Abstract
The sodium/iodide symporter (NIS) stimulates iodide uptake in normal lactating breast, but is not known to be active in nonlactating breast or breast cancer. We studied NIS gene regulation and iodide uptake in MCF-7 cells, an estrogen receptor (ER)-positive human breast cancer cell line. All-trans retinoic acid (tRA) treatment stimulated iodide uptake in a time- and dose-dependent fashion up to ≈9.4-fold above baseline. Stimulation with selective retinoid compounds indicated that the induction of iodide uptake was mediated by retinoic acid receptor. Treatment with tRA markedly stimulated NIS mRNA and immunoreactive protein (≈68 kDa). tRA stimulated NIS gene transcription ≈4-fold, as shown by nuclear run-on assay. No induction of iodide uptake was observed with RA treatment of an ER-negative human breast cancer cell line, MDA-MB 231, or a normal human breast cell line, MCF-12A. The iodide efflux rate of tRA-treated MCF-7 cells was slow (t1/2 = 24 min), compared with that in FRTL-5 thyroid cells (t1/2 = 3.9 min), favoring iodide retention in MCF-7 cells. An in vitro clonogenic assay demonstrated selective cytotoxicity with 131I after tRA stimulation of MCF-7 cells. tRA up-regulates NIS gene expression and iodide uptake in an ER-positive breast cancer cell line. Stimulation of radioiodide uptake after systemic retinoid treatment may be useful for diagnosis and treatment of some differentiated breast cancers.
Differentiated thyroid cancer has been effectively treated by using radioiodine (131I) therapy for over 40 years (1). In this treatment, 131I is given orally, absorbed into the blood stream, and transported by the sodium/iodide symporter (NIS) into susceptible tissues. In normal thyroid tissue, NIS is expressed on the plasma membrane at the basolateral surface of follicular cells (2, 3). NIS is responsible for the ability of the thyroid gland to concentrate iodide (4, 5) and depends on the electrochemical gradient of Na+, which is maintained by the activity of Na+-K+ ATPase (4, 6). Iodide trapped in thyroid follicular cells is organified as thyroglobulin by the action of thyroperoxidase and used to synthesize thyroid hormone. NIS gene expression in thyroid cells is regulated by thyrotropin (TSH) via the cAMP-dependent pathway (4, 7, 8). Thyroid cancer requires high levels of TSH to induce or to maximally stimulate 131I uptake (1). In some differentiated thyroid cancer, treatment with retinoic acid (RA), in addition to TSH stimulation, has been reported to induce NIS mRNA expression (9) and NIS activity (10). The results of clinical pilot studies have suggested that iodide uptake may be stimulated by RA treatment into metastases of thyroid cancer that do not take up 131I under standard treatment conditions (10).
Iodide accumulation in normal breast tissue was reported over 40 years ago (11–14). In the mouse lactating mammary gland, iodide is concentrated 6- to 15-fold in milk, relative to the plasma iodide concentration (13), less than the magnitude of iodine concentration in the thyroid. Trapped iodide is secreted into the milk, and ≈20% is organified, as a result of the action of peroxidase expressed in the alveolar cells of the breast (14–16). Recent studies have demonstrated expression of NIS in the mammary gland (17–19). NIS activity in the breast is stimulated by prolactin (PRL) (20, 21). It has been reported that some hormone-dependent breast cancers also concentrate iodide, 5- to 7-fold, compared with plasma iodide concentration (13, 22). Recently, NIS mRNA expression was shown in human breast cancer (6 of 7 specimens) (19). The iodide concentration in breast cancer specimens, however, was significantly reduced compared with that in thyroid tissue or benign breast adenomas (19).
RA plays a pivotal role in development, differentiation, and cell growth. The action of RA is mediated through two families of nuclear receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs). RARs and RXRs both are expressed in MCF-7 cells, an estrogen receptor (ER)-positive human breast cancer cell line (23). All-trans RA (tRA) inhibits cell cycle progression and induces apoptosis in many tumor cell lines (24), including MCF-7 (25–28). In clinical studies, tRA and its analogues have proved useful for treatment of a number of cancers (reviewed in ref. 24).
We hypothesized that RA could induce iodide uptake activity in breast cancer cells and that NIS regulation in cancer would differ from the hormonal regulation of NIS in normal breast tissue.
Materials and Methods
Chemicals, Cells, and Vectors.
All cell lines were obtained from the American Type Culture Collection and maintained according to the recommended conditions. tRA, 9-cis RA, and 4-[E2–5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl-1-propenyl] benzoic acid (TTNPB), sheep PRL, and synthetic oxytocin (OT) were purchased from Sigma. The human NIS expression vector was kindly provided by T. Endo and T. Onaya (Yamanashi Medical University, Yamanashi, Japan). The PDS (Pendred's syndrome gene) cDNA was kindly provided by P. Kopp (Northwestern University Medical School, Chicago). A pBluescript vector containing human β-actin cDNA was obtained from the American Type Culture Collection.
Iodide Uptake Assay.
Unless otherwise noted, cells were grown in 12-well dishes, washed with Hanks' balanced salt solution (HBSS), and incubated for 2 h at 37°C with 500 μl HBSS containing ≈0.1 μCi carrier-free Na125I (Amersham Pharmacia Biotech) and 10 μM NaI. The specific activity under these conditions was 20 mCi/mmol. After incubation, the cells were washed twice with ice-cold HBSS and scraped from each well, and radioactivity was measured in a γ-counter. The radioactivity was normalized to the cell number at the time of the assay. For kinetic studies, cells were incubated with 0.1–600 μM NaI and 20 mCi/mmol Na125I at 37°C for 5 min.
Northern Blot Analysis.
Total RNA was prepared with Trizol reagent (Life Technologies, Grand Island, NY), and Northern blot was performed as described (29). For analysis of NIS, blots were hybridized with a human NIS cRNA probe prepared by the in vitro transcription of pcDNA3 containing the human NIS cDNA (8) with SP6 RNA polymerase (Promega). For analysis of PDS gene expression, PDS cDNA was labeled by a random-primed labeling method. Blots were rehybridized with a β-actin probe as a control for RNA content.
Nuclear Run-On Assay.
Isolation of nuclei and in vitro transcription were carried out as described (30). Linearized plasmids containing the target cDNAs (22 μg) dissolved in 0.4 M NaOH were immobilized onto a Hybond-N+ nylon membrane (Amersham Pharmacia) and hybridized with the labeled RNA as described (30). Blots were exposed to x-ray film, and intensities of signals were scanned and quantitated by using the National Institutes of Health image program version 1.62 (available on the Internet at http://rsb.info.nih.gov/nih-image/).
Western Blot Analysis.
Postnuclear membrane fractions were prepared and Western blot analysis was performed as described (7) with a polyclonal anti-human NIS antibody (31) (provided by T. Onaya and T. Endo) and horseradish peroxidase-conjugated anti-rabbit IgG (Boehringer Mannheim). Quantitation analysis was performed as well as in nuclear run-on assay.
Iodide Efflux Assay.
The procedure has been described (32, 33). Briefly, cells in 12-well dishes were incubated with HBSS containing 10 μM NaI and 20 mCi/mmol Na125I at 37°C for 1 h, and the medium was replaced every 5 min with fresh HBSS without NaI. The content of 125I in the collected supernatant was measured by γ-counter. After the last time point (60 min), the cells were extracted with 400 μl ethanol to count residual radioactivity.
Measurement of Iodide Organification and Peroxidase Activity.
Cells, grown in 12-well dishes, were incubated for 2 h at 37°C with 500 μl HBSS containing ≈1.0 μCi carrier-free Na125I and 100 μM NaI. Contents of organified iodide in the cells were determined as described (34). The value was normalized to the cellular protein content measured in the same cells with Bio-Rad protein assay. The activity of peroxidase was measured as described (35).
131I-Cytotoxic Assay.
The procedure was carried out as described (33) with minor modifications. Briefly, cells grown in 75-cm2 flasks were treated with or without 1 μM tRA for 48 h. The cells then were incubated for 7 h in 5% CO2 at 37°C with 5 ml of HBSS containing 0, 10, or 100 μCi/ml Na 131I (NEN) and 0, 1, or 10 μM NaI, respectively. The reaction was terminated by removing the 131I-containing medium and washing cells twice with HBSS. The cells then were trypsinized, counted, and plated at densities of 250 and 1,000 cells/well with growth medium in 6-well plates. Uptake of 131I- was confirmed by a Geiger Mueller counter before the plating. Cells were grown for 10 days, fixed with 3:1 methanol/acetic acid, and stained with crystal violet, and the number of macroscopic colonies were counted. The survival rate was calculated as the percentage of cell colonies in plates treated with 131I- compared with those treated with only HBSS.
Statistical Analysis.
All numerical data are expressed as the mean ± SD. Statistical significance of differences was determined by conducting a paired Student's t test.
Results
tRA Induces Iodide Uptake Activity in MCF-7 Cells.
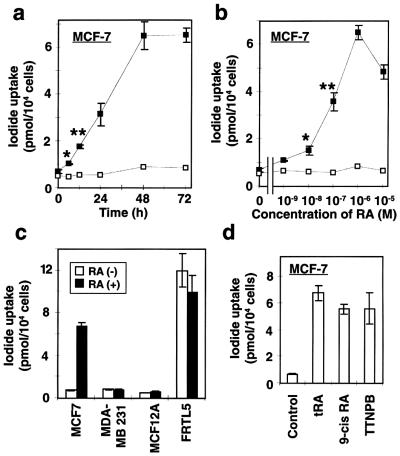
MCF-7 cells concentrated only a small amount of 125I (net amount was 0.18 pmol/104 cells) at baseline (Fig. 1a). The addition of 1 μM of tRA, however, significantly increased iodide uptake at 6 h (approximately 1.5-fold above baseline, P < 0.05) and reached the maximum (approximately 9.4-fold) at 48 h (Fig. 1a). The uptake was increased in a dose-dependent manner (Fig. 1b) and was completely blocked by 30 μM KClO4 (Fig. 1 a and b, □), a specific inhibitor of iodide transport via NIS (4, 36). tRA, however, did not stimulate 125I uptake in a less differentiated ER-negative human breast cancer cell line, MDA-MB-231, or a normal human breast tissue-derived cell line, MCF-12A (Fig. 1c).
Figure 1.
Effects of RA on iodide uptake in breast cancer cells. (a) Time course of RA-induced up-regulation of iodide uptake in MCF-7 cells. Cells were incubated with 1 μM of tRA for the indicated time and then iodide uptake assay was performed with 500 μl of HBSS containing ≈0.1 μCi Na125I and 10 μM NaI. The reactions with (□) or without (■) 30 μM KClO4 were terminated at 2 h, and the content of iodide in the cells was determined with γ-counter. (b) Dose dependency of tRA on iodide uptake in MCF-7 cells. Cells were incubated with the indicated concentration of tRA for 48 h, and iodide uptake assay was performed with (□) or without (■) 30 μM KClO4. (c) Iodide uptake activity in breast tissue-derived cell lines treated with (■) or without (□) 1 μM tRA for 48 h. MCF-7, ER-positive breast cancer cell line; MDA-MB 231, ER-negative breast cancer cell line; MCF-12A, normal breast tissue-derived cell line; FRTL-5, rat thyroid cell line. (d) Effects of several retinoid compounds on iodide uptake by MCF-7 cells: 1 μM of tRA, 9-cis RA, or TTNPB (4-[E2–5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl-1-propenyl] benzoic acid) was added to the growth medium, and iodide uptake assay was performed after 48 h. Values are expressed as means ± SD (n = 3). *, P < 0.05; **, P < 0.01, when compared with untreated cells.
FRTL-5, a rat thyroid cell line, is a well-characterized in vitro standard for iodide uptake in normal thyroid cells (36, 37). The amount of iodide concentrated in tRA-treated MCF-7 cells was ≈56% of that seen in FRTL-5 cells (Fig. 1c). The cell/medium iodide concentration ratio (C/M ratio) in tRA-treated MCF-7 cells was ≈14, whereas that in FRTL-5 cells was ≈25. tRA treatment of FRTL-5 cells did not stimulate iodide uptake (Fig. 1c).
The retinoid receptors in MCF-7 cells include RARα (32 fmol/mg nuclear protein), RARγ (35 fmol/mg), and RXRα (60 fmol/mg) (23). To evaluate whether RAR mediates the induction of iodide uptake, we assayed iodide uptake in response to several retinoid compounds. The RAR-specific ligand, TTNPB (4-[E2–5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl-1-propenyl] benzoic acid), induced iodide uptake activity in MCF-7 cells to the same level as tRA (Fig. 1d), indicating that RAR mediates the effect (38). 9-cis RA, which stimulates both RAR and RXR (38), also was not different from tRA stimulation (Fig. 1d).
RA Stimulates NIS Gene Expression.
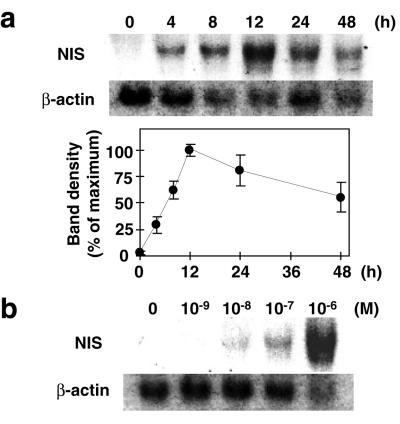
The expression of NIS mRNA in MCF-7 cells, with and without RA treatment, was analyzed by Northern blot. No NIS mRNA was detected by Northern blot analysis in MCF-7 cells before RA treatment (Fig. 2a, lane 0 h). tRA stimulated NIS mRNA with a detectable band at 4 h, reaching a maximum at 12 h (Fig. 2a). The levels of mRNA fell to ≈59% of maximum at 48 h (Fig. 2a). tRA stimulation of NIS mRNA was dose dependent with maximum stimulation at 1 μM tRA (Fig. 2b). Nuclear run-on analysis was performed to determine whether tRA increased the transcription rate of the NIS gene. The transcription rate of the NIS gene was increased a maximum of 3.65 ± 0.58-fold 12 h after tRA stimulation (Fig. 3) and fell to ≈62% of maximum at 48 h (Fig. 3). These findings are consistent with down-regulation of the transcriptional response with continuous RA stimulation.
Figure 2.
Effect of tRA on NIS mRNA level in MCF-7 cells. (a) Time course of tRA-induced up-regulation of NIS mRNA expression in MCF-7 cells. Cells were incubated with 1 μM tRA for indicated time and then Northern blot analysis was performed with 25 μg of total RNA. Radioactivity of the blots was quantitated, and the intensity of NIS mRNA was normalized to β-actin mRNA (Lower). Values are mean ± SD (n = 3). (b) Dose dependency of tRA on NIS mRNA induction in MCF-7 cells. Cells were incubated with indicated concentration of tRA for 12 h and then Northern blot analysis with 25 μg of total RNA was performed. These experiments are representative of three experiments performed with similar results.
Figure 3.
Nuclear run-on analysis after treatment of MCF-7 cells with tRA. Nuclei were prepared from cells incubated with 1 μM tRA for time periods as indicated. Transcription of the isolated nuclei was analyzed by hybridization of 32P-labeled RNA to 22 μg of human NIS and human β-actin cDNAs immobilized on nylon membranes. (a) Representative of three experiments performed with similar results. (b) Densitometric analysis of three independent run-on assays. Signals of NIS were quantified and normalized to those of β-actin. Data at 0 h are arbitrarily assigned a value of 1, and intensities of NIS are expressed as a fold increase over that basal value. Values are means ± SD (n = 3). P < 0.03, at 12 h when compared with the group at 0 h.
RA Stimulates NIS Protein Expression.
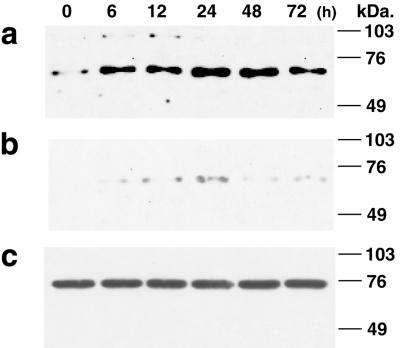
We examined the expression of NIS protein in MCF-7 cells in response to RA treatment. Human NIS in the thyroid is a glycosylated protein of 90–97 kDa (3). Western blot analysis of the postnuclear membrane fraction of MCF-7 cells with anti-human NIS antibody (31) demonstrated a weak band of ≈68 kDa before the RA treatment (Fig. 4a, lane 0 h). In RA-treated MCF-7 cells, two species of immunoreactive NIS protein, ≈90 kDa and ≈68 kDa, were detected (Fig. 4a). The major immunoreactive protein in MCF-7 cells, ≈68 kDa, was increased by tRA treatment, reaching a maximum (≈10.9-fold basal level) at 24 h (Fig. 4a). Reduced glycosylation of NIS protein in NIS-transfected Chinese hamster ovary cells recently has been reported (3). The ≈68-kDa protein detected in MCF-7 cells is the same as that recently reported to be deglycosylated NIS (3). NIS function is preserved with partial or even absent N-linked glycosylation (39). Although only a faint band of NIS protein was detected in ER-negative MDA-MB 231 cells, there was a modest induction of the ≈90-kDa and ≈68-kDa protein after the tRA treatment (Fig. 4b). In contrast, MCF-12A cells, derived from normal breast tissue, contained abundant immunoreactive protein (≈74 kDa), but protein levels were not changed by tRA treatment (Fig. 4c). MCF-12A cells expressed NIS protein to the same level seen in RA-treated MCF-7 cells (Fig. 4c), but they did not take up iodide (Fig. 1c). The discordance between high NIS protein levels and low iodide uptake also is seen in primary thyroid cells grown in monolayer culture (T.K., G.A.B., and J. M. Hershman, unpublished data). Normal breast cells, as observed with primary thyroid cells, may require induction of secondary structure and cell polarity for the NIS protein to be functional.
Figure 4.
Effect of tRA on NIS protein levels in three human breast tissue-derived cell lines. MCF-7 cells (a), MDA-MB-231 cells (b), or MCF-12A cells (c) were treated with 1 μM tRA for the indicated times, and the postnuclear membrane fraction was prepared. Protein (150 μg) was applied to each lane, and Western blot analysis was performed with anti-human NIS antibody.
Characterization of RA-Induced Iodide Uptake in MCF-7 Cells.
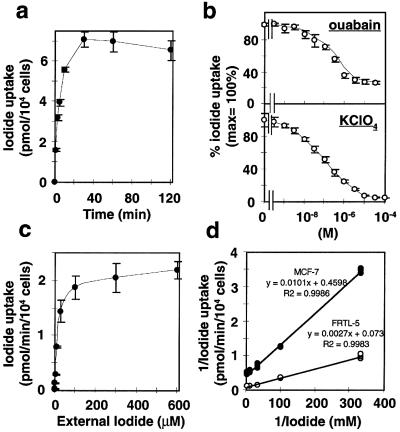
The kinetics of iodide uptake in MCF-7 cells was analyzed and compared with FRTL-5 cells. tRA stimulated MCF-7 cells took up iodide rapidly and reached a half-maximal level of activity in 10 min and saturation at ≈30 min (Fig. 5a). The iodide uptake was inhibited by ouabain, an inhibitor of Na+-K+ ATPase, in a dose-dependent manner (Fig. 5b Upper), indicating that the iodide transport is facilitated by the Na+-K+ ATPase in MCF-7 cells, as has been shown in thyroid tissue (4, 6). The suppression of iodide uptake by ouabain in MCF-7 cells was approximately 69–75% of maximal levels, and the maximum suppression was obtained at ≈10 μM (Fig. 5b). These data closely agree with ouabain suppression of iodide uptake in the FRTL-5 rat thyroid cell line (6). The persistent iodide uptake activity in MCF-7 cells (25–31%), independent of Na+-K+ ATPase, may be mediated by another Na+ transporter. tRA-induced iodide uptake also was inhibited by KClO4 in a dose-dependent manner (Fig. 5b Lower), consistent with previous findings in FRTL-5 cells (36).
Figure 5.
Characterization of iodide uptake by tRA-treated MCF-7 cells. Cells were treated with 1 μM of tRA for 48 h before assay. (a) Time course of iodide uptake in tRA-treated MCF-7 cells. Iodide uptake was initiated by incubating MCF-7 cells with 500 μl of HBSS containing ≈0.1 μCi Na125I and 10 μM NaI. The reactions were terminated at the indicated times, and the content of iodide in the cells was determined. Values are means ± SD (n = 3). (b) Inhibition of RA-induced iodide uptake by ouabain and KClO4. (Upper) Indicated concentration of ouabain was added to the growth medium containing RA 30 min before the assay. In iodide uptake assay, cells were incubated for 30 min at 37°C with 500 μl of HBSS containing ≈0.1 μCi Na125I, 10 μM NaI, and the various concentrations of ouabain. (Lower) Inhibition of RA-induced iodide uptake by KClO4. Iodide uptake assay was performed by 30-min incubation with ≈0.1 μCi Na125I, 10 μM NaI, and indicated concentration of KClO4. (c and d) Dependency of initial velocity of iodide uptake (5 min) on the extracellular iodide concentration in tRA-treated MCF-7 cells (c), and comparison of the kinetics of iodide uptake between MCF-7 cells and FRTL-5 rat thyroid cells (d). Cells were incubated at 37°C for 5 min with 500 μl of HBSS containing 20 mCi/mmol 125I- and indicated concentration of NaI. Trapped 125I- was measured by γ-counter and normalized to the cell number. Nonspecific binding of 125I- was determined in duplicate assays in the presence of 30 μM KClO4, and this value was normalized to the cell number and subtracted from the values measured (36). (c) The subtracted data (net uptake velocity) are expressed as means ± SD (n = 3). (d) The data of 3–300 μM NaI, which were chosen according to previous studies (33, 36, 41), are graphed as Lineweaver-Burk plot. All points of triplicate wells are plotted. ●, MCF-7 cells; ○, FRTL-5 cells.
The initial velocity of iodide uptake was determined by incubation for 5 min with 0.1–600 μM NaI (Fig. 5c). Excess external iodide (> 100 μM) saturated the iodide transport. Lineweaver-Burk double-reciprocal plots yielded the Km and Vmax values (Fig. 5d). The apparent Km for iodide was 21.9 ± 5.1 μM (the mean ± SD) in MCF-7 cells, which is less than that in FRTL-5 cells (37.0 ± 5.2 μM). These results are consistent with the range of values reported in FRTL-5 cells (33, 36) and NIS expressed in nonthyroidal cells (5, 33, 40, 41). The Vmax of iodide uptake in MCF-7 (≈2.17 pmol/min per 104 cells) was significantly lower than that in FRTL-5 cells (≈13.7 pmol/min per 104 cells), indicating reduced expression of NIS in MCF-7 cells compared with thyroid-derived cells.
Iodide Efflux Is Relatively Low in MCF-7 Cells Compared with FRTL-5 Cells.
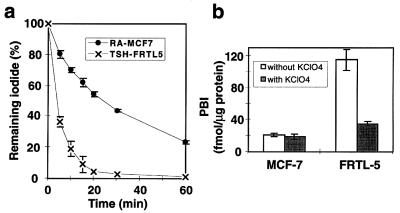
Although iodide uptake is essential for maximal effectiveness of 131I therapy, the retention of iodide, as reflected by iodide efflux, is also a critical variable. Iodide efflux was determined in tRA-treated MCF-7 cells and FRTL-5 rat thyroid cells (Fig. 6a). In FRTL-5 cells, more than 60% of the cellular radioactivity was released into the medium during the initial 5 min (t1/2 = 3.9 min), and no activity was detected at 60 min. These data are in agreement with previous data with FRTL-5 cells (32, 33, 42). In contrast, the MCF-7 cells exhibited a slow iodide release with only 20% released during the initial 5 min and 24% remaining at 60 min (t1/2 = 24 min). The same efflux rate was obtained with various concentration of trapped iodide in the cells (0.3–6.0 nmol/105 cells; data not shown). To investigate whether the slow efflux in MCF-7 cells was the result of iodide organification, trichloroacetic acid precipitation of cell lysate was performed after the incubation of the cells with 1 μM Na125I. As shown in Fig. 6b, no iodide organification was observed in MCF-7 cells. Only a small amount of iodide was organified in FRTL-5 cells (Fig. 6b), although the efflux rate was relatively high. The rate of iodide organification in FRTL-5 cells was consistent with previous reports (34). It has been reported that peroxidase activity is correlated with the ability to iodinate, and casein-like proteins in milk were the major sites where iodination occurred within the rat mammary gland (14). Because the lack of iodide organification in the MCF-7 cells might be caused by insufficient substrate, we measured peroxidase activity with guaiacol as a substrate (35), but did not detect peroxidase activity (data not shown). Based on recent reports of pendrin promoting iodide efflux in the thyroid (43), we probed a Northern blot from MCF-7 cells with and without tRA stimulation and did not detect PDS mRNA, which encodes pendrin, although it was detectable in thyroid (data not shown). Reduced efflux in MCF-7 cells compared with FRTL-5 may be the result of the absence of pendrin in MCF-7 cells.
Figure 6.
Iodide efflux is reduced in MCF-7 cells compared with FRTL-5 rat thyroid cells. (a) Iodide efflux from tRA-treated MCF-7 cells compared with that from FRTL-5 rat thyroid cells. MCF-7 cells were treated with 1 μM tRA for 48 h before the assay. FRTL-5 cells were maintained with 1 milliunit/ml TSH. ●, MCF-7 cells; x, FRTL-5 cells. (b) Iodide organification in tRA-treated MCF-7 cells and FRTL-5 cells. Cells were incubated for 2 h at 37° C with 500 μM HBSS containing ≈1.0 μM Na125I and 100 μM NaI. Nonspecific binding of 125I- was determined in duplicate assays in the presence of 30 μM KClO4. The radioactivity of trichloroacetic acid-precipitate was normalized to the cellular protein content measured in the same cells. Data are means ± SD (n = 3).
Cytotoxic Clonogenic Assay in 131I-Treated MCF-7 Cells.
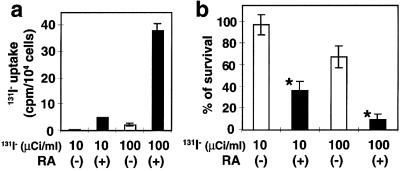
We used a previously established assay (33) to investigate whether 131I had selective cytotoxic activity in RA-stimulated MCF-7 cells compared with cells without tRA stimulation. MCF-7 cells were incubated with 1 μM tRA for 48 h, exposed to 10 or 100 μCi Na 131I for 7 h, and colony formation was assessed after 10 days. MCF-7 cells took up 131I in response to tRA treatment (Fig. 7a). As shown in Fig. 7b, the survival rate, based on the clonogenic assay, was markedly reduced in response to 131I (P < 0.0001). Treatment of tRA-stimulated cells with 131I resulted in a dose-dependent reduction in colony formation with 36.6 ± 7.9% (the mean ± SD) remaining after 10 mCi 131I and 9.65 ± 4.6% after 100 mCi 131I. In contrast, survival of control cells (without tRA treatment) was 97.5 ± 9.4% after 10 mCi 131I and 68.0 ± 9.9% after 100 mCi 131I. Treatment with only tRA did not affect the survival rate as reflected in colony formation (data not shown). These results demonstrate that cytotoxicity from 131I treatment of tRA-stimulated MCF-7 cells is similar in magnitude to that reported in tumor cell lines transfected with a NIS-expressing vector (33).
Figure 7.
131I treatment significantly reduced the survival rate of RA-treated MCF-7 cells. Cells were treated with or without 1 μM tRA for 48 h, and then incubated with HBSS containing 0, 10, or 100 μCi/ml Na 131I and 0, 1, or 10 μM NaI, respectively for 7 h. Cells were harvested and applied to clonogenic assay as described (33). (a) Uptake of 131I- by MCF-7 cells. After the incubation with 131I, β-ray activity of the trapped 131I was counted by a Geiger Mueller counter. The count was normalized to the cell number. (b) The survival rate of MCF-7 cells after the 131I treatment. The rate was calculated as a percentage of cell colonies treated with 131I- compared with those treated with only HBSS. Solid columns, RA-treated MCF-7 cells; open columns, unstimulated MCF-7 cells. Values are means ± SD (n = 6). *, P < 0.0001, when compared with the group of unstimulated MCF-7 cells.
Forskolin, OT, or PRL Does Not Induce Iodide Uptake in MCF-7 Cells.
It has been reported that the iodide uptake in thyroid cells is stimulated by TSH via the cAMP/adenylate cyclase pathway (4, 7, 8, 37). Treatment of MCF-7 cells with 0.1–100 μM forskolin, an agonist of adenylate cyclase, however, did not increase the iodide uptake (data not shown).
The stimulatory effect of PRL on iodide uptake in mouse has been reported (20, 21). OT may increase iodide uptake in breast tissue because iodide uptake is stimulated in lactating mammary gland (13). OT (10–1,000 nM) and PRL (10–10,000 ng/ml), however, did not significantly stimulate the iodide uptake in MCF-7 cells (data not shown), and these hormones did not change the iodide uptake induced by tRA (data not shown). The effect of PRL on iodide uptake in breast requires synthesis of mRNA and protein, likely NIS (21). The signaling pathway for PRL or OT to stimulate NIS gene expression may be impaired in breast cancer compared with lactating breast cells.
Effect of RA on Iodide Uptake Is Cell Selective.
NIS mRNA expression has been reported in a number of human tissues, in addition to thyroid and mammary gland, including prostate, placenta, and lung (17, 18). We, therefore, tested tRA-stimulated iodide uptake in four human cancer cell lines: an androgen receptor-positive prostate cancer cell line (LNCaP), a choriocarcinoma cell line (JEG-3), and two nonsmall cell lung cancer cell lines (A549 and H460). No induction of iodide uptake, however, was found in these cell lines in response to tRA treatment (data not shown).
Discussion
We have shown that tRA rapidly induces iodide uptake activity (in 6 h) after the up-regulation of NIS gene expression mediated by RAR. The up-regulation of NIS by TSH in thyroid cells has been well characterized (4, 7, 37) and contrasts with tRA stimulation in MCF-7 cells. NIS mRNA levels are rapidly increased in TSH-stimulated FRTL-5 rat thyroid cells; however, there is a 12-h latency before the onset of increased iodide uptake and maximum uptake is observed at 72 h (7, 37). In contrast, in MCF-7 cells, tRA increased NIS mRNA, NIS protein, and iodide uptake levels by 6 h, reaching a maximum at 12, 24, and 48 h, respectively, and no latency period of iodide uptake was observed. One possible explanation for the difference in time course between TSH-treated FRTL-5 cells and tRA-treated MCF-7 cells is the requirement for de novo protein synthesis for TSH-stimulated NIS gene expression. In FRTL-5 cells, the induction of NIS mRNA by TSH requires de novo protein synthesis (7). RA directly stimulates NIS mRNA expression via endogenous RARs in MCF-7 cells without a requirement for new protein synthesis.
NIS gene expression in thyroid cells is regulated by transcription factors, such as TTF-1 (thyroid transcription factor-1) (44), Pax-8 (Paired domain transcription factor) (45), and NTF-1 (NIS TSH-responsive factor-1) (46), whose expression is enriched in the thyroid. These are important for up-regulation of NIS gene expression by TSH, and the accumulation of cAMP after TSH stimulation is required to activate these factors (45, 46). In contrast, cAMP agonists decrease iodide uptake in mouse mammary tissue culture (21). We have confirmed that forskolin, an adenylate cyclase agonist, does not induce iodide uptake activity in MCF-7 cells. In contrast to tRA stimulation of iodide uptake in ER-positive breast cancer cells, tRA decreases iodide uptake in FRTL-5 cells (ref. 9 and our data). These findings also indicate that NIS gene regulation differs in the thyroid and breast.
We have demonstrated tRA induction of iodide uptake in ER-positive MCF-7 cells, but not in ER-negative MDA-MB 231 cells. The MDA-MB 231 cell line is known as an RA “resistant” breast cancer cell line and the expression of RARα and RARβ is reduced compared with MCF-7 (47). Because the induction of NIS in MCF-7 cells by tRA is mediated by RARs, the reduced expression of NIS in MDA-MB 231 is likely caused by the reduced RAR content. Studies in MCF-7 cells show that estradiol increases RAR expression and down-regulates RXR (23), a profile that would be favorable for a NIS response. Recent studies show that in FRTL-5 rat thyroid cells, estradiol down-regulates NIS gene expression (48). Additionally, it has been reported that RA inhibits activation of ER in MCF-7 cells (49, 50).
Efflux of iodide from MCF-7 cells was prolonged (t1/2 = 24 min) compared with that in FRTL-5 cells (t1/2 = 3.9 min). The rapid efflux rate in FRTL-5 cells has been reported (32, 42), whereas a low efflux rate was seen when a vector expressing the rat NIS cDNA was transfected into Chinese hamster ovary cells (41, 42). In patients with total iodination defects in the thyroid, trapped iodide is completely released within 1 h after administration of ClO4- (51). Iodide organification is, therefore, one of the important factors for the thyroid gland to retain trapped iodide. In lactating mammary gland, and some hormone-dependent mammary tumors, iodination of protein was observed (13, 14, 16). Our results of iodide organification, however, indicated no iodination activity in MCF-7 cells, whereas a small amount of iodide was organified in FRTL-5 cells. Very recently, the cDNA of another iodide transporter, pendrin, has been cloned as a chloride/iodide transporter (52), which may function in thyroid gland to transport iodide into the follicular lumen before its organification (43). PDS mRNA, which encodes pendrin, is found exclusively in the thyroid, including FRTL-5 cells (43). In MCF-7 cells, no PDS mRNA was detected by Northern blot before or after treatment with tRA. Therefore, it is possible that a thyroid-specific iodide trap and release system involving pendrin may be responsible for the high iodide efflux rate in FRTL-5 thyroid cells. Nonthyroidal cells, such as MCF-7 cells and Chinese hamster ovary cells, have a slower efflux rate, likely because of the absence of pendrin.
Although no iodide organification was found in MCF-7 cells, our cytotoxicity study with 131I demonstrated a significant effect of 131I treatment after stimulation with RA in MCF-7 cells. The results are significant, because monolayer cells were used in our study, in which β-energy emitted from trapped 131I is deposited outside of the monolayer cells, but washed away in our assay (33). Our assay, therefore, likely underestimates cytotoxicity caused by 131I. Most differentiated thyroid cancers have varying degrees of impaired iodide organification (53–55), but 131I therapy is still effective in most thyroid cancer, even when organification is diminished or absent.
In conclusion, we report here the stimulatory effect of tRA on iodide uptake in MCF-7 cells, after up-regulation of the NIS gene mediated by RAR. This effect is selective for ER-positive breast cancer. Iodide efflux rate in tRA-treated MCF-7 cells was prolonged compared with the thyroid, and 131I treatment after tRA stimulation was selectively toxic for breast cancer cells. Stimulation of radioiodine uptake after systemic retinoid treatment may be useful for diagnosis and treatment of some differentiated breast cancer.
Acknowledgments
We are grateful to Drs. J. M. Hershman, M. Sugawara, T. Onaya, S. Dubinett, and A. Lichtenstein for helpful discussions and guidance. This work was supported by Veterans Affairs Medical Research Funds, the Veterans Affairs Research Enhancement Award Program in Cancer Gene Medicine, Thyroid Center of Excellence Grant from Knoll Pharmaceutical Company, and Thomas B. Rosenberg.
Abbreviations
- NIS
sodium/iodide symporter
- RA
retinoic acid
- tRA
all-trans retinoic acid
- PRL
prolactin
- OT
oxytocin
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- ER
estrogen receptor
- TSH
thyrotropin
- PDS
Pendred's syndrome gene
- HBSS
Hanks' balanced salt solution
- 131I
radioiodine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140217197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140217197
References
- 1.Mazzaferri E L. In: The Thyroid. Braverman L E, Utiger R D, editors. Philadelphia: Lippincott–Raven; 1996. pp. 922–945. [Google Scholar]
- 2.Caillou B, Troalen F, Baudin E, Talbot M, Filetti S, Schlumberger M, Bidart J M. J Clin Endocrinol Metab. 1998;83:4102–4106. doi: 10.1210/jcem.83.11.5262. [DOI] [PubMed] [Google Scholar]
- 3.Castro M R, Bergert E R, Beito T G, Roche P C, Ziesmer S C, Jhiang S M, Goellner J R, Morris J C. J Endocrinol. 1999;163:495–504. doi: 10.1677/joe.0.1630495. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco N. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 5.Dai G, Levy O, Carrasco N. Nature (London) 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 6.Eggo M C, Bachrach L K, Mak W, Burrow G N. Horm Metab Res. 1986;18:167–172. doi: 10.1055/s-2007-1012262. [DOI] [PubMed] [Google Scholar]
- 7.Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Endocrinology. 1997;138:2227–2232. doi: 10.1210/endo.138.6.5189. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Endo T, Kawaguchi A, Ikeda M, Nakazato M, Kogai T, Onaya T. J Clin Endocrinol Metab. 1997;82:3331–3336. doi: 10.1210/jcem.82.10.4269. [DOI] [PubMed] [Google Scholar]
- 9.Schmutzler C, Winzer R, Meissner-Weigl J, Kohrle J. Biochem Biophys Res Commun. 1997;240:832–838. doi: 10.1006/bbrc.1997.7715. [DOI] [PubMed] [Google Scholar]
- 10.Simon D, Koehrle J, Reiners C, Boerner A R, Schmutzler C, Mainz K, Goretzki P E, Roeher H D. World J Surg. 1998;22:569–574. doi: 10.1007/s002689900436. [DOI] [PubMed] [Google Scholar]
- 11.Brown-Grant K. J Physiol (London) 1957;135:644–654. doi: 10.1113/jphysiol.1957.sp005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskin B A, Parker J A, Bassett J G, George D L. Obstet Gynecol. 1974;44:398–402. [PubMed] [Google Scholar]
- 13.Thorpe S M. Int J Cancer. 1976;18:345–350. doi: 10.1002/ijc.2910180312. [DOI] [PubMed] [Google Scholar]
- 14.Strum J M. Anat Rec. 1978;192:235–244. doi: 10.1002/ar.1091920204. [DOI] [PubMed] [Google Scholar]
- 15.Etling N, Gehin-Fouque F. Pediatr Res. 1984;18:901–903. doi: 10.1203/00006450-198409000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Shah N M, Eskin B A, Krouse T B, Sparks C E. Proc Soc Exp Biol Med. 1986;181:443–449. doi: 10.3181/00379727-181-42279. [DOI] [PubMed] [Google Scholar]
- 17.Ajjan R A, Kamaruddin N A, Crisp M, Watson P F, Ludgate M, Weetman A P. Clin Endocrinol (Oxford) 1998;49:517–523. doi: 10.1046/j.1365-2265.1998.00570.x. [DOI] [PubMed] [Google Scholar]
- 18.Spitzweg C, Joba W, Eisenmenger W, Heufelder A E. J Clin Endocrinol Metab. 1998;83:1746–1751. doi: 10.1210/jcem.83.5.4839. [DOI] [PubMed] [Google Scholar]
- 19.Kilbane M T, Ajjan R A, Weetman A P, Dwyer R, McDermott E W, O'Higgins N J, Smyth P P. J Clin Endocrinol Metab. 2000;85:1245–1250. doi: 10.1210/jcem.85.3.6442. [DOI] [PubMed] [Google Scholar]
- 20.Rillema J A, Yu T X. Am J Physiol. 1996;271:E879–E882. doi: 10.1152/ajpendo.1996.271.5.E879. [DOI] [PubMed] [Google Scholar]
- 21.Rillema J A, Rowady D L. Proc Soc Exp Biol Med. 1997;215:366–369. doi: 10.3181/00379727-215-44145. [DOI] [PubMed] [Google Scholar]
- 22.Briand P. Anticancer Res. 1983;3:273–281. [PubMed] [Google Scholar]
- 23.Titcomb M W, Gottardis M M, Pike J W, Allegretto E A. Mol Endocrinol. 1994;8:870–877. doi: 10.1210/mend.8.7.7984149. [DOI] [PubMed] [Google Scholar]
- 24.Evans T R J, Kaye S B. Br J Cancer. 1998;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira C, Pratt M A. Mol Endocrinol. 1997;11:1191–1202. doi: 10.1210/mend.11.9.9977. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Stetler-Stevenson M, Steeg P S. Oncogene. 1997;15:107–115. doi: 10.1038/sj.onc.1201142. [DOI] [PubMed] [Google Scholar]
- 27.Toma S, Isnardi L, Raffo P, Dastoli G, De Francisci E, Riccardi L, Palumbo R, Bollag W. Int J Cancer. 1997;70:619–627. doi: 10.1002/(sici)1097-0215(19970304)70:5<619::aid-ijc21>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Mangiarotti R, Danova M, Alberici R, Pellicciari C. Br J Cancer. 1998;77:186–191. doi: 10.1038/bjc.1998.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown T. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. New York: Wiley; 1994. pp. 4.9.1–4.9.14. [Google Scholar]
- 30.Greenberg M E, Bender T P. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. New York: Wiley; 1994. pp. 4.10.1–4.10.11. [Google Scholar]
- 31.Saito T, Endo T, Kawaguchi A, Ikeda M, Katoh R, Kawaoi A, Muramatsu A, Onaya T. J Clin Invest. 1998;101:1296–1300. doi: 10.1172/JCI1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss S J, Philp N J, Grollman E F. Endocrinology. 1984;114:1108–1113. doi: 10.1210/endo-114-4-1108. [DOI] [PubMed] [Google Scholar]
- 33.Mandell R B, Mandell L Z, Link C J., Jr Cancer Res. 1999;59:661–668. [PubMed] [Google Scholar]
- 34.Urabe M, Hershman J M, Pang X P, Murakami S, Sugawara M. Endocrinology. 1991;129:807–814. doi: 10.1210/endo-129-2-807. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara M, Sugawara Y, Wen K. Thyroid. 1999;9:513–518. doi: 10.1089/thy.1999.9.513. [DOI] [PubMed] [Google Scholar]
- 36.Weiss S J, Philp N J, Grollman E F. Endocrinology. 1984;114:1090–1098. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- 37.Weiss S J, Philp N J, Ambesi-Impiombato F S, Grollman E F. Endocrinology. 1984;114:1099–1107. doi: 10.1210/endo-114-4-1099. [DOI] [PubMed] [Google Scholar]
- 38.Allegretto E A, McClurg M R, Lazarchlk S B, Clemm D L, Kerner S A, Elgort M G, Boehm M F, White S K, Pike J W, Heyman R A. J Biol Chem. 1994;268:26625–26633. [PubMed] [Google Scholar]
- 39.Levy O, De la Vieja A, Ginter C S, Riedel C, Dai G, Carrasco N. J Biol Chem. 1998;273:22657–22663. doi: 10.1074/jbc.273.35.22657. [DOI] [PubMed] [Google Scholar]
- 40.Eskandari S, Loo D D, Dai G, Levy O, Wright E M, Carrasco N. J Biol Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 41.Kosugi S, Sasaki N, Hai N, Sugawa H, Aoki N, Shigemasa C, Mori T, Yoshida A. Biochem Biophys Res Commun. 1996;227:94–101. doi: 10.1006/bbrc.1996.1473. [DOI] [PubMed] [Google Scholar]
- 42.Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Endocrinology. 1997;138:4493–4496. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- 43.Royaux I E, Suzuki K, Mori A, Katoh R, Everett L A, Kohn L D, Green E D. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 44.Endo T, Kaneshige M, Nakazato M, Ohmori M, Harii N, Onaya T. Mol Endocrinol. 1997;11:1747–1755. doi: 10.1210/mend.11.11.0012. [DOI] [PubMed] [Google Scholar]
- 45.Ohno M, Zannini M, Levy O, Carrasco N, di Lauro R. Mol Cell Biol. 1999;19:2051–2060. doi: 10.1128/mcb.19.3.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohmori M, Endo T, Harii N, Onaya T. Mol Endocrinol. 1998;12:727–736. doi: 10.1210/mend.12.5.0101. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Lee M-O, Wang H-G, Li Y, Hashimoto Y, Klaus M, Reed J C, Zhang Z-K. Mol Cell Biol. 1996;16:1138–1149. doi: 10.1128/mcb.16.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furlanetto T W, Nguyen L Q, Jameson J L. Endocrinology. 1999;140:5705–5711. doi: 10.1210/endo.140.12.7197. [DOI] [PubMed] [Google Scholar]
- 49.Demirpence E, Balaguer P, Trousse F, Nicolas J C, Pons M, Gagne D. Cancer Res. 1994;54:1458–1464. [PubMed] [Google Scholar]
- 50.Pratt M A C, Deonarine D, Teixeira C, Novosad D, Tate B F, Grippo J F. J Biol Chem. 1996;271:20346–20352. doi: 10.1074/jbc.271.34.20346. [DOI] [PubMed] [Google Scholar]
- 51.de Vijlder J J, Vulsma T. In: The Thyroid. Braverman L E, Utiger R D, editors. Philadelphia: Lippincott–Raven; 1996. pp. 749–755. [Google Scholar]
- 52.Scott D A, Wang R, Kreman T M, Sheffield V C, Karnishki L P. Nat Genet. 1999;21:440–443. doi: 10.1038/7783. [DOI] [PubMed] [Google Scholar]
- 53.Field J B, Larsen P R, Yamashita K, Mashiter K, Dekker A. J Clin Invest. 1973;52:2404–2410. doi: 10.1172/JCI107430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas-Morvan C, Nataf B, Tubiana M. Ann Radiol (Paris) 1977;20:739–742. [PubMed] [Google Scholar]
- 55.Paul S J, Sisson J C. Endocrinol Metab Clin North Am. 1990;19:593–612. [PubMed] [Google Scholar]