Abstract
The information processing capacity of the human mind is limited, as is evidenced by the attentional blink—a deficit in identifying the second of two targets (T1 and T2) presented in close succession. This deficit is thought to result from an overinvestment of limited resources in T1 processing. We previously reported that intensive mental training in a style of meditation aimed at reducing elaborate object processing, reduced brain resource allocation to T1, and improved T2 accuracy [Slagter, H. A., Lutz, A., Greisschar, L. L., Frances, A. D., Nieuwenhuis, S., Davis, J., et al. Mental training affects distribution of limited brain resources. PloS Biology, 5, e138, 2007]. Here we report EEG spectral analyses to examine the possibility that this reduction in elaborate T1 processing rendered the system more available to process new target information, as indexed by T2-locked phase variability. Intensive mental training was associated with decreased cross-trial variability in the phase of oscillatory theta activity after successfully detected T2s, in particular, for those individuals who showed the greatest reduction in brain resource allocation to T1. These data implicate theta phase locking in conscious target perception, and suggest that after mental training the cognitive system is more rapidly available to process new target information. Mental training was not associated with changes in the amplitude of T2-induced responses or oscillatory activity before task onset. In combination, these findings illustrate the usefulness of systematic mental training in the study of the human mind by revealing the neural mechanisms that enable the brain to successfully represent target information.
INTRODUCTION
A major limitation in human information processing concerns the ability of the brain to process two temporally close meaningful stimuli. This limitation is illustrated by the attentional-blink paradigm: When the second of two target stimuli (T1 and T2) follows the first one in close temporal proximity (within 500 msec) in a rapid stream of distracter events, it often goes unnoticed (Ward, Duncan, & Shapiro, 1996; Raymond, Shapiro, & Arnell, 1992). Many theoretical accounts of this so-called attentional-blink deficit have relied on some form of limited attentional resource that is allocated to the leading target at the expense of the trailing target (Jolicoeur & Dell’Acqua, 1998; Ward et al., 1996; Chun & Potter, 1995; Shapiro, Raymond, & Amell, 1994). For instance, it has been postulated that when many resources are devoted to T1 processing, too few may be available for subsequent T2 processing, rendering its representation vulnerable to distracter interference (Shapiro et al., 1994). In line with this idea, several recent event-related potential (ERP) studies (Kranczioch, Debener, Maye, & Engel, 2007; Sessa, Luria, Verleger, & Dell’acqua, 2007; Slagter et al., 2007; Martens, Elmallah, London, & Johnson, 2006; Martens, Munneke, Smid, & Johnson, 2006; Shapiro, Schmitz, Martens, Hommel, & Schnitzler, 2006; Sergent, Baillet, & Dehaene, 2005) have shown that the ability to accurately identify T2 is related to the latency and/or amplitude of the T1-elicited P3b, a brain-potential index of resource allocation (Wickens, Kramer, Vanasse, & Donchin, 1983). For example, a delayed or larger T1-evoked P3b has been observed in trials in which T2 was missed (i.e., blink trials) versus detected (i.e., no-blink trials) (Sergent et al., 2005). These electrophysiological findings support the notion that the amount of resources invested in T1 processing markedly influences the processing capacity available for T2, and thus, whether T2 will be consciously perceived or not.
The attentional blink does not represent an absolute bottleneck in information processing. This is most clearly indicated by the fact that most participants are able to identify both targets on at least a portion of the trials (e.g., Martens, Munneke, et al., 2006). At present, it is unclear why physically identical information sometimes does and sometimes does not reach awareness. It is notable in this respect that the attentional blink has been observed to get smaller when measures are taken that prevent an overinvestment of attentional resources in stimulus processing. For example, T2 detection has been found to benefit from a diffusion of attention (Arend, Johnston, & Shapiro, 2006; Olivers & Nieuwenhuis, 2005, 2006). Moreover, we recently found that 3 months of intensive training in a style of meditation, which allegedly reduces elaborate object processing (Lutz, Slagter, Dunne, & Davidson, 2008), reduced brain resource allocation to T1, as indexed by a smaller T1-elicited P3b, and improved T2 detection, with no impairment in T1 detection (Slagter et al., 2007). This common style of open monitoring (OM) meditation, also known as Vipassana meditation, consists in being attentive moment by moment to anything that occurs in experience, whether it be a sensation, thought, or feeling (Lutz, Slagter, et al., 2008). Usually, one starts by focusing or stabilizing concentration on an object such as the breath. Then one broadens one’s focus, cultivating a nonreactive form of awareness. This form of awareness is nonreactive in the sense that, ideally, one does not become caught up in judgments and affective responses about sensory or mental stimuli. Although initially the practitioner frequently “clings” to objects in a way that takes up resources available to process information related to current experience, eventually, a trait is thought to emerge such that one can sustain the “non-clinging” state in which one is attentive to the content of experience from moment to moment. As participants were not engaged in formal meditation during task performance, our observations that intensive OM meditation reduced T1 capture and improved T2 detection are in line with the idea that one long-term effect of this style of meditation is a reduction in the propensity to “get stuck” on an object. Together with previous behavioral findings (Olivers & Nieuwenhuis, 2005, 2006), these data support the idea that the attentional blink is due to an overinvestment of attentional resources in stimulus processing, and that this suboptimal processing mode can be counteracted by manipulations promoting a less object-focused state of attention.
Recent electroencephalography (EEG) work has shown that the attentional state of the observer is reflected in ongoing or baseline neural activity prior to stimulus presentation, and can predict subsequent visual perception performance (e.g., Hanslmayr et al., 2007; Thut, Nietzel, Brandt, & Pascual-Leone, 2006; Linkenkaer-Hansen, Nikulin, Palva, Ilmoniemi, & Palva, 2004). To our knowledge, only one previous attentional-blink study has examined the relationship between baseline neural activity and T2 perception (Martens, Munneke, et al., 2006). In this study, mean EEG activity in a 1024-msec fixation period before task onset was compared between a group of participants with a relatively large attentional blink (“blinkers”) and a group of participants with a relatively small attentional blink (“nonblinkers”). No differences between groups in mean baseline EEG activity were observed, but the nonblinkers showed an earlier T1-elicited P3b than the blinkers. These findings might be taken as evidence that the state of the system right before task onset does not strongly determine the ability of the system to process T1 efficiently, thus arguing against the idea that the observer’s mental state influences conscious target perception. However, an across-subjects comparison, as was used in the Martens, Munneke, et al. (2006) study, may have obscured small differences in baseline mental state related to successful T2 detection. Furthermore, Martens et al. only looked at the amplitude (or power), not phase, of oscillatory activity, whereas phase synchrony might provide a more sensitive measure of attentional state (see, for example, Hanslmayr et al., 2007). This possibility is substantiated by findings from other EEG studies, which have reported within-subject differences in synchronous EEG activity between blink and no-blink trials in the alpha (Kranczioch et al., 2007), beta (Gross et al., 2004), and gamma (Nakatani, Ito, Nikolaev, Gong, & van Leeuwen, 2005) frequency ranges, not only after T1 presentation but also right before and during distracter presentation. This variation of findings showing differential processing of the distracter stimuli points to differences in attentional state or the way attention is directed prior to the presentation of T1. It also highlights an important role for phase synchrony in the attentional blink, in line with recent work indicating that phase synchrony is essential in the formation of transient neuronal assemblies that constitute the meta-representations required by sensory awareness (Singer, 1999, 2002) and in the communication therein (Fries, 2005). Of particular importance, some of this work has associated sensory awareness with increased consistency across trials in the phase of oscillatory EEG activity in the delta (e.g., Lakatos, Karmos, Mehta, Ulbert, & Schroeder, 2008) and alpha (e.g., Palva, Linkenkaer-Hansen, Naäätänen, & Palva, 2005) frequency bands, as indexed by the phase-locking factor (PLF; Tallon-Baudry & Bertrand, 1999). Phase consistency may thus provide a particularly useful measure in the study of conscious target perception.
The aim of the current study was to further examine the impact of intensive OM training on brain function and behavior, and investigate why physically identical information sometimes does and sometimes does not reach awareness. To this end, we evaluated the time course and correlation of neuronal oscillations associated with T2 perception by analyzing unreported aspects of EEG data from our previous study (Slagter et al., 2007). Specifically, the current study tested two hypotheses. The first hypothesis concerned the effects of the previously observed reduction in brain resource allocation to T1 on the overall readiness of the system to be disturbed by a new target stimulus (i.e., T2). The second hypothesis was based on the idea that attentional processing of T1 can be predicted by the baseline mental state of the observer. These two hypotheses and corresponding predictions are described in detail below.
As mentioned above, training in OM meditation is thought to leave the system more open to, or ready to process, whatever thoughts, feelings, or sensations may arise, without grasping to an explicit object. Based on this description, we hypothesized that not only will such training reduce elaborate T1 processing, as we previously observed (Slagter et al., 2007), but it will also render the system more susceptible to T2, as resources should be more consistently available to process new information. As mentioned above, it is possible to reveal whether the recorded EEG signals at a given latency show a consistent or nonrandom phase relationship to a presented stimulus across trials using the PLF (Tallon-Baudry & Bertrand, 1999). Using this measure of stimulus-locked phase variability, we examined whether intensive training in OM meditation decreased trial-to-trial variability in the recruitment of processes related to the conscious perception of T2. We also directly investigated the relationship between the previously observed reduction in brain-resource allocation to T1 (as indexed by T1-elicited P3b amplitude) and the ability of the system to be perturbated by a new target stimulus (i.e., T2). Our specific prediction was that those individuals who showed the greatest reduction in brain-resource allocation to T1 would show the greatest increase in T2-locked phase consistency. In addition to effects of intensive mental training on phase variability, we also explored the possibility that such training affected the amplitude of neuronal oscillations.
To address the second hypothesis, the current study examined whether the observed reduction in brain-resource allocation to T1 was associated with any changes in baseline mental state. As mentioned above, the mental state of the observer has been found to influence the attentional blink, with a less object-focused state of attention promoting a smaller attentional blink, presumably by reducing the propensity to overinvest resources in T1 processing (Olivers & Nieuwenhuis, 2005, 2006). Our second hypothesis therefore was that intensive mental training would be associated with changes in baseline mental state, as reflected by the state of oscillatory brain activity before task onset. We predicted that such changes would be predictive of target perception. In contrast to the Martens, Munneke, et al. (2006) study, the relationship between baseline mental state and conscious perception was interrogated within the same individual, and effects of intensive mental training on the amplitude as well as phase of baseline oscillatory activity were examined.
Data were collected from 17 participants at the beginning and end of a 3-month meditation retreat during which they meditated for 10 to 12 hr/day (practitioner group). Control data were collected from 23 matched participants interested in learning about meditation (novice group), who received a 1-hr meditation class and were asked to meditate for 20 min daily for 1 week prior to each session. In each session, participants performed an attentional-blink task in which they had to identify two targets (both numbers) embedded in a rapid stream of distracter letters (Figure 1) while their EEG was recorded. T2 could follow T1 either within (334 msec; short-interval trial) or outside (672 msec; long-interval trial) the time window of the attentional blink. Participants were not engaged in formal meditation during task performance.
Figure 1.
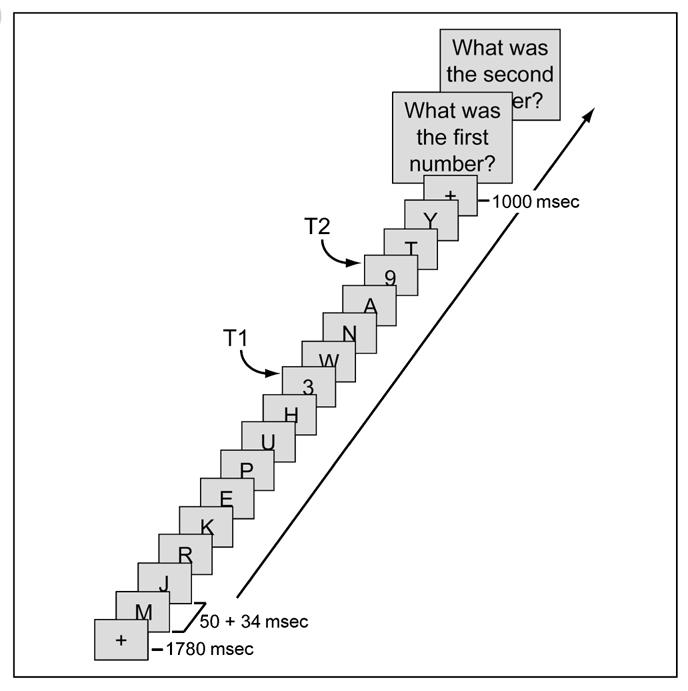
Attentional-blink task. On every trial, between 15 and 19 items were presented at the center of the screen, preceded by a 1780-msec fixation cross. Most of the items were letters, presented for 50 msec each and followed by a 34-msec blank. On T2-present trials, there were two target numbers (T1 and T2) among the items, which participants had to detect and report at the end of the trial. The temporal distance between T1 and T2 could be short (336 msec) or long (672 msec).
METHODS
Participants
Seventeen practitioners (7 men; median age = 41 years, range = 22–64 years, median education = 18 years) were recruited prior to the start of a 3-month meditation retreat at the Insight Meditation Society in Barre, MA. Twenty-three matched controls (9 men; median age = 41 years, range = 20–62 years, median education = 17 years) with no prior meditation experience were recruited via advertisements in local newspapers directed at individuals interested in learning about meditation. The participants had no history of mental or neurological illness and gave informed consent to participate. Participants were trained in Vipassana meditation, a style of OM meditation (Lutz, Slagter, et al., 2008), which cultivates concentration and “bare” attention (see Introduction). Practitioners also received some training in “metta,” a loving kindness and compassion meditation.
The practitioners self-selected for the meditation group and all had prior experience with meditation. As mentioned in Slagter et al. (2007), no relationship was observed between prior meditation experience (i.e., number of days in a retreat prior to our study) and attentional-blink task performance at Time 1. Prior meditation experience also did not interact with the meditation intervention, as there was no significant relationship between prior meditation experience and the change over time in attentional-blink task performance. The absence of an association between the amount of prior meditation training and our study results (see below) may be due to the fact that there was great variation across the practitioners in the styles and traditions of the previously learned meditation. Longitudinal research examining and comparing the effects of different styles of meditation on brain and mental function and the duration of such effects is needed.
Stimuli and Task
Stimuli were presented in black on a gray (40 cd/m2) background at the center of a computer screen. Each trial started with a 1780-msec task preparation period during which a fixation cross (0.5° × 0.5°) was shown. This period was followed by a rapid serial stream of 15 or 19 letters (0.8° × 0.8°) (Figure 1). Each letter was randomly drawn (without replacement) from the alphabet (except B, I, O, Q, and S) and presented for 50 msec, followed by a 34-msec blank. On each trial, one or two letters were replaced with a number, randomly drawn (without replacement) from the Sets 2 to 9. When only one letter was replaced by a number, a second letter was replaced with a blank screen (T2-absent trials). The temporal distance between the first (T1) and second (T2) number (or the blank screen) could be short (336 msec) or long (672 msec). T2 and the blank screen were presented at Temporal Positions 3–5 from the end of the stream. To prevent the saliency of T2-absent trials, each distracter could be replaced by an empty screen with a 20% probability, except those surrounding T1 and T2 and the last distracter in the sequence (cf. Sergent & Dehaene, 2004).
Participants were informed that there could be one or two numbers in the letter stream, and were asked to report these numbers 1000 msec after the stream ended by typing the numbers in order on a keyboard. Participants were instructed to guess T2 if they thought that T2 had been presented, but were not entirely sure about its identity. If they were absolutely sure that no T2 was presented, they entered zero for this number. A new trial began 200 msec after the second response. After a short practice block, participants performed four blocks of 102 trials each, consisting of 192 short-interval/T2-present trials, 72 long-interval/T2-present trials, 72 short-interval/T2-absent trials, and 72 long-interval/T2-absent trials, all intermixed within blocks.
Study Procedure and Data Acquisition
In each session, after practicing the task first for 34 trials, participants performed four blocks of 102 trials of the attentional-blink task while their EEG was recorded. EEG was recorded at 512 Hz from 64 Ag—AgCl electrodes using the Active-Two System (BioSemi, Amsterdam, Netherlands). Additional electrodes recorded the right and left mastoid process and the electrooculogram. Participants were instructed not to engage in meditation at the beginning of the session.
Data Analysis
Behavioral Data Analysis
T1 and T2 accuracy data were submitted to separate repeated measures ANOVAs with interval (short, long) and session (Time 1, Time 2) as within-subject factors, and group (novices, practitioners) as a between-subject factor. T2 accuracy was based only on those trials in which T1 was correctly reported.
EEG Data Analysis
EEGLAB (Delorme & Makeig, 2004) and Matlab (Mathworks, Natick, MA) were used for off-line EEG data processing. Due to technical problems, the EEG data of two novices could not be analyzed. Data of the remaining participants were high-passed filtered (1 Hz), rereferenced to the average of both mastoids, and cleaned of large movement-related artifacts. ICA was then used to remove ocular artifacts (Jung et al., 2000). For each condition of interest, downsampled (256 Hz) data were epoched into segments of 5000 msec duration, in synchrony with the onset of the stimulus stream (-1782 to 3214 msec), and baseline-corrected (whole interval). Trials with remaining artifacts (exceeding ±80 μV) were removed (16% of trials on average). After artifact correction, trials were further epoched in synchrony with T1 (-1000 to 1500 msec) and baseline corrected (200 msec preceding T1).
Phase-locking Factor
We investigated stimulus locking of ongoing cortical activity using the PLF, which quantifies the nonuniformity of a phase distribution (Palva et al., 2005). PLF ranges from 0 to 1, with the value 1 indicating a perfect phase locking and values approaching 0 indicating nearly random phases. If phases are uncorrelated and randomly drawn from a uniform distribution, the PLFs of different sets obey the Rayleigh distribution p(λ) = λσ-2exp [-0.5λ2σ-2], where λ denotes the PLF for a given realization, σ = λ λm(0.5π)-0.5, with λm being the mean of the distribution. The null hypothesis that the samples were drawn from a uniform distribution was tested using the Rayleigh statistic in which λm is simply defined by the number of trials (i.e., lm = 0.5log(N)). Because the Rayleigh distribution is a function of its mean λm only, PLFs were normalized by λm to express the strength of phase locking directly in a statistically meaningful manner (cf. Palva et al., 2005). PLF/λm > 1.95 corresponds to p < .05 and PLF/λm > 2.42 to p < .01.
For the time—frequency analyses, the continuous phase of ongoing activity was obtained by convolving the data x(t) with a family of Morlet wavelets w(t, f0) = Aexp(-t2/ 2σt2)exp(2iπf0t), where σt = m/2πf0, i is the imaginary unit, m defines the compromise between time and frequency resolution, and f0 is the center frequency of the wavelet. The convolution gives a complex vector, of which the angle is the phase of the signal in a frequency band with a center frequency of f0 and a frequencydomain SD of σf = f0/m. In this study, m was 5.5, and the wavelet family was composed of 14 center frequencies, separated by ∼1σf and ranging from 5 to 55.5 Hz (cf. Palva et al., 2005). For a 10-Hz wavelet, the time-domain SD (σt) is 87.5 msec, and the time-domain envelope half-width at half-height is 103 msec (cf. Yamagishi et al., 2003).
To examine our first prediction that intensive training in OM meditation decreased trial-to-trial variability in the recruitment of processes related to the conscious perception of T2, the phase locking to T1 and T2 was estimated for each trial type of interest (i.e., short-interval no-blink, blink, and T2-absent trials, and long-interval no-blink trials), session (Time 1, Time 2), and participant separately. In-house simulations1 showed that a minimum of 40 trials is necessary to obtain reliable phase-locking estimates. Yet, many participants did not have 40 or more artifact-free trials of every trial type of interest at both time points. In particular, due to the fact that many practitioners showed a relatively small attentional blink after 3 months of intensive meditation (see Results section), only 5 (out of 17) practitioners still had more than 40 blink trials at Time 2. Therefore, a direct examination of effects of intensive mental training on target phase locking in no-blink compared to blink trials was not feasible and the following two-step approach was taken. As we previously showed that effects of mental training were selective to those trials in which T2 was accurately identified (i.e., no-blink trials; Slagter et al., 2007), effects of mental training on target locking of the ongoing cortical activity were first examined for short-interval no-blink trials. Fourteen (out of 17) practitioners and 20 (out of 21) novices had enough no-blink trials for reliable phase-locking estimates at both time points to be included in this analysis. Second, whenever mental training-related changes in phase variability in no-blink trials were observed, two additional analyses were run to determine whether thus observed effects were related to differential T1 or T2 processing. The first of these analyses focused on short-interval T2-absent trials in which neural activity was not confounded by T2-related processing. The same 14 practitioners and 20 novices had enough trials for reliable phase-locking estimates at both time points to be included in this analysis. The second analysis examined target locking in trials in which T2 was missed (i.e., blink trials). This latter analysis allowed us to examine whether mental training-related changes in target locking observed in no-blink trials may reflect differences in processes related to the conscious versus unconscious perception of T2. As relatively few participants had enough blink trials for reliable phase-locking estimates at both time points (see above), here we collapsed across groups. Thirteen participants (7 practitioners) had enough trials for reliable phase-locking estimates at both time points to be included in this analysis. Finally, we also examined phase locking for long-interval no-blink trials, to determine whether or not observed effects were specific to the time window of the attentional blink.
To ensure that equal numbers of trials were used to calculate PLFs for each session, for each participant and trial type separately, we first determined whether one session had more (artifact-free) trials of a given trial type (e.g., no-blink trials) than the other session. If this was the case, the surplus number of trials was then randomly removed from the dataset. For the short-interval no-blink trial analysis, the average number of trials included in the analysis for each session was: novices, 98 trials; practitioners, 93 trials. For the T2-absent trial analysis, the average number of trials included in the analysis for each session was: novices, 50 trials; practitioners, 49 trials. For the blink trial analysis, the average number of trials included in the analysis for each session was: 56 blink trials. For the long-interval no-blink trial analysis, the average number of trials included in the analysis for each session was: novices, 46 trials; practitioners, 48 trials. t Tests revealed that the number of trials used in each analysis did not differ significantly between groups (all ps > .71).
The target-induced amplitude fluctuations were determined by averaging across trials the amplitude envelopes given by Morlet wavelets (cf. Palva et al., 2005; Tallon-Baudry & Bertrand, 1999). Mental training-related changes in target-induced amplitude related to conscious T2 perception were examined by comparing changes in non-target-locked (i.e., induced) activities over time in no-blink and blink trials between groups for each frequency band separately. Participants that had 15 or more artifact-free blink and no-blink trials at both time points were included in this analysis (cf. Slagter et al., 2007; 10 practitioners and 12 novices).
To examine our second prediction that intensive training in OM meditation would result in changes in baseline mental state, changes in oscillatory brain activity were examined in a 500-msec time window of 700 to 200 msec before the onset of the stimulus stream, separately for each frequency band, trial type of interest, and participant. Amplitude fluctuations were determined by averaging across trials the amplitude envelopes given by Morlet wavelets, as described above. Thus, the complete epoch length of 5000 msec was used to obtain baseline amplitude measures so that the interval of interest did not interfere with invalid edge effects. To examine effects of mental training on baseline activity, we only looked at the 500-msec window during the prestream fixation period. Participants that had 15 or more artifact-free blink and no-blink trials at both time points were included in this analysis (cf. Slagter et al., 2007; 10 practitioners and 12 novices). Given the higher number of trials required to obtain reliable phase-locking estimates, the phase locking to the onset of the task preparation period was analyzed using the same two-step approach described above for the target-locked phase-locking analysis.
In all analyses, the presence of effects on the group level was evaluated using binomial statistics (PB; cf. Palva et al., 2005). The significance of the difference in change in (non)target-locked responses over time between groups was assessed with the Wilcoxon rank sum test (PS). Whenever Time-by-Group or Cond-by-Time-by-Group interactions were observed, the significance of the difference in target-locked responses between sessions was estimated with the Wilcoxon signed-rank test (PW) for each group (novices, practitioners), separately. For all analyses, a significance criterion of p < .05 for at least four adjacent electrodes for at least one σt (i.e., theta = 175.1 msec; alpha = 83.5 msec; beta = 47.9 msec; gamma = 27.5 msec) was used (cf. Slagter et al., 2007).
RESULTS
Behavior
The behavioral results are reported in full detail in Slagter et al. (2007). Here, we provide an overview of the main results. As can be seen in Figure 2, T2 accuracy was significantly lower in short- compared to long-interval trials, indicating an attentional blink. In line with previous reports (e.g., Martens, Munneke, et al., 2006), the behavioral results further showed large variability between individuals of both groups in attentional-blink size at Time 1: Three practitioners and one novice performed at or near-chance level at both time points (i.e., T2 accuracy around 14%). Regardless of whether or not these participants were included in the analysis, a significant reduction in attentional-blink magnitude over time was observed for the practitioner group compared to the novice group, as reflected by a three-way interaction between interval (short, long), group (practitioners, novices), and session (Time 1, Time 2) [Figure 2; at-chance participants included: F(1, 38) = 4.5, p = .040; at-chance participants excluded: F(1, 34) = 4.3, p = .045]. Post hoc analyses confirmed that only the practitioner group showed a significantly smaller attentional blink at Time 2 [Interval by Time interaction; Practitioners: F(1, 16) = 16.1, p = .001; Novices: F(1, 22) = 1.12, p = .31]. In addition, the mental training-related improvement in T2 accuracy was selective to the time window of the attentional blink [Group by Time interaction; short-interval trials: F(1, 38) = 7.4, p = .010; long-interval trials: F(1, 38) = 0.7, p = .41].
Figure 2.
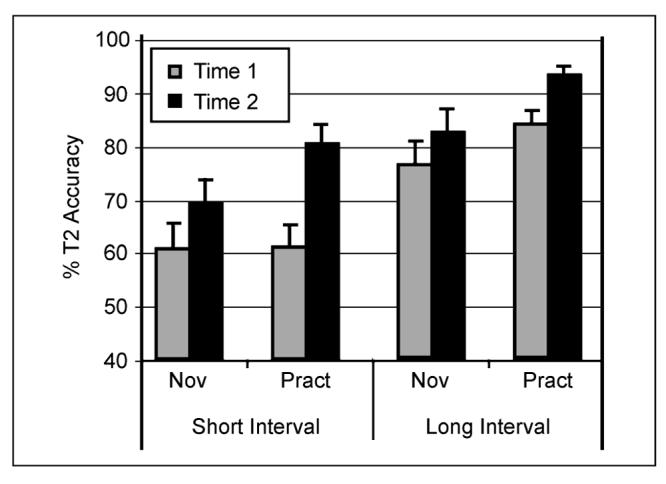
Effects of intensive mental training on the attentional blink. Average T2 accuracy (plus standard error) for each session, T1—T2 interval, and group (at-chance participants excluded). Note that both groups showed an attentional blink at Time 1: lower T2 accuracy at short- compared to long-interval trials. Note further that, as predicted, the practitioner group showed a significantly larger reduction in attentional-blink size over time than the novice group. Adapted from Slagter et al. (2007).
T1 accuracy was not significantly different between groups and improved slightly over time [F(1, 38) = 8.1, p = .007], indicating that improved T2 detection did not impair T1 detection. At Time 1, the practitioners and novices accurately identified T1 on 78% and 79% of the short-interval trials, respectively, and on 88% and 88% of the long-interval trials, respectively. At Time 2, the practitioners and novices accurately identified T1 on 83% and 82% of the short-interval trials, respectively, and on 91% and 91% of the long-interval trials, respectively. The design also included T2-absent trials in which only T1 was presented and T2 was replaced by a blank (see Methods). Average correct report of T2 absence showed no main effect of session or any significant interactions including group, and will not be discussed further.
EEG Data
Mental Training-related Changes in Target-locked Phase Variability
Because training in OM meditation is thought to enhance moment-to-moment awareness and has been shown to reduce brain-resource allocation to T1 (Slagter et al., 2007), our first hypothesis was that at the time of T2 presentation, brain resources would be more consistently available to process T2. We predicted that this would be reflected by a mental training-related decrease in variability in the phase of T2-induced EEG responses. The PLF was used as an index of phase variability (see Methods; cf. Palva et al., 2005; Tallon-Baudry & Bertrand, 1999). Mental training-related changes in the phase of target-induced EEG responses were only observed for the theta (4.1–8.5 Hz) frequency band. Figure 3 shows target locking of the theta phase for the various trial types at Time 1 and Time 2, separately for the practitioners and novices. As can be seen in this figure, in no-blink trials, in which both T1 and T2 were consciously perceived, two peaks in theta phase locking were observed at frontal scalp sites: one around 320 msec post-T1 and one around 320 msec post-T2 (or around 660 msec post-T1 in short-interval trials: Figure 3A; and around 990 msec post-T1 in long-interval trials: Figure 3E). However, when only T1 was correctly identified and T2 was missed (i.e., blink trials; Figure 3C) or replaced by a blank (i.e., T2-absent trials; Figure 3D), only the first of these peaks was present. Together, these observations indicate that neural activity in the theta frequency-band phase-locked robustly to target stimuli only when they were consciously perceived.
Figure 3.
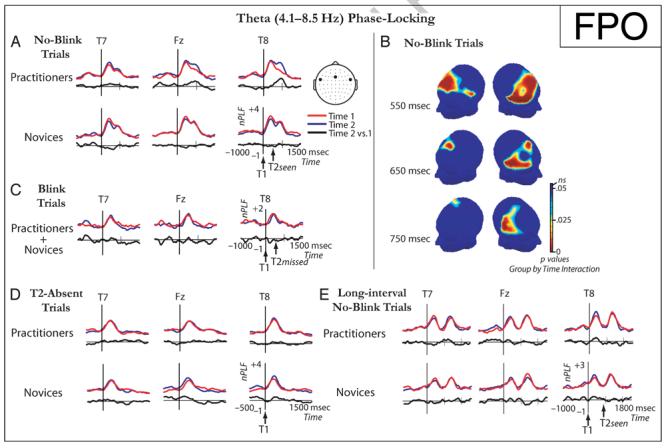
Effects of intensive mental training on target phase locking. (A, C, D, E) Target locking of the theta frequency-band phase at electrodes FT7, Fz, and FT8, time-locked to T1 onset, shown separately for short-interval no-blink (A), blink (C), and T2-absent (D) trials and long-interval no-blink trials (E), and for each session and group. In combination, these data indicate that neural activity in the theta frequency band phase-locked robustly to consciously perceived target stimuli over frontal scalp regions. In addition, they show a significant mental training-related increase in T2 phase locking over midline frontal and right lateral frontal and centro-parietal scalp regions (B). This increase in phase consistency over time was only observed for the practitioner group, indicating that intensive mental training may have reduced trial-to-trial variability in the recruitment of processing leading toward the conscious perception of T2.
Interestingly, and in line with our prediction, in short-interval no-blink trials, a mental training-related increase in phase locking of the theta frequency band was observed during the time window of the second peak, as reflected by a significant interaction between session (Time 1, Time 2) and group (practitioners, novices) (Figure 3A and B). This effect started around 121 msec post-T2 over right ventrolateral and midline frontal scalp regions [121–501 msec post-T2, maximal at FC6 at 242 msec: p(min) = .00026, and at Fz at 266 msec: p(min) = .0039], and was accompanied slightly later in time by increased theta phase locking over right ventro-lateral central scalp sites [309–558 msec post-T2, maximal at T8 at 382 msec: p(min) = .000034]. Importantly, this increase in theta phase locking over right ventrolateral, midline frontal, and central scalp regions was only observed for the practitioner group [significant main effect of session (Time 1, Time 2); FC6 at 242 msec post-T2: p = .004; Fz at 266 msec: p = .007; T8 at 382 msec: p = .0004], and not for the novice group [FC6 at 242 msec post-T2: p = .09; Fz at 266 msec: p = .12; T8 at 382 msec: p = .28] (see Figure 3B). As mental training affected theta phase locking during the time window of the second peak, which was only present when T2 was detected (i.e., not in blink trials), mental training may have affected processes related to the conscious perception of T2. This interpretation receives further support from an additional analysis showing that the mental training-related changes in theta phase locking observed in no-blink trials were not found for short-interval T2-absent trials, in which T2 was replaced by a blank (see Figure 3D; FC6 at 242 msec post-T2: p = .87; Fz at 266 msec: p = .99; T8 at 382 msec: p = .24).2 Interestingly, in contrast to short-interval no-blink trials, in long-interval no-blink trials, no mental training-related increase in frontal phase locking of the theta frequency band was observed during the time window of the second peak (all ps > .05). It thus appears that effects of mental training on target processing were selectively expressed in trials in which T2 was detected and followed T1 within the time window of the attentional blink. Altogether, the above results indicate, as predicted based on the conception that OM meditation renders the system more ready to process information related to current experience (Lutz, Slagter, et al., 2008; Lutz, Dunne, & Davidson, 2006), that intensive training in this style of meditation reduced trial-to-trial variability in the recruitment of processes related to the detection of T2.
As Figure 3B shows, the observed mental training-related increase in phase locking of the theta frequency band was only significant over right, but not left, ventral frontal and central scalp sites. Formal tests confirmed that this effect was right-lateralized: A significant interaction between group (novice, practitioner), session (Time 1, Time 2), and hemisphere (left, right) was observed both at frontal [FC6 vs. FC5; 269–527 msec post-T2, p(max) = .007] and at central [T8 vs. T7; 296–437 msec post-T2, p(max) = .007] ventral scalp regions.
We specifically hypothesized that the state of those individuals who showed the greatest reduction in brain-resource allocation to T1 over time (as indexed by the amplitude of the T1-elicited P3b; see Slagter et al., 2007) would be more ready to be perturbated again by a new target stimulus (as indexed by T2-locked phase variability). Indeed, correlation analyses revealed that those individuals—practitioner or novice—that showed the largest decrease in brain-resource allocation to T1 over time generally showed the greatest increase in phase consistency: A reliable, negative cross-subject correlation was observed in no-blink trials between the mental training-related decrease in T1-elicited P3b amplitude (mean amplitude 420–440 msec post-T1 at Pz; cf. Slagter et al., 2007) and the corresponding change in phase consistency (mean normalized PLF 586–622 msec post-T1 at Fz; r = -0.34; p = .046; mean normalized PLF 702–738 msec post-T1 at T8; r = -.37; p = .029) (see Figure 4). Note, however, that as predicted, only the practitioners showed this resource-sharing mechanism: The P3b amplitude reduction to T1 and the subsequent increase in phase consistency of T2-induced EEG responses3 were present only for this group and not for the novices (see above). No relationship was found between the mental training-related change in phase consistency at Fz, FC6, or T8 and the corresponding change in T2 accuracy (all ps > .29).
Figure 4.
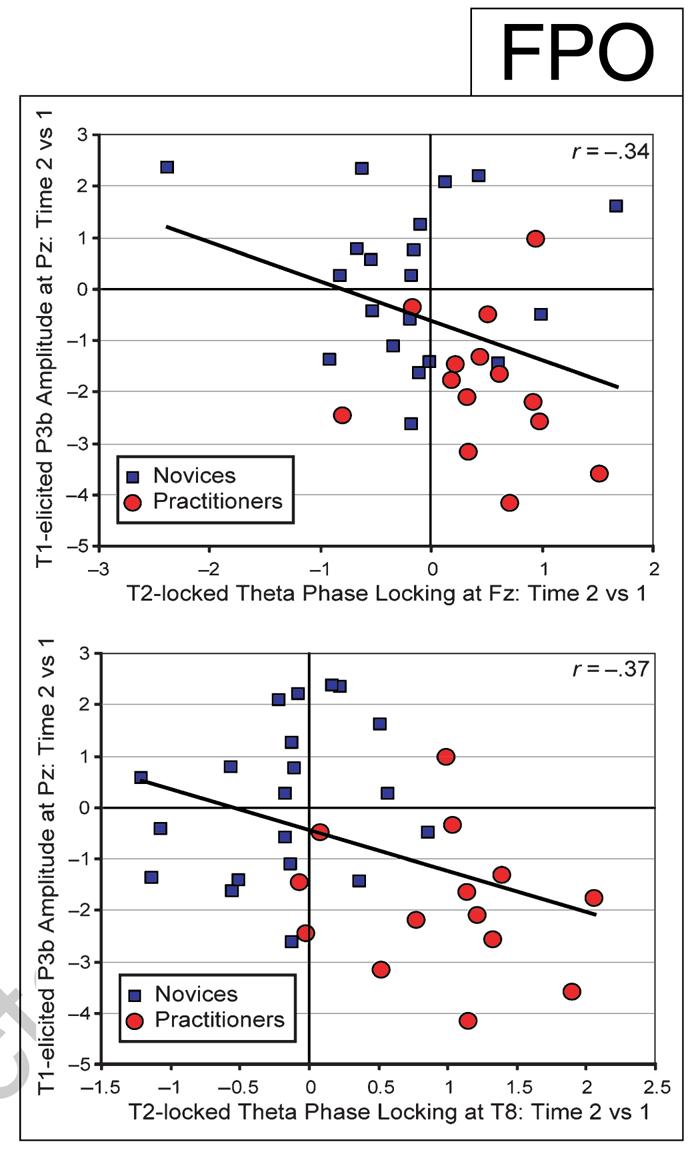
The efficient processing of T1 reduces variability in the recruitment of processes related to conscious T2 perception. Relationship between the change in T1-elicited P3b amplitude and the corresponding change in T2 locking of the theta frequency-band phase at electrodes Fz (top) and T8 (bottom) (for no-blink trials). Note that those individuals that showed the largest decrease in T1-elicited P3b amplitude over time generally showed the largest reduction in theta phase variability over time.
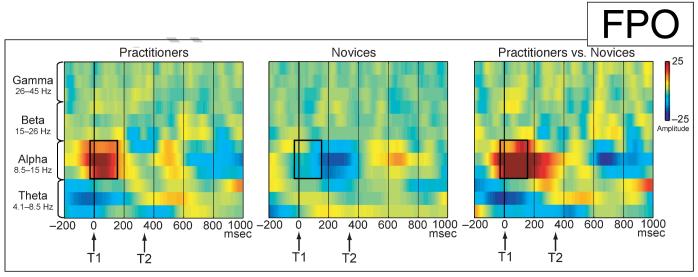
Mental training-related changes in target phase locking were not observed for any of the other frequency bands [i.e., alpha (8.5–15 Hz), beta (15–26 Hz), and gamma (26–45 Hz)]. Interestingly, however, a mental training-related change in phase locking of the alpha frequency band was observed in a time window of several hundred milliseconds prior to T1 onset during distracter presentation over midline occipital scalp sites, as reflected by a significant interaction between group (practitioners, novices) and session (Time 1, Time 2) [-414 to -214 msec, maximal at Oz at -292 msec: p(min) = .003]. In this time window, the practitioners, but not the novices, showed a significant reduction in alpha phase locking in no-blink trials over occipital brain areas (practitioners: Oz at -292 msec: p = .01; novices: Oz at -292 msec: p = .16). Notably, in the attentional-blink paradigm, stimuli are usually presented at a rate that falls within the alpha frequency band (e.g., one every hundred milliseconds). One previous study reported reduced phase locking to distracter stimuli at the stimulus-presentation frequency in no-blink compared to blink trials prior to T1 presentation (Kranczioch et al., 2007). It is thus possible that the here observed mental training-related reduction in alpha phase locking prior to T1 presentation reflected differential processing of the distracter stimuli.
Time—Frequency Analysis of Amplitude Dynamics
Mental Training-related Changes in Target-induced Amplitude of Oscillatory Activity
Although changes in phase variability may reflect the active formation and uncoupling of neuronal assemblies, their activation may be reflected by amplitude changes in oscillatory activity (for a review, see Tallon-Baudry & Bertrand, 1999). We therefore explored effects of intensive mental training on the amplitude (or power) of non-target-locked (i.e., induced) oscillatory neural activities. More specifically, changes in target-induced amplitude over time in no-blink versus blink trials were compared between the two groups, separately for each frequency band (see Methods for details). The only significant mental training-related change in target-induced responses was observed in the alpha frequency band between -31 and 160 msec post-T1 (see Figure 5). In this time window, the practitioners showed a significant increase in alpha power over time over occipital scalp regions in no-blink trials, as expressed by a significant Group (practitioners, novices) by Condition (blink, no-blink) by Session (Time 1, Time 2) interaction (maximal at POz at 102 msec post-T1: p = .003; Practitioners: p = .037; Novices: p =.30). No significant mental training-related changes in target-induced responses related to the detection of T2 were observed for any of the other frequency bands examined. Note that we focused on effects of mental training on induced activity and that our (null) findings therefore cannot directly be compared to that of previous attentional-blink studies, which examined differences in induced activity between blink and no-blink trials.
Figure 5.
Changes in target-induced amplitude over time. Changes in target-induced amplitude (μV) over time in no-blink versus blink trials are shown for each group (practitioners, novices) and frequency band separately, for electrode POz. Between —31 msec and 160 msec post-T1 (black rectangular box), the practitioners compared to the novices showed a significant increase in alpha power over time over occipital scalp regions in no-blink compared to blink trials. No other significant mental training-related changes in target-induced responses related to the detection of T2 were observed.
Mental Training-related Changes in Baseline Oscillatory Activity
As mentioned in the Introduction, one major aim of OM meditation is to remain only in a monitoring state, attentive moment by moment to anything that occurs in experience, without focusing on any explicit object. As it has been shown that the general mental state of the observer can influence the magnitude of the attentional blink (Olivers & Nieuwenhuis, 2005, 2006), our second hypothesis was that mental training would result in changes in baseline mental state, which may have affected subsequent target processing. Baseline mental state was indexed by ongoing brain activity (amplitude and phase) in the different frequency-band ranges in a 500-msec task preparation period before the onset of the stimulus stream. In contrast to our hypothesis, but in line with previous findings (Martens, Munneke, et al., 2006), intensive mental training was not associated with any significant changes in baseline mental state when mean EEG power in the different frequency bands was directly compared between blink and no-blink trials and groups. In addition, in this period, mental training did not produce any changes in phase variability locked to the onset of the task preparation period in any of the examined frequency bands.
DISCUSSION
The main findings of our study can be summarized as follows. First, successful target detection was associated with enhanced theta phase locking. This novel finding indicates a role for local phase synchrony of oscillatory theta activity in conscious target perception. Second, intensive mental training was associated with enhanced theta phase locking after successfully detected T2s, in particular, for those individuals who showed the greatest reduction in T1-elicited P3b amplitude. In line with theoretical accounts of the attentional blink (Shapiro, Arnell, & Raymond, 1997), these data reveal a direct relationship between the amount of resources devoted to T1 processing, as indexed by T1-elicited P3b amplitude, and the ability of the system to react to new target information, as reflected by theta phase locking to T2. They also provide support for the idea that the attentional blink is due to an overinvestment of attentional resources in stimulus processing (Shapiro et al., 1994), a suboptimal processing mode that can be counteracted by manipulations that counteract such an overinvestment (Arend et al., 2006; Olivers & Nieuwenhuis, 2005, 2006). In addition, these results support the notion that purely mental training can reduce the propensity to “get stuck” on a target, and thereby render the system more open to process information related to momentary experience (Lutz, Slagter, et al., 2008; Lutz et al., 2006). Third, mental training was not associated with changes in baseline mental state, as defined by ongoing oscillatory activity in a 500-msec fixation period right before task onset. Below we will discuss these three principal findings and their implications in more detail.
Conscious Target Perception and Theta Phase Synchrony
In both the practitioner and novice groups, neural activity in the theta frequency-band range phase-locked robustly to consciously perceived target stimuli only. No such phase locking occurred when T2 went undetected or was replaced by a blank. These novel observations indicate that local phase synchrony of oscillatory theta activity may provide a neural correlate of conscious target perception. As such, they extend previous findings from EEG studies examining neural synchrony between two brain areas during attentional-blink task performance (Kranczioch et al., 2007; Nakatani et al., 2005; Gross et al., 2004) and prior research investigating the role of synchronization of oscillatory responses in sensory awareness (Engel & Singer, 2001). Although many EEG investigations have examined event-related changes in theta power (e.g., Bastiaansen & Hagoort, 2003), relatively few studies have examined event-related changes in theta phase in humans. Interestingly, two recent intracranial EEG studies have reported a theta phase resetting at many distributed recording sites during the periods when probe items have to be identified and compared to target representations stored in working memory (Rizzuto et al., 2003, 2006). It is thus possible that the observed theta phase locking to successfully detected targets reflects a memory comparison process, in line with the well-known role of theta in memory (Bastiaansen & Hagoort, 2003; Kahana, Seelig, & Madsen, 2001). This interpretation also fits with theoretical accounts of the attentional blink, which have linked this deficit to capacity limitations of working memory (Shapiro et al., 1997).
Effects of Mental Training on Resource Availability
Intensive mental training was associated with enhanced theta phase locking after successfully detected T2s, in particular, for those individuals who showed the greatest reduction in T1-elicited P3b amplitude. These data suggest that meditation practice can significantly affect the way stimuli are processed and perceived. Specifically, as participants were not engaged in formal meditation during task performance, together with our previous findings (Slagter et al., 2007), the current data support the notion that intensive mental training, as cultivated by OM meditation, can reduce the propensity to “get stuck” on a target, as reflected by a reduction in T1-elicited P3b amplitude. This appears to leave resources more rapidly available to process information related to momentary experience, as reflected by both reduced variability in subsequent processes related to successful T2 detection and improved T2 accuracy. It should be noted, however, that the correlation between the observed mental training-related reduction in T1-elicited P3b amplitude and increase in T2-related phase consistency was relatively modest. This may indicate that the two effects reflect different, somewhat independent effects of mental training. Interestingly, as mentioned above, it is thought that although initially, the Vipassana practitioner still has the tendency to “cling” to objects in a way that takes up resources available to process information related to current experience, eventually, a trait emerges such that one can sustain the “nonclinging” state in which one is attentive to the content of experience from moment to moment. Perhaps the reduction in P3b amplitude reflects reduced “clinging,” whereas the increase in theta phase locking may reflect the development of the ability to monitor experience from moment to moment.
Our findings also corroborate the idea, based on studies investigating differences in mental and neural processing between expert meditators and novices (e.g., Lutz, Brefczynski-Lewis, Johnstone, & Davidson, 2008; Brefczynski-Lewis, Lutz, Schaefer, Levinson, & Davidson, 2007; Holzel et al., 2007; Lutz, Greischar, Rawlings, Richard, & Davidson, 2004), that mental processes are flexible skills that can be trained through purely mental training. Several other longitudinal studies have previously reported positive effects of training of variable duration and in a variety of meditation styles on various mental skills (Farb et al., 2007; Jha, Krompinger, & Baime, 2007; Tang et al., 2007). Future longitudinal studies are needed to examine the amount of training necessary in a given practice to produce demonstrable effects and to compare the effects of different styles of meditation on mental function.
Notably, intensive mental training enhanced theta phase locking only after T2s that were successfully detected and followed T1 within the time window of the attentional blink. As mentioned above, it is possible that the observed theta phase locking to T2 reflects a memory comparison process, in line with the well-known role of theta in memory (Bastiaansen & Hagoort, 2003; Kahana et al., 2001) and the notion that the attentional-blink deficit reflects capacity limitations of working memory (Shapiro et al., 1997). Thus, one possibility is that by reducing brain resource allocation to the first target, mental training increased the capacity of the system to process T2 in working memory.
An alternative interpretation of the observed theta phase locking to successfully detected T2s is in terms of a T2-driven reorienting process. It is notable that the mental training-related increase in theta phase locking occurred over right ventral frontal and central, as well as midline frontal scalp areas. According to an influential neurobiological model of selective attention (Corbetta & Shulman, 2002), a right-lateralized ventral network of brain areas, which includes the inferior frontal cortex, the temporo-parietal junction, and midline frontal regions, is specialized for the detection of behaviorally relevant stimuli, particularly when they are salient or unexpected. This right-lateralized “ventral system” is thought to direct attention in a bottom—up manner to salient events, thereby disrupting ongoing cognitive activity so that salient information can be selected for higher-order processing. Bearing in mind that EEG records neural activity at the scalp, the scalp topography of the here observed mental training-related increase in theta phase locking to T2 may therefore signify a greater overall readiness of this ventral system to be perturbated by a new target stimulus. An interpretation of this effect in terms of a stimulus-driven orienting response is in line with the fact that it was observed prior to the occurrence of the T2-elicited P3b, which usually peaks between 450 and 650 msec post-T2, is completely suppressed for undetected T2s, and has been related to higher-order stimulus processing (e.g., Vogel, Luck, & Shapiro, 1998). It also substantiates the idea that OM meditation cultivates a form of bare attention, in which attention remains in an open state so it can be directed to sensations, thoughts, emotions, and memories whenever they arise (Lutz, Slagter, et al., 2008; Lutz et al., 2006).
Interestingly, lesion studies (Hillstrom, Husain, Shapiro, & Rorden, 2004; Shapiro, Hillstrom, & Husain, 2002; Husain, Shapiro, Martin, & Kennard, 1997), split-brain patient studies (Giesbrecht & Kingstone, 2004), and transcranial magnetic stimulation studies (Cooper, Humphreys, Hulleman, & Praamstra, 2004) all support a critical role for a right-lateralized system in attentional-blink tasks. For example, Husain et al. (1997) found that patients with right ventral frontal or right ventral parietal lesions showed an abnormally long-lasting attentional blink. Thus, a right-lateralized system that serves to orient attention to unpredictable, salient events may critically determine performance in attentional-blink tasks. The overall readiness of this system to be perturbated in a bottom—up manner may be reflected by agreement across trials of the phase of oscillations in the theta frequency band after target events. However, the possibility bears further investigation.
It should be noted that our data are not only consistent with limited-capacity accounts of the attentional blink (Shapiro et al., 1997), but also with more recent models of the attentional blink, which assign a critical role to the distracter immediately following T1 (e.g., Olivers & Meeter, in press; Nieuwenstein, 2006; DiLollo, Kawahara, Ghorashi, & Enns, 2005). In these models, T1 does not drain resources, as limited-capacity accounts of the attentional blink typically assume (Shapiro et al., 1997), but elicits a strong attentional enhancement, that is also carried over to the post-T1 distracter. It is thought that the accidental encoding of this distracter stimulus subsequently hampers T2 selection. For example, according to the boost and bounce theory (Olivers & Meeter, in press), T1 elicits transient excitatory feedback activity meant to provide access to working memory. However, accidentally, the post-T1 distracter is also boosted, resulting in a subsequent strong inhibitory feedback response, which, in effect, closes the gate to working memory. Our data are not inconsistent with this scenario, if one considers the P3b to be the neural correlate of the attentional enhancement response (see also Olivers & Meeter, in press). Reduced attentional enhancement of T1 would also reduce attentional enhancement of the post-T1 distracter, reducing its disruptive effects and increasing the possibility that T2 is detected. Thus, our data do not dissociate between these more recent models and limited-capacity models, but are consistent with both types of models.
Effects of Mental Training on Baseline Mental State
In contrast to our second hypothesis, intensive mental training was not associated with changes in baseline mental state related to the conscious perception of T2, as defined by ongoing brain activity in the different frequency bands in a 500-msec fixation period before task onset. This null finding is in accordance with a previous study by Martens, Munneke, et al. (2006). They did not observe any differences in mean baseline EEG activity between participants with a relatively large attentional blink (“blinkers”) and participants with a relatively small attentional blink (“nonblinkersh), even though the nonblinkers showed an earlier T1-elicited P3b than the blinkers. Together, these findings may indicate that the mental state of the observer before stimulus presentation is not critical for successful attentional-blink task performance.
Yet, behavioral findings have shown that manipulations promoting a diffusion of attention can significantly reduce the magnitude of the attentional blink (Olivers & Nieuwenhuis, 2005, 2006), indicating a critical role for the mental state of the observer in the attentional blink. In addition, previous EEG studies have reported differences in synchronous neural activity between blink and no-blink trials in the alpha (Kranczioch et al., 2007), beta (Gross et al., 2004), and gamma (Nakatani et al., 2005) frequency ranges right before T1 presentation, during distracter presentation. This variation of findings showing differential processing of the distracter stimuli also points to differences in attentional state or the way attention is directed prior to the presentation of T1. In the current study, intensive mental training was also associated with differential processing of distracter stimuli before T1 presentation, as reflected by enhanced phase locking of the alpha frequency band over occipital scalp regions, further suggesting differences in pre-T1 attentional state. Interestingly, alpha activity is thought to reflect active functional inhibition (Klimesch, Sauseng, & Hanslmayr, 2007; Jensen, Gelfand, Kounios, & Lisman, 2002), and it is thus possible that mental training resulted in enhanced inhibition of the distracter stimuli in the stream. However, post hoc analyses showed that the observed effects in the alpha frequency band range were not strongest for the stimulus presentation frequency, arguing against this possibility. It is unclear what other process the observed increase in alpha phase locking prior to T1 might reflect.
In light of our and previous observations of differential distracter processing prior to T1 presentation, which are indicative of differences in attentional settings between no-blink and blink trials, our null finding of no mental training-related changes in baseline state is somewhat surprising. As to the absence of mental training-related changes in phase locking to the onset of the task preparation period, it is possible that the way attention was directed was not strongly temporally related to the onset of this period. It is also possible that, although we used a more sensitive within-subject contrast (as opposed to the across-subject contrast used in the Martens, Munneke, et al., 2006 study), our measures of baseline mental state were not sensitive enough. Future studies are necessary to determine to what extent the state of the system right before task onset affects the ability to process T1 efficiently and detect both targets.
Conclusions
In conclusion, we found that agreement across trials of the phase of oscillations in the theta frequency-band range after unpredictable target events was associated with their successful detection. In addition, in line with theoretical accounts of the attentional blink (Shapiro et al., 1997), a direct relationship was observed between the amount of resources devoted to T1 processing, as indexed by T1-elicited P3b amplitude, and the ability of the system to react to new target information, as reflected by theta phase locking to T2. Together with our previous data (Slagter et al., 2007), the current data suggest that purely mental training can affect how limited attentional resources are distributed. Specifically, they suggest that training in OM meditation can render the system more rapidly available to process new target information. Intensive meditation training was not associated with changes in the amplitude of T2-induced responses or changes in baseline mental state, as indexed by oscillatory activity before task onset. In combination, these findings illustrate the usefulness of systematic mental training in the study of the human mind by revealing the neural mechanisms that enable the brain to successfully process target information.
Acknowledgments
We thank the Barre Insight Meditation Society, and P. Babe, A. D. Francis, S. Harkness, Z. Moran, and N. Rawlings for assistance in data collection. We thank J. Davis for teaching the novices meditation. We also thank the Mind and Life Institute for helping to facilitate this work. This work was supported by grants from the National Institute of Mental Health (R01-MH43454 and P50-MH069315-03 to R. J. D.), the National Center for Complementary and Alternative Medicine (U01AT002114-01A1 to A. L.), the Netherlands Organization for Scientific Research (S. N.), and gifts from Bryant Wangard and Ralph Robinson, Keith and Arlene Bronstein, and the John W. Kluge Foundation (to R. J. D.).
Footnotes
UNCITED REFERENCES Bullock, McClune, & Enright, 2003 Sinkkonen, Tiitinen, & Näätänen, 1995
This simulation used the EEG data from 17 participants that had at least 80 no-blink trials at Time 1 (7 practitioners) and focused on the theta frequency band (4.1–8.5 Hz) and a time period of 300–350 msec post-T1. This was done as preliminary analyses using all participants showed phase locking of this band to T1 for this time window at Time 1 (see also below). For each participant, normalized PLF values were calculated based on different subsets of trials varying in size (n = 20, 30, 40, 50, 60, 70, or 80). These trials were randomly selected from the total number of trials available. Analyses revealed that although the pattern of results was unstable (i.e., which electrodes showed significant phase locking) when 20 or 30 trials were included, when 40 or more trials were included, the same pattern of results emerged in each analysis. Although the inclusion of more than 40 trials increased normalized PLF values, the overall scalp topography of significant theta phase-locking effects did not differ appreciably between the analyses using 40 or more trials. Thus, a minimum of 40 trials appears necessary to obtain reliable phase-locking estimates.
This conclusion is further strengthened by an additional analysis, which directly compared target phase locking in no-blink and blink trials at Time 1. Due to our requirement of at least 40 trials per trial type for reliable phase-locking estimates (see Methods), only 14 participants could be included in this analysis. We therefore collapsed across groups. A significant main effect of condition (blink, no-blink) was observed over the same midline frontal (maximal at Fz at 266 msec post-T2; p = .023) and right ventrolateral frontal (maximal at FC6 at 406 msec post-T2: p = .005) scalp regions that showed a mental training-related increase in theta phase locking in no-blink trials over time. This effect reflected greater theta phase locking in no-blink compared to blink trials, providing important support for our claim that the second peak in theta phase locking over frontal scalp regions reflects processes related to conscious T2 perception.
There is debate in the literature as to whether phase locking reflects transient, stimulus-evoked activity or an actual phase reset of ongoing oscillatory activity. The first account predicts that phase locking should be accompanied by post-T2 increases in oscillatory power in the theta frequency band (e.g., Hanslmayr et al., 2007). Contrary to this prediction, additional analyses of non-stimulus-locked amplitude fluctuations obtained by averaging across trials the amplitude envelopes given by Morlet wavelets (cf. Palva et al., 2005; Tallon-Baudry & Bertrand, 1999) did not reveal any changes in posttarget power in the theta frequency-band range (see section “Mental Training-related Changes in Non-target-locked Amplitude of Oscillatory Activity”). The here observed phase locking at frontal sites may thus have arisen from an actual reset of ongoing oscillations in the theta frequency-band range, in line with the second account. Yet, as reported in the section “Mental Training-related Changes in Baseline Mental State,” no mental training-related changes in power in this band were found in the prestream baseline period. Our data thus do not disambiguate between an actual phase reset and the initiation of a new oscillation.
REFERENCES
- Arend I, Johnston S, Shapiro K. Task-irrelevant visual motion and flicker attenuate the attentional blink. Psychonomic Bulletin & Review. 2006;13:600–607. doi: 10.3758/bf03193969. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39:967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, McClune MC, Enright JT. Are the electroencephalograms mainly rhythmic? Assessment of periodicity in wide-band time series. Neuroscience. 2003;121:233–252. doi: 10.1016/s0306-4522(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Cooper ACG, Humphreys GW, Hulleman J, Praamstra P. Trans-cranial magnetic stimulation (TMS) to right parietal cortex modifies the attentional blink. Experimental Brain Research. 2004;155:24–29. doi: 10.1007/s00221-003-1697-9. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Neuroscience Reviews. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- DiLollo V, Kawahara J, Ghorashi SMS, Enns JT. The attentional blink: Resource depletion or temporary loss of control? Psychological Research. 2005;69:191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends in Cognitive Sciences. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: Mindfulness meditation reveals distinct neural modes of self reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Kingstone A. Right hemisphere involvement in the attentional blink: Evidence from a split-brain patient. Brain and Cognition. 2004;55:303–306. doi: 10.1016/j.bandc.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, et al. Modulation of long-range neural synchrony reflects temporal limitations of attention in humans. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesh W, Herrmann CS, Bauml K. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;17:1–8. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hillstrom A, Husain M, Shapiro KL, Rorden C. Spatiotemporal dynamics of attention in visual neglect: A case study. Cortex. 2004;40:433–440. doi: 10.1016/s0010-9452(08)70137-0. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neuroscience Letters. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385:154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective & Behavioral Neuroscience. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Dell’Acqua R. The demonstration of short-term consolidation. Cognitive Psychology. 1998;32:138–202. doi: 10.1006/cogp.1998.0684. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinion in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Research, Brain Research Reviews. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Maye A, Engel AK. Temporal dynamics of access to consciousness in the attentional blink. Neuroimage. 2007;37:947–955. doi: 10.1016/j.neuroimage.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainement of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. Journal of Neuroscience. 2004;24:10186–10190. doi: 10.1523/JNEUROSCI.2584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis JA, Johnstone T, Davidson RJ. Voluntary regulation of the neural circuitry of emotion by compassion meditation: Effects of expertise. PLoS One. 2008;3:e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness: An introduction. In: Zelazo P, Moscovitch M, Thompson E, editors. Cambridge handbook of consciousness. 2006. [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Richard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Elmallah K, London R, Johnson A. Cuing and stimulus probability effects on the P3 and the AB. Acta Psychologica. 2006;123:204–218. doi: 10.1016/j.actpsy.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Martens S, Munneke J, Smid H, Johnson A. Quick minds don’t blink: Electrophysiological correlates of individual differences in attentional selection. Journal of Cognitive Neuroscience. 2006;18:1423–1438. doi: 10.1162/jocn.2006.18.9.1423. [DOI] [PubMed] [Google Scholar]
- Nakatani C, Ito J, Nikolaev AR, Gong P, van Leeuwen C. Phase synchronization analysis of EEG during attentional blink. Journal of Cognitive Neuroscience. 2005;17:1969–1979. doi: 10.1162/089892905775008706. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR. Top–down controlled, delayed selection in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:973–985. doi: 10.1037/0096-1523.32.4.973. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Meeter M. (in press). A boost and bounce theory of temporal attention Psychological Review [DOI] [PubMed] [Google Scholar]
- Olivers CN, Nieuwenhuis S. The beneficial effect of concurrent task-irrelevant mental activity on temporal attention. Psychological Science. 2005;16:265–269. doi: 10.1111/j.0956-7976.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Nieuwenhuis S. The beneficial effects of additional task load, positive affect, and instruction on the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:364–379. doi: 10.1037/0096-1523.32.2.364. [DOI] [PubMed] [Google Scholar]
- Palva S, Linkenkaer-Hansen K, Naäätänen R, Palva JM. Early neural correlates of conscious somatosensory perception. Journal of Neuroscience. 2005;25:5248–5258. doi: 10.1523/JNEUROSCI.0141-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nature Neuroscience. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Sergent C, Dehaene S. Is consciousness a gradual phenomenon? Evidence for an all-or-none bifurcation during the attentional blink. Psychological Science. 2004;15:720–728. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Sessa P, Luria R, Verleger R, Dell’acqua R. P3 latency shifts in the attentional blink: Further evidence for second target processing postponement. Brain Research. 2007;1137:131–139. doi: 10.1016/j.brainres.2006.12.066. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: A versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Singer W. Consciousness from neurobiological perspective. In: Metzinger T, editor. Neural correlates of consciousness. MIT Press; Cambridge, MA: 2002. pp. 121–137. [Google Scholar]
- Sinkkonen J, Tiitinen H, Naäätänen R. Gabor filters: An informative way for analysing event-related brain activity. Journal of Neuroscience Methods. 1995;56:99–104. doi: 10.1016/0165-0270(94)00111-s. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Hillstrom AP, Husain M. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Current Biology. 2002;12:1320–1325. doi: 10.1016/s0960-9822(02)01040-0. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Schmitz F, Martens S, Hommel B, Schnitzler A. Resource sharing in the attentional blink. NeuroReport. 2006;17:163–166. doi: 10.1097/01.wnr.0000195670.37892.1a. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Arnell KA, Raymond JE. The attentional blink. Trends in Cognitive Sciences. 1997;1:291–296. doi: 10.1016/S1364-6613(97)01094-2. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Raymond JE, Amell KM. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:357–371. doi: 10.1037//0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greisschar LL, Frances AD, Nieuwenhuis S, Davis J, et al. Mental training affects distribution of limited brain resources. PloS Biology. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- Ward R, Duncan J, Shapiro K. The slow time-course of visual attention. Cognitive Psychology. 1996;30:79–109. doi: 10.1006/cogp.1996.0003. [DOI] [PubMed] [Google Scholar]
- Wickens C, Kramer A, Vanasse L, Donchin E. Performance of concurrent tasks: A psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Goda N, Anderson SJ, Yoshida Y, Kawato M. Attentional modulation of oscillatory activity in human visual cortex. Neuroimage. 2003;20:98–113. doi: 10.1016/s1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]