Abstract
We recently discovered that progestin in hormone replacement therapy (HRT) for postmenopausal women has detrimental effects on the ear and central auditory system (Guimaraes et al., Proc. Nat. Acad. Sci. – USA, 103: 14246-9). To start determining the generality and neural bases of these human findings, the present study examined the effects of combination HRT (estrogen+progestin) and estrogen alone on hearing in perimenopausal mice. Specifically, auditory brainstem responses (ABRs-sensitivity of the auditory system) and distortion product otoacoustic emissions (DPOAEs-cochlear outer hair cell system) were employed. Middle age female CBA mice received either a time-release, subcutaneous implanted pellet of estrogen+progestin, estrogen alone, or placebo. Longitudinal comparisons of ABR threshold data obtained at 4 months of treatment revealed statistically significant declines in auditory sensitivity over time for the combined estrogen+progestin treatment group, with the estrogen only group revealing milder changes at 3, 6 and 32 kHz. DPOAE testing revealed statistically significant differences for the estrogen+progestin treatment group in the high and middle frequency ranges (15–29 and 30–45kHz) after as early as 2 months of treatment (p<0.01 and p<0.001, respectively). Statistically significant changes were also seen at 4 months of treatment across all frequencies for the combined HRT group. These data suggest that estrogen+progestin HRT therapy of 4 months duration impairs outer hair cell functioning and overall auditory sensitivity. These findings indicate that estrogen+progestin HRT may actually accelerate age-related hearing loss, relative to estrogen monotherapy; findings that are consistent with the clinical hearing loss observed in aging women that have taken combination HRT.
Keywords: Aging, Hearing Loss, Presbycusis, HRT, Otoacoustic Emissions, Progestin, Progesterone, Estrogen, Auditory Brainstem Response, Sex Hormones
Introduction
Presbycusis, hearing loss with increasing age, is often characterized by an initial decrease in hearing sensitivity in the high frequencies, with later progression into the lower frequencies. As our population continues to age, the number of individuals with presbycusis, and ultimately its negative effects on speech perception, cognition and language comprehension grows rapidly. Specifically, presbycusis is the foremost communication disorder, and one of the top three chronic medical conditions of our aged population (Pleis and Lethbridge-Çejku, 2007).
Several studies have indicated that decreased hearing acuity can have extensive psychosocial consequences such as depression, isolation and decreases in cognitive functioning among the elderly (Mader 1984; Mulrow et al. 1990). Coupled with the difficulties understanding speech in background noise characteristic of presbycusis, efforts have been made to broaden our understanding of the underlying neural mechanisms (Willott 1991, Frisina and Walton, 2001; Frisina and Rajan, 2005), hopefully leading to better preventative and therapeutic interventions. Crucial to this understanding is determining the potential roles that pharmacotherapy, including use of hormone therapies, may play in interventions aimed at slowing or reducing the progression of presbycusis.
Recent research has indicated that the use of hormone replacement therapy (HRT) has many negative effects on women’s health (e.g., Pradhan et al., 2002; Hulley et al., 2002); however its effect on the auditory nervous system is still not clear. The confirmed presence of estrogen α and β receptors within the inner ear sensory cell nuclei suggests a physiological role for sex hormones in auditory perception (Stenberg et al., 1999, 2003). Related studies have found that estrogen may have a central nervous system role in sensory processing by decreasing auditory brainstem response (ABR) neurophysiological latencies, thereby increasing temporal processing capabilities. While the effects of estrogen on higher cognition and conditions such as Alzheimer’s disease remains unclear, we have recently elucidated the negative effects of combination HRT on hearing sensitivity and central auditory speech processing in background noise, the number one complaint of hearing-impaired persons (Guimaraes et al., 2006). In contrast, some studies have suggested that increased levels of serum estrogen are associated with positive effects on the hearing sensitivity of simple sounds of postmenopausal women (Kim et al., 2002; Kilicdag et al., 2004; Hultcrantz et al., 2006). Studies investigating physiological testing end points, such as the ABR have confirmed differences in absolute waveform latencies and interpeak latencies between controls and postmenopausal women taking estrogen therapy, suggesting the auditory pathway is sensitive to hormonal replacement, and confirming the functional importance of estrogen receptors for auditory processing (Caruso et al., 2003).
The aim of the present investigation was to examine the effects of estrogen+progestin combined HRT and estrogen alone on age-related changes in auditory processing using neurophysiological testing measures, including ABRs and distortion-product otoacoustic emissions (DPOAEs) for the perimenopausal CBA mouse model of presbycusis. Progestin is the synthetic version of progesterone, with the former most often used clinically as a component of combination HRT. Comparisons with the human clinical condition allow for assessment of this useful animal model for investigations of hormonal effects on sensory aging.
Material and Methods
This prospective study involved 25 female middle-aged (15–17mon) CBA mice with ovaries intact and 7 males in the same age group. Twenty-two of these animals were obtained from National Institute of Aging and the remainder were bred in-house, and housed under institutional protocol. Females were randomized into treatment or placebo groups and baseline measures of DPOAEs and ABRs were obtained for all groups, including males. Females then received a subcutaneous placement, via syringe injection directly in the skin behind the shoulder (no anesthetic required), of two consecutive 60-day time released pellets (Innovative Research of America, Sarasota, FL) of either 17β estradiol (n=6, 0.006 mg/day), progestin + 17β estradiol (n=9, 0.4 mg/day + 0.006 mg/day respectively), placebo (n=10). Males (n=7) were untreated. These doses were selected to provide physiological concentrations of estrogen and progestin to achieve the same concentrations as previous studies (Albertson et al., 1975; Sharpe et al., 1991). Longitudinal data for DPOAE amplitudes and ABR thresholds were obtained monthly over 4 mon, using procedures similar to our previous reports (Jacobson et al. 2003; Guimaraes et al. 2004, Varghese et al., 2005).
Neurophysiological Recording Measures
Ipsilateral acoustic stimulation and simultaneous measurement of DPOAEs was accomplished with a Tucker-Davis (TDT, Alachua, FL) BioSig System III. Stimuli were digitally synthesized at 200 kHz using SigGen software applications with the ratio of f2/f1 constant at 1.25, and L1=65 dB and L2=50 dB SPL as calibrated in a 0.1cc coupler simulating the mouse ear canal. After synthesis, F1, F2 and the wideband noise were each passed through an RP2.1 D/A converter to PA5 programmable attenuators. Following attenuation, the signals went to ED1 speaker drivers which fed into the EC1 electrostatic loudspeakers coupled to the ear canal via short flexible tubes with rigid plastic tapering tips. For DPOAE measurements, resultant ear canal sound pressure was recorded with an ER10B+ Low Noise microphone and probe (Etymotic, Elk Grove Village, IL) housed in the same coupler as the F1 and F2 speakers. The output of the ER10B+ amplifier was input to an MA3 microphone amplifier, whose output went to an RP2.1 A/D converter for sampling at 200 kHz. An FFT was performed on the resultant waveform. The magnitude of F1, F2, the 2f1–f2 distortion product and the noise floor of the frequency bins surrounding the 2f1–f2 component were measured from the FFT. The procedure was repeated for geometric mean frequencies ranging from 5.6 to 44.8 kHz to adequately assess the functional range of mouse hearing.
Mice with clearly visualized, healthy tympanic membranes were included. Mice were anesthetized with a mixture of ketamine/xylazine (120 and 10 mg/kg body weight, respectively, intraperitoneal injection) prior to all experimental sessions. All recording sessions were completed in a soundproof acoustic chamber. Prior to recording, the stimulus probe and microphone were placed near the TM with the aid of the operating microscope. The stimulus was administered monotically under quiet conditions to obtain the DPOAE.
ABRs were recorded with subcutaneous platinum needle electrodes placed at the vertex (non-inverting input), right mastoid prominence (inverted input), and tail (indifferent site). EEG activity was differentially amplified (50k or 100k; Grass Instruments Model P511 EEG amplifier, West Warwick, RI), then input to an A/D converter (TDT AD1), and digitized at 50 kHz. Each averaged response is based on 300–500 stimulus repetitions recorded over 10 ms epochs, for 3, 6, 12, 24, 32 and 48 kHz. Contamination by muscle and cardiac activities was prevented by rejecting data epochs in which the single trace EEG contained peak-to-peak amplitudes of exceeding 50 microV. During this procedure, a general anesthetic, Avertin (Tribomoethanol, 5 mg/10 gm body weight IP) was used to anesthetize the mice. Duration of testing was approximately one hour/animal.
Statistical Analysis
Results for each animal were averaged by frequency and applied towards group mean data. Statistical analysis utilized a repeated measure two-way analysis of variance (ANOVA) across placebo, estrogen (E), estrogen+progestin (E+P) and the male subject groups. When an ANOVA main effect was statistically significant at the p<0.05 level or better, then Bonferroni’s post hoc t tests, corrected for multiple comparisons, were run to determine if statistically significant differences existed between specific groups.
Results
Auditory Brainstem Response – Peripheral Auditory System Sensitivity
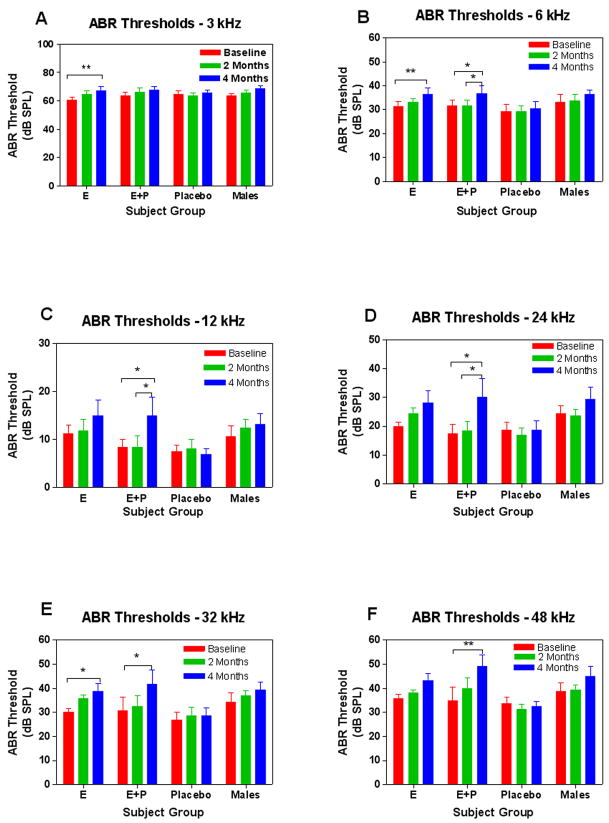
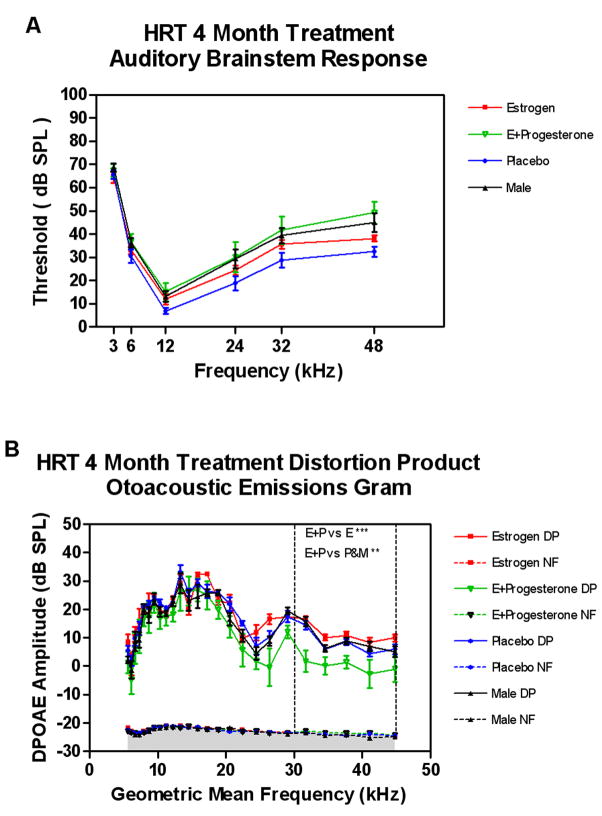
Subject groups were compared longitudinally across the four-month HRT treatment interval. Analysis of individual treatment data for ABR thresholds, revealed statistically significant differences across all frequencies above 3 kHz over time for the E+P group, as shown in Figure 1. Individuals treated with E only, showed significant changes over time at 3, 6 and 32 kHz only, while the placebo and male groups did not display these corresponding changes. These data showed higher thresholds across all frequencies for the E+P treatment group, with a less significant alteration in the E only group. As a general trend, larger changes can be observed with increasing frequencies in most groups, with the E+P group revealing the strongest trend for age-progressive hearing impairment, particularly at the 4 month time point. Group threshold data were then compared across baseline and 4 month conditions. No significant differences between groups were observed at baseline, but a statistically significant difference between the E+P and placebo groups for the 32 and 48 kHz conditions was observed at 4 months of treatment, as displayed in Figure 2A. In addition, a trend toward increasing thresholds over time was present in the male group vs. placebo at 48 kHz. Full statistical details can be viewed in Table 1.
Figure 1.
Longitudinal comparisons among groups revealed statistically significant changes to ABR thresholds at 4 mon of treatment when compared to baseline, across 6–48 kHz for the E+P group. The estrogen only treatment group revealed statistically significant changes at 3, 6 and 32 kHz only, with no corresponding changes in either the placebo or male groups. Error bars represent standard error of the mean (SEM).
Figure 2.
A) Comparisons of ABR absolute threshold changes at 4 mon of treatment revealed a general trend toward increasing threshold changes with increasing frequency with the largest trend seen in the E+P treatment group. A two way ANOVA for HRT treatment at 4 mon revealed significant differences in ABR thresholds when comparing E+P vs. Placebo at 32 and 48 kHz, and for Placebo vs. Male at 48 kHz. B) A one-way ANOVA for HRT treatment at 4 mon treatment revealed statistically significant differences in otoacoustic emissions amplitudes when comparing the E+P vs. Estrogen only, Placebo and male groups within the high frequency range (dashed vertical lines). DP – DPOAE amplitude; NF – noise floor amplitude. Error bars represent standard error of the mean (SEM).
Table 1.
Two-Way Repeated Measures ANOVA for ABR Thresholds (Subject Group × Time)
| 3 kHz |
6 kHz |
||||
|---|---|---|---|---|---|
| F time(2,52)=8.48 | p=0.0007 | F time(2,52)=12.2 | p<0.0001 | ||
| Bonferroni Post-test | Pre vs. 4 Mon | 2 Mon vs. 4 Mon | Bonferroni Post-test | Pre vs. 4 Mon | 2 Mon vs. 4 Mon |
| E | t=3.21, p<0.01 | ns | E | t=3.35, p<0.01 | ns |
| E+P | ns | ns | E+P | t=2.90, p<0.05 | t=2.90, p<0.05 |
| Placebo | ns | ns | Placebo | ns | ns |
| Male | ns | ns | Male | ns | ns |
|
| |||||
|
12 kHz |
24 kHz |
||||
| F time(2,52)=4.02 | p=0.024 | F time(2,52)=7.48 | p=0.0014 | ||
|
| |||||
| Bonferroni Post-test | Pre vs. 4 Mon | 2 Mon vs. 4 Mon | Bonferroni Post-test | Pre vs. 4 Mon | 2 Mon vs. 4 Mon |
|
| |||||
| E | ns | ns | E | ns | ns |
| E+P | t=2.67, p<0.05 | t=2.67, p<0.05 | E+P | t=3.09, p<0.05 | t=2.89, p<0.05 |
| Placebo | ns | ns | Placebo | ns | ns |
| Male | ns | ns | Male | ns | ns |
|
| |||||
|
32 kHz |
48 kHz |
||||
| F time(2,52)=8.40 | p=0.0007 | F time(2,52)=8.83 | p<0.0005 | ||
|
| |||||
| Bonferroni Post-test | Pre vs. 4 Mon | 2 Mon vs. 4 Mon | Bonferroni Post-test | Pre vs. 4 Mon | 2 Mon vs. 4 Mon |
|
| |||||
| E | t=2.82, p<0.05 | ns | E | ns | ns |
| E+P | t=3.02, p<0.05 | ns | E+P | t=3.82, p<0.01 | ns |
| Placebo | ns | ns | Placebo | ns | ns |
| Male | ns | ns | Male | ns | ns |
ANOVA: Analysis of variance, Pre: Pretreatment, Mon: Post-treatment month, ns: Not significant
Distortion Product Otoacoustic Emissions – Outer Hair Cell System
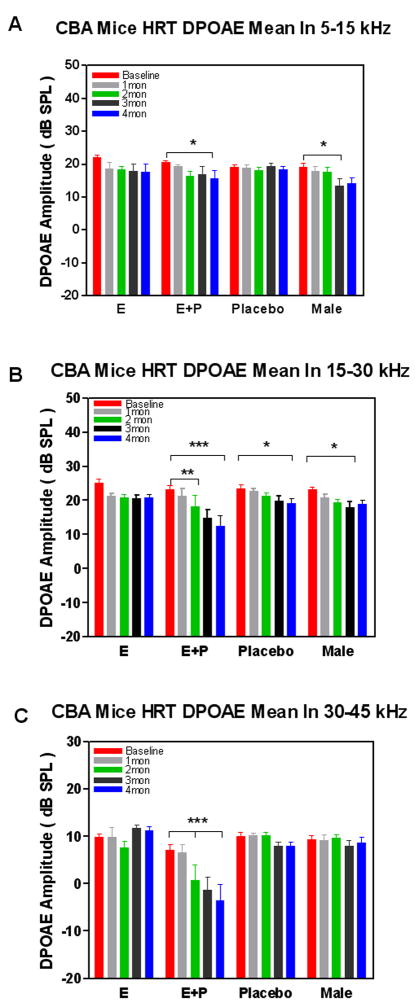
For DPOAEs, groups were compared within their respective treatment groups at baseline and 1–4 months of treatment (Figure 3). Analysis of the middle frequency (15–30 kHz) and high frequency (30–45 kHz) range for DPOAEs at 2 months of treatment revealed a statistically significant difference when compared to baseline in the E+P group only. In addition, analysis at 4 months also revealed a statistically significant difference in DPOAE amplitude in the high frequencies for the E+P treatment group when compared to the baseline and 2-month values (Figure 3C). However, there were no corresponding statistically significant changes observed in the E and placebo groups in this frequency range. Measures obtained in the mid frequency range (15–30 kHz) showed significant changes when compared longitudinally within all groups, except the E only group (Figure 3B). The E+P group again revealed the most dramatic change over time with DPOAE amplitude changes observed as early as 2 months, and then again at 4 months. Similar comparisons in the placebo and male groups revealed significant aging changes at the 4 month condition only. There were no comparable changes in the estrogen group. In the low frequency region (5–15 kHz) significant differences were observed in the E+P and male groups at 4 months when compared to their baseline values (Figure 3A). There were no corresponding changes observed in the E only and placebo groups within this frequency range. Statistical data are summarized in Table 2. A ANOVA of the DPOAE-gram data comparing baseline amplitude across frequencies and between groups revealed no statistically significant changes across the entire 5–45 kHz frequency range for the subject groups of the present investigation. Comparisons made after 4 months of treatment revealed statistically significant changes in the high frequency range (30–45 kHz) when comparing the E+P vs. E group and the E+P vs. placebo and male groups (Figure 2B, between vertical dashed lines).
Figure 3.
Longitudinal comparisons of otoacoustic emissions amplitudes among groups revealed statistically significant changes across: A) low (5–15 kHz), B) middle (15–30 kHz) and C) high (30–45 kHz) frequency ranges for the E+P treatment group at 4 mon of treatment, and as early as 2 mon in the middle and high frequency ranges. Less significant changes were seen within the middle frequency range at 4 mon for the placebo and male groups. Error bars represent standard error of the mean (SEM).
Table 2.
Two-Way Repeated Measures ANOVA for DPOAEs (Subject Group vs. Time)
| 5–15kHz | |||
|---|---|---|---|
| F time (4,112) = 5.267 | p = 0.0006 | ||
| Bonferroni Post-test | Pre vs. 2 Mon | Pre vs. 3 Mon | Pre vs. 4 Mon |
| E | ns | ns | ns |
| E+P | ns | ns | t=2.872, p< 0.05 |
| Placebo | ns | ns | ns |
| Male | ns | t=2.836, p< 0.05 | t=2.562, p< 0.05 |
| 15–30kHz | |||
|---|---|---|---|
| F time (4,112) =14.81 | p < 0.0001 | ||
| Bonferroni Post-test | Pre vs. 2 Mon | Pre vs. 3 Mon | Pre vs. 4 Mon |
| E | ns | ns | ns |
| E+P | t=3.104, p<0.01 | t=5.057, p<0.001 | t=6.496, p<0.001 |
| Placebo | ns | ns | t=2.717, p<0.005 |
| Male | ns | t=2.759, p<0.05 | ns |
|
| |||
| Bonferroni Post-test | 1 Mon vs. 3 Mon | 1 Mon vs. 4 Mon | 2 Mon vs. 4 Mon |
|
| |||
| E | ns | ns | ns |
| E+P | t=3.930, p<0.001 | t=5.369, p<0.001 | t=3.392, p<0.01 |
| Placebo | ns | ns | ns |
| Male | ns | ns | ns |
| 30–45kHz | |||
|---|---|---|---|
| F time (4,112) =4.687 | p = 0.0016 | ||
| Bonferroni Post-test | Pre vs. 2Mon | Pre vs. 3 Mon | Pre vs. 4 Mon |
| E | ns | ns | ns |
| E+P | t=3.862, p<0.001 | t=5.088, p<0.001 | t=6.408, p<0.001 |
| Placebo | ns | ns | ns |
| Male | ns | ns | ns |
|
| |||
| Bonferroni Post-test | 1 Mon vs. 2 Mon | 1 Mon vs. 3 Mon | 1 Mon vs. 4 Mon |
|
| |||
| E | ns | ns | ns |
| E+P | t=3.494, p<0.01 | t=4.720, p<0.001 | t=6.039, p<0.001 |
| Placebo | ns | ns | ns |
| Male | ns | ns | ns |
|
| |||
| Bonferroni Post-test | 2 Mon vs. 4 Mon | ||
|
|
|||
| E | ns | ||
| E+P | t=2.545, p<0.05 | ||
| Placebo | ns | ||
| Male | ns | ||
ANOVA: Analysis of variance, Pre: Pretreatment, Mon: Post-treatment month, ns: Not significant
Discussion
Combination HRT Negatively Affects Hearing
Consistent with our previous discovery of the negative influences of progestin+estrogen on the human auditory system of post-menopausal women (Guimaraes et al., 2006), the results of the current investigation in perimenopausal mice revealed worse hearing in the E+P group, relative to E alone and the placebo mice. For ABR thresholds, a small negative effect of the E alone was observed (Figures 1A, 1B, 1E, 2B), adding to the controversy of whether estrogen has a positive or negative impact on hearing when administered alone. Interestingly, this smaller negative effect revealed by the ABR thresholds for estrogen, was not observed for the DPOAE amplitudes. In fact, at middle and high frequencies, where ABR thresholds were elevated by estrogen, some improvement in DPOAE amplitudes occurred. This suggests that any detrimental effects of estrogen alone in the cochlea may not significantly involve the outer hair cell system. Indeed, outer hair cells may benefit from the estrogen via their estrogen receptors, as reflected by the DPOAE amplitude measurements for the estrogen-alone subject group. The males showed some modest declines in DPOAE amplitudes, and a trend towards higher ABR thresholds, over the time course of the study. These are likely indicative of normal aging of these males over that time period.
The hearing measures of the present study are assays of peripheral auditory functioning (cochlea of the inner ear, hair cells) and of early auditory brainstem processing, guiding the way for more detailed future studies of the neural bases of the mechanisms of action of sex hormones on the auditory system. One puzzling aspect of the possible underlying mechanisms is that the presence or absence of progesterone receptors has not been reported for hair cells of the organ of Corti. If these receptors are not present, it implies that more complicated hormonal pathways for progestin to reduce auditory functioning via administration of combination HRT may be involved.
Efficacy of the Animal Model for Hormone Studies on Sensory Systems
In animal models, previous studies have compared hearing sensitivity, such as ABR, in ovariectomized rats (Coleman et al., 1994), but no study has yet appeared comparing additional physiological hearing measures, such as distortion product otoacoustic emissions (DPOAE), in mouse models while carefully controlling HRT administration. DPOAEs are noninvasive, frequency specific measurements used to determine the integrity of outer hair cell functioning (Kemp, 1978; Gifford and Guinan, 1987). In contrast, ABR testing measures and quantifies neurophysiological activity generated by the auditory portion of the inner ear (cochlea) and brainstem pathways, providing measures of inner hair cell and auditory brainstem sensitivity and functionality. Utilizing these methodologies, Guimaraes et al. (2004) reported that premenopausal female mice have better hearing than males, an advantage that goes away in old age (post-menopausal).
The present investigation analyzed the effects of HRT utilizing these objective, physiologically-based hearing tests. For DPOAE testing, statistically significant differences were observed within the high frequency (30–45 kHz) and middle frequency (15–30 kHz) ranges after as soon as 2 months of combination (E+P) treatment, and at 4 months in the low frequencies. Results for ABR testing revealed a trend toward increasing thresholds over time in all groups, but with significantly worse thresholds in the E+P group, specifically in the high frequencies for the 4-month condition. Taken together, these findings indicate that combined E+P HRT actually impairs inner ear (outer hair cell) functioning, having deleterious effects even before a measurable effect on overall hearing sensitivity takes place (ABR thresholds at 4 months). The E+P combination HRT accelerated hearing loss seen here for the mice appears to model many aspects of the accelerated presbycusis observed in aged women for combination HRT (Guimaraes et al., 2006), although discrepancies between the human and mouse results may related to species differences. For example, in the human study, the E alone group was similar to the control group, whereas in a few cases the E alone group showed some cases of elevated ABRs relative to the mouse placebo group.
Sex Hormones and the Auditory System
The role of progesterone in the auditory system is currently not well understood. Animal studies in the rat have found the presence of progesterone receptors within the inner ear (auditory and vestibular) such as the endolymphatic sac and stria vascularis. These structures help to regulate endolymph and cochlear blood supply, respectively, and therefore play essential roles in underlying hearing mechanisms. Studies in the guinea pig have suggested that both estrogen and progesterone may produce enhanced inflammatory responses in the cochlea, which may lead to observable hearing deficits in a dose-sensitive manner (Bittar et al., 2001). Combination HRT may also have negative effects on the auditory efferent feedback system from the medial superior olivary complex to the outer hair cells (Zhu et al., 2004).
In the normal menstrual cycle, progesterone peaks during the luteal phase. This sex hormone is produced mainly by the corpus luteum following ovulation and returns to baseline measures if no pregnancy is achieved. Studies of regular cycling (ovulatory) women have shown cyclical, reversible and predictable changes in auditory processing, including decreases in long-latency potentials (P2 and N2) and ABR latencies during the mid-to-late luteal phases of the cycle when progesterone levels are maximized. These findings suggest a possible negative interaction of progesterone on auditory functioning (Walpurger et al., 2004). DPAOE testing throughout the menstrual cycle has not revealed these same changes, with amplitudes staying generally consistent (Yellin and Stillman, 1999). However, these DPOAE measures were made on a weekly basis likely reducing its sensitivity to detect changes during the functional periods of the menstrual cycle, specifically the mid-luteal phase, approximately 5 days in duration, when progesterone levels peak. More frequent and sensitive testing of DPOAE levels during the menstrual cycle has not been reported. Lastly, in contrast to the present investigation, and that of Guimaraes et al. (2006) in post-menopausal women, Hederstierna et al. (2007) recently reported some improvements at two frequencies (2–3 kHz) of the pure-tone audiogram for pre-, peri- and post-menopausal women using combination HRT. Differences in exact ingredients and dosages between European and US HRT regimens might account for the relative benefits of combination HRT reported in these studies.
Proposed mechanisms for progesterone’s negative effects on hearing are diverse. One possible mechanism centers on progesterone’s ability to down regulate estrogen receptors in breast and uterine tissue, balancing the effects of estrogen. While the progesterone’s direct effects on the cochlea remains unknown at present, continuous progestin therapy, such as occurs for HRT or some birth control pills, without the typical withdrawal seen in normal monthly menstrual cycling may produce on ongoing down-regulation of the estrogen receptor or possible irreversible receptor damage that might prevent circulating estrogens from properly binding. In normal cycling, progesterone levels would quickly fall making estrogen receptors available again for beneficial physiological activity.
For another possible mechanism, bioidentical progesterones (currently available in some combined HRT regimens) mobilize extracellular fluids and antagonize the retention effects of estrogen by exerting a powerful diuretic property and possibly enhancing endogenous hormone sensitivity, such as to angiotensin II, in the cochlea. Previous studies have reported observable reductions in cochlear blood flow, putting the inner ear at risk for the damaging effects of hypoxia or hypoglycemia (Laugel et al., 1988). In addition, cochlear fluids are known to be sensitive to diuretic use. Continuous use of progesterone therapy might result in abnormal diuretic responses, disrupting delicate inner ear sodium and potassium ionic balances, including disruption of the endocochlear potential, necessary for proper sensory transduction and neural processing of auditory stimuli. In addition, HRT has failed to protect against age-related decline in vasomotor function, underscoring the possible negative effects of progesterone receptor stimulation from combination HRT (Sorensen et al., 1998). A limitation of the current investigation was the lack of a progesterone-only subject group, which will be an important aspect of follow-up studies to the findings of this report.
Summary
The negative effects of combined estrogen+progestin therapy on the auditory system have been observed here and in post-menopausal women who have take combination HRT (Guimaraes et al., 2006), though the exact mechanisms of progestin’s negative actions on the auditory system remains elusive. Research has suggested that progesterone receptors are present within the inner ear, and combination HRT may contribute to cochlear blood supply vascular problems, disrupt inner ear potassium ionic balances, enhance damaging inflammatory processes within the auditory system, or cause the system to become less sensitive to circulating estrogens, by down regulating or modifying estrogen receptors within the inner ear or portions of the brain used for hearing.
Conclusion
A major finding here, is that female perimenopausal CBA mice, with ovaries intact, who are receiving continuous release combined estrogen+progestin HRT, have significantly reduced DPOAE amplitudes with as little as 2 months of treatment. The DPOAEs measure inner ear outer hair cell functioning, an essential component of the auditory system sensory transduction mechanism. DPOAE testing may provide evidence of changes to cochlear electromechanics well before they are detected on overall auditory tests, such as the ABR. Further analysis of the histology of progesterone receptors in the inner and brain may provide essential information regarding the biological mechanisms of progesterone and its functions in sensory systems. In the wake of the Women’s Health Initiative, the list of negative effects of combined progestin+estrogen therapy continues to grow, raising serious and important concerns for women taking HRT and physicians who prescribe combined HRT.
Acknowledgments
We thank John Housel for technical assistance and Enza Daugherty for project support. Supported by NIH Grant P01 AG09524 from the National Institute on Aging, and P30 DC05409 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson BD, Bradley EL, Terman CR. Plasma progesterone concentration in prairie deermice (Peromyscus maniculatus bairdii) from experimental laboratory populations. J Reprod Fert. 1975;42:407–414. doi: 10.1530/jrf.0.0420407. [DOI] [PubMed] [Google Scholar]
- Bittar RS, Cruz OL, Lorenzi MC, Marone SA, Miniti A. Morphological and functional study of the cochlea after administration of estrogen and progesterone in the guinea pig. Int Tinnitus J. 2001;7:41–5. [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Serra A. Auditory brainstem response in premenopausal women taking oral contraceptives. Human Reproduction. 2003;18:85–89. doi: 10.1093/humrep/deg003. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–15. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Rajan R. Inferior Colliculus: Aging and plasticity. In: Winer J, Schreiner C, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 559–584. Ch. 18. [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JP, editor. Handbook of Mouse Auditory Res.: From Behavior to Molecular Biology. New York: CRC Press; 2001. pp. 339–379. [Google Scholar]
- Gifford ML, Guinan JJ., Jr Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Frisina ST, Mapes F, Tadros SF, Frisina DR, Frisina RD. Progestin negatively affects hearing in aged women. Proc Nat Acad Sci – PNAS. 2006;103:14246–14249. doi: 10.1073/pnas.0606891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Hederstierna C, Hultcrantz M, Collins A, Rosenhall U. Hearing in women at menopause. Prevalence of hearing loss, audiometric configuration and relation to hormone replacement therapy. Acta Oto-laryngol. 2007;127:149–155. doi: 10.1080/00016480600794446. [DOI] [PubMed] [Google Scholar]
- Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, Vittinghoff E, Hunninghake D. Non-cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and estrogen/progestin replacement study follow-up (HERS II) J Am Med Assoc. 2002;288:58–64. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: A summary of recent investigations. Acta Oto-Laryngol. 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Jacobsen M, Kim S, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion-product otoacoustic emissions declines with age: a comparison of findings in CBA mice with human listeners. Laryngoscope. 2003;113:1707–1713. doi: 10.1097/00005537-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emission from the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erikan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. Am J Obst Gynecol. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang BM, Chae HD, Kim CH. The association between serum estradiol level and hearing sensitivity in postmenopausal women. Obst Gynecol. 2002;5:726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]
- Laugel GR, Wright JW, Dengerink HA. Angiotensin II and progesterone effects on laser doppler measure of cochlear blood flow. Acta Otolaryngol (Stockh) 1988;106:34–9. doi: 10.3109/00016488809107368. [DOI] [PubMed] [Google Scholar]
- Mader S. Hearing impairment in elderly persons. J Am Geriatric Soc. 1984;32:548–53. doi: 10.1111/j.1532-5415.1984.tb02245.x. [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Augilar C, Endicott JE, Velez R, Tuley MR, Charlip WS, et al. Association between hearing impairment and the quality of life in elderly individuals. J Am Geriatric Soc. 1990;38:45–50. doi: 10.1111/j.1532-5415.1990.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Pleis JR, Lethbridge-Çejku M. Vital Health Stat. 235. Vol. 10. US Government Printing Office; Washington, DC: 2007. Summary health statistics for U.S. adults: National Health Interview Survey, 2006. National Center for Health Statistics. [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective Analysis from the Women’s Health Initiative Observational Study. J Am Med Assoc. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Bertero MC, Vernon MW. Detection of a progesterone-induced secretory protein synthesized by the uteri but not the endometriotic implants of rates with induced endometriosis. Fertility Sterility. 1991;55:403–409. [PubMed] [Google Scholar]
- Sorensen KE, Dorup I, Hermann AP, Mosekilde L. Combined hormone replacement therapy does not protect women against the age-related decline in endothelium-dependent vasomotor function. Circulation. 1998;97:1234–1238. doi: 10.1161/01.cir.97.13.1234. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Simonoska R, Stygar D, Sahlin L, Hulcrantz Effect of estrogen and antiestrogens on the estrogen receptor content in the cochlea of ovariectomized rats. Hear Res. 2003;182:19–23. doi: 10.1016/s0378-5955(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, Hulcrantz M. Mapping of estrogen receptors α and β in the inner ear of mouse and rat. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Varghese GI, Zhu X, Frisina RD. Age-related declines in contralateral suppression of distortion product otoacoustic emissions utilizing pure tones in CBA/CaJ mice. Hear Res. 2005;209:60–67. doi: 10.1016/j.heares.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Walpurger V, Pietrowsky R, Kirschbaum C, Wolf OT. Effects of menstrual cycle on auditory event-related potentials. Hormones Behav. 2004;46:600–606. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the auditory system: anatomy, physiology, and psychoacoustics. Singular Publishing; San Diego: 1991. [Google Scholar]
- Yellin MW, Stillman RD. Otoacoustic emissions in normal cycling females. J Am Acad Audiol. 1999;10:400–408. [PubMed] [Google Scholar]
- Zhu X, Kim SH, Frisina RD. Sex differences in age-related decline of the auditory efferent system in CBA mice. Assoc Res Otolaryngol Abstr. 2004;27 [Google Scholar]