Williams; University of Michigan, Ann Arbor: N. Foster, J. Lord, N. Johnson; University of Minnesota, Minneapolis: A. Hochhalter; University of Nevada, School of Medicine: C. Bernick, G. Vranesh, D. Munic, P. LeBlanc; University of New Mexico: J. Adair, S. McClelland; University of Pennsylvania: C. Clark, K. Gravanda, V. Cotter, J. Nuñez, E. Ryan-Ripp; University of Pittsburgh: S. DeKosky, L. Smith-Macedonia, T. Baumgartner, AL Kane; University of Rochester Medical Center: P. Tariot, B. Goldstein, L. Terwilliger; University of South Florida, Tampa: E. Pfeiffer, B. Luhn, D. Baxter, J. Hunter; University of Southern California: L. Schneider, N. Taggart, K. Stevens-Dagerman; University of Texas Southwestern Medical Center: M. Weiner, K. Martin-Cook, T. Ninman, S. Pierce; University of Washington: E. Peskind, M. Raskind, R. Wood, N. Brown, J. O'Connell, N. Pham; Veterans Affairs Medical Center, Augusta: M.E. Nichols, C. Bailie, D. Hillesland; Vanderbilt University: R. Margolin, D. Kent, L. McFarland; Washington University School of Medicine: J.C. Morris, S. Stiening, A. Dromerick, C. Dyer; Wien Center: R. Duara, P.D. Roberts; Yale University School of Medicine: C. Van Dyck, M. MacAvoy, L. Cretella, T. Rightmer, L. Zeiser.
Address correspondence and reprint requests to Dr. Po H. Lu, Mary S. Easton Center for Alzheimer's Disease Research, 10911 Weyburn Avenue, Suite 200, Los Angeles, CA 90095-7226 plu@mednet.ucla.edu.
*Members of The Alzheimer's Disease Cooperative Study Group are listed in the appendix.
Supported by grants from the National Institute on Aging (K23-AG028727), Alzheimer's Association (NIRG-07-60424), the Alzheimer's Disease Cooperative Study (U01 AG010483), the Alzheimer's Disease Research Center (P50 AG-16570 from the National Institute on Aging), Alzheimer's Disease Research Center of California, Jim Easton, and the Sidell Kagan Foundation.
Medications: Donepezil (Aricept; Pfizer, New York, NY, and Eisai, Tokyo, Japan).
Disclosure: Author disclosures are provided at the end of the article.
Presented in part at the International Conference on Alzheimer's Disease, Chicago, IL, 2008.
Received August 22, 2008. Accepted in final form March 13, 2009.
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 2.Geslani DM, Tierney MC, Herrmann N, Szalai JP. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer's disease. Dement Geriatr Cogn Disord 2005;19:383–389. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388. [DOI] [PubMed] [Google Scholar]
- 4.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004;63:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry 2006;63:916–924. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol 2006;63:693–699. [DOI] [PubMed] [Google Scholar]
- 8.Encinas M, De Juan R, Marcos A, et al. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer's disease. Eur J Nucl Med Mol Imaging 2003;30:1473–1480. [DOI] [PubMed] [Google Scholar]
- 9.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228–234. [DOI] [PubMed] [Google Scholar]
- 10.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475–1483. [DOI] [PubMed] [Google Scholar]
- 11.Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis Assoc Disord 2004;18:17–21. [DOI] [PubMed] [Google Scholar]
- 12.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer's disease. Dement Geriatr Cogn Disord 2007;24:253–259. [DOI] [PubMed] [Google Scholar]
- 13.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol 2004;61:1290–1293. [DOI] [PubMed] [Google Scholar]
- 14.Gabryelewicz T, Styczynska M, Luczywek E, et al. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry 2007;22:563–567. [DOI] [PubMed] [Google Scholar]
- 15.Robert PH, Berr C, Volteau M, et al. Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer's disease: a one-year follow-up study. Clin Neurol Neurosurg 2006;108:733–736. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry 1999;56:261–266. [DOI] [PubMed] [Google Scholar]
- 17.Rozzini L, Chilovi BV, Trabucchi M, Padovani A. Depression is unrelated to conversion to dementia in patients with mild cognitive impairment. Arch Neurol. 2005;62:505; author reply 505–506. [DOI] [PubMed]
- 18.Holmes C, Wilkinson D, Dean C, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology 2004;63:214–219. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer's disease. Am J Psychiatry 2004;161:532–538. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale, Revised: Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 22.Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988;24:637–639. [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278–296. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 26.Zubenko GS, Moossy J. Major depression in primary dementia. Clinical and neuropathologic correlates Arch Neurol 1988;45:1182–1186. [DOI] [PubMed] [Google Scholar]
- 27.Forstl H, Levy R, Burns A, Luthert P, Cairns N. Disproportionate loss of noradrenergic and cholinergic neurons as cause of depression in Alzheimer's disease–a hypothesis. Pharmacopsychiatry 1994;27:11–15. [DOI] [PubMed] [Google Scholar]
- 28.Zweig RM, Ross CA, Hedreen JC, et al. The neuropathology of aminergic nuclei in Alzheimer's disease. Ann Neurol 1988;24:233–242. [DOI] [PubMed] [Google Scholar]
- 29.Craig AH, Cummings JL, Fairbanks L, et al. Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neurol 1996;53:1116–1120. [DOI] [PubMed] [Google Scholar]
- 30.Hirono N, Mori E, Ishii K, et al. Frontal lobe hypometabolism and depression in Alzheimer's disease. Neurology 1998;50:380–383. [DOI] [PubMed] [Google Scholar]
- 31.Apostolova LG, Akopyan GG, Partiali N, et al. Structural correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord 2007;24:91–97. [DOI] [PubMed] [Google Scholar]
- 32.Li YS, Meyer JS, Thornby J. Longitudinal follow-up of depressive symptoms among normal versus cognitively impaired elderly. Int J Geriatr Psychiatry 2001;16:718–727. [DOI] [PubMed] [Google Scholar]
- 33.Adler G, Chwalek K, Jajcevic A. Six-month course of mild cognitive impairment and affective symptoms in late-life depression. Eur Psychiatry 2004;19:502–505. [DOI] [PubMed] [Google Scholar]
- 34.Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet 2004;363:2105–2115. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer's disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry 2001;71:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007;6:501–512. [DOI] [PubMed] [Google Scholar]
- 37.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 2002;51:145–155. [DOI] [PubMed] [Google Scholar]
- 38.Ikonomovic MD, Mufson EJ, Wuu J, Cochran EJ, Bennett DA, DeKosky ST. Cholinergic plasticity in hippocampus of individuals with mild cognitive impairment: correlation with Alzheimer's neuropathology. J Alzheimer Dis 2003;5:39–48. [DOI] [PubMed] [Google Scholar]
- 39.Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer's disease: the cholinergic hypothesis revisited. Neurology 1996;47:876–883. [DOI] [PubMed] [Google Scholar]
- 40.Enns MW, Larsen DK, Cox BJ. Discrepancies between self and observer ratings of depression: the relationship to demographic, clinical and personality variables. J Affect Disord 2000;60:33–41. [DOI] [PubMed] [Google Scholar]


