Abstract
Objective
To evaluate the effect of patients’ perceptions of providers’ communication on medication adherence in hypertensive African Americans.
Methods
Cross-sectional study of 439 patients with poorly-controlled hypertension followed in community-based healthcare practices in the New York metropolitan area. Patients’ rating of their providers’ communication was assessed with a perceived communication style questionnaire,while medication adherence was assessed with the Morisky self-report measure.
Results
Majority of participants were female, low-income, and had high school level educations, with mean age of 58 years. Fifty-five percent reported being nonadherent with their medications; and 51% rated their provider’s communication to be non-collaborative. In multivariate analysis adjusted for patient demographics and covariates (depressive symptoms, provider degree), communication rated as collaborative was associated with better medication adherence (β = -.11, p = .03). Other significant correlates of medication adherence independent of perceived communication were age (β = .13, p = .02) and depressive symptoms (β = -.18, p = .001).
Conclusion
Provider communication rated as more collaborative was associated with better adherence to antihypertensive medications in a sample of low-income hypertensive African-American patients.
Practice Implications
The quality of patient-provider communication is a potentially modifiable element of the medical relationship that may affect health outcomes in this high-risk patient population.
Keywords: Patient-provider communication, medication adherence, African American, Hypertension
I. Introduction
African Americans have the highest prevalence of hypertension, making it a major contributor to cardiovascular morbidity and mortality in this population [1]. Poor adherence to prescribed antihypertensive medications has been implicated as a major barrier to poor blood pressure control in African Americans [2]. However, potentially modifiable barriers to medication adherence in this patient population, such as patient-provider communication, have not been well studied.
According to the Institute of Medicine report, Unequal Treatment, the perceived quality of interpersonal communication within the patient-physician relationship is a potential mechanism for the worse health outcomes noted in minority populations [3]. Patients’ perception of their physicians’ communication, including the ability to listen and show respect during the medical encounter, are important determinants of patient satisfaction and health care utilization [4-7]. Further, patients who engage in shared decision-making with providers are more likely to be informed about their condition, more likely to be satisfied with the interpersonal and technical aspects of their care, and more likely to adhere to recommended treatment [8-10]. Minority patients, however, are least likely to engage in a participatory relationship with their physicians [11]. Similarly, physicians tend to deliver less information and supportive talk to minority patients, as well as those of lower socioeconomic status [12, 13].
Despite the mounting evidence that minority patients receive a lower quality of interpersonal care and, thus, are less satisfied with the relationship they have with their providers [6, 14], few studies have assessed the effect of communication on intermediate clinical outcomes, such as medication adherence, in this patient population [15, 16]. Further, of the studies conducted to date, none have examined the mechanisms through which such effects occur, specifically in a community-based sample of largely low-income, hypertensive African Americans, who experience the highest burden of hypertension-related outcomes of any other racial group. Thus, the objective of this study was to evaluate the effect of patients’ perceptions of their providers’ communication on medication adherence, among hypertensive African Americans followed in community-based primary care practices.
2. Methods
2.1. Participants
This study was conducted as part of an ongoing group randomized controlled trial, Counseling African Americans to Control Hypertension (CAATCH), in Community/Migrant Health Centers (C/MHCs). The purpose of CAATCH is to evaluate the effectiveness of a multilevel intervention in improving blood pressure (BP) control among hypertensive African Americans. The present cross-sectional study was designed to assess patients’ perception of their providers’ communication on medication adherence in a cohort of patients currently enrolled in CAATCH. All questionnaires for this cross-sectional study were administered at the baseline visit prior to the inception of the intervention, thus eliminating any influence it may have had on the present study’s primary measures. To be eligible for CAATCH, patients had to be: a) self-identified as African American/Black and receiving care in the participating C/MHC sites; b) carry a diagnosis of hypertension (ICD: 401-401.9) on at least two previous clinic visits in the previous year; c) have uncontrolled blood pressure (BP); d) taking at least one anti-hypertensive medication; e) 18 years of age or older; and f) fluent in English. Additionally, patients were excluded if they: a) were unable to give informed consent, or b) refused to participate. Eligible providers were enrolled if they were: a) an attending healthcare provider in the participating C/MHC sites, and b) providing care to at least five patients with uncontrolled hypertension. In this study, uncontrolled HTN was defined as an average SBP ≥ 140 mm Hg or DBP ≥ 90 mmHg (for those without comorbidity); or average SBP ≥ 130 mm Hg or DBP ≥ 80 mm hg (for those with diabetes or kidney disease) [17]. Following the consent procedures, the RA completed an eligibility checklist to confirm that the patient met all inclusion and exclusion criteria. To be eligible for this study, patients must have had uncontrolled HTN, which was determined by two methods: a) having uncontrolled BP readings at two previous clinic visits to their provider as indicated by their medical charts, and b) an overall averaged uncontrolled BP reading at the consent visit (based on the mean of the final 2 of the 3 measurements). The BP readings at the consent visit were taken by trained Research Assistants (RA) using a validated automated blood pressure monitor BPTru device (Snoqualmie, WA, USA) [18]. This provides a reliable means of ensuring that the participants have uncontrolled HTN upon enrollment into the study. Upon enrollment, all participants provided written informed consent approved by the Institutional Review Board of Columbia University Medical Center.
2.2. Measures
2.2.1 Demographic data
At the provider-level, demographic data collected include gender, age, duration of practice at the C/MHC site, medical degree and specialty. At the patient-level, data on gender, age, marital status, employment status, education and income level (SES), insurance status, medical comorbidity, mean systolic blood pressure (SBP),diastolic blood pressure (DBP), and number of antihypertensive medications were collected. Research assistants (RAs) measured patients’ baseline SBP and DBP using the BPTru device and following standard American Heart Association guidelines [18, 19]. All RAs were trained to use the BpTRU monitors by the principal investigator (PI) of the parent trial (GO), who is a hypertension specialist and internist. The trainings included: review of steps for proper machine use; responding to error messages; and role-plays with use of BpTRU. To promote quality control, RAs attended quarterly booster trainings by the PI to review protocol for use of the BpTRU.
2.2.2 Medication adherence
Self-reported adherence was assessed with the well-validated scale developed by Morisky et al. [20] This questionnaire asks patients to respond “yes” or “no” to the following questions: a) “Have you ever forgotten to take your blood pressure medicine?” b) “Are you sometimes careless in regards to your medicine?” c) “Do you skip your medicine when you are feeling well?” and d) “When you feel badly due to the medicine, do you skip it [19]?” The scale was scored as a continuous measure and coded such that patients received a score of “1” for each negative response. Higher scores indicated better adherence (range: 0 – 4). This scale was found to be reliable in a study of inner-city hypertensive patients [21]. In our sample, the Cronbach’s alpha was .67, which is consistent with reliability estimates reported by Morisky et al. [20]
2.2.3. Provider communication
Patients’ rating of their providers’ communication was assessed with a measure derived from a study assessing the effect of physicians’ initial and follow-up communication styles on the beliefs and behaviors of patients with depression [22]. This measure was chosen to assess perceived physician communication because it was one of the few theoretically-based scales available at the start of the parent trial that directly tested the effects of physician communication on medication-taking behavior. Using concepts from the Health Communication Model, this scale assesses patients’ perception of the quality of their physicians’ communication and the extent to which the physician encourages patient participation in the treatment process. Note that the 13-item follow-up communication scale was administered in the present study because it assessed the extent to which physicians’ monitored patients’ medication use, an essential component of a collaborative patient-physician relationship.
Responses to the first eleven questions are based on a Likert-type scale. Sample questions include “To what degree was your doctor: 1 = friendly during the visit? and To what extent did your doctor: 2 = ask if you had questions and concerns?” The remaining two questions require categorical (yes/no) responses and ask whether written information about the medication was given to patients and if a follow-up appointment was scheduled. The responses to questions 1 to 11 were scored as 1 = not at all to 4 = very much. The last two items were scored as 0 = no or 1 = yes. Given that different metrics (e.g., categorical) were used for the final two items, each response on the 13-item scale was converted into a z-score and then summed as a continuous measure to create a composite score. Bultman and Svarstad [22] reported a Cronbach’s alpha of.73 for this scale; while its reliability in our sample was a much-improved .92. Because of the negatively skewed distribution of the range of responses, several statistical techniques were employed to standardize the measure and preserve the nature of the distribution for parametric analysis. Of the non-parametric tests performed, reverse scoring and transforming the data into a natural log scale fit the data best, and thus was used for all further analyses. As a result, the lower scores we report are indicative of a more collaborative communication.
2.3. Covariates
2.3.1. Health literacy
A health literacy assessment was included as a covariate in the data analysis because of the negative effect of poor health-literacy skills on medication adherence [23]. Health literacy was assessed with the Rapid Estimate of Adult Literacy in Medicine (REALM) [24]. It consists of 66 common medical terms, and patients are asked to pronounce each word. Correct pronunciations are given a score of 1 whereas mispronunciations and non-attempts are scored as 0. The codes are summed to form a continuous score for each patient, with higher scores indicating better health literacy scores [24]. The Cronbach’s alpha for the REALM was .98 in our sample.
2.3.2. Depressive symptoms
A measure of depressive symptoms was included as a covariate in this study because of the negative effect of depression on medication adherence [25]. Depressive symptoms were assessed using the Patient Health Questionnaire-9 (PHQ-9) [26]. Responses for the first eight questions range from 0 = not at all, to 4 = nearly everyday, while the final question’s responses range from 0 = not at all difficult, to 3 = extremely difficult. The scale was scored as a continuous measure, ranging from 0 to 35. Total scores ≤ 4 suggest mild depressive disorder while scores from 5 to 14 suggest moderate depressive disorder, and scores > 15 imply major depressive disorder [26]. The PHQ-9 Cronbach’s alpha was .87 in this study sample.
2.3.3. Medical comorbidity
The number of comorbid conditions was recorded using the Charlson Comorbidity Index, which is a weighted index designed to evaluate the longitudinal risk of mortality attributable to comorbid disease [27].
2.4. Statistical Analyses
Patient characteristics were described with frequency distributions. The number of screened, eligible, and ineligible patients was documented. Reasons for ineligibility were also documented. Descriptive statistics were used to describe all variables measured in the study. Measures of central tendency and dispersion were used to identify outliers and skewness of the data. Bivariate analyses were used to examine the relationship between the selected covariates (depressive symptoms, health literacy) and the dependent variable (medication adherence). Covariates were included in the multivariate analyses if they were significantly associated with medication adherence.
In anticipation of nesting in the data, a three-level analytic model was created (patients nested within providers, who are nested within sites) and intra-cluster correlations (ICC) were computed for the perceived communication and medication adherence variables. The degree of dependency in perceived communication and medication adherence was quite small (ICC = .009 and .01, respectively) and did not reach significance, indicating that medication adherence and perceived communication can be viewed as independent variables across providers and sites. Therefore, hierarchical linear regression was used in all further analyses. Patient demographics and variables found significant in the bivariate analyses were included in the multivariate analysis to determine whether their inclusion explained any of the variance between patients’ ratings of their providers’ communication and medication adherence. For each of the variables, standardized regression coefficients (β) were calculated to assess their unique contribution to the given model. All analyses were computed using SPSS version 14 and significance levels were set at p ≤ .05.
3. Results
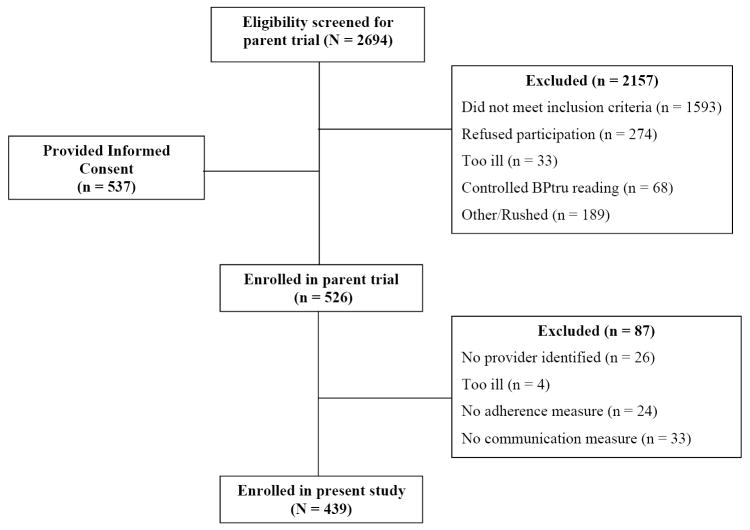
A total of 72 providers who enrolled in the parent trial were included in this cross-sectional study. A majority of the participating providers were female, had a mean age of 45 years, were internists, and on average had practiced 7 years at their respective C/MHC (Table 1). The patient flow in the study is shown in Figure 1. As shown, 2,694 patients were screened, of whom 1,593 (59%) did not meet the inclusion criteria. Of the eligible 1,101 patients, 564 (51%) were excluded for various reasons (declined to participate, did not the have time to participate, were too ill, or blood pressure was controlled at the screening visit). Of the final 526 eligible patients, 87 (17%) patients were excluded from this cross-sectional study because their providers were not participating in the parent trial, were too ill, or had incomplete data. The excluded patients did not differ significantly from patients in the final sample in terms of age, gender, SES, insurance status, baseline SBP and DBP, level of medication adherence, or medical comorbidity. Thus, data for this study were obtained and analyzed for a final sample of 439 patients.
Table 1.
Provider Characteristics
| Characteristics (N = 72) | |
|---|---|
| Mean Age (± SD) | 44.67 (10.9) |
| Range: 26 – 67 years | |
| Female: n (%) | 41 (57) |
| Race: n (%) | |
| Caucasian | 15 (21) |
| African American | 27 (38) |
| Hispanic | 6 (8) |
| Asian | 10 (14) |
| East Indian | 14 (19) |
| Degree: n (%) | |
| MD | 60 (83) |
| NP | 5 (7) |
| PA | 7 (10) |
| Specialty: n (%) | |
| Internal Medicine | 54 (75) |
| Family Medicine | 18 (25) |
| Mean years providing care at C/MHC | 6.94 (7.03) |
| Range: 3 months – 27 years |
Figure 1.
Patient flow through the study
Patient demographics are shown in Table 2. Sixty-eight percent of patients were female, with a mean age of 58 years. Approximately half had Medicaid, one-third had less than a high school education, two-thirds were unemployed, and most reported a household income of less than $20,000. One third of the patients had a Charlson Comorbidity Index score ≥ 3 with about 30 percent reporting some form of target organ damage. Mean SBP and DBP was 151.27 mm Hg and 90.94 mm Hg, respectively. The mean self-reported medication adherence score was 2.95 (range: 0 – 4, with higher scores indicative of better adherence); 55 percent were categorized as non-adherent, i.e. responded “yes” to at least one of the self-report items. Fifty-one percent of patients rated their provider’s communication to be non-collaborative.
Table 2.
Patient characteristics
| Characteristics (N = 439) | |
|---|---|
| Mean Age (± SD) | 57.69 (12.1) |
| Range: 25 – 98 years | |
| Female: n (%) | 300 (68) |
| Marital Status: n (%) | |
| Single | 118 (26) |
| Married | 111 (25) |
| Divorced/Separated | 141 (32) |
| Widowed | 68 (16) |
| Education: n (%) | |
| Elementary | 166 (38) |
| High School | 151 (34) |
| Some College | 121 (27) |
| Unemployed: n (%) | 290 (66) |
| Income (%) | |
| ≤$20,000 | 323 (74) |
| Insurance Status: n (%) | |
| None | 62 (14) |
| Private | 56 (13) |
| Medicare | 109 (25) |
| Medicaid | 203 (47) |
| Comorbidity: n (%) | |
| 0 | 77 (18) |
| 1 | 114 (26) |
| 2 | 99 (23) |
| 3 or more | 149 (34) |
| Comorbity: n (%) | |
| CHF | 53 (12) |
| Stroke | 34 (8) |
| MI | 31 (7) |
| Diabetes | 132 (31) |
| Mean Blood Pressure | |
| SBP (± SD) | 151.27 (18.06) |
| Range: 114 – 211mm Hg | |
| DBP (± SD) | 90.94 (11.78) |
| Range: 67 – 141 mm Hg | |
| Mean N of antihypertensive meds (± SD) | 2.13 (0.97) |
| Range: 1 - 4 | |
| Patient Measures | |
| Mean adherence score (± SD)a | 2.95 (1.20) |
| Non-adherent: n (%) | 243 (55) |
| Non-collaborative communication: n (%) | 191 (51) |
| Mean PHQ-9 score (± SD) | 4.39 (4.38) |
| Range: 0 - 35 | |
| Mean REALM score (± SD) | 57.09 (12.17) |
| Range: 0-66 | |
Higher scores are indicative of better adherence.
3.1. Bivariate Analyses
The unadjusted bivariate relationships between medication adherence, patient ratings of physician communication and the covariates were calculated. Physician communication rated by patients to be more collaborative was significantly associated with better medication adherence (r = -.15, p = .003). Higher levels of depressive symptoms (r = -.23, p < .001) were significantly associated with worse medication adherence and physician communication rated by patients as less collaborative (r = -.12, p = .018). Better medication adherence was significantly associated with having a provider with a medical degree (MD) compared to a nurse practitioner (NP) or physician’s assistant (PA) (r = -.12, p = .02). However, there was no relationship between provider degree and patient ratings of physician communication. In contrast, health literacy and number of years of practice were not associated with medication adherence or physician communication. Due to the significant influence of provider degree and depressive symptoms on medication adherence, these variables were included as covariates in the multivariate analysis.
3.2. Hierarchical Linear Regression Analyses
In multivariate analyses, adjusting for patient demographics, depressive symptom scores and provider degree, patients who rated their providers’ communication to be more collaborative were significantly more likely to report better medication adherence than patients who rated their provider’s communication as non-collaborative (β = -.11, p = .03; see Table 3). Thus, confirming our hypothesis, patients’ perception of providers’ collaborative communication was positively correlated with medication adherence. Similarly, younger age and presence of depressive symptomology were also independently associated with worse medication adherence (β = .13, p = .02 and β = -.18, p = .001, respectively). A test of the moderation effect revealed no significant interaction between patients’ ratings of provider communication and depressive symptoms or between patients’ ratings of provider communication and patient age on medication adherence (p = .75 and p = .64, respectively; Results not shown).
Table 3.
Results of Hierarchical Linear Regression Testing the Effects of Patient’s Rating of Provider Communication on Medication Adherence (N = 439)
| Variable | β (SE) | p |
|---|---|---|
| Communication | -.11 (0.007) | .03* |
| Age | .13 (0.005) | .02* |
| Gender | -.05 (0.13) | .31 |
| Education | .01 (0.08) | .83 |
| Income | -.01 (0.07) | .84 |
| Comorbidity | -.07 (0.06) | .21 |
| Provider degree | .05 (0.19) | .26 |
| Depressive symptoms | -.18 (0.11) | .001* |
| R2 | .098 |
4. Discussion and Conclusion
4.1. Discussion
Findings from our study indicate that patients’ ratings of their provider’s communication perceived as more collaborative was associated with better adherence to prescribed antihypertensive medications in hypertensive African Americans. We also found that younger age and the presence of depressive symptoms was significantly associated with worse medication adherence in this study’s patient population, which is consistent with previous studies in hypertensive patients [25, 28-30]. The patient-physician relationship is at the heart of an effective interaction between patients and physicians in primary care practices. The quality of communication between the patient and physician represents an essential and potentially modifiable component of this relationship. Although the specific mechanism underlying this relationship is unclear, this study supports a growing and compelling body of evidence showing the positive relationships between collaborative patient-physician communication and health behaviors in patients with chronic disease [7, 31]. For instance, Schneider et al. [32] found that higher ratings of the adherence dialogue, assessed as the quality of provider’s information-sharing techniques, ability to understand problems, and offer help with medications, explained most of the variance between perceived quality of the patient-provider relationship and adherence to antiretroviral medications [32]. In a sample of diabetic outpatients, participants that perceived their physician to possess effective general and diabetes-specific communication behaviors demonstrated an 11 percent absolute improvement in adherence to hypoglycemic medications as compared to those patients that perceived their physician to be an ineffective communicator [33]. Similarly, Heisler et al. [34] found that a higher level of perceived satisfaction with provider’s communication about patient’s illness and treatment was significantly associated with adherence to oral hypoglycemics [34].
While our findings corroborate those of previous studies [15, 16, 22, 32-35], a major strength of this study is that it is one of the first to investigate the relationship between patients’ ratings of provider’s communication and medication adherence in a community-based sample of largely underserved, low-income hypertensive African Americans, who receive care in low-resource community-based primary care practices. This patient population is at much greater risk for poor adherence to treatment that other populations [36]. Previous studies, on the other hand, have focused largely on Caucasian, privately insured patients, who received care in medical sub-specialty practices.
An additional strength of this study was in its adjustment for important covariates such as depressive symptoms and medical comorbidity that may help to explain the underlying mechanism between patient-provider communication and medication adherence. This is important, because patients’ decision to adhere to their medication depends on a myriad of factors, of which the quality of the patient-physician relationship is only one component of the decision-making process [37, 38]. Previous studies ignored the role of these important covariates.
Several limitations are worth noting. First, the providers participating in this study may not be representative of those in Managed Care organizations and private practices. Providers who work with underserved patients typically prefer a biopsychosocial approach to their medical practice, and are particularly motivated by issues of social justice and equity in healthcare and thus, may exhibit more collaborative communication with patients [39]. Second, we did not assess the duration of the medical relationship between the patient and provider. The importance of continuity of care is well-documented in that patients who report having a regular primary care provider are significantly more satisfied with the diagnostic and interpersonal aspects of their care than those who do not [33]. The cross-sectional nature of this study makes a causal interpretation difficult. Although, it is unlikely that the patients’ reported medication adherence affects perceptions of their provider’s communication, longitudinal studies are needed to assess the nature of this relationship.
Medication adherence was assessed through self-report, which may have resulted in an overestimation of adherence levels. However, our data showed that 55 percent of the patients reported being non-adherent, which is similar to the estimated 50 to 70 percent range of non-adherence rates documented by the World Health Organization [40]. For our study, we compared patients’ adherence status to their baseline blood pressure. Although not significant, patients categorized as adherent had lower SBP and DBP at baseline compared to patients identified as non-adherent (SBP: 150 mm Hg versus 153 mm Hg, p = .53, respectively; DBP: 89 mm Hg versus 92 mm Hg, p = .95, respectively). Furthermore, we found a positive correlation between medication adherence and blood pressure (p = .01). Future studies will do well to utilize a more objective measure of adherence, such as electronic monitoring devices to assess the relationship between the quality of patient-physician communication and medication adherence.
With regards to the quality of provider’s communication, this assessment relied on patients’ self-reported perception of the interaction thus; scores may reflect characteristics specific to the patient such as personality traits or self-efficacy, rather than the actual dialogue. Despite this limitation of a self-report communication measure, the importance of understanding the patient’s perspective of the patient-physician relationship cannot be understated. As acknowledged by Epstein [41], “patients notice different things than physicians” about the medical interaction, and it is these perceptions that they use to make decisions about their behaviors. For example, in an assessment of patient-centered care on health outcomes, patients’ perception of the relationship predicted positive health outcomes and better recovery from concerns while communication scores based on audio-taped analyses showed no relationship [42]. Future research would benefit from combining the strengths of both subjective and objective assessments to identify the specific components of the patient-provider relationship that produce actual behavioral outcomes. Similarly, more research is needed to examine both the patient and physician-level characteristics (e.g., personality traits, race, gender) that contribute to patients’ assessment of the quality of their physician’s communication to understand how these perceptions influence health behaviors.
4.2. Conclusion
In conclusion, we found that provider communication rated by patients as being more collaborative was associated with better medication adherence in a sample of hypertensive African-American patients followed in community-based primary care practices.
4.3. Practice Implications
The implications of this finding reinforce the need to develop effective uses of health communication that foster collaborative patient-physician communication, if we are to attain the goals of Healthy People 2010 to reduce health disparities and increase the quality and years of a healthy life. This requires the development of interventions targeted not just at the disease outcomes, but also at this aspect of the physician-patient communication during routine practice. This is important because interpersonal communication is reciprocal and dynamic; the patient influences the quality of dialogue within the medical relationship equally as much as the physician. Patient activation training delivered in waiting-rooms is one such strategy that has been shown to increase patient self-efficacy, assertiveness and shared-decision making, as well as improve communication skills and health outcomes in patients with chronic diseases [43].
To foster collaborative communication by providers, communication-skills training should be formally and systematically implemented into graduate medical education. By increasing opportunities for medical students to interview patients in the preclinical years, incorporating narratives and open discussions into the curricula, and encouraging self-reflection during residency, instructors ultimately support the development of a confident and satisfied physician prepared to face the challenges of a complex healthcare system [44]. Medical students that use narratives are more likely to value shared decision-making and be confident when utilizing these skills during patient interactions [45]. In terms of healthcare practices, evidence-based teaching models that provide healthcare providers with multiple avenues to learn, as well as practice, tangible, and effective communication skills should be integrated and reinforced into the culture of the organization. The Kaiser Permanente program serves as one such example of a large healthcare maintenance organization that has successfully achieved this goal [46].
Acknowledgments
We gratefully acknowledge Andrea Cassells, MPH and Chamanara Khalida, MD for all of their help with data management.
Role of Funding Source This study was supported by F31 HL081926-01, R01 HL 078566, and R24 HL 76857 from the National Heart, Lung, and Blood Institute. The funding agency played no role in the design, conduct, or reporting of the study, or in the decision to submit this manuscript for publication.
Footnotes
Conflict of Interest All authors declare that there are no competing or financial relationships that may lead to a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 2.Bosworth HB, Dudley T, Olsen MK, Voils CI, Powers B, Goldstein MK, Oddone EZ. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119:70 e9–15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Unequal Treatment: Confronting racial and ethnic disparities in healthcare. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 4.Beach MC, Sugarman J, Johnson RL, Arbelaez JJ, Duggan PS, Cooper LA. Do patients treated with dignity report higher satisfaction, adherence, and receipt of preventive care? Ann Fam Med. 2005;3:331–8. doi: 10.1370/afm.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper-Patrick LA, Gallo JJ, Gonzales JJ, Vu HT, Powe NR, Nelson C, Ford DE. Race, gender, and partnership in the patient-physician relationship. J Amer Med Assoc. 1999;282:583–9. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 6.Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159:997–1004. doi: 10.1001/archinte.159.9.997. [DOI] [PubMed] [Google Scholar]
- 7.Stewart MA. Effective physician-patient communication and health outcomes: A review. CMAJ. 1995;152:1423–33. [PMC free article] [PubMed] [Google Scholar]
- 8.Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15:25–38. [PubMed] [Google Scholar]
- 9.Bertakis KD, Roter D, Putnam SM. The relationship of physician medical interview style to patient satisfaction. J Fam Pract. 1991;32:175–81. [PubMed] [Google Scholar]
- 10.Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–63. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan SH, Gandek B, Greenfield S, Rogers W, Ware J. E Patient and visit characteristics related to physicians’ participatory decision-making style. Results from the Medical Outcomes Study. Med Care. 1995;33:1176–87. doi: 10.1097/00005650-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Roter DL, Stewart M, Putnam SM, Lipkin M, Jr, Stiles W, Inui TS. Communication patterns of primary care physicians. J Amer Med Assoc. 1997;277:350–6. [PubMed] [Google Scholar]
- 13.Willems S, De Maesschalck S, Deveugele M, Derese A, De Maeseneer J. Socioeconomic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns. 2005;56:139–46. doi: 10.1016/j.pec.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907–15. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 15.Beach MC, Duggan PS, Moore RD. Is patients’ preferred involvement in health decisions related to outcomes for patients with HIV? J Gen Intern Med. 2007;22:1119–24. doi: 10.1007/s11606-007-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006;21:661–5. doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 18.Wright JM, Mattu GS, Perry TL, Gelferc ME, Strange KD, Zorn A, Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood Press Monit. 2001;6:161–5. doi: 10.1097/00126097-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 Pt 1):2460–70. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 20.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health. 1992;82:1607–12. doi: 10.2105/ajph.82.12.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bultman DC, Svarstad BL. Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Educ Couns. 2000;40:173–85. doi: 10.1016/s0738-3991(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 23.Gazmararian JA, Kripalani S, Miller MJ, Echt KV, Ren J, Rask K. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006;21:1215–21. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, Crouch MA. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–5. [PubMed] [Google Scholar]
- 25.Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–11. doi: 10.1046/j.1525-1497.2002.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120:26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Morris AB, Li J, Kroenke K, Bruner-England TE, Young JM, Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006;26:483–92. doi: 10.1592/phco.26.4.483. [DOI] [PubMed] [Google Scholar]
- 30.Kim MT, Han HR, Hill MN, Rose L, Roary M. Depression, substance use, adherence behaviors, and blood pressure in urban hypertensive black men. Ann Behav Med. 2003;26:24–31. doi: 10.1207/S15324796ABM2601_04. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan SH, Greenfield S, Ware JE., Jr Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27:S110–27. doi: 10.1097/00005650-198903001-00010. [DOI] [PubMed] [Google Scholar]
- 32.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19:1096–103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18:624–33. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17:243–52. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuertes JN, Mislowack A, Bennett J, Paul L, Gilbert TC, Fontan G, Boylan LS. The physician-patient working alliance. Patient Educ Couns. 2007;66:29–36. doi: 10.1016/j.pec.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Hyre AD, Krousel-Wood MA, Muntner P, Kawasaki L, DeSalvo KB. Prevalence and predictors of poor antihypertensive medication adherence in an urban health clinic setting. J Clin Hypertens (Greenwich) 2007;93:179–86. doi: 10.1111/j.1524-6175.2007.06372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiMatteo MR. Patient adherence to pharmacotherapy: the importance of effective communication. Formulary. 1995;30:596–8. 601–2, 605. [PubMed] [Google Scholar]
- 38.Roter DL, Hall JA. Health education theory: an application to the process of patient-provider communication. Health Educ Res. 1991;6:185–93. doi: 10.1093/her/6.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Li LB, Williams SD, Scammon DL. Practicing with the urban underserved. A qualitative analysis of motivations, incentives, and disincentives. Arch Fam Med. 1995;4:124–33. doi: 10.1001/archfami.4.2.124. discussion 134. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Adherence to long-term therapies: Evidence for action. World Health Organization; Geneva: 2003. [Google Scholar]
- 41.Epstein RM. Making communication research matter: what do patients notice, what do patients want, and what do patients need? Patient Educ Couns. 2006;60:272–8. doi: 10.1016/j.pec.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 43.Cegala DJ, Post DM, McClure L. The effects of patient communication skills training on the discourse of older patients during a primary care interview. J Am Geriatr Soc. 2001;49:1505–11. doi: 10.1046/j.1532-5415.2001.4911244.x. [DOI] [PubMed] [Google Scholar]
- 44.Beach MC, Inui T. Relationship-centered care. A constructive reframing. J Gen Intern Med. 2006;21:S3–8. doi: 10.1111/j.1525-1497.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobie S. Viewpoint: reflections on a well-traveled path: self-awareness, mindful practice, and relationship-centered care as foundations for medical education. Acad Med. 2007;82:422–7. doi: 10.1097/01.ACM.0000259374.52323.62. [DOI] [PubMed] [Google Scholar]
- 46.Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58:4–12. doi: 10.1016/j.pec.2005.01.014. [DOI] [PubMed] [Google Scholar]