Summary
There is an increasing awareness of the role of inflammation in cancer. Immune responses can limit the growth of some tumors, but paradoxically, may promote the growth of others. Cytokines are critical mediators of both the innate and the adaptive immune responses. In this chapter, we will describe several methods for the detection of inflammatory cytokines. First, we will describe a protocol for quantification of cytokine mRNA by real-time quantitative PCR. In addition, we will describe detection of cytokine proteins by ELISA as well as by novel cytokine bead arrays. Finally, a method will be described for in situ detection of cytokine production by immunohistochemistry.
Keywords: Cytokines, Inflammation, Cancer, Real-time PCR, ELISA, Cytokine bead array, Immunohistochemistry
1. Introduction
Inflammation plays a dual role in malignancies. In some situations, immune reactivity against tumors can help limit their growth and even lead to their eradication (1). On the other hand, inflammation can contribute to the creation of a favorable environment for tumor progression (2, 3). Detection of the spectrum of inflammatory and anti-inflammatory cytokines has therefore become increasingly important in the study of cancer, both in mouse models and in human patients. In this chapter, we describe several methods for measuring in vivo cytokine production in mice. In principle, the same methods can be used for detection of human cytokines. Each of these methods has advantages as well as limitations, which we will discuss.
The first method, real-time quantitative polymerase chain reaction (Q-PCR), involves measurement of cytokine mRNA transcript abundance. This method is relatively straightforward and quantitative, and allows for the detection of many different cytokines from relatively small sample amounts. One of its major disadvantages is that the presence of RNA does not always accurately reflect protein levels. For example, secretion of some cytokines [such as interleukin (IL)-4 and IL-10] is (partially) regulated at the translational level (4, 5), while others are even regulated post-translationally (such as IL-1 and IL-18) (6). Another disadvantage of RNA-based detection of cytokines is that identification of the cellular sources of the cytokines requires isolation of different cell types, which may be difficult. Finally, although PCR is highly sensitive, if only a small proportion of the cells present in the tissue sample produce the cytokine(s) of interest, the threshold for detection may not be reached.
Secondly, we will discuss enzyme-linked immunosorbance assay (ELISA), a method allowing detection of secreted cytokines at the protein level. This protocol is straightforward and quantitative. On the other hand, it has the disadvantage that sufficient quantities of tissue fluids are not always easily obtained and that, due to cellular consumption of cytokines, actual cytokine levels may be underestimated.
The third method we will describe is the cytokine bead array, which is advantageous as it allows for detection of a whole panel of cytokines in a multiplex fashion using small sample volumes. The major disadvantages of this method are high cost, possible low sensitivity, and (as with ELISA) possible underestimation of cytokine production due to consumption.
While none of these methods allows for identification of the cytokine-producing cell types, the last protocol to be described in this chapter, immunohistochemistry, overcomes this limitation. This method also allows for detection of small numbers of cytokine-producing cells in a tissue, which might not produce enough cytokine to be detected by the other methods. Yet, in contrast to the first three protocols, this method is poorly quantitative and can suffer from low sensitivity for detection of secreted proteins. Alternatively, in situ hybridization on fresh frozen tissue sections or paraffin sections can be performed to detect the localization of cytokine mRNA species. This technique will reveal the type and localization of cytokine-producing cells. However, similar to immunohistochemistry, this method is not quantitative and the presence of RNA does not always accurately reflect protein levels. For in situ hybridization protocols, we refer the reader to some excellent books on the subject (e.g., In situ hybridization, principles and practice, by Polak and McGee, Oxford University Press. 1998; Methods in Molecular Biology, In situ hybridization protocols, edited by Darby, Humana Press, 2000).
2. Materials
2.1. Real-Time PCR Assay
2.1.1. RNA Isolation
Ultrapure DNase/RNase-free distilled water (Invitrogen #10977-049) (see Note 1).
Trizol reagent (Invitrogen, #15596-026).
Chloroform.
Isopropanol.
RNeasy microkit (Qiagen, #7400).
2-mercaptoethanol.
Spectrophotometer capable of measuring A260 and A280.
Eppendorff centrifuge.
Polytron homogenizer (PT MR2100, Kinematica AG, Switzerland).
DNA-free kit (Ambion, AM1906).
2.1.2. cDNA Reaction
Ultrapure DNase/RNase-free distilled water (Invitrogen #10977-049).
Superscript II (Invitrogen, #18064-022) or Superscript III (Invitrogen #18080-093) (includes 5× first-strand buffer and 0.1 M DTT) (see Note 2).
Oligo dT12–18 (Invitrogen #18418-012) (see Note 3).
RNaseOUT (Invitrogen #10777-019) (optional).
dNTP mix (10 mM) (Invitrogen #18427-013).
PCR apparatus or water bath.
2.1.3. Real-Time PCR
Ultrapure DNase/RNase-free distilled water (Invitrogen #10977-049).
5′ and 3′ primers.
Fluorescent probes or SYBR green (see Note 4).
AmpliTaq Gold (Applied Biosystems #4311816) (see Note 5).
GeneAmp 10× buffer (included with Amplitaq Gold).
MgCl2 (25 mM) (included with Amplitaq Gold).
dNTP mix (10 mM) (Invitrogen #18427-013).
Real-time PCR apparatus (see Note 6), e.g., ABI 7500 Real-Time PCR system (Applied Biosystems).
Eppendorff tubes.
96-well Optical Reaction Plates (Applied Biosystems #4306737) (see Note 7).
MicroAmp Optical Adhesive Film (Applied Biosystems #4311971) (see Note 7).
2.1.4. Validated Primer-Probe Sets (for Mouse Cytokines) (See Notes 8 and 9)
-
Hprt (7)
FW: 5′-CTGGTGAAAAGGACCTCTCG-3′
RV: 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′
Probe: 5′-FAM-TGTTGGATACAGGCCAGACTTTGTT-GGAT-BHQ-3′
-
β-actin (8)
FW: 5′-GAAGTCCCTCACCCTCCCAA-3′
RV: 5′-GGCATGGACGCGACCA-3′
FAM: 5′-AGCCACCCCCACTCCTAAGAGGAGG-BHQ-3′
-
Tnf-α (9)
FW: 5′-CTCCAGGCGGTGCCTATGT-3′
RV: 5′-GAAGAGCGTGGTGGCCC-3′
Probe: 5′-FAM-CAGCCTCTTCTCATTCCTGCTTGT-GGC-BHQ-3′
-
Ifn-α (9)
FW: 5′-CTTCCACAGGATCACTGTGTACCT-3′
RV: 5′-TTCTGCTCTGACCACCTCCC-3′
Probe: 5′-FAM-AGAGAGAAGAAACACAGCCCCTGT-GCC-BHQ-3′
-
Ifn-β (9)
FW: 5′-CTGGAGCAGCTGAATGGAAAG-3′
RV: 5′-CTTCTCCGTCATCTCCATAGGG-3′
Probe: 5′FAM-CAACCTCACCTACAGGGCGGACT-TCAAG-BHQ-3′
-
Ifn-γ (7)
FW: 5′-GGATGCATTCATGAGTATTGC-3′
RV: 5′-CCTTTTCCGCTTCCTGAGG-3′
Probe: 5′-FAM-TTTGAGGTCAACAACCCACAG-GTCCA-BHQ-3′
-
Tgf-β
FW: 5′-CCCGAAGCGGACTACTATGC-3′
RV: 5′-ATAGATGGCGTTGTTGCGGT-3′
Probe: 5′FAM-AGAGGTCACCCGCGTGCTAATGGTG-BHQ-3′
-
Il-4 (7)
FW: 5′-AGATCATCGGCATTTTGAACG-3′
RV: 5′-TTTGGCACATCCATCTCCG-3′
Probe: 5′-FAM-TCACAGGAGAAGGGACGCCATGC-BHQ-3′
-
Il-5 (7)
FW: 5′-CGCTCACCGAGCTCTGTTG-3′
RV: 5′-CCAATGCATAGCTGGTGATTTTT-3′
Probe: 5′-FAM-CAATGAGACGATGAGGCTTCCT-GTCCC-BHQ-3′
-
Il-6 (9)
FW: 5′-CCAGAAACCGCTATGAAGTTCC-3′
RV: 5′-TCACCAGCATCAGTCCCAAG-3′
Probe: 5′-FAM-TCTGCAAGAGACTTCCATCCAGTT-GCCT-BHQ-3′
-
Il-12 p40
FW: 5′-CTCAGGATCGCTATTACAATTCCTC-3′
RV: 5′-TTCCAACGTTGCATCCTAGGATC-3′
Probe: 5′-FAM-TGCAGCAAGTGGGCATGTGTTCC-BHQ-3′
-
Il-13 (7)
FW: 5′-GCTTATTGAGGAGCTGAGCAACA-3′
RV: 5′-GGCCAGGTCCACACTCCATA-3′
Probe: 5′-FAM-CAAGACCAGACTCCCCTGTGCAACG-BHQ-3′
-
Il-17a (10)
FW: 5′-CTCCAGAAGGCCCTCAGACTAC-3′
RV: 5′-AGCTTTCCCTCCGCATTGACACAG-3′
Probe: 5′-FAM-TCTGGGAAGCTCAGTGCCGCCAC-CAGC-BHQ-3′
-
Il-17f (10)
FW: 5′-GAGGATAACACTGTGAGAGTTGAC-3′
RV: 5′-GAGTTCATGGTGCTGTCTTCC-3′
Probe: 5′-FAM-AGTTCCCCATGGGATTACAACAT-CACTC-BHQ-3′
-
Il-21 (11)
FW: 5′-ATCCTGAACTTCTATCAGCTCCAC-3′
RV: 5′-GCATTTAGCTATGTGCTTCTGTTTC-3′
Probe: 5′-FAM-AAGCCATCAAACCCTGGAAACAATAA-GACA-BHQ-3′
2.2. ELISA (See Note 10)
ELISA plates, e.g., Maxisorp 96-well flat-bottom plates from Nunc #442404
-
0.05 M Carbonate Coating Buffer pH 9.6: 8 ml of 0.2 M Na2CO3 (0.2 M = 21.2 g/l)
17 ml of 0.2 M NaHCO3 (0.2M–16.8g/l)
75ml H2O
PBSB (PBS with 1% BSA)
-
Blocking solution: 1× PBS
3%BSA
-
Wash buffer: 1× PBS
0.05%Tween20
-
Capture and detection antibodies (see Note 11): We have had good success with the following antibody pairs:
– IFN-γ (#551216 and #554410, BD Pharmingen)
– IL-1a (#14-7011-85 and #13-7111-85, eBiosciences)
– IL-1b (cat#MAB401 clone 30311 and cat#BAF401 at 100 ng/ml, R + D systems)
– IL-2 (#554424 and #554426, BD Pharmingen)
– IL-4 (554387 and #554390, BD Pharmingen)
– IL-5 (554393 and #554397, BD Pharmingen)
– IL-6 (#554400 and #554402, BD Pharmingen)
– IL-9 (#500-P59 and #500-P59BT, Peprotech)
– IL-10 (#551215 and #554423, BD Pharmingen)
– IL-12 p40 (#551219 and #554476, BD Pharmingen)
– IL-17 (#10215-01and #10214-08, Southern Biotech)
– IL-18 (#D047-3 clone 74 and #D048-6 clone 93-10C, MBL, Woburn MA)
– TNF-α (#14-7325-85 and #13-7326-85, eBiosciences
Recombinant interleukins (BD Pharmingen, eBiosciences)
Avidin-HRP (see Note 12)
SureBlue TMB substrate (Kirkegaard & Perry Laboratories) (see Note 12)
Stop solution (e.g., 3 M NaOH)
2.3. Cytometric Bead Assay
-
BD CBA kits containing:
Antibody-conjugated capture beads (for each cytokine there is one vial of beads)
Cytometer Setup Beads
PE-detection reagent
Standard recombinant proteins (one single standard mixture is provided to generate standard curves for all the analytes tested). Each kit contains two vials.
PE-positive control detector
FITC-positive control detector
Wash buffer
Assay diluent
A flow cytometer equipped with a 488-nm laser capable of detecting and distinguishing fluorescence emissions at 576 and 670 nm. We have good experience with the BD FACSCalibur (BD Biosciences) and BD CellQuest Software.
Sample acquisition tubes for a flow cytometer, 12 × 75 mm (BD Falcon Cat.No. 352008).
BD CBA Software (BDbiosciences, Cat.No. 550065).
2.4. Immunohistochemistry
2.4.1. Perfusion
Isofluorane (30%, diluted in propylene glycol).
Absorbent cloth or cotton ball.
1× phosphate-buffered saline (PBS).
hemostats (Roboz #RS-7291 and #RS-7231).
Dissection scissors (Roboz #RS-5914SC).
Forceps (Roboz #RS-5135).
60-ml syringe.
Butterfly needle, 23-gauge (Becton Dickinson #36-7283).
2.4.2. Tissue Handling and Sectioning
15-ml conical-type screw-top tubes.
4% (w/v) paraformaldehyde (PFA), diluted in PBS.
Sucrose, 10, 20, and 30%, diluted in PBS.
Superfrost Plus Gold slides (Fisher).
Freezing microtome.
Microtome blades.
Optimal cutting temperature compound (OCT, Sakura #4583).
Standard cryomolds (Sakura # 4557).
2.4.3. Tissue Staining and Analysis
Mini PAP pen (Zymed, #00-8877).
Humidified chamber.
Coplin jar or staining dish.
Serum-free protein block (Dako #S3022).
Primary antibodies, purified.
Secondary antibodies, conjugated with Alexa Fluors (Invitrogen).
Fluorescence-mounting media (ProLong Gold with DAPI, Invitrogen #P-36931).
Glass cover slips, 22 × 40, 50, or 60 mm as appropriate.
Fluorescence microscope.
3. Methods
3.1. Real-Time PCR
3.1.1. Detection Method and Principle
Quantitative measurement of RNA concentrations relies on real-time detection of amplified cDNA targets (amplicons) generated by successive rounds of PCR amplification. Amplicons are detected on the basis of fluorescence, which increases proportionally with the PCR product. Quantification is determined by comparing the number of cycles required per sample to cross a certain threshold of fluorescence (Ct). This threshold is set in the linear phase of the reaction, such that the difference between samples in the number of cycles required to cross this threshold reflects the relative difference in the starting amount of the target sequence. Although real-time PCR could, in principle, be used to obtain an absolute value for the number of mRNA transcripts in the starting material, this can be tricky (see Note 13). Rather, most researchers use this technique to measure relative differences between different samples, for which this is an accurate method. This chapter is mostly dedicated to the practical aspects of measurement of cytokine mRNA quantity, and more elaborate descriptions of the principle behind real-time PCR are beyond its scope. For this, we refer the reader to an excellent article published elsewhere (12).
Two different methods for detection are widely used. First, the DNA intercalating minor groove-binding fluorophore SYBR green is used, which only produces a strong signal when incorporated into double-stranded DNA. As the template cDNA used for these assays is single stranded and is therefore not bound by SYBR green, this dye selectively detects the double-strand amplicon. SYBR green works well, is very sensitive (due to the fact that many fluorophore molecules bind to each molecule of PCR product), and is the cheapest method available. However, there are a few caveats. First, care must be taken in primer design, to avoid self-associating primers, as these would generate a double-strand DNA product which would be indiscriminately bound by SYBR green. Furthermore, the specificity of the PCR reaction must be high, as nonspecific products also contribute to the overall signal. Specific amplification of a single product can be tested by performing a melt curve analysis (see later). Another check that is sometimes used is to run the real-time PCR reaction to completion, and to then subject products to gel electrophoresis to ensure that only one band corresponding to the predicted amplicon molecular weight is present. Thus, while SYBR green is a good detection method for quantitative PCR, it often requires optimization before one can have confidence in the results.
A much greater chance of instant success is obtained by using nested fluorescent probes for detection. These probes are designed to anneal to a specific sequence within the amplicon. Generation of nonspecific products or primer dimer is, therefore, much less of a problem than with SYBR green, as these products are not likely to bind the probe. The principle behind the detection with these probes is as follows: probes contain a fluorescent label on one end and a quencher on the other, which prevents the fluorophore from emitting. Free probe molecules are therefore not detected. However, fluorochrome is released from the quencher when probe molecules bound to their target sequence are degraded due to exonuclease activity of the DNA polymerase as it is filling in the sequence. Fluorescence is therefore directly proportional to the amount of specific product.
In this chapter, we discuss the use of probes labeled with FAM. However, probes can be labeled with other fluorochromes as well. This makes it possible to measure several different products simultaneously in the same sample (multiplexing), using differently labeled probes. Multiplexing can be quite useful when a specific set of cytokines must be measured frequently from small sample volumes. It is important that all primer-probe sets are compatible with one another. This obviously depends on the specific set of cytokines being tested. Since many different combinations are possible, a detailed discussion of multiplexing is beyond the scope of this chapter.
3.1.2. Probe Design Guidelines
Design of probes is reasonably forgiving:
The melting temperature must be higher than that of the primers to ensure optimal occupancy by probe when polymerization starts (i.e., higher than 65 °C).
Ideally, the probe does not form hairpins or homo duplexes, as these properties reduce sensitivity.
Probes should not be longer than 30 nucleotides, as that reduces the efficiency of quenching.
Since G residues quench fluorescence, these should be avoided at the 5′ end (this residue remains attached to the fluorophore after hydrolysis of the probe).
Continuous stretches of four or more identical nucleotides (especially G) can influence probe conformation and reduce hybridization efficiency.
Often, probes are designed to cross the intron–exon boundary and thereby help avoid detection of contaminating genomic DNA.
Custom probes can be ordered from several companies. We have good experience with probes made by Biosearch Technologies. Using custom-made probes is a good option for genes that will be measured many times. Premade primer-probe sets are provided by Applied Biosystems. These are cost effective when only a limited number of analyses will be performed. While we have had good success with these sets, two disadvantages are that (1) no information is provided about the sequences of the primers and probes, and (2) the cost per sample is higher than ordering custom probes in larger quantity.
3.1.3. Primer Design Guidelines
A list of validated primer-probe sets is provided in Subheading 2.1.4. A further list of validated real-time PCR assays can be found at http://medgen.ugent.be/rtprimerdb/. In some cases, it will be necessary to design new assays. Primer design should be particularly meticulous when using SYBR green to detect amplification, for the reasons outlined in Subheading 3.1.1. Several considerations should be followed when designing primers:
The real-time program cycles between 60 and 94 °C and annealing as well as extension must occur during the 60 °C step. This temperature is below the optimal temperature for most polymerases. Therefore, long amplicons may not be amplified well. Ideally, the amplicon should be smaller than 150 nucleotides. Longer amplicons may work, but likely result in reduced sensitivity.
In case oligo dT was used for priming for the first-strand cDNA reaction, it is best to choose primers annealing at the 3′ end of the cDNA. Because oligo dT priming starts at the 3′ end, that end is always represented better than more 5′ sequences. Of course, if the only unique sequence is found at the 5′ end, then this is the only option.
Although it is good practice to clean up RNA preps with DNase I before making cDNA, it is still a good idea to choose primers in different exons (separated by an intron of over 1 kB), such that measurement of contaminating genomic DNA can be excluded. Specificity can be enhanced using a nested fluorescent probe that anneals to the two different exons (that would be separated by an intron in genomic DNA, precluding probe annealing).
The melting temperature of the primers to their target sequence should be 60 °C or higher.
Ideally, neither the GC nor AT content should exceed 60%, and stretches of more than three continuous identical nucleotides should be avoided.
Avoid primers that form homo- or hetero-duplexes or hairpins. It is a good idea to use a program such as MacVector to help design primers.
It is advisable to perform a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) with the primer sequences to minimize the chances of amplifying something other than the desired sequence. Also, check the target sequence by BLAST, align the obtained sequences, and avoid primers annealing in regions containing polymorphisms.
When using SYBR green to detect the PCR product, it is important to test the reliability of the primers in a melt curve analysis (see Subheading 3.1.10). To this end, first run the real-time PCR program using a DNA mixture known to contain the target sequence. After completion of the program, run the melt curve analysis. In general, the most practical approach is to design several primer pairs and test them this way, side by side. Then, choose the best pair from this panel. If good primer pairs are not identified this way, titration of primer concentrations (in four twofold steps) often allows one to identify a pair amplifying just a single product.
An excellent program to help design assays is offered at http://www.biosearchtech.com/products/probe_design.asp. This site links to the NCBI databases to perform BLAST searches as well as to the NCBI ePCR site, where a virtual PCR can be performed to test the specificity of the assay.
3.1.4. Reference Genes
For every sample, the concentration of a reference gene, which is (presumed to be) similarly expressed under the conditions tested, should be measured. The concentrations for the experimental genes can then be expressed relative to that of the internal reference, thereby controlling for differences between samples in starting RNA concentrations and efficiency of the RT reaction. Often-used reference genes include hprt, gapdh, β-tubulin, and β-actin. Unfortunately, it is not always possible to predict whether expression of these genes will in fact be constant, especially when novel conditions are used. In that case, it is best to test several reference genes and use the one that shows the least variation under the conditions used. Although some researchers use the amount of input RNA to normalize, this is not a good method, since it does not control for differences in the RT reaction. It is essential to measure the reference gene and experimental gene in material derived from the same cDNA reaction. Some researchers find it helpful to even assay the reference gene side by side on the same plate with the cytokine cDNAs being tested, which may minimize error from repeated cDNA sample handling.
3.1.5. Tissue Sample Preparation
Freshly harvested tissue should be immediately frozen in liquid nitrogen and stored at −80 °C. Keep snap-frozen tissue samples at −80 °C before extraction. Avoid repeated freeze-thaw cycles. Tissue cytokine levels in highly vascularized tissues can be obscured by cytokines present in “contaminating” blood. Presence of blood in tissue samples can be minimized by performing cardiac perfusion (see Note 14) of mice with PBS prior to isolation of tissues. For RNA extraction, it is recommended to use tissue pieces of about 5 mm in diameter.
3.1.6. RNA Preparation
While commercially available kits for isolation of RNA, such as Qiagen’s RNeasy (Qiagen, #7400), give good results in many cases, some researchers prefer to use the Trizol method. This method tends to result in higher RNA yields and is significantly cheaper than the use of kits, making it a method of choice when funds are limited. However, the Trizol preparation protocol does harbor the risk of carryover of small amounts of phenol, which inhibit reverse transcriptase, thereby adversely affecting cDNA yield. Therefore, we routinely use RNeasy columns to clean up the RNA obtained from the Trizol procedure. This way, we get the highest yields of high-quality RNA. For RNA preparation we refer the reader to the Trizol and RNeasy manuals. Add Trizol reagent while keeping the samples at −80 °C. For RNA extraction from tissues, homogenization by polytron (PT MR2100, Kinematica AG, Switzerland) is necessary. Incomplete homogenization could result in degradation of the isolated RNA due to the inability of the Trizol to reach the inner cells in an organ or tissue clump. In general, it is very important to use RNase-free materials. Gloves should be worn throughout the isolation procedure. Most plastics, such as tips and Eppendorff tubes, are clean, if taken straight from the box. Set aside a fresh box for each of these and do not reach inside the box. Pick up tips and tubes with gloves or forceps. RNA samples can be stored at −20 °C for several weeks and at −80 °C for years.
3.1.7. DNase Treatment (Optional)
Most RNA purification methods (including RNeasy) fail to eliminate all genomic DNA from the preparation. Unless the specific PCR primers were chosen to exclude detection of genomic DNA (see Subheading 3.1.3, step 3), it is important to remove contaminating genomic DNA. When using SYBR green for detection, removal of genomic DNA is always recommended. The standard method is treatment with DNase, followed by removal of the DNase. We have had good results with the Ambion DNA-free kit (AM1906). For the specifics of DNA removal, we refer the reader to the manual supplied with the kit.
3.1.8. cDNA Preparation
We have had good experience with the Superscript II and III first-strand synthesis systems for RT-PCR (Invitrogen, #11904-018 and #18080-093, respectively) (see Note 2). The generation of first-strand cDNA can be primed using oligo dT primers, random hexamers, or with sequence-specific primers. We prefer the use of oligo dTs (see Note 15), as such primers allow for generation of good-quality cDNA and limit reverse transcription to (polyadenylated) mRNAs, resulting in lower complexity of the cDNA mixture than is obtained using random hexamers (see Note 3). At the same time, this method allows for detection of all cytokines from the same cDNA reaction, in contrast to cDNA made with sequence-specific primers. For a detailed cDNA synthesis protocol we refer the reader to the manual for the Superscript II (or III) first-strand synthesis system for PCR. cDNA samples can be stored at −20 °C for several weeks and at −80 °C for years.
3.1.9. Real-Time PCR Protocol
It is best to measure individual samples at least in duplicate. Especially for low abundance targets, it is useful to perform measurements in triplicate, as the intrinsic error of the Q-PCR measurement is relatively large in this case.
Always include a standard titration (in duplicate) in the same reaction (see Note 16). The standard curve should start at a concentration higher than any of the experimental samples and proceed below the lowest sample, as this will allow the investigator to ascertain whether experimental values fall within the linear range of the amplification curve. There are several different possible sources for standards (see Note 17). When using positive control cDNA, start with the highest possible concentration (see Note 18) and then do seven fivefold dilutions. Always include a control sample without template (in duplicate).
-
Make a 2× master mix containing per sample (see Note 19) (make enough of this common mix for two extra reactions to account for pipetting error):
-
Into each well of a 96-well PCR plate add:
– 10 μl master mix
– 10 μl diluted cDNA
Total volume: 20 μl (see Note 19)
It is best to release the cDNA into the common mix solution, as this ensures that all of the solution from the tip ends up in the well. When releasing against the wall of the tube, sometimes a small volume stays behind in the pipette or clings to the side of the pipette. Of course, putting the tip into the common mix solution necessitates using separate tips for different replicates of the same sample. If many samples are to be tested, put the diluted cDNAs into strip tubes and load the samples into the plate using a multichannel pipette. This significantly reduces the chances of getting confused.
Seal the plate with an optical-grade self-adhesive transparent cover, making sure not to touch the cover without gloves (to avoid making greasy stains which might interfere with transmission of the light).
Vortex the plate lightly (at 30% of maximum speed).
Spin the plate for 30 s at 1,000 rpm.
-
Run the samples in your Real-time PCR machine using the following protocol:
– One cycle: 2 min hot start at 94 °C.
– 40 cycles: 30 s 94 °C
– 30 s 60 °C
For programming the PCR machine, we refer the reader to the manual supplied with the specific apparatus. Make sure to program your apparatus to detect FAM (or SYBR green) and to mark the wells containing samples, standard titration, and no template controls. Some machines (e.g., the Bio-Rad icycler) will only record measurements taken from the wells marked before the run, while others will record the entire plate.
When marking the standard titration, enter concentrations. Since usually only relative amounts are measured, the numbers here can start at an arbitrary number (i.e., if fivefold dilutions were used, the starting concentration could be 78,125, followed by fivefold dilutions, such that the lowest concentration ends up as one).
3.1.10. Melt Curve (Optional)
While melt curves are typically not run when using primer-probe sets, when using SYBR green to detect the PCR product, it is critical to run a melt curve at the end of the PCR reaction. The melting temperature of double-stranded DNA depends on its length and GC contents. Different products thus tend to melt at different temperatures. This property is exploited during melt curve analysis. In this analysis, the temperature is gradually increased at the end of the PCR reaction. When the melting temperature for a double-strand DNA product is reached, this results in a loss of fluorescence, since SYBR green only fluoresces when bound to double-strand DNA. The loss of fluorescence is generally displayed as the rate of change in fluorescence against the temperature, producing a peak around the melting temperature. A single sharp peak indicates the presence of a single product, whereas a broad peak or the presence of more than one peak demonstrates the presence of multiple products. The quantification can only be trusted if a single peak is obtained. If a broad peak or more than one sharp peak is obtained, the results cannot be trusted and the assay must be repeated, possibly after assay optimization.
3.1.11. Data Analysis
When fluorescence is plotted against the number of cycles, four phases can be identified in the real-time PCR reaction: (1) the background phase, (2) the exponential phase, (3) the linear phase, and (4) the plateau phase. Quantification occurs on the basis of the number of cycles required to cross a certain fluorescence level (known as a threshold) (Fig. 1A). This threshold is set in the exponential phase, as that is when the PCR reaction occurs with optimal efficiency, such that with each cycling (approximately) a doubling of product occurs. Correctly setting the threshold is therefore an important parameter in quantification. Software default settings are useful, but may sometimes have to be adjusted manually (e.g., if there is high noise during the exponential phase). The following guidelines should be followed in analyzing the data:
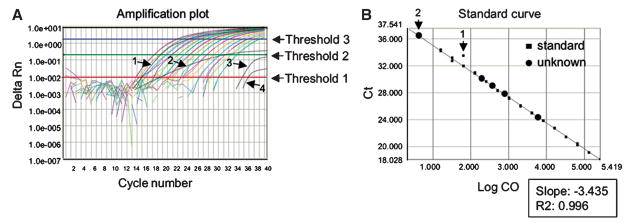
Fig. 1.
Real-time PCR plots. (A) Amplification plot. Fluorescence increase is shown as a function of cycle number. Delta Rn represents the normalized reporter (Rn) fluorescence intensities after subtraction of baseline intensities. Baseline cycles are those before a steady upward trend in fluorescence occurs. Generally, the baseline cycles are determined by a program software and need not be adjusted. This threshold is set automatically by program software, but sometimes, manual adjustments can improve data quality. The threshold must be set within the exponential phase of the curve, as that is the phase of the reaction when components are not limiting and close to a doubling of product occurs with each cycle. For this reason, it is easiest to plot the Delta Rn on a logarithmic scale, since this magnifies the exponential phase compared to a linear view. In this example, the threshold could be set a bit lower, such that it would include trace number 3. However, threshold 1 is set too low, because it fails to exclude nonspecific signal generated at the first four cycles of the reaction, when no specific product is present yet. Trace number 4 cannot reliably be included in the analysis, therefore. On the other hand, threshold 3 is set too high, as it no longer falls within the exponential phase. Reliable amplification curves all run parallel to one another and have similar shapes. Trace number 1 looks reliable, but trace number 2 cannot be trusted, because of its deviant shape. (B) Standard curve. The cycle at which a sample crossed the threshold (threshold cycle, Ct) is plotted against the starting concentration (on a logarithmic scale-Log CO). The slope of the standard curve (closed squares) is between 3.1 and 3.5, indicating almost perfect exponential kinetics of the PCR reaction and the reliability of the curve fit (R2) is high. The standard curve could still be improved by excluding standard sample number 2, which is a clear outlier. Measurements of most experimental samples (unknowns, closed circles) can be trusted, except for sample number 2, which falls outside the standard curve.
In a graph plotting fluorescence against cycle number, the shapes of all curves should be similar. The only difference should be in their horizontal position in the graph. Samples yielding atypical curves should be excluded from the analysis (Fig. 1A).
The standard curve should be examined, in which the starting concentration is plotted against the threshold cycle (Ct) (Fig. 1B). The slope of this curve should be between −3.2 and −3.5 (if the efficiency of the reaction is optimal, it takes approximately 3.3 doublings to achieve a tenfold increase in product) and the correlation coefficient (r2) should be greater than 98%. If the standard curve does not meet these criteria, determine whether there are obvious outliers. If, for instance, one of the duplicate samples fits perfectly, while the other does not, it is appropriate to exclude the outlier (Fig. 1B). Also, one may find that all of the samples fall within a certain range of the standards, allowing the other standards to be excluded (e.g., if all samples fall within the last five standards, the first two can be omitted), and this can often improve the precision of the results. Sometimes, it is necessary to adjust the threshold (see later). Only samples within the range covered by the standard can be reliably quantified, (Fig. 1B) and samples that are to be compared must be analyzed with the same threshold setting.
Sometimes, the default settings exclude quantification of certain samples, which nonetheless can visually be seen to yield real product at higher cycles (Fig. 1A). It is often possible to include these samples by reducing the threshold. To this end, it is best to plot the fluorescence on a logarithmic scale, as that way the exponential phase of the reaction is visually drawn out and appears linear, allowing more subtle determination of where to set the new level.
If manual reduction of the threshold results in a good standard curve that encompasses the range of concentrations of the previously excluded samples, the samples can be reanalyzed with this Ct.
When SYBR green has been used, the melt curve must be examined to ensure that only a single product (read out as a single sharp peak) was generated. If this is not the case, data analysis cannot be reliably conducted.
All values should be normalized by dividing them by those obtained for the value of the reference gene (see Subheading 3.1.4) for those samples. Afterward, values can be directly compared. It is often convenient to divide all normalized samples by the normalized value for a control sample or the lowest sample. Thereby, that sample is arbitrarily set to one and all other samples are expressed as fold increases.
3.2. ELISA
3.2.1. Basic Principle
ELISA assays allow quantitative measurement of antigens in biological samples. A wide variety of ELISA assay principles can be used. In this chapter, we will focus on the sandwich ELISA – an assay suitable to quantify cytokine levels in samples. Briefly, a capture antibody with specificity for the cytokine of interest is immobilized on microtiter wells. Biological samples and standard samples (containing a known concentration of recombinant cytokine) are then allowed to react with the immobilized capture antibody. Unbound protein is removed through extensive washing, after which a second specific antibody, conjugated to an enzyme such as horseradish peroxidase (HRP) or alkaline phosphatase (AP), is added to form a sandwich with the captured cytokine. Sometimes, this second antibody is conjugated to biotin, and detection occurs by subsequent addition of streptavidin linked to HRP or AP. A chromogenic substrate is added, which is chemically converted by the enzyme coupled to the detection antibody, resulting in a color change. The intensity of the color is proportional to the amount of cytokine bound to the capture antibody. Finally, the optical density of the reaction is measured with a spectrophotometer and compared with the optical density of the known standard samples to determine protein concentrations.
A modified version of the ELISA immunoassay is the enzyme-linked immunosorbent spot (ELISPOT) assay. This method allows the visualization and quantification of the secretory product (e.g., cytokines) of individual activated or responding cells within a cell population. For this method, we refer the reader to articles published elsewhere (e.g., Methods in Molecular Biology; Handbook of ELISPOT: methods and protocols, by A.E. Kalyuzhny, Humana Press. 2005).
3.2.2. Sample Preparation
Serum Samples
Blood should be collected from mice using standard methods (e.g., cardiac puncture, orbital or tail bleed). Collect blood samples in Eppendorf tubes and allow to clot for 2 h at room temperature or overnight at 4 °C. Spin samples for 20 min at 1,200 rpm and collect supernatant (=serum). Alternatively, blood can be collected in commercial tubes (e.g., Greiner vacuette serum tubes with clot activator, Greiner Bio-One #454320). Aliquot serum samples (10–50 μl) and store at −80 °C. Avoid repeated freeze-thaw cycles.
Tissue Samples (See Note 24)
Freshly harvested tissue should be immediately frozen in liquid nitrogen and stored at −80 °C. During the entire protein isolation method, keep tissue on ice as much as possible.
Protein extraction:
Weigh samples.
Thaw samples on ice.
Place tissue in a glass petri dish on ice, and mince tissue using two scalpels.
Transfer tissue pieces to cold Eppendorff tube.
-
Add lysis buffer. Protein yields vary depending on the lysis buffer used. It is important to use a lysis buffer that does not contain SDS, as this might affect stability of cytokines.
-
1× lysis buffer:
10 mM Tris–HCl (pH 8.0)
150 mM NaCl
1% NP-40
10% Glycerol
5 mM EDTA
Protease inhibitor cocktail (Roche)
We use the following formula to calculate the volume of lysis buffer: 5× weight tissue in mg = volume of lysis buffer in μl
-
Smoothly homogenize the tissue sample using an Eppendorf-fitting pestle.
Incubate the lysates for 1 h at 4 °C, while rotating.
Sonicate the lysates. We have good experience with bath sonication, using a 7-min regimen with 0.5 min of intermittent on and off sonification periods using the Diagenode Bioruptor (www.diagenode.com) with a cooling water pump. Optimal sonicator settings must be determined empirically for each type of machine and tissue. Make sure the samples remain at 4 °C during sonication.
Centrifuge samples for 15 min at 4 °C at 14,000 rpm.
Collect supernatant, determine protein concentration, aliquot, and store at −80 °C. Avoid repeated freeze-thaw cycles.
3.2.3. ELISA Protocol
Coat plates with 100 μl of capture antibodies at 1 μg/ml in coating buffer (see Notes 25 and 26). Make sure all wells are completely covered with liquid by gently tapping the sides of the plates. Incubate for 3 h at room temperature or overnight at 4 °C (see Note 27). Make sure to coat enough wells to perform at least duplicate measurements of the standard and experimental samples. For latter, it is best to measure different replicates of the original biological experiments. If only single samples are available, measure these in duplicate.
Dump the coating antibodies and add 200 μl of blocking solution to block free binding sites on the plate (see Note 28). Incubate for 1 h (minimally) at room temperature.
Wash four times by flicking off the plate and adding 250 μl per well of wash buffer each time. Commercially available ELISA plate washers work well for this purpose. However, washing can also be carried out by submerging the plates one by one in a container with wash buffer, by using a squirt bottle, or by manual pipetting.
After the final wash, blot the plates dry on a piece of paper towel (see Note 28).
Prepare dilutions of standard samples and samples in PBSB (see Note 29). For new experiments, it is a good idea to test the samples undiluted as well as include a few dilutions, e.g., fivefold, 25-fold, and 125-fold. In most cases this should give values that fall within the standard curve at least at one of the dilutions (see Note 30). The standard curve should start at 20 ng/ml. Six 3-fold dilutions will titrate the cytokine levels below the threshold for reliable detection of most ELISA antibody sets (50 pg/ml).
Add 100 μl (see Note 31) per well of samples (supernatants, tissue fluids, tissue lysates) and standards. Set up duplicate wells for each sample or standard (see Subheading 3.2.3, step 1). Also include negative samples containing only PBSB as blank.
Incubate overnight at 4 °C. Shorter incubations will work, but can reduce the sensitivity of the assay. Some researchers do 1- or 2-h incubations while gently rocking the plate at room temperature.
Wash four times by flicking off the plate and adding 200 μl per well of wash buffer each time. After the final wash, blot the plates dry on a piece of paper towel (see Note 28).
Add 100 μl per well of biotinylated detection antibodies (see Note 32) diluted in PBSB to a concentration of 1 μg/ml (see Note 25).
Incubate for 1–2 h at room temperature.
Wash four times by flicking off the plate and adding 200 μl per well of wash buffer each time. After the final wash, blot the plates dry on a piece of paper towel (see Note 28).
Add 100 μl per well of Avidin-HRP diluted 2,000-fold in PBSB
Incubate 30 min at room temperature. Longer incubation times are not recommended as these result in higher background.
Wash four times by flicking off the plate and adding 200 μl per well of wash buffer each time. After the final wash, blot the plates dry on a piece of paper towel (see Note 28).
Add 100 μl of TMB substrate solution per well. Signal will show up as a blue color, which turns yellow after addition of Stop solution. In some cases, the signal can come up very rapidly (see Note 33). It is therefore important to have a container with Stop solution ready such that the reaction can be stopped immediately after adding substrate solution. It is best to add substrate solution using a multichannel pipette and to work at a steady pace. Importantly, the Stop solution should be added in the same order as the substrate solution at the same pace, to avoid false differences.
Absorbance is measured at 450 nm and is stable for up to 1 h after addition of Stop solution. Please note that air bubbles may interfere with absorbance measurement. Therefore, it is important to remove air bubbles from wells prior to analysis.
3.2.4. Data Analysis
For the settings of the ELISA plate reader, we refer the reader to the instrument manual. Make sure to choose the correct wavelength (some machines can measure the full spectral range, whereas other machines contain a limited number of standard filters). In general, software provided with the ELISA plate reader can also be used for data analysis. We refer the reader to the software manual for details. In general, the first analysis step is to make a standard curve using the serial dilutions of protein standards. This analysis models protein concentration as a function of the OD. Since the standard curve tends to be sigmoid, linear regression is not recommended. Point-to-Point, Cubic Spline, or four Parameter are the most accurate curve-fitting routines. If linear regression must be used, the experimenter should only include the portion of the standard curve that falls within the linear range. Before proceeding to the analysis of the unknown samples, make sure the standard curve is reliable, as judged by an R2 value greater than 0.98. Outliers are frequently found at higher concentrations, where saturation has occurred. These should be discarded. If one of a duplicate standard obviously falls outside the standard curve, it is also best to discard that sample and recalculate the curve. Subsequently, ensure that experimental samples fall within the range of the assay. Please note that near the asymptotes of the standard curve, the uncertainty of the protein concentration increases. Many ELISA reader programs will automatically exclude samples outside the reliable area of the standard curve. If the O.D. of the experimental sample is above the standard curve, the experiment has to be repeated with more dilute samples. Conversely, if the value is below the standard curve, the test has to be repeated with more concentrated samples. The next step is to calculate the protein concentrations of the biological samples, using the corresponding standard curves. Please be aware that protein concentration estimates are uncertain, due to processing error and biological variability. Sufficient replication within and across experiments is important for making precise estimates of both concentrations and errors.
3.3. Cytometric Bead Array
3.3.1. Basic Principle
One limitation of conventional ELISA assays is that it is not possible to detect multiple cytokines in one assay. As a consequence, multiple ELISA assays need to be developed and performed to detect multiple cytokines in a single sample. This is time-consuming and laborious, and requires relatively large volumes of tissue samples. This problem can be circumvented by employing a multiplex bead array system, such as the Cytometric Bead Array (CBA) system, developed by BD Biosciences. It should be noted that this system is only one of a variety of multiplex bead arrays available, and using the CBA system requires a flow cytometer. Another available system utilizes the Luminex 100 bead reader and bead arrays kits available from Bio-Rad or Upstate. Given that flow cytometers are widely available to both clinical and basic science departments, we will focus on the CBA system in this chapter.
The BD CBA system combines the sensitivity of the flow cytometer with a multiplex particle-based immunoassay. Here, we focus on preconfigured BD CBA kits. It is also possible to design your own bead-based immunoassay using BD CBA Flex Sets. These Flex Sets are formulated to be mixed to any size plex of analytes of interest. For more details, we refer the reader to www.bdbiosciences.com. Currently, two different mouse BD CBA kits are available: the mouse inflammation kit (IL-6, IL-10, MCP-1, IFN-γ, TNF, IL-12 p70) and the mouse Th1/Th2 kit (IL-2, IL-4, IL-5, TNF, IFN-γ). We refer the reader to the manufacturers’ website (www.bdbiosciences.com/pharmingen/cba) for updated information and for information about human CBA kits. The BD Cytometric Bead Array (CBA) system employs a set of different suspended particles with discrete fluorescence intensities to simultaneously detect multiple soluble analytes, such as cytokines, in one sample with a flow cytometer. This technique allows fast determination of cytokine profiles in small sample volumes. Each subset of suspended beads is coated with different capture antibodies, and has a distinct fluorescent intensity in the FL3 channel, allowing them to be distinguished. For the assay, briefly, the different capture bead populations are mixed, incubated with recombinant protein standards or test samples, and subsequently incubated with PE-conjugated detection antibodies (measured in FL2) to form sandwich complexes. The standard and test samples are analyzed using the flow cytometer (we have good experience with the FACS caliber) and BD CBA Analysis Software.
3.3.2. Sample Preparation
See Subheadings 3.2.2, step 1 and 3.2.2, step 2
3.3.3. Bead Array Protocol
The Instruction Manual provided with the BD CBA kit gives excellent step-by-step instructions. Briefly:
Reconstitute a vial of lyophilized standard with 0.2 ml of Assay Diluent and allow it to equilibrate for at least 15 min. Please note: following reconstitution, use the Standard within 12 h.
Prepare serial standard dilutions according to the manufacturer’s recommendations.
Pool the individual capture beads immediately before use by vortexing and mixing an aliquot of 10 μl of each capture bead suspension for each test sample and standard sample.
Transfer 50 μl of mixed beads to each assay tube.
Prepare test samples; depending on the cytokine levels in the test samples, it may be necessary to dilute test samples with Assay Diluent to ensure that fluorescence intensity falls within the range of the standard curve. We obtained best results with undiluted tissue samples which were prepared according to the protocol described in Subheading 3.2.2, step 2.
Add 50 μl standard and test samples to the tubes containing mixed Capture beads.
Add 50 μl of PE-detection antibodies.
Incubate the tubes for 2 h at RT protected from light.
-
Meanwhile, perform the Cytometer Setup procedure:
(b) Add 50 μl Cytometer Setup beads to setup tubes A, B, and C.
(c) Add 50 μl of FITC-positive control to tube B and 50 μl of PE-positive control to tube C.
(d) Incubate tubes A, B, and C for 30 min at RT protected from light.
(e) Add 450 μl of Wash Buffer to tube A, and 400 μl of Wash Buffer to tubes B and C.
Proceed with the Standard and Test samples:
Add 1 ml of Wash buffer to each assay tube and centrifuge at 200 × g for 5 min.
Discard the supernatant.
Resuspend the bead pellet in 300 μl Wash buffer. The samples are now ready for analysis on a flow cytometer. For Cytometer Setup and Data acquisition, we refer the reader to the instruction manual of the BD CBA kit.
3.3.4. Data Analysis
BD CBA Software is essential for the data analysis. We refer the reader to the step-by-step software manual for details. In general, the Mean Fluorescence Intensities (MFIs) of the serially diluted standard samples are calculated by the software and used to generate the standard curves of each cytokine. The standard curves model the protein concentration as a function of the MFI. Before proceeding to analysis of the unknown samples, make sure the unknowns fall within the range of the standard curve. If the MFI is above the standard curve, the experiment has to be repeated with more dilute samples. Conversely, if the MFI is below the standard curve, the test has to be repeated with more concentrated samples. Any individual value that appears to be a clear outlier may be excluded and tested again in a following experiment. The software automatically calculates cytokine concentrations present in the test samples, using the corresponding standard curves and dilution factors. Sufficient replication within and across experiments is important for making precise estimates of both concentrations and errors.
3.4. Immunohistochemistry
3.4.1. Detection Method and Principle
There are two widely used methods for immunohistochemical detection of proteins. The first method relies on colorimetric detection (typically using secondary antibodies conjugated with biotin, a horseradish peroxidase-labeled avidin-biotin-complex reagent, and 3,3′-diaminobenzidine as a substrate). This method produces a brown or black (in the presence of nickel chloride) reaction deposit, which can be viewed using bright-field microscopy. The main advantage of this method is that it provides excellent morphology of tissue samples. However, key disadvantages are that this technique is time consuming and detection is monochromatic and can be used for only one antigen. One way to overcome the latter limitation is to combine this reaction with an alkaline phosphatase system, which yields a reaction product with red color, thus allowing simultaneous detection of two antigens. Yet, this not an optimal solution, as it can be difficult to separate red and brown signals, especially under conditions of intense staining.
The second method relies on indirect immunofluorescence detection. The principle involves incubating tissue sections with (1) a primary antibody for the cytokine of interest, and (2) a secondary antibody conjugated with fluorescent molecules and directed against the host immunoglobulin of the primary antibody. After staining, tissue sections are mounted with fluorescence-mounting medium (which protects fading of fluorescent molecules) and viewed under dark field with a fluorescence microscope. Key advantages of this method are its high sensitivity and the ability to separate different wavelengths of fluorescent emitted light (limited by the number of filter/mirror units on the fluorescence microscope, often up to four). Thus, up to three antigens plus a nuclear counterstain can typically be imaged on the same tissue section. A disadvantage is that cellular morphology is more difficult to discern than with the colorimetric detection system described earlier. However, for detection of cytokines, the advantages of this method usually outweigh the disadvantages, and we therefore present this method in detail in the present chapter. If the reader is also interested in the colorimetric method, we refer him/her to an excellent protocol supplied by Vector laboratories at http://www.vectorlabs.com/data/descriptions/pdf/PK7200.pdf.
3.4.2. Choosing Primary Antibodies
Choice of primary antibodies is often predetermined by testing and quality control offered by antibody suppliers. For example, companies such as Serotec, Santa Cruz Biotechnology, CALTAG laboratories, Chemicon International, and Becton Dickinson typically offer information on antibody data sheets as to their suitability for immunohistochemistry. This is sometimes accompanied by dilution information and incubation times. This latter information should, of course, be taken as a starting point and not as an optimized solution. A few points should be kept in mind when choosing primary antibodies:
Some cytokines are synthesized in an inactive form (e.g., TNF-α, IL-1β, and TGF). If the active (cleaved) forms of these cytokines are the focus, then appropriate antibodies against the active forms should be used, if available.
As with most assays, good negative and positive controls should be obtained to initially titrate and optimize primary antibodies. Companies will sometimes supply these controls as additional catalog items.
If multiple formats are offered for primary antibodies, always choose a purified format (see Note 34).
Make sure that all antibodies are from different hosts when using more than one primary antibody.
If antisera (e.g., serum taken from immunized rabbits and directly used for immunostaining) are to be used instead of purified primary antibodies, it is good practice to use a “normal serum” negative control (i.e., serum from nonimmunized animals, preferably even preimmune serum from the same animal) to account for nonspecific background.
Expect to titrate primary antibodies before drawing experimental conclusions; as mentioned earlier, company-supplied information can be a useful starting point (see Note 35).
3.4.3. Choosing Secondary Antibodies
There is now a wide variety of fluorescent-tagged secondary antibodies directed against immunoglobulins of various species. Older fluorophores (e.g., FITC, Cy3, PE, TRITC, Texas red, Cy5, Cy5.5) are still widely available and are commonly used for immunofluorescence. Yet, newer-generation fluorophores have become available that are often brighter and offer higher signal-to-noise ratios than their older-generation counterparts. For example, Alexa Fluors (developed by Molecular Probes, now Invitrogen) are now commonly conjugated to secondary antibodies and are available in almost any format that one would need. A few issues should be kept in mind when selecting fluorescent-labeled secondary antibodies:
Care should be taken to choose a fluorescence color combination of secondary antibodies that can be optimally detected by the filter/mirror units equipped with the available fluorescence microscope. These filter/mirror units often have “white pages” that detail their excitation and emission wavelength maxima. Such values can be compared with the candidate fluorophores to determine if detection would be optimal with a particular microscope configuration.
Fluorophores that have overlapping emission spectra should be avoided. If one of these fluorophores is particularly bright, there is a chance that it could bleed into the other fluorescence channel. This risk is minimized if their excitation spectra are sufficiently different and nonoverlapping.
Make sure that all antibodies are host compatible when revealing more than one primary antibody. For example, do not use a combination of goat antimouse and rabbit antigoat secondary antibodies, as these will crossreact. Unlike primary antibodies, secondary antibodies can be from the same host – and this is even the preferred scenario.
Try to avoid secondary antibodies conjugated with fluorophores that emit in the blue portion of the visible light spectrum (e.g., cascade blue, pacific blue, Alexa Fluor 350, AMCA). Many of these fluorophores are difficult to excite and are therefore poor emitters. Also, the blue channel is typically reserved for nuclear counterstaining with DAPI (which is easily excited and therefore a strong emitter).
3.4.4. Immunofluorescence Protocol
Processing samples for immunofluorescence begins with obtaining the tissues of interest, which can then be fixed, cryoprotected, stained, and mounted for viewing under dark field. Specific steps are outlined as follows:
Isofluorane is the anesthesia of choice, as it is fast acting and inexpensive. However, the experimenter must work quickly once the mouse is under anesthesia, as isofluorane wears off in a matter of minutes (see Note 36). Anesthetize mice by placing a small amount of 30% isofluorane on an absorbent cloth or cotton ball in a bell jar, and then cap the jar once the mouse is placed in it. Mice should be in deep anesthesia (slow breathing) if they are being perfused. If organs/tissues are being isolated without perfusion, then animals can be euthanized by isofluorane overdose, and the breathing rate of the animals (i.e., depth of anesthesia) need not be monitored.
While not an absolutely necessary step, it is good practice (see Note 37) to begin by perfusing mice (see Note 14).
Organs/tissues should be rapidly harvested and placed into ice-cold 4% PFA (where PFA volume is enough to completely immerse the sample). Samples are fixed overnight at 4 °C; the general rule of thumb is that it takes about 1 h for every 1 mm of tissue fixation. Generally, if the size of the organ/tissue sample is larger than what will easily fit into a 15-ml conical tube, the sample should be cut into pieces that will neatly fit.
The next day, 4% PFA is removed from the conical tube and processed for cryoprotection (see Note 38) by replacing the PFA with 10% sucrose diluted in PBS. When the samples fall to the bottom of the tube (or overnight at 4 °C), this is replaced with 20% and then 30% sucrose (where replacement is determined again by when the samples fall or overnight at 4 °C).
Samples are then embedded in OCT by orienting the organs/tissues in cryomolds and filling them with OCT. A small amount (one drop) of OCT is first placed into the cryomold to hold the organ/tissue in place.
Sample blocks are cut on a freezing microtome and applied to slides. Typically, a thickness setting of 10–25 μm is used. Thicker sections (e.g., 25–40 μm) allow for better sensitivity (and are well suited for deconvolution or confocal microscopy, where z-stacks are acquired), but make focus more difficult by epifluorescence microscopy (where a thickness of 10–25 μm is preferred). There is no substitute for practice when it comes to operation of the freezing microtome. With practice, it should be relatively straightforward to apply cryosections to slides without cutting artifacts such as uneven sectioning, curling of frozen sections, or bubbles under the sections.
Slides are air-dried for 5 min, and a PAP pen is applied to the border of the specimen to prevent solutions from leaking (see Note 39). One application of the PAP pen should be sufficient for the entire procedure – but if not, it should be reapplied as necessary. Slides are then placed into a humidified chamber (see Note 40).
Samples are preblocked with serum-free protein block for 30 min at ambient temperature (see Note 41).
The blocking reagent is poured off onto paper towels, and appropriate dilutions of the primary antibodies are added. The volume should be enough to completely cover the sample (typically 125–200 μl). Primary antibodies are incubated overnight at 4 °C in the humidified chamber, although incubation time/temperature can vary. For example, 1–2 h incubation at ambient temperature may be used.
Slides are rinsed three times for 5 min each in ambient-temperature PBS in a Coplin jar or staining dish.
Excess PBS is poured off of slides, and appropriate dilutions of the secondary antibodies are added. Slides are then incubated in a humidified chamber for 1 h at ambient temperature. Do not forget to cover the humidified chamber with aluminum foil to protect samples from light.
Slides are rinsed three times for five min each in ambient-temperature PBS in a Coplin jar or staining dish.
Slides are dipped into distilled H2O (to desalt the specimens/slides) and subsequently placed in a dark space to air dry. Once dried, slides are mounted with fluorescence-mounting medium (see Note 42) and appropriately sized cover slips. After overnight curing, slides can be viewed under dark field.
3.4.5. Fluorescence Microscopy: Acquiring and Interpreting Images
A detailed discussion of microscopic technique is beyond the scope of this chapter, and for that we refer the reader to an excellent textbook on the subject (Basic Methods in Microscopy: Protocols and Concepts from Cells: a Laboratory Manual by David L. Spector, Robert D. Goldman 2006, Cold Spring Harbor Laboratory Press). We will, however, briefly cover the topic as related to cytokine immunofluorescence.
Most modern fluorescence microscopes are equipped with a CCD camera system, which can generally be classified as (1) color, (2) black and white (grayscale), or (3) black and white with enhanced infrared sensitivity. In general, color CCD cameras are the least sensitive to fluorescence, and black-and-white cameras are the more sensitive. Black-and-white cameras with enhanced infrared capability are different from their black-and-white camera counterparts only in that they are more sensitive to picking up far-red wavelength emissions. If blue, green, and red fluorophores are being used exclusively, then there is no need for the enhanced infrared capability. However, if far-red fluorophores are being used (such as Alexa Fluor 647 or Cy5.5), then this type of camera is a must-have. Because of their greater sensitivity, black-and-white cameras (with or without extended infrared sensitivity) are often the cameras of choice for fluorescence microscopy, and color cameras are most often used for bright-field microscopy. With black-and-white cameras, it should be noted that fluorescent images need to be acquired separately in each channel, and then false-colored and merged using software such as Adobe Photoshop.
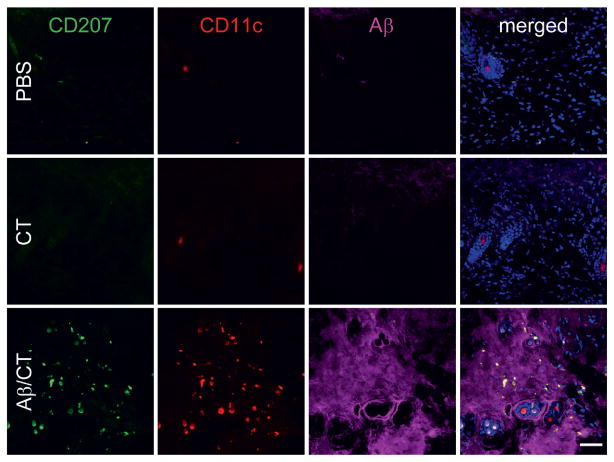
Interpretation of microscopic images in general can be difficult, and there is no substitute for experience. Interpretation of cytokine immunofluorescence poses additional difficulties. This is owed to the fact that cytokines are soluble molecules and are therefore more difficult to detect than membrane-bound proteins. Tissue samples may show (1) extracellular signal, (2) intracellular (vesicular) signal, or (3) a combination of both. The signal from cytokines will often have a punctate appearance, especially when intracellular. Having a nuclear counterstain (e.g., DAPI) is invaluable for establishing the location of the signal, as it reveals the region of the specimen occupied by the nucleus (and where cytokine signal should not be found). The experimenter should be acutely aware of possible artifacts and train him/herself to distinguish valid signal from artifact. For example, antibody precipitation artifact appears as a punctate pattern of immunoreactivity; but this pattern will be scattered in and around the tissue section in a haphazard fashion. Another common artifact is background signal, which can be difficult to discriminate from valid signal in some cases. Yet, a good negative control can be very helpful, as any signal in this sample would most likely be background. Specifically, a negative control should not contain the cytokine of interest. Perhaps the best example of a good negative control would be an organ/tissue from a cytokine-deficient (i.e., a gene knockout) mouse. For representative fluorescent images, see Fig. 2, where mice have been immunized via a transcutaneous route with a cytokine-like peptide (Aβ, a 42 amino acid-soluble peptide that is cleaved from a ~110-KDa transmembrane protein) plus the adjuvant cholera toxin (CT). In this experiment, the Aβ/CT group serves as a positive condition for detection of the Aβ peptide, while the CT alone or PBS control groups serve as negative controls. Note the lack of Aβ peptide signal in the DAPI-positive regions.
Finally, it should be mentioned that microscopic images can be quantified, although this approach is not as highly quantitative as others discussed in this chapter (e.g., real-time PCR, ELISA). There are a number of “freeware” programs available for this purpose, such as NIH Image (http://rsb.info.nih.gov/nih-image/) and Image J (http://rsb.info.nih.gov/ij/). These software packages will allow determination of positive pixels and total pixels, which can be represented as a ratio to measure staining intensity. Also, macros can be written for these software programs to allow counting of positive signals and even more advanced quantification such as length, diameter, and shape of signals. It should be stressed that, if quantitative microscopy is the goal, all images should be acquired using the same microscope settings (including filter and exposure parameters), and image acquisition should be performed from samples processed side by side.
Fig. 2.
Skin confocal micrographs of a cytokine-like peptide (Aβ) and immune cell-surface markers after transcutaneous immunization. To characterize dermal immune responses to Aβ plus cholera toxin (Aβ/CT) transcutaneous (t.c.) immunization, skin tissues were prepared from nontransgenic C57BL/6 mice t.c.-immunized for 18 h with PBS (control, Top), CT alone (Middle), or Aβ/CT (Bottom) as indicated and then analyzed by laser scanning confocal microscopy with the indicated antibodies (antibody 4G8 was used to reveal Aβ). Note the presence of CD207 + CD11c+ Langerhans cells in Aβ-positive regions in the Aβ/CT t.c.-immunized group. DAPI (blue signal) was used as a nuclear counterstain in merged images shown to the right. (Scale bar: 50 μm.). This figure and figure legend was reproduced with permission from Proceedings of the National Academy of Sciences of the United States of America.
5. Conclusion
We have presented a number of techniques in this chapter aimed at measuring expression of cytokines in samples derived from in vivo sources. As with most methodologies, each of these has associated advantages and disadvantages, which we have discussed. One approach to overcoming limitations of any one protocol is to use more than one method for each experiment. For example, both real-time PCR and immunohistochemistry can be used to allow highly quantitative mRNA results (real-time PCR) and localization of protein within the organ/tissue (immunohistochemistry). Another example would be using 50% of an organ to homogenize and assay by ELISA, and the other 50% for immunohistochemistry. In conclusion, the type of cytokine data that the experimenter is interested in obtaining must often serve as a guide for determining which method is best suited for gleaning results.
Acknowledgments
The authors would like to thank Dr. J. Magarian Blander for critical reading of the manuscript and help with the section on real-time PCR primer-probe sets. We further thank A. Antov for providing template real-time PCR graphs. T.T. is supported by an Alzheimer’s Association grant and a National Institutes of Health “Pathway to Independence” award (1K99AG029726 and 4R00AG029726). D.A. is supported by an AMC fellowship and by an award from the Landsteiner Foundation. K.E.dV is supported by a grant from the Dutch Cancer Society (NKI 2006–3715).
Footnotes
Traditionally researchers have been using DEPC-treated water. We routinely use Ultrapure DNase/RNase-free distilled water from Invitrogen (#10977-049), which is a more convenient alternative.
Superscript III has greater thermal stability than Superscript II. Therefore, the cDNA reaction can be performed at a higher temperature (60 °C instead of 72 °C), allowing better read through of RNAs with complex secondary structures (which are relaxed at higher temperatures). Nonetheless, we have had very good experiences with Superscript II as well. Since Superscript III is superior for certain templates, all samples to be compared in an experiment must be primed with the same enzyme, to avoid false differences.
Random hexamers can be used for priming of the cDNA reaction instead of (or together with) Oligo d(T). This results in a more complex cDNA mixture, also representing ribosomal as well as immature RNA (which are not polyadenylated). However, this is usually not a problem. The use of random hexamers is recommended in cases where the target sequence for the PCR is on the 5′ end of the RNA, as these regions tend to be under-represented in Oligo d(T)-primed cDNA.
Avoid exposing fluorescent probes to light. Store probes at −70 °C. Probes should be HPLC purified.
Other polymerases can be used. Amplitaq Gold is convenient, as in this preparation the enzyme is kept inactive, until the PCR reaction is initiated with a hot start. Thus, spurious polymerization on nonspecific targets at low temperatures is avoided. Other enzymes are available with similar properties, such as Platinum Taq (Invitrogen #10966-018). Regular polymerases can also be used, as long as they have 5′–3′ exonuclease activity, which is necessary for cleaving the quencher off of the probe. We have, for instance, used Tsg successfully. However, when using such enzymes the researcher must use a program with a hot start and keep samples at 4 °C until right before putting the samples into the (hot) PCR apparatus.
Go to http://www.biocompare.com for a comparison of several instruments.
It is best to use the PCR plates and optical adhesive film recommended by the manufacturer of the real-time PCR apparatus, as not all plates work well in all machines.
RTPrimerDB (http://medgen.ugent.be/rtprimerdb/), Primer-Bank (http://pga.mgh.harvard.edu/primerbank/index.html), and Real-Time PCR Primer Sets (http://www.realtimeprimers.org) provide lists of validated quantitative PCR assays.
These primers all use BHQ as quencher. We have also had good results with TAMRA-linked probes.
Many commercially available ELISA kits with precoated ELISA plates are available. Such ready-to-use ELISA kits are a good option if limited numbers of samples need to be tested. However, for large sample numbers, it is more cost effective to buy individual antibodies and perform the ELISA as detailed in the protocol described here.
Detection and capture antibody must recognize different epitopes. Polyclonal antibodies for ELISA are suboptimal, as these recognize multiple antigens and therefore lack specificity and yield high backgrounds. It is best to use directly conjugated (e.g., with biotin, HRP or AP) detection antibodies. If these are not available, it is recommended to conjugate the detection antibody using a commercially available conjugation kit for instance to biotin, available from many manufacturers (e.g., Sigma or Pierce).
Although we have consistently had good results using HRP-based detection with TMB as substrate, some researchers prefer to use alkaline phosphatase (AP) for detection. P-nitrophenyl phosphate (PNPP) is used as substrate for this enzyme. Signal will show up as a yellow color. Absorbance may be read directly at 405 nm, or the reaction may be stopped by adding Stop solution. Stopping this reaction also results in a yellow end product that can be read at 405 nm.
Absolute quantification of mRNA abundance would require having a reliable standard of known quantity. Using a DNA standard does not give reliable results, because there are significant differences in the kinetics of the PCR reaction between samples containing DNA template or a complex mixture of cDNAs. The closest approximation of absolute quantity could be obtained by mixing a DNA standard with a cDNA sample known not to express the gene of interest (for instance yeast cDNA). Even then, the quantity of transcripts is likely to be underestimated given that the efficiency of copying each RNA molecule into cDNA of sufficient length to be measured is probably below 100%.
For cardiac perfusion mice must be anesthesized first. Isofluorane is the anesthesia of choice, as it is fast acting and inexpensive. However, the experimenter must work quickly once the mouse is under anesthesia, as isofluorane wears off in a matter of minutes. Anesthetize mice by placing a small amount of 30% isofluorane (diluted in polypropylene glycol) on an absorbent cloth or cotton ball in a bell jar, and then capping the jar once the mice are placed in it. One can also prepare a 15-ml conical tube with cotton balls soaked in 30% isofluorane. This can be placed over the nose of the mouse to keep the animal in deep anesthesia. Mice should be in deep anesthesia (slow breathing). Alternatively, more accurate anesthesia can be obtained by an anesthesia machine using a 1:1 mixture of isoflurane and oxygen. The chest is carefully opened (taking care not to lacerate any major blood vessels), and a hemostat (Roboz #RS-7291 and #RS-7231) is used to maintain access to the chest cavity. Perfusion is accomplished by injecting 60 ml of ice-cold PBS into the heart. The syringe is filled with the PBS, a butterfly needle (23 gauge, Becton Dickinson #36-7283) is attached, and any air bubbles are released by depressing the plunger (it is important to remove any air bubbles from the syringe prior to perfusion as these will become trapped in capillaries/capillary beds and greatly reduce the efficiency of perfusion.). The butterfly needle is then inserted into the left ventricle and clamped into place with a hemostat. The right atrium is then lacerated, and the PBS is slowly pushed through the heart (the 60 ml should be injected over 12–15 min). Ideally, this should not be done by hand, but with an infusion pump for cardiac perfusion (e.g., KDS100, kdScientific Inc.).
Paraffin tissue sections cannot use oligo dT, because formaldehyde fixation results in loss of polyA tails.
While, in theory, the difference in concentration between two samples could be calculated from the difference in cycles required to cross the threshold (every cycle representing a doubling of the amplicon), this is often not accurate. Titration of a standard allows one to draw a standard curve and use the slope of the curve to determine the true difference between different samples.
One strategy is to use a purified plasmid containing the cytokine gene of interest, where the plasmid quantity has been determined by a spectrophotometer (in ng). This plasmid can then be serially diluted (typically seven 10-fold dilutions beginning with 1 ng) and used as a standard alongside experimental samples. Alternatively, the cytokine cDNA (or just the region containing the amplicon for real-time PCR) can be amplified by regular PCR, isolated, and its concentration measured by spectrophotometry. It is important to realize, however, that amplification of single-strand targets in a mixed cDNA obtained from reverse transcription of cellular RNA does not necessarily have the same kinetics as amplification of a purified DNA template. A good solution is to use a single-strand cDNA sample obtained from positive control cells for this titration. Good sources for positive controls for measurement of IL-2, IFN-γ, IL-6, IL-10, IL-13 and IL-17 would be cDNA from in vitro skewed T helper cells; for TNF-α, IL-6, IL-12, and type I IFN, macrophages or dendritic cells activated in vitro with 50 ng/ml of LPS for 3 h could be utilized.
The Superscript II cDNA synthesis protocol recommends using no more than 1/10 of a cDNA reaction per sample in a 50-μl PCR, as this can actually result in lower PCR efficiency. This means that 1/25 can be used maximally in a 20-μl PCR reaction. As 5 μg is the maximum amount of input RNA for a 20-μl first-strand cDNA reaction, the maximum amount of cDNA per PCR reaction is 0.2 μg worth of total RNA
While most manufacturers recommend 50-μl volumes for each sample, we routinely do our measurements in 20 μl, significantly increasing economy.
Applied Biosystems also sells 2× premixes of Amplitaq Gold, buffer and MgCl2 dNTPs and ROX with (#4309155) and without (#4370048) SYBR green.
The MgCl2 concentration is very important for the performance of the PCR. The higher the MgCl2, the greater the yield of PCR product. However, nonspecific products are often also better amplified at higher MgCL2 concentrations. Using a MgCl2 concentration of 2.5 mM works well for many real-time PCRs. When problems are encountered (too little signal or nonspecific products), titrating the MgCl2 in the window between 1 and 4 mM (using 0.5 mM increments) sometimes helps. Different polymerases can have different optima, but Tsg and Taq (and its derivatives) works well at 2.5mM MgCl2.
dNTPs are not very stable. Small stocks (200 ml) should be stored at −20 °C. Stocks can be refrozen 3–4 times, but must be kept at 4 °C when thawed.
ROX is a fluorescent dye, which can be included in the reaction as an inert reference. As the signal by this dye does not change during the reaction, it can be used to control for pipetting errors. Perfectly good results can be obtained without this reference, however.
In highly vascularized tissues, tissue-cytokine levels can be obscured by cytokine levels present in “contaminating” blood. To obtain the most accurate measurement of tissue cytokine levels, presence of blood can be minimized by cardiac perfusion of mice with PBS prior to isolation of tissues (See Note 14).
While several of the capture and detection antibodies might work at lower concentrations, this concentration works well for all antibody sets listed in Subheading 2.2.
Some researchers prefer to use a bicarbonate buffer for coating, but we had also good success with PBS.
Shorter incubations work, but may reduce the sensitivity of the assay.
Prevent plates from drying out during the protocol. Therefore, don’t leave plates standing around without liquid for more than just one minute.
A good way is to prepare twofold concentrated dilutions of both samples and standard samples. That way, the plates can be filled with 50 μl medium first, to avoid drying out. Samples and standards are added later in 50 μl.
Range of cytokine ELISA values from in vivo sources (e.g., tissue/organ homogenates, serum/plasma) can vary widely – from pg to ng – depending on the particular experiment and the source of the sample. For example, if measuring cytokines in mouse serum/plasma, one may need to dilute the sample anywhere from 1:4 to 1:200. For this reason, it is often preferable to do an initial check of dilution factors using known positive control sample(s). Once a suitable dilution factor is identified, experimental samples can then be assayed en masse at this dilution point. Keep in mind that more concentrated samples will have higher background, and so a good negative control should be used to establish background levels of the cytokines being assayed.
50-μl volumes work well too, if sample amount is limiting. In that case, also use 50 μl of capture antibody coating as well as of detection antibodies, substrate, and stop solution.
Secondary antibodies directly coupled to enzymes are available for some cytokines. However, the use of biotinylated detection antibodies tends to allow for more sensitivity, since a single antibody can carry multiple biotin moieties, resulting in amplification of the signal.
Assays that develop very rapidly may yield artificial differences between samples, due to small differences in the development time of different samples. In that case, one should perform lower dilutions. Alternatively, alkaline phosphatase can be used for detection, as this system tends to develop more slowly (see Note 12).
Generally, purified antibodies are preferred for indirect immunofluorescence, as these offer most flexibility in terms of colors for fluorescent-tagged secondary antibodies. Indirect immunofluorescence is preferred to a direct method (i.e., using fluorescent-labeled primary antibodies without a secondary antibody) for detection of cytokines as it will offer enhanced sensitivity. Also, biotinylated primary antibodies can be used. These can be used along with substituting streptavidin conjugated with a fluorophore in place of a species-directed secondary antibody, if desired. These also offer enhanced sensitivity due to the presence of multiple Biotin moieties per antibody, each of which can bind streptavidin.
If information is provided for antibody dilution, it is common practice to “bracket” dilutions, including two below and two above the suggested dilution for titration. For example, if 1:200 is provided, include 1:50, 1:100, 1:200, 1:500, and 1:1000 in the dilution series. Positive control tissue should be used for the titration.
If isofluorane anesthesia wears off prematurely, one can prepare a 15-ml conical tube with cotton balls soaked in 30% isofluorane. This can be placed over the nose of the mouse to keep the animal in deep anesthesia.
Perfusion removes contaminating blood cells from the tissue of interest. This is important as it (1) removes staining signal from these cells, which is likely not of interest, and (2) removes background signal from red blood cells (RBCs). RBCs autofluoresce and are therefore a source of high background.
Cryoprotection is a good practice as it greatly improves the morphology of the tissue sample. If morphology is not a concern, then this step can be omitted.
The PAP pen “ink” is actually a waxy glue that needs time to air dry. 5 to 10 min is typically long enough to accomplish this.
Humidified chambers for histology can be purchased from commercial sources. They can also be “home made” by obtaining a pyrex casserole dish, taping parallel serological pipettes, 2–3 in. apart, to the bottom of the dish, and placing moist paper towels on the bottom. Slides can then be placed on the serological pipettes and the entire apparatus can be covered by plastic wrap and aluminum foil (to keep the inside dark during fluorescent antibody steps). It is very important that tissue sections do not dry out during the course of the procedure.
If cost is an issue, blocking solution can be made up by diluting 10% serum of the host of the secondary antibody. However, we have found that the commercially available serum-free protein block is a more effective blocking reagent.
Fluorescence-mounting medium can also be “home made” (for a description of how to make this, see “Basic Methods in Microscopy: Protocols and Concepts from Cells: a Laboratory Manual by David L. Spector, Robert D. Goldman 2006, Cold Spring Harbor Laboratory Press”). However, in our experience, ProLong Gold works exceptionally well, is easy to apply, and hardens (cures) overnight to prevent the cover slip from moving.
References
- 1.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 4.Le T, Leung L, Carroll WL, Schibler KR. Regulation of interleukin-10 gene expression: possible mechanisms accounting for its upregulation and for maturational differences in its expression by blood mononuclear cells. Blood. 1997;89:4112–4119. [PubMed] [Google Scholar]
- 5.Scheu S, Stetson DB, Reinhardt RL, Leber JH, Mohrs M, Locksley RM. Activation of the integrated stress response during T helper cell differentiation. Nat Immunol. 2006;7:644–651. doi: 10.1038/ni1338. [DOI] [PubMed] [Google Scholar]
- 6.Petrilli V, Papin S, Tschopp J. The inflammasome. Curr Biol. 2005;15:R581. doi: 10.1016/j.cub.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 7.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 8.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 12.Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protocol. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]