Abstract
Lipolysis of stored triacylglycerols provides lipid precursors for the assembly of apolipoprotein B (apoB) lipoproteins in hepatocytes. Abhydrolase domain containing 5 (ABHD5) is expressed in liver and facilitates the lipolysis of triacylglycerols. To study the function of ABHD5 in lipoprotein secretion, we silenced the expression of ABHD5 in McA RH7777 cells using RNA interference and studied the metabolism of lipids and secretion of apoB lipoproteins. McA RH7777 cells deficient in ABHD5 secreted reduced amounts of apoB, triacylglycerols, and cholesterol esters. Detailed analysis of liquid chromatography-mass spectrometry data for the molecular species of secreted triacylglycerols revealed that deficiency of ABHD5 significantly reduced secretion of triacylglycerols containing oleate, even when oleate was supplied in the culture medium; the ABHD5-deficient cells partially compensated by secreting higher levels of triacylglycerols containing saturated fatty acids. In experiments tracking the metabolism of [14C]oleate, silencing of ABHD5 reduced lipolysis of cellular triacylglycerols and incorporation of intermediates derived from stored lipids into secreted triacylglycerols and cholesterol esters. In contrast, the incorporation of exogenous oleate into secreted triacylglycerols and cholesterol esters was unaffected by deficiency of ABHD5. These findings suggest that ABHD5 facilitates the use of lipid intermediates derived from lipolysis of stored triacylglycerols for the assembly of lipoproteins.
Keywords: Lipolysis, triacylglycerols, cholesterol esters, RNA interference, McArdle hepatoma, liquid chromatography-mass spectrometry
1. Introduction
Hepatic secretion of apolipoprotein B (apoB)-containing lipoproteins is regulated primarily by the availability of lipids for the assembly of lipoprotein particles [1;2]. The association of apoB with lipids initiates lipoprotein formation [3]; when the availability of lipids is limited, apoB is targeted for degradation, and the secretion of apoB lipoproteins decreases [1;4]. In hepatocytes, stored triacylglycerols (TAG) provide lipid precursors for the assembly of apoB-lipoproteins [5;6]. These TAG are not incorporated en bloc, but instead, undergo a cycle of lipolysis and re-esterification before being packaged into lipoproteins [7-9]. Therefore, in hepatocytes, the lipolysis of stored TAG is an important step in the formation of lipoproteins, although the enzymes that catalyze this lipolysis and the steps between lipolysis and lipoprotein assembly are poorly characterized.
Abhydrolase domain containing 5 (ABHD5), also called CGI-58 for comparative genome identification 58, is a protein that facilitates lipolysis of TAG. Mutations in the gene that encodes ABHD5 cause Chanarin-Dorfman Syndrome (CDS), which is characterized by accumulation of TAG in various tissues [10]. Hepatosteatosis is a commonly observed trait in individuals with CDS [10-12], suggesting that ABHD5 is necessary for the maintenance of triacylglycerol homeostasis in hepatocytes. Furthermore, the expression of ABHD5 in liver of both humans and mice [13;14] suggests that ABHD5 may play a role in the metabolism of stored TAG for lipoprotein assembly. In assays conducted in vitro, addition of exogenous ABHD5 increased triacylglycerol hydrolase activity of cytosolic fractions from liver [14]. ABHD5 lacks triacylglycerol hydrolase activity [14], yet increases the activity of adipose triglyceride lipase (ATGL) in vitro [14;15]. It has been proposed that ABHD5 acts as a co-activator of ATGL, although the mechanism is poorly understood [14;15]. Since ATGL is expressed in liver [16], it may hydrolyze stored TAG to mobilize lipid substrates for lipoprotein assembly; ABHD5 may contribute to this process. A recent report shows that ABHD5 displays lysophosphatidic acid acyltransferase activity [17] (also Montero-Moran, G. and Brasaemle, D. L., unpublished data). Thus, ABHD5 is an enzyme that may be involved in biosynthetic pathways for TAG, phospholipids, or both. It is currently unclear whether the role of ABHD5 in assisting ATGL-mediated hydrolysis of TAG is related to utilization of fatty acids released from TAG hydrolysis in the synthesis of phosphatidic acid, or via a separate mechanism potentiated through a protein-protein interaction of ABHD5 with ATGL [18]
Since the lipolysis of stored TAG is necessary for the assembly of lipoproteins [5;6] and ABHD5 facilitates TAG hydrolysis [14;19], we hypothesized that ABHD5 plays a role in the formation of lipoproteins. We established a loss-of-function model using RNA interference (RNAi) to reduce ABHD5 expression in McA RH7777 rat hepatoma cells. Lipolysis of intracellular TAG and secretion of TAG, cholesterol esters (CE), and apoB were studied. Liquid chromatography-mass spectrometry (LC-MS) methods were developed to quantify the small amounts of lipids secreted by McA RH7777 cells. We provide evidence that ABHD5 is important for the assembly and subsequent secretion of lipoproteins in hepatocytes.
2. Materials and Methods
2.1. Design of small hairpin-RNA and production of adenoviral vectors
RNAi sequences targeting ABHD5 were selected using algorithms from Ambion, Genscript, and Dharmacon; sequence specificity was evaluated by a homology search against the mouse and rat genomic database using BLAST. The sequence GAAGTAGTAGACCTAGGTT, corresponding to nucleotides 347 to 365 of the ABHD5 coding sequence, was selected. The scrambled sequence TTACATGTTAAGAGAGGCG was selected for use as a control sequence. Adenoviral vectors directing the expression of small hairpin RNA (shRNA) and a green fluorescent protein reporter were generated using pAdtrack-H1.1 (GenScript) and AdEasy XL Adenoviral Vector System (Stratagene). The adenoviral vectors were purified by polyethylene glycol precipitation and cesium chloride banding [20]. The titers of the adenoviral preparations were determined by plaque assay.
2.2. Transduction of McA RH7777 cells with adenoviral vectors
McA RH7777 rat hepatoma cells (American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 20% horse serum and 5% fetal bovine serum (culture medium) using collagen-coated vessels. Transductions were performed by incubating the cells in culture medium containing the adenoviral vectors for 24 h followed by re-plating to avoid cell overgrowth. The cell incubations were conducted 48 h after transduction, when protein levels of ABHD5 were optimally reduced (Supplemental Fig. 1) and the cells were ~90% confluent.
The efficiency of adenoviral transduction was assessed by determination of the percentage of cells expressing green fluorescent protein by cell counting using a Nikon Diaphot-TMD Inverted Microscope with TMD-EF epi-fluorescence equipment. Cell viability was determined by trypan blue exclusion [21] and cell membrane integrity was assessed using a lactate dehydrogenase-based in vitro toxicology assay (Sigma, TOX-7). A multiplicity of infection of 25 plaque forming units per cell was selected for subsequent adenoviral transductions based on an efficiency of transduction of greater than 90% of the cells with no detectable toxicity.
2.3. Cell incubations
Radiolabeled [14C]oleic acid (OA) (Amersham) was used to track the synthesis, storage, metabolism, and secretion of lipids; these experiments had three incubation periods designated pulse, washout, and chase. The experimental design was similar to a protocol described by Gilham et al. [22]. During the pulse period, cells were incubated for 4 h in culture medium containing 0.4 mM [14C]OA complexed to fatty acid-free bovine serum albumin (BSA) (Sigma, A6003) at a 4:1 molar ratio. Subsequently, during the washout period, the cells were incubated in culture medium containing 5 g fatty acid-free BSA per liter of medium for 2 h. This incubation provides time for the secretion of lipoproteins assembled during the pulse period, prior to the beginning of the chase period [22-24]. Finally, during the chase period, the cells were incubated in culture medium containing 5 g fatty acid-free BSA per liter of medium for 4 h. Separate samples of cells and media were collected after the pulse, pulse and washout, or pulse, washout, and chase periods. The experiments conducted to analyze apoB secretion followed a similar protocol, but media used for the pulse period contained unlabeled OA. The incubation with OA (designated “+OA”) was analogous to the pulse period. Similarly, the incubation designated “-OA” was equivalent to the chase period. Experiments conducted to analyze the secretion of lipids using LC-MS followed a protocol identical to that used for the analysis of apoB secretion, except that the media lacked serum.
2.4. Analysis of proteins by immunoblotting
Cells were suspended in 20 mM Tris-HCl, pH 8, 1 mM EDTA, 10 mM NaF, 100 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 10 mg/l leupeptin, 500 μM benzamidine, and lysed by probe sonication. Protein concentrations of lysates were determined using Coomassie Plus Reagent (Pierce). Proteins from cell homogenates were solubilized in gel loading buffer, resolved by SDS-PAGE, and transferred to nitrocellulose membranes. ABHD5 was detected using polyclonal antisera raised in rabbits against recombinant mouse ABHD5 [13] (1:2,500), horseradish peroxidase-conjugated anti-rabbit antibodies (Sigma A0545) (1:100,000), and SuperSignal chemiluminescence reagent (Pierce). The antisera raised against mouse ABHD5 detects rat ABHD5, which has 99% identity with the mouse protein. The antiserum binds non-specifically to a ~116 kDa protein in McA RH7777 cell lysates; the 116 kDa protein was used as a loading control. Actin was detected using a monoclonal antibody raised in mice against β-actin (Sigma A5316) (1:5000) followed by horseradish peroxidase-conjugated anti-mouse antibodies (Sigma A4416) (1:5,000), and enhanced chemiluminescence reagents.
2.5. Measurement of mass of cellular TAG
Lipids were extracted from cell homogenates [25] and the mass of TAG was determined using Infinity Triglyceride Liquid Stable Reagent (ThermoElectron) [26;27].
2.6. Analysis of secreted apoB
ApoB content of culture media from cell incubations was determined by competitive RIA employing a mouse monoclonal antibody raised against rat apoB which is equally reactive with B100 and B48 (N-terminal epitope) [28]. Because there is one molecule of apo B per lipoprotein, this assay quantifies lipoprotein particle secretion on a molar basis. ApoB standards were prepared using rat VLDL diluted in culture medium. Rates of apoB secretion were calculated as ng apoB secreted into the medium per hour per mg cell protein.
2.7. Analysis of secreted lipids by LC-MS
Lipids in the media from cell incubations were extracted [29] after addition of trinonanoin (Nu-Chek Prep) as an internal standard. Lipids were analyzed on a system consisting of an UltiMate 3000 HPLC (Dionex) coupled to a 4000 QTRAP® mass spectrometer (Applied Biosystems). Lipids were separated using a Spherisorb® S5W 4.6 × 100 mm silica column (Waters) and the tertiary normal phase solvent gradient described by Homan and Anderson [30], with HPLC-grade solvents (Fisher Scientific). Ionization was carried out with the PhotoSpray™ ion source operated in positive ion mode with toluene as the dopant. CE and TAG were measured during period 1 (0 to 5.75 min) in Enhanced Mass Spectrometry mode with a scan rate of 1000 amu/s and collision energy set at 10 V. Cholesterol was monitored in period 2 (5.75 to 24.25 min) using Enhanced Resolution mode. Mass measurements were made by comparing peak areas in the samples to areas generated using standard curves of triolein, cholesterol, and cholesteryl palmitate (Sigma).
2.8. Analysis of radiolabeled lipids
Cellular and medium lipids were extracted [31] and resolved by TLC on Silica Gel HLF plates (Analtech) developed with hexane:ethyl ether:acetic acid 80:20:2 (v:v:v) [32]. Radioactivity was detected and quantified using a Storm System phosphorimager (Amersham). The low radioactivity of [14C]CE was quantified by recovery of the lipids from the TLC plates and liquid scintillation counting.
2.9. Statistical analysis
Experiments included three to five replicate samples and were repeated two to five times, as detailed in each figure legend. Data correspond to the means and standard error of the mean (SEM) of average values obtained from replicate samples from each experiment, unless otherwise noted. Statistical analyses were performed by t-test or ANOVA using GraphPad Prism software and the differences were considered significant when the p value was less than 0.05.
3. Results
3.1. McA RH7777 cells deficient in ABHD5 accumulate TAG
To reduce ABHD5 expression, we transduced McA RH7777 cells with an adenoviral vector directing the expression of shRNA targeting ABHD5, which we designated ABHD5-shRNA. For control conditions, we used an adenoviral vector directing the expression of a scrambled-sequence shRNA, which we designated control-shRNA. Transduction of McA RH7777 cells with ABHD5-shRNA reduced the expression of ABHD5 protein by ~90% when compared to either cells transduced with control-shRNA or non-transduced cells (Fig. 1). In contrast, protein levels of β-actin (Fig. 1B) and calnexin (data not shown) were comparable in cells incubated with ABHD5-shRNA relative to either type of control cell. Transduction of cells with ABHD5-shRNA did not decrease cell viability, or reduce total cellular protein content (data not shown). The reduction in protein levels of ABHD5 increased cellular TAG by 41% under normal culture conditions when compared to either cells transduced with control-shRNA or non-transduced cells (Fig. 2).
Figure 1. ABHD5-shRNA reduced protein levels of ABHD5 by 90%.
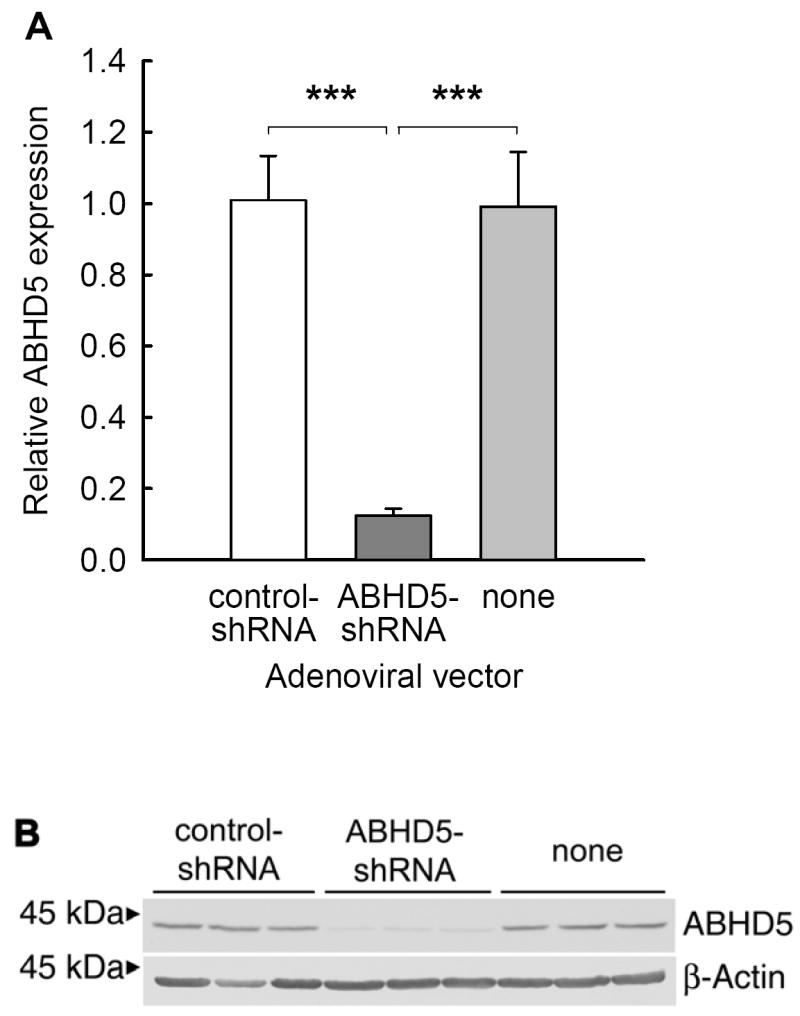
McA RH7777 cells were transduced with either ABHD5-shRNA or control-shRNA, or were not transduced. Cells were harvested after 48 hours of growth in serum-containing culture medium without supplemental fatty acids. The expression of ABHD5 and β-actin were analyzed by immunoblotting (B). Expression of ABHD5 was quantified by scanning densitometry and expressed relative to controls (A). The results represent the means and standard deviations from a representative experiment with triplicate samples. The experiment was repeated five times with similar results. Statistical analysis was performed using one-way ANOVA and Bonferroni’s post-hoc test; *** indicates significant differences at p < 0.001.
Figure 2. Reduction of ABHD5 expression increased TAG by 41%.
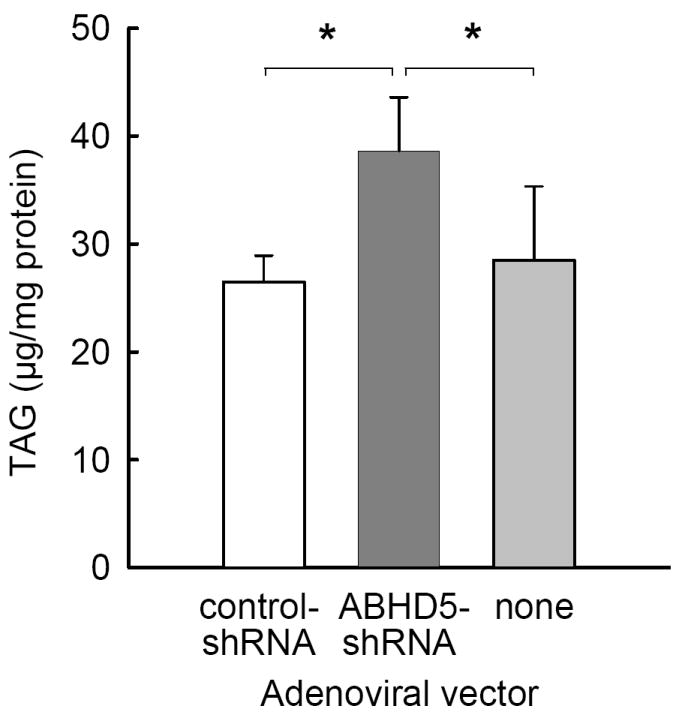
McA RH7777 cells were transduced with either ABHD5-shRNA or control-shRNA, or were not transduced. Cells were harvested after 48 hours of growth in serum-containing culture medium without supplemental fatty acids. The mass of TAG in solvent extracts of the cells was quantified. The results represent the means and SEM from four independent experiments. Statistical analysis was performed using one-way ANOVA and Tukey’s post-hoc test; * indicates significant differences at p < 0.05.
3.2. The secretion of apoB-VLDL is reduced in McA RH7777 cells deficient in ABHD5
To assess the role of ABHD5 in the assembly of lipoproteins, we measured apoB secreted into the culture medium by McA RH7777 cells deficient in ABHD5. McA RH7777 cells use both exogenous fatty acids and lipids derived from stored TAG to assemble lipoproteins [22;33]. Thus, we analyzed the apoB content of media from McA RH7777 cells incubated in the presence or absence of added OA. During 4 h incubation with 0.4 mM OA, the apoB content of media was comparable between McA RH7777 cells deficient in ABHD5 and control cells (Fig. 3); thus, the transduction of cells with ABHD5-shRNA has no direct effect on the secretion of apoB. To measure apoB secretion in the absence of added OA, McA RH7777 cells were first incubated with OA to increase cellular TAG stores, submitted to a washout period to provide time for secretion of lipoproteins assembled during the OA incubation, and then incubated for 4 h in culture medium without added OA. During incubation without OA, the apoB content of media was 19% lower in cells deficient in ABHD5 than in control cells (Fig. 3). Thus, when exogenous fatty acids are available, ABHD5 is not essential for the assembly and secretion of apoB lipoproteins. However, in the absence of added OA, ABHD5-deficient cells secrete fewer apoB-containing particles.
Figure 3. The secretion of apoB was decreased in McA RH7777 cells deficient in ABHD5 when supplemental oleate was removed from the culture medium.
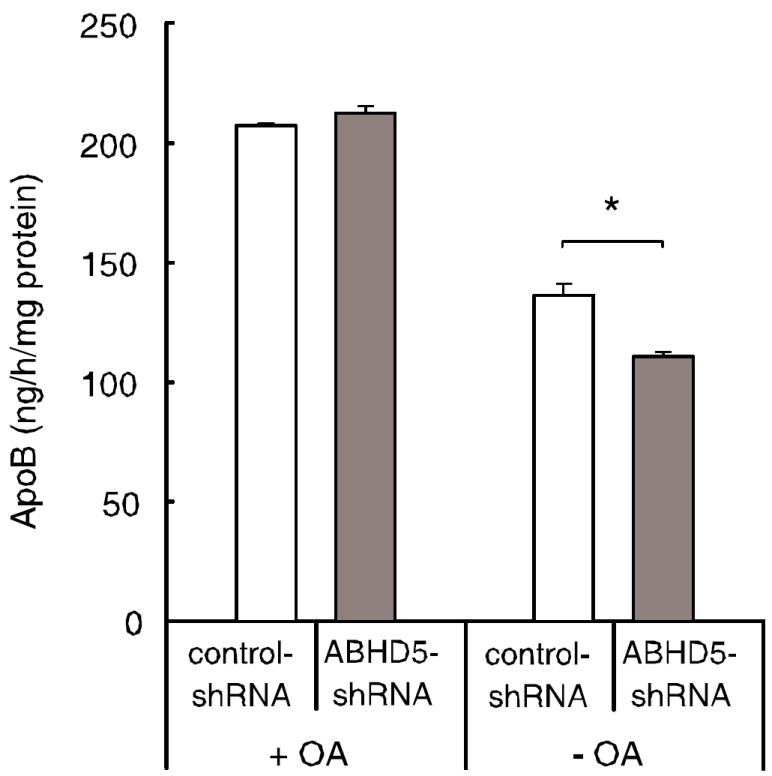
McA RH7777 cells transduced with ABHD5-shRNA or control-shRNA were incubated for 4 h with medium containing 0.4 mM OA (+OA). Additionally, McA RH7777 cells that had been pre-incubated with OA to increase cellular TAG stores were incubated for 4 hours in medium without added OA (-OA). Total apoB secreted into the medium was quantified by RIA. The results represent the means and SEM from two experiments performed with 3 or 5 replicate samples per condition. Statistical analysis was performed using two-way ANOVA and Bonferroni’s post-hoc test; * p < 0.05.
3.3. The secretion of TAG and CE is decreased in ABHD5-deficient cells
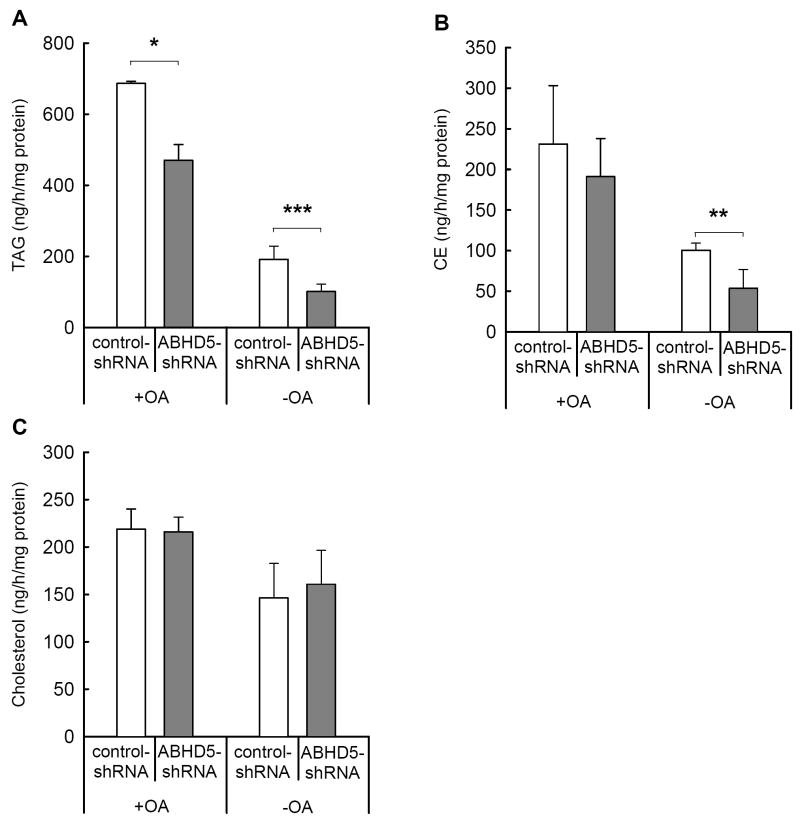
The decreased amount of apoB-lipoproteins secreted by ABHD5-deficient cells suggests a concurrent reduction in the lipid components of those lipoproteins. To study lipid secretion, we incubated ABHD5-deficient and control cells for 4 h either with or without OA, as previously described, and measured the mass of neutral lipids in the incubation media. We employed highly sensitive LC-MS methods with photospray ionization of samples; these procedures allow quantification of as little as 50 pg of an individual lipid. During incubation with OA, McA RH7777 cells deficient in ABHD5 secreted 32% less TAG than control cells (Fig. 4A). A trend toward lower secretion of CE in ABHD5-deficient cells reached statistical significance in one of two experiments conducted (Fig. 4B). This decreased lipid mass was likely associated with the same number of apoB-containing particles of reduced lipid content.
Figure 4. The secretion of TAG and CE was reduced in cells deficient in ABHD5.
McA RH7777 cells transduced with ABHD5-shRNA or control-shRNA were incubated for 4 h in medium either with 0.4 mM OA (+OA) or, following preincubation with 0.4 mM OA, without OA (-OA). TAG (A), CE (B), and free cholesterol (C) secreted into the medium were analyzed by LC-MS. Data represent the means and SEM from two experiments performed with four or five replicate samples. Statistical analyses were performed using two-way ANOVA; * indicates significant differences at p < 0.05; **, p < 0.01; and ***, p < 0.001.
Removal of OA from the culture medium significantly reduced the secretion of both TAG and CE in both control and ABHD5-deficient cells. During incubation without OA, cells deficient in ABHD5 secreted 47% less TAG and 47% less CE than control cells (Fig. 4A and B). In contrast, the values for cholesterol in the media were similar for both ABHD5-deficient and control cells under both conditions (Fig. 4C).
LC-MS analysis of lipids in the media facilitated identification of the molecular species of TAG (Table 1, Supplemental Table 1 and Supplemental Figure 2). Use of photospray ionization with enhanced mass spectrometry supported the highest sensitivity of detection, but identification of component fatty acids was limited to those found in diacylglycerol ions derived from TAG. The major species of diacylglycerol detected under all conditions was diolein (C18:1/18:1), which represented 60-75% of the total mass of TAG in the culture medium. Under both +OA and −OA conditions, deficiency of ABHD5 reduced secretion of species of TAG containing one or more oleate (C18:1) moieties relative to that of cells expressing ABHD5 (controls); these differences were greatest under −OA conditions. Interestingly, ABHD5-deficient cells secreted significantly higher levels of TAG with saturated fatty acids (C16:0/16:0 and C16:0/C18:0) than control cells in +OA conditions. Although these species of triacylglycerol are minor components of the secreted lipoproteins, they increased from 1% of total TAG in control cells to 3.5% of total TAG in ABHD5-deficient cells. Similarly, in −OA conditions, levels of these species were below detection in medium from control cells, but at higher, detectable levels in medium from ABHD5-deficient cells. In summary, ABHD5 deficiency decreases the secretion of TAG and CE; the effect is greatest in the absence of exogenous fatty acids, consistent with a role for ABHD5 in facilitating utilization of stored TAG for lipoprotein assembly.
Table 1. Effects of silencing of ABHD5 on triacylglycerol secretion by McA RH7777 cells.
LC-MS analysis of TAG secreted in the experiment depicted in Figure 4. The diacylglycerol ions observed using photospray ionization are [M+ H+ -H2O]. The diacylglycerol molecule shown is the most probable member of the group. See supplemental Table 1 for a description of each group.
| Secretion Rate, ng/hour/mg protein | ||||||||
|---|---|---|---|---|---|---|---|---|
| + Oleic Acid (+OA) | -Oleic Acid (-OA) | |||||||
| TAG Group1 | Control-shRNA | ABHD5-shRNA | % Change | P value3 | Control-shRNA | ABHD5-shRNA | % Change | P value |
| 16:0/16:0 | 3.33±1.012 | 7.90±1.86 | 137.3% | 0.001 | 0.004 | 0.73±0.69 | NA5 | NA |
| 16:1/18:1 | 22.40±2.17 | 15.96±2.37 | -28.7% | 0.002 | 5.29±1.03 | 3.06±0.52 | -42.2% | 0.003 |
| 16:0/18:1 | 57.81±5.21 | 45.39±5.16 | -21.5% | 0.005 | 19.07±2.96 | 14.40±1.38 | -24.5% | 0.013 |
| 16:0/18:0 | 4.29±0.64 | 6.49±1.68 | 51.2% | 0.026 | 0.00 | 1.10±0.83 | NA | NA |
| 18:1/18:2 | 26.75±2.58 | 19.06±2.32 | -28.7% | 0.001 | 8.41±0.71 | 4.71±0.61 | -43.9% | 0.001 |
| 18:1/18:1 | 520.75±77.7 | 277.28±45.90 | -46.8% | 0.001 | 96.46±15.45 | 44.73±4.13 | -53.6% | 0.001 |
| 18:0/18:1 | 41.82±6.15 | 27.40±3.80 | -34.5% | 0.002 | 9.96±1.93 | 5.15±0.66 | -48.2% | 0.001 |
| 18:0/18:0 | 1.69±1.00 | 2.70±0.77 | 60.1% | >0.05 | 0.00 | 0.00 | NA | NA |
| 18:1/20:1 | 23.84±3.52 | 12.33±1.87 | -48.3% | 0.001 | 8.84±1.84 | 2.67±0.27 | -69.8% | 0.001 |
| Total TAGs | 702.67 | 414.52 | -41.0% | 148.026 | 76.56 | -48.3% | ||
TAG Group includes the likely diacylglycerol from the observed mass.
Values are means ± standard deviation of 5 determinations from one representative experiment of two.
Statistical analysis was carried out with t-tests.
0.00 indicates the TAG were below the detection limit of 50 pg/injection.
NA = not available
The secretion rate for total TAG for control cells under −OA conditions was 21% the rate under +OA conditions.
3.4. The secretion of TAG and CE synthesized from stored lipids is diminished in cells deficient in ABHD5
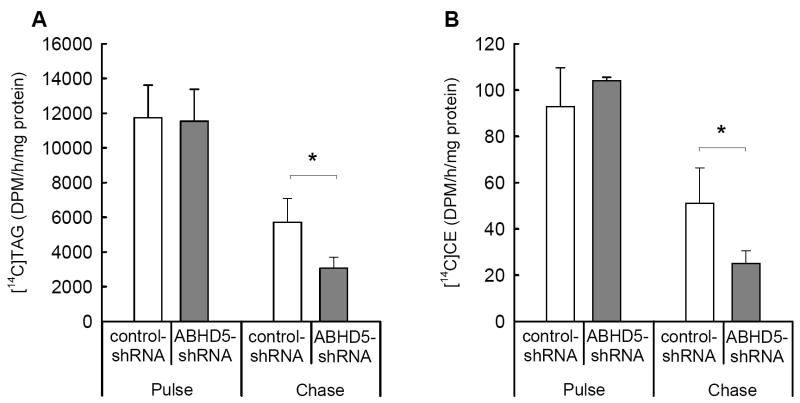
To directly assess the use of precursors derived from stored lipids for the assembly of lipoproteins, we incubated cells with [14C]OA and analyzed lipids secreted into the medium during both 4 h pulse and 4 h chase periods. These analyses discriminate between the contributions of exogenous and stored sources of fatty acids to the assembly of lipoproteins. During the pulse period, when exogenous [14C]OA provides the most abundant source of radiolabeled precursors for the synthesis of lipids, the content of [14C]TAG and [14C]CE was similar in media from cells deficient in ABHD5 and from controls (Fig. 5A and B). In contrast, during the chase period, when stored lipids provide the majority of radiolabeled precursors, ABHD5-deficient cells secreted 46% less [14C]TAG and 51% less [14C]CE into the medium than control cells (Fig 5A and B). Thus, deficiency of ABHD5 decreases the use of precursors derived from stored lipids for the synthesis of TAG and CE that are then secreted, presumably within lipoproteins.
Figure 5. The secretion of TAG and CE synthesized from precursors derived from stored lipid was decreased in McA RH7777 cells deficient in ABHD5 under chase conditions.
McA RH7777 cells transduced with ABHD5-shRNA or control-shRNA were incubated with [14C]OA for 4 h (pulse), incubated in medium without [14C]OA for 2 h (washout), and then incubated in fresh medium without [14C]OA for 4 h (chase). The secretion of [14C]TAG (A) and [14C]CE (B) into the culture medium was analyzed during the pulse and chase periods. Data correspond to the means and SEM from three (pulse) or four (chase) independent experiments, each of which included triplicate samples. Statistical analyses were performed using two-way ANOVA and Bonferroni’s post-hoc test; * indicates significant differences at p < 0.05.
3.5. The lipolysis of cellular TAG is decreased in McA RH7777 cells deficient in ABHD5
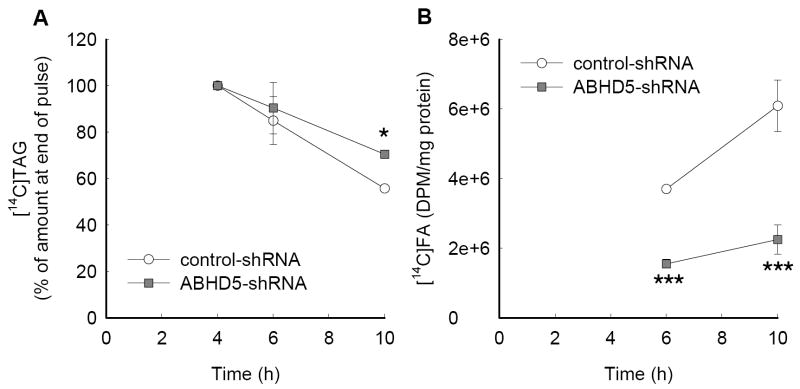
To evaluate the lipolysis of stored TAG under conditions that allow lipoprotein assembly, ABHD5-deficient and control cells were incubated with [14C]OA for 4 h to load the cells with a traceable pool of TAG. More than 75% of the [14C]OA taken up by the cells was incorporated into cellular TAG in both ABHD5-deficient and control cells, whereas less than 2% of [14C]OA was incorporated into CE, with the remainder in phospholipids (data not shown). During subsequent incubation in the absence of added [14C]OA (4 h to 10 h), [14C]TAG decreased less in ABHD5-deficient cells than in control cells (31% versus 44%, respectively, at 10 h) (Fig. 6A). The lipolysis of TAG produces fatty acids, some of which are re-esterified into TAG or CE for lipoprotein assembly, and some of which are released into the culture medium. Under conditions when fatty acids released during lipolysis of stored TAG are available for lipoprotein assembly, free [14C]OA released into the medium was 59% and 73% lower in ABHD5-deficient cells at 6 and 10 hours, respectively, when compared to control cells (Fig. 6B). Nonetheless, the specific radioactivity of cellular TAG was comparable between ABHD5-deficient and control cells throughout the incubations (Supplemental Fig. 3). Thus, [14C]OA was esterified and equilibrated into the cellular pool of stored TAG; radioactivity recovered in the culture media represented metabolism of this pool. In summary, the lipolysis of cellular TAG is decreased in McA RH7777 cells deficient in ABHD5.
Figure 6. The lipolysis of cellular TAG was reduced in McA RH7777 cells deficient in ABHD5.
McA RH7777 cells transduced with ABHD5-shRNA or control-shRNA were incubated with [14C]OA for 4 h (pulse) and subsequently incubated in medium without [14C]OA for 6 h. (A) Cellular [14C]TAG were quantified and normalized to the amount present at 4 h, which was expressed as 100%. (B) [14C]Fatty acids released into media were quantified. Each data point represents the mean and SEM from two to four experiments performed with triplicate samples; some error bars are hidden by the symbols. Statistical analyses were performed using ANOVA and Bonferroni’s post-hoc test; * indicates significant differences at p < 0.05 and ***, p < 0.001.
4. Discussion
The main finding of this paper is that ABHD5 plays an important role in the efficient use of lipids derived from lipolysis of stored TAG for assembly of apoB-lipoproteins. This finding is supported by the following alterations observed in McA RH7777 cells deficient in ABHD5: (a) the lipolysis of cellular TAG was reduced leading to increased storage of TAG; (b) the utilization of lipids released from stored TAG to synthesize TAG and CE in the secretory pathway was decreased resulting in reduced mass of TAG and CE in the incubation media; and (c) the secretion of apoB-containing lipoproteins was reduced. Decreases in secretion of apoB, TAG and CE were greatest when cells were cultured without addition of supplemental fatty acids. In contrast, when OA was added to the culture medium, these changes were reduced.
The secretion of apoB-lipoproteins is regulated primarily by the availability of lipids for particle assembly [1;3-5]. ApoB transcription is mostly constitutive [4;34]; regulation of apoB expression occurs at the translational and post-translational levels. During translation, apoB associates with lipids in the lumen of the endoplasmic reticulum to form a lipid-poor precursor; this lipidation of apoB is essential to prevent degradation by proteosomal and non-proteosomal mechanisms [1]. Subsequently, the bulk of lipids are added to form a fully-lipidated VLDL particle [2]. Stored TAG constitute a major source of lipid precursors for the assembly of lipoproteins in hepatocytes [5;6]. Lipolysis of cytoplasmic stores of TAG is followed by re-esterification in the endoplasmic reticulum and incorporation of TAG and CE into lipoproteins [7]. Our findings indicate that ABHD5 facilitates the lipolysis of stored TAG and the provision of lipid precursors for incorporation into the lipid core of apoB lipoproteins.
Deficiency of ABHD5 significantly reduced the secretion of oleate-containing TAG both when supplemental oleate was provided in the culture medium, and when the medium contained no supplemental fatty acids. However, when radiolabeled oleate was used to track the metabolism of the supplemental fatty acid, deficiency of ABHD5 reduced secretion of TAG containing radiolabeled oleate only during chase conditions following withdrawal of the fatty acid supplement. These observations suggest that stored TAG supply a substantial portion of lipid substrates for lipoprotein assembly, even when exogenous fatty acids are abundant. Detailed analysis of the molecular composition of secreted TAG revealed an increase in secretion of TAG with saturated fatty acids when ABHD5 expression was reduced in cells that were incubated either in the presence or absence of exogenous oleate. These data suggest that altered lipid flux resulting from depletion of ABHD5 induces compensatory mechanisms favoring the incorporation of saturated fatty acids, potentially derived from de novo synthesis, into TAG in the secretory pathway. These studies of the molecular composition of secreted TAG underscore the complexity of metabolic pathways leading to lipoprotein assembly.
The function of ABHD5 has not been fully elucidated. Although ABHD5 has lysophosphatidic acid acyltransferase activity [17] (also Montero-Moran, G. and Brasaemle, D.L., unpublished data), ABHD5 has also been shown to increase triacylglycerol hydrolase activity of purified ATGL or cytosolic extracts from cells overexpressing ATGL [14;35]. It has been hypothesized that ABHD5 serves as an activating cofactor for ATGL [14;15;35]. ABHD5 interacts with ATGL through a protein-protein interaction [18], and may therefore induce a conformational change in ATGL that activates the lipase. Alternatively, ABHD5-catalyzed incorporation of fatty acids released during lipolysis into phosphatidic acid may prevent end product inhibition of ATGL. Regardless of the mechanism, deficiency of ABHD5 may reduce ATGL activity, and consequently, decrease lipolysis of stored TAG and the assembly and secretion of lipoproteins. The effect of depletion of ABHD5 in reducing secretion of CE suggests that fatty acids derived from the hydrolysis of TAG are used for subsequent CE synthesis. Further investigation is needed to determine whether levels of stored CE are also affected by ABHD5 deficiency.
The importance of ATGL in lipoprotein secretion, although not expressly studied, is suggested by reduced VLDL TAG in the plasma of fasted ATGL null mice [19]. However, these mice also have low levels of free fatty acids in plasma, presumably due to the absence of ATGL in adipose tissue [19]. Thus, it is unclear whether the decrease in plasma TAG is due primarily to the absence of ATGL in liver or secondarily to the decrease in plasma fatty acids [19]. Overexpression of ATGL in mouse liver significantly increases hydrolysis of stored TAG, yet does not affect the secretion of apoB-containing lipoproteins [36]. Interestingly, clinical evidence comparing characteristics of humans with mutations in ABHD5 to characteristics of humans with mutations in PNPLA2, the gene that encodes ATGL, suggests that ABHD5 functions independently from ATGL in some tissues. Patients with mutations in PNPLA2 have prominent cardiomyopathy, which is absent in CDS patients [10], whereas CDS patients have ichthyosis, which is absent in patients with mutations in PNPLA2 [37]. Additional investigation is required to ascertain whether the function of ABHD5 in lipoprotein assembly is related to its putative function as a cofactor of ATGL or to acyltransferase activity.
Surprisingly, most reports describe normal plasma lipid concentrations in individuals with CDS [11;12]. However, the range of plasma lipid concentrations considered normal is extremely broad [38], and moderately decreased plasma lipid levels may still fall within the normal range. Two of approximately forty reported cases of CDS had hypertriglyceridemia; these rare observations may indicate that this hypertriglyceridemia is unrelated to CDS [39;40]. Neither the compositions nor the metabolism of lipoproteins in CDS patients have been described. Observations of hepatosteatosis in the majority of CDS patients suggest that triacylglycerol homeostasis in liver is disrupted by deficiency in ABHD5.
Our results extend those of Brown and co-workers [41], published while these studies were underway. In experiments using radiolabeled OA, Brown and co-workers showed that the secretion of lipoprotein-TAG decreased in McA RH7777 cells deficient in ABHD5. Our data obtained using radioactive OA support those findings. We have extended these studies by utilizing LC-MS to detect changes in the mass of secreted TAG and CE, and to characterize the fatty acid composition of the secreted TAG. Moreover, we have demonstrated that the secretion of apoB was reduced in cells deficient in ABHD5. The studies of Brown and co-workers [41] also demonstrated reduction in β-oxidation of radiolabeled OA in ABHD5-deficient McA RH7777 cells, providing an additional mechanism which promotes accumulation of TAG, beyond reduced lipoprotein secretion. Finally, these studies showed a modest 10% increase in secretion of radio-labeled TAG from McA RH7777 cells with overexpressed ABHD5, adding support to the importance of ABHD5 in lipoprotein assembly.
In summary, ABHD5 facilitates the utilization of stored TAG to provide lipid precursors for assembly of apoB-lipoproteins in hepatocytes. These results contribute to our knowledge about the processes of assembly and secretion of lipoproteins. The data suggest that modest pharmacological modulation of ABHD5 may provide important benefits in altering the lipid composition of VLDL and reducing plasma levels of TAG.
Supplementary Material
Acknowledgments
We thank Drs. R. Ariel Igal and Judith Storch for useful discussions, Dr. Vidya Subramanian and Amy Marcinkiewicz for helpful discussions and critical review of the manuscript, Diana Johnson for helpful discussions and technical advice, Monica McAndrews-Hill for advice on ABHD5 nomenclature, and Deanna L. Russell, Jenny Jokinen, and Serguei Krasnoiartsev for technical assistance. Research was supported by a postdoctoral fellowship from the American Heart Association, Heritage Affiliate to JMC, NIH R01 DK54797 and an Established Investigator Award from the American Heart Association to DLB, NIH R01 HL047586 to JLD, and NIH R01 DK50376 to JS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fisher EA, Ginsberg HN. Complexity in the Secretory Pathway: The Assembly and Secretion of Apolipoprotein B-containing Lipoproteins. Journal of Biological Chemistry. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 2.Rustaeus S, Lindberg K, Stillemark P, Claesson C, Asp L, Larsson T, Boren J, Olofsson SO. Assembly of Very Low Density Lipoprotein: A Two-Step Process of Apolipoprotein B Core Lipidation. J Nutr. 1999;129:463S–466S. doi: 10.1093/jn/129.2.463S. [DOI] [PubMed] [Google Scholar]
- 3.Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. Journal of Internal Medicine. 2005;258:395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JL, Ginsberg HN. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. Journal of Lipid Research. 1993;34:167–179. [PubMed] [Google Scholar]
- 5.Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density lipoprotein. Biochem Soc Trans. 2004;32:59–64. doi: 10.1042/bst0320059. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins D, Gibbons GF. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992;284:457–462. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lankester DL, Brown AM, Zammit VA. Use of cytosolic triacylglycerol hydrolysis products and of exogenous fatty acid for the synthesis of triacylglycerol secreted by cultured rat hepatocytes. Journal of Lipid Research. 1998;39:1889–1895. [PubMed] [Google Scholar]
- 9.Yang LY, Kuksis A, Myher JJ, Steiner G. Origin of triacylglycerol moiety of plasma very low density lipoproteins in the rat: structural studies. J Lipid Res. 1995;36:125–136. [PubMed] [Google Scholar]
- 10.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud’homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pena-Penabad C, Almagro M, Martinez W, Garcia-Silva J, Del Pozo J, Yebra MT, Sanchez-Manzano C, Fonseca E. Dorfman-Chanarin syndrome (neutral lipid storage disease): new clinical features. Br J Dermatol. 2001;144:430–432. doi: 10.1046/j.1365-2133.2001.04051.x. [DOI] [PubMed] [Google Scholar]
- 12.Igal RA, Rhoads JM, Coleman RA. Neutral lipid storage disease with fatty liver and cholestasis. J Pediatr Gastroenterol Nutr. 1997;25:541–547. doi: 10.1097/00005176-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A Mediates the Reversible Binding of CGI-58 to Lipid Droplets in 3T3-L1 Adipocytes. Journal of Biological Chemistry. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 14.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 16.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul S. Desnutrin, an Adipocyte Gene Encoding a Novel Patatin Domain-containing Protein, Is Induced by Fasting and Glucocorticoids: ECTOPIC EXPRESSION OF DESNUTRIN INCREASES TRIGLYCERIDE HYDROLYSIS. Journal of Biological Chemistry. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 19.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 20.Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, Newgard CB. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 21.Phelan MC. Basic Techniques in Mammalian Cell Tissue Culture. In: Bonifacino JS, editor. Current protocols in cell biology. John Wiley; New York, NY: 2007. pp. 1.1.5–1.1.8. [DOI] [PubMed] [Google Scholar]
- 22.Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- 23.Boren J, Rustaeus S, Olofsson SO. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. Journal of Biological Chemistry. 1994;269:25879–25888. [PubMed] [Google Scholar]
- 24.Francone OL, Kalopissis AD, Griffaton G. Contribution of cytoplasmic storage triacylglycerol to VLDL-triacylglycerol in isolated rat hepatocytes. Biochim Biophys Acta. 1989;1002:28–36. doi: 10.1016/0005-2760(89)90060-x. [DOI] [PubMed] [Google Scholar]
- 25.Coleman R, Bell RM. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. Journal of Biological Chemistry. 1976;251:4537–4543. [PubMed] [Google Scholar]
- 26.Schwartz DM, Wolins NE. A simple and rapid method to assay triacylglycerol in cells and tissues. Journal of Lipid Research. 2007;48:2514–2520. doi: 10.1194/jlr.D700017-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. Journal of Lipid Research. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Sparks JD, Bolognino M, Trax PA, Sparks CE. The production and utility of monoclonal antibodies to rat apolipoprotein B lipoproteins. Atherosclerosis. 1986;61:205–211. doi: 10.1016/0021-9150(86)90139-5. [DOI] [PubMed] [Google Scholar]
- 29.Slayback JR, Cheung LW, Geyer RP. Quantitative extraction of microgram amounts of lipid from cultured human cells. Anal Biochem. 1977;83:372–384. doi: 10.1016/0003-2697(77)90046-x. [DOI] [PubMed] [Google Scholar]
- 30.Homan R, Anderson MK. Rapid separation and quantitation of combined neutral and polar lipid classes by high-performance liquid chromatography and evaporative light-scattering mass detection. Journal of Chromatography B: Biomedical Sciences and Applications. 1998;708:21–26. doi: 10.1016/s0378-4347(97)00651-8. [DOI] [PubMed] [Google Scholar]
- 31.Bligh E, Dyer W. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 32.Kates M. Techniques of lipidology: isolation, analysis, and identification of lipids. Elsevier, Amsterdam; The Netherlands: 1986. pp. 232–254. [Google Scholar]
- 33.Lehner R, Vance DE. Cloning and expression of a cDNA encoding a hepatic microsomal lipase that mobilizes stored triacylglycerol. Biochem J. 1999;343:1–10. [PMC free article] [PubMed] [Google Scholar]
- 34.Zannis VI, Kan HY, Kritis A, Zanni EE, Kardassis D. Transcriptional regulatory mechanisms of the human apolipoprotein genes in vitro and in vivo. Curr Opin Lipidol. 2001;12:181–207. doi: 10.1097/00041433-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. Journal of Lipid Research. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Jr, Huang LS. Hepatic Overexpression of Hormone-sensitive Lipase and Adipose Triglyceride Lipase Promotes Fatty Acid Oxidation, Stimulates Direct Release of Free Fatty Acids, and Ameliorates Steatosis. Journal of Biological Chemistry. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 38.Cox RA, Garcia-Palmieri MR. Cholesterol, Triglycerides, and Associated Lipoproteins. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods. Butterworths; Boston, MA: 1980. pp. 153–160. [PubMed] [Google Scholar]
- 39.Duzovali O, Ikizoglu G, Turhan AH, Yilgor E. Dorfman-Chanarin syndrome: a case with hyperlipidemia. Turk J Pediatr. 2006;48:263–265. [PubMed] [Google Scholar]
- 40.Williams ML, Koch TK, O’Donnell JJ, Frost PH, Epstein LB, Grizzard WS, Epstein CJ. Ichthyosis and neutral lipid storage disease. Am J Med Genet. 1985;20:711–726. doi: 10.1002/ajmg.1320200417. [DOI] [PubMed] [Google Scholar]
- 41.Brown JM, Chung S, Das A, Shelness GS, Rudel LL, Yu L. CGI-58 facilitates the mobilization of cytoplasmic triglyceride for lipoprotein secretion in hepatoma cells. Journal of Lipid Research. 2007;48:2295–2305. doi: 10.1194/jlr.M700279-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.