Abstract
Mice of the C57BL/6J (B6) inbred strain exhibit genetic progressive sensorineural hearing loss and have been widely used as a model of adult-onset hearing loss and presbycusis. Males and females exhibit similar degrees of hearing loss until about 3 months of age, after which, the loss accelerates in females. This paper reviews research on how the B6 auditory system is affected by sex, gonadectomy (i.e., a reduction of gonadal hormone levels), and nightly exposure to moderately intense augmented acoustic environments (AAEs) – a low-frequency noise band (LAAE) or high-frequency band (HAAE). Several findings indicate a negative effect of ovarian hormones on the female B6 auditory system. Whereas the sex difference in high-frequency hearing loss was not significantly affected by gondadectomies, the female disadvantage in ABR thresholds at lower frequencies was erased by ovariectomy. Moreover, exposure to the LAAE or HAAE caused losses of hair cells that were more severe in intact females than in ovariectomized females or in males. Finally, intact females had more severe loss of neurons in the low-frequency region of the anterior ventral cochlear nucleus (AVCN) than other groups. In contrast, the presence of androgens had beneficial effects. Loss of hair cells and AVCN neurons after AAE exposure were more severe in orchidectomized males than in intact males. Ideas, hypotheses, and potential mechanisms concerning the findings are discussed.
1. Introduction
Mice of the C57BL/6J (B6) inbred strain exhibit genetic progressive sensorineural hearing loss and have been widely used as a model of adult-onset sensorineural hearing loss and presbycusis (for reviews see Erway et al., 1993, 2001; Henry, 1983; Frisina and Walton, 2001; Johnson et al., 1997; Li and Borg, 1991; Willott, 1996; Zheng et al., 2005). In B6 mice, hearing is near-normal in young adults (2 months of age), but by 5–6 months of age, high-frequency hearing loss is substantial, becoming severe by 9–12 months. There is a sex difference in the rate and severity of sensorineural hearing loss in B6 mice and this is one focus of the present review. Males and females exhibit similar degrees of hearing loss until about 3 months of age, when the loss begins to accelerate in females. By 6 months of age ABR thresholds for high frequencies (32 kHz +) are 20–30 dB higher in females. By 9 months of age, thresholds for lower frequencies have also become more elevated in females (Henry, 2002, 2004; Willott and Bross, 2004; Willott et al., 2006b). Well into the second year of life, both males and females become profoundly deaf.
The earliest and most severe histopathology in B6 mice is a loss of outer hair cells (OHCs) beginning in the high-frequency cochlear base. OHCs loss progresses, with mid-cochlea being the least affected. Inner hair cells (IHCs) show similar but less severe attrition. A loss of spiral ganglion cells (SGCs) occurs, and this is also most severe in the basal cochlea. Degeneration of other cochlear tissue and central auditory neurons occurs as well (Di Palma et al., 2001a; Frisina and Walton, 2001; Hequembourg and Liberman, 2001; Idrizbegovic et al., 2003; Ohlemiller and Gagnon, 2004; Spongr et al., 1997; Willott, 1996; Willott and Bross, 1996; Willott et al., 1987). Sensorineural hearing loss in B6 mice is the result of a gene, named Ahl (age-elated hearing loss) by Erway et al. (1993), that codes for a hair-cell specific cadherin and affects stereocilia (Di Palma et al., 2001b; Johnson et al., 1997; Noben-Trauth et al., 2003).
In addition to the actions of the Ahl gene, cochlear sensitivity and histopathology of B6 mice are also affected by exposure to augmented acoustic environments (AAEs). In a series of experiments in our laboratory, AAE treatments have consisted of nightly 12-hr exposures to repeated, 200 msec noise bursts of 70 dB sound pressure level (SPL re: 20μPa) at a rate of 2 per sec. Treatment overlapped the period when progressive hearing loss occurs in B6 mice, age 3 weeks to middle age. Earlier studies (Willott and Turner, 1999; Willott and Bross, 2004) employed an AAE whose spectral peak was in the mouse’s “middle frequencies” (MAAE: 8–20 kHz). The MAAE treatment caused a lessening and/or slowing of age-related hearing loss at frequencies roughly corresponding with the MAAE spectrum, as sensorineural cochlear damage was ameliorated. The relative benefits of MAAE treatment were similar for males and females. More recent studies (Willott et al., 2006, 2008) employed either a “low frequency” AAE (LAAE: 2–8 kHz) or a “high frequency” AAE (HAAE: half- octave band centered at 20 kHz). These treatments had some negative effects, exacerbating the strain-typical hearing loss and cochlear damage. The LAAE and HAAE studies also used gonadectomized males and females, allowing sex differences to be examined in more detail. The roles of sex, gonadal hormones, and LAAE/HAAE treatment in hearing loss provide a second focus of the review.
The reduction of cochlear sensitivity and auditory-evoked afferent neural activity associated with sensorineural hearing loss results in secondary effects in the central auditory system of B6 mice. For example, degenerative changes occur in the anterior ventral cochlear nucleus (AVCN) as hearing declines. A third focus of the present review concerns the effects of sex, gonadectomy, and AAE treatment on neuron death in the AVCN of B6 mice.
For this special issue of Hearing Research, data from previous studies (e.g., Willott and Bross, 2004; Willott et al., 2006b, 2008) have been reorganized and reanalyzed to emphasize the effects of sex and gonadectomy in greater depth. In addition, some new material is included. Where feasible, potential, broader implications of the findings are suggested.
1.2. Subject groups
Most of the material to be reviewed involves mice that were gonadectomized at age 2–3 weeks by highly experienced surgeons at the Jackson Laboratory before being shipped to our laboratory. Mice served in one of 12 subject groups based on sex (M, F), gonadal status (gonadally intact [int], ovariectomized [OVX], orchidectomized [ORX]), and AAE treatment (high AAE [HAAE], low AAE [LAAE], control [con]): intFcon, intMcon, OVXcon, ORXcon, intFHAAE, intMHAAE, OVXHAAE, ORXHAAE, intFLAAE, intMLAAE, OVXLAAE, ORXLAAE. For AAE treatment, weanling mice were placed in a plastic cage (12 × 13 × 30 cm), 4 mice per cage. Here they received consecutive 12-hour nights of AAE treatment through 9 months of age.
2. ABR thresholds
ABR thresholds provide a good indication of hearing sensitivity, and this is the measure that has revealed the sex differences in B6 mice (Henry, 2002, 2004; Willott and Bross, 2004). Mean ABR thresholds for intact and gonadectomized 9-month-old non-exposed control mice are shown in Figure 1. Group X Frequency 2-way ANOVAs, followed by Fishers LSD (protected t) tests were performed on the data to confirm the following observations. All testing and data analysis was done by a technician who was “blind” with respect to subjects’ group identity.
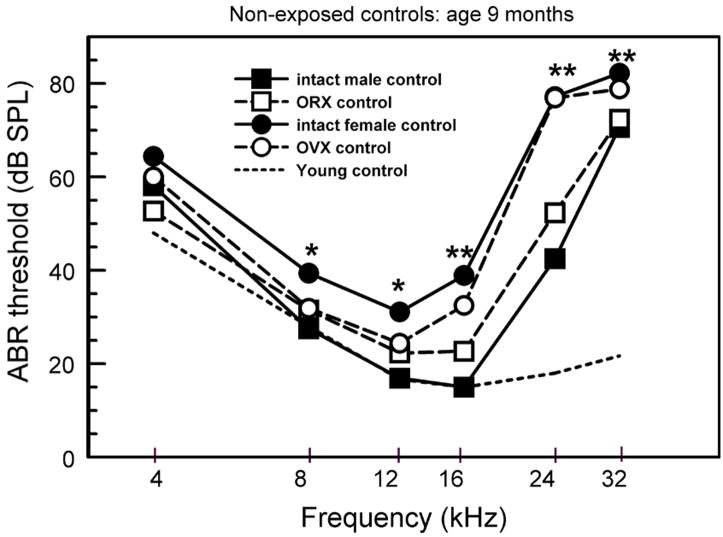
Figure 1.
ABR thresholds for non-exposed control mice. (asterisks: intact female controls differed significantly from the other groups. Double asterisks: intact and OVX female controls differed significantly from the other groups. There were 5 to 9 mice per group; standard errors did not overlap when differences were significant (see Willott et al., 2006b for ANOVAs and actual SEMs).
2.1 Non-exposed controls
Among non-exposed control groups, ABR thresholds of intact females (filled circles) were significantly higher than those of intact males (filled squares) for tones of 8–32 kHz. Thresholds of OVX females (unfilled circles) were higher than those of intact male controls for only for higher frequencies of 16–32 kHz (double asterisk: both female groups higher than males; single asterisk: only intact females are higher). Thresholds of ORX males (unfilled squares) did not differ significantly from those of intact males.
The data indicate that the sex difference persisted for higher frequencies despite ovariectomy. However, at lower frequencies, OVX females did not differ from males. This suggests that the presence of estrogen and/or progesterone (ovarian hormones) had some influence on ABR thresholds – exacerbating hearing loss at lower frequencies in females. No role of male hormones is evident with respect to ABR threshold elevations.
2.2. AAE treatment
It was previously reported that LAAE and HAAE treatment of B6 mice caused threshold elevations at some frequencies (Willott et al., 2006b, 2008). To parse the role of gonadal hormones in these elevations, ABR data for males (Fig. 2) and females (Fig. 3) are presented as a function of surgical status and AAE condition. The overall conclusion from examination of these figures is that gonadectomy had little effect on the relative change in ABR thresholds brought about by AAE exposure when gonadectomized and intact mice are viewed separately.
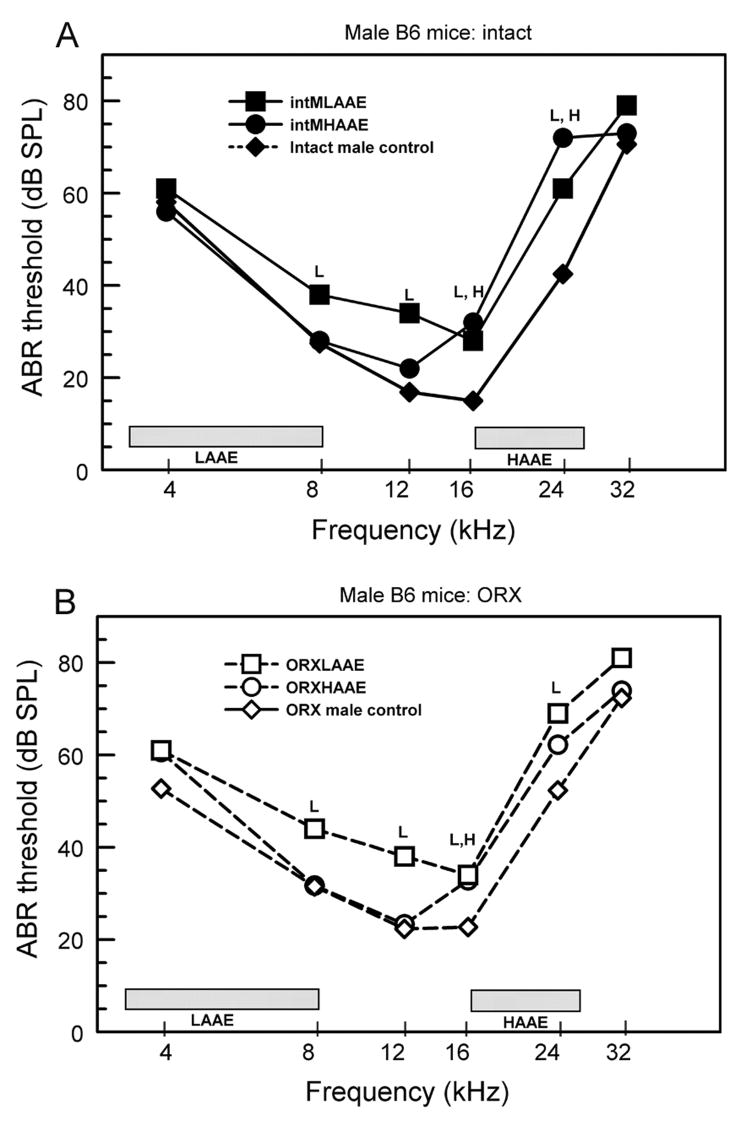
Figure 2.
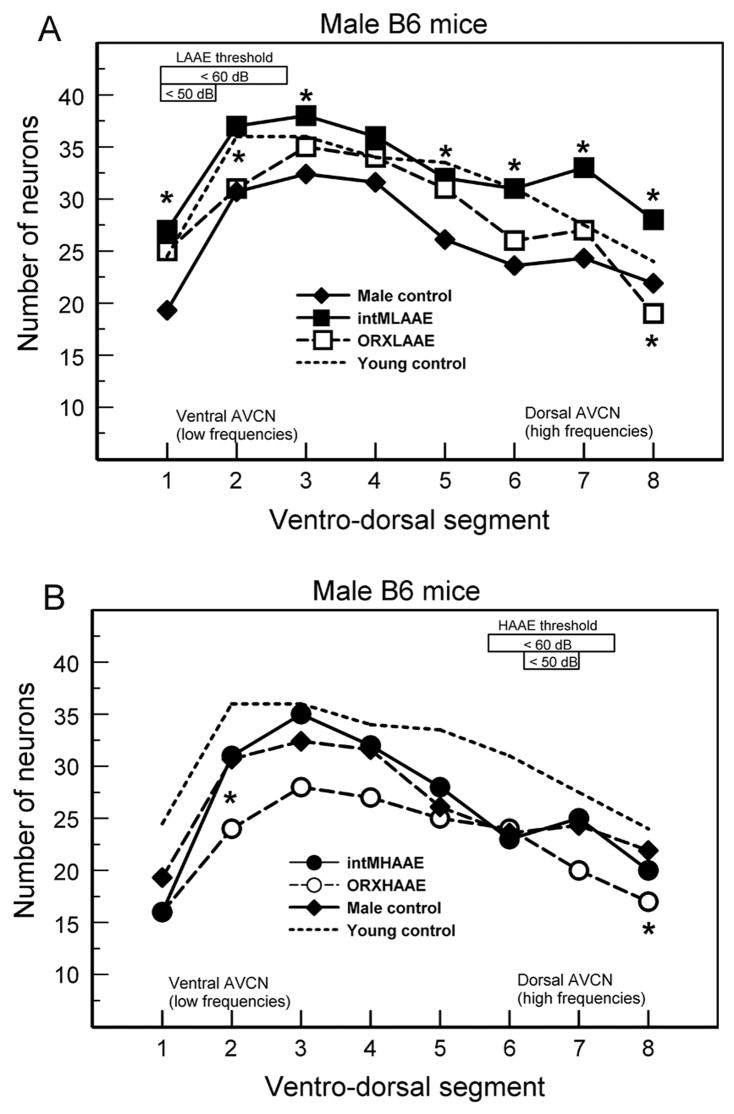
ABR thresholds for male B6 mice. The frequency bands of the AAE noise are shown by the shaded bars. A. Gonadally intact males. B. ORX males. L= LAAE-treated mice differed significantly from controls; H = HAAE mice differed significantly from controls. There were 5 to 9 mice per group (see Willott et al., 2006b and 2008 for ANOVAs and SEMs).
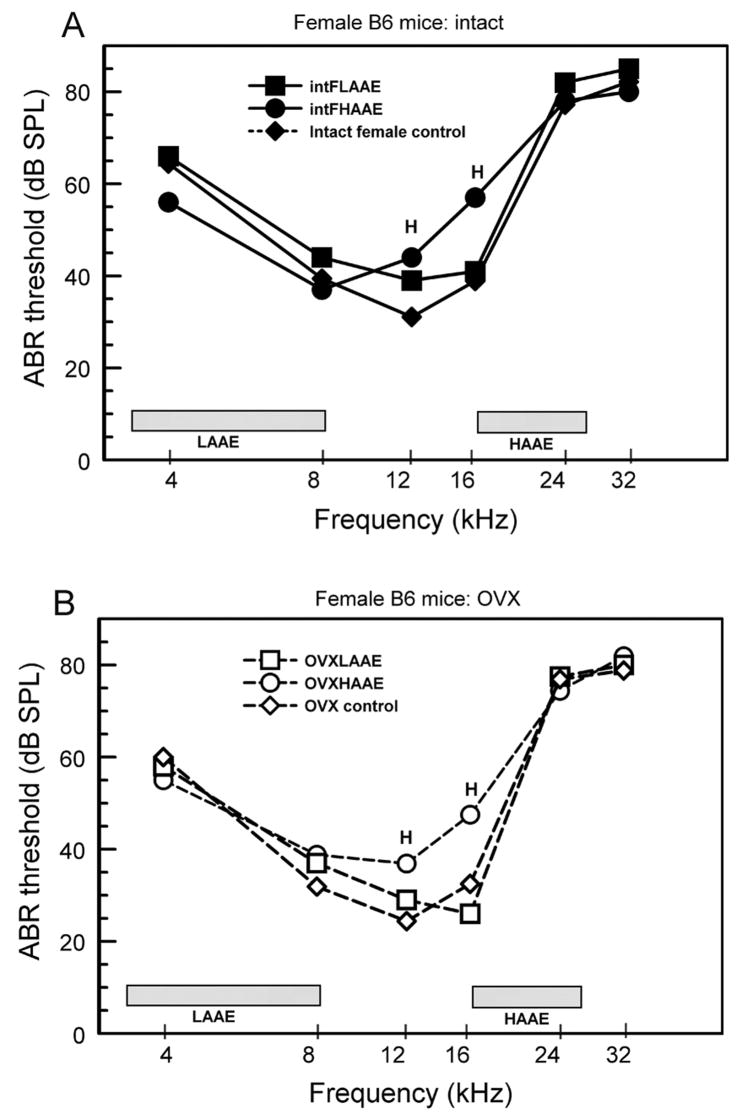
Figure 3.
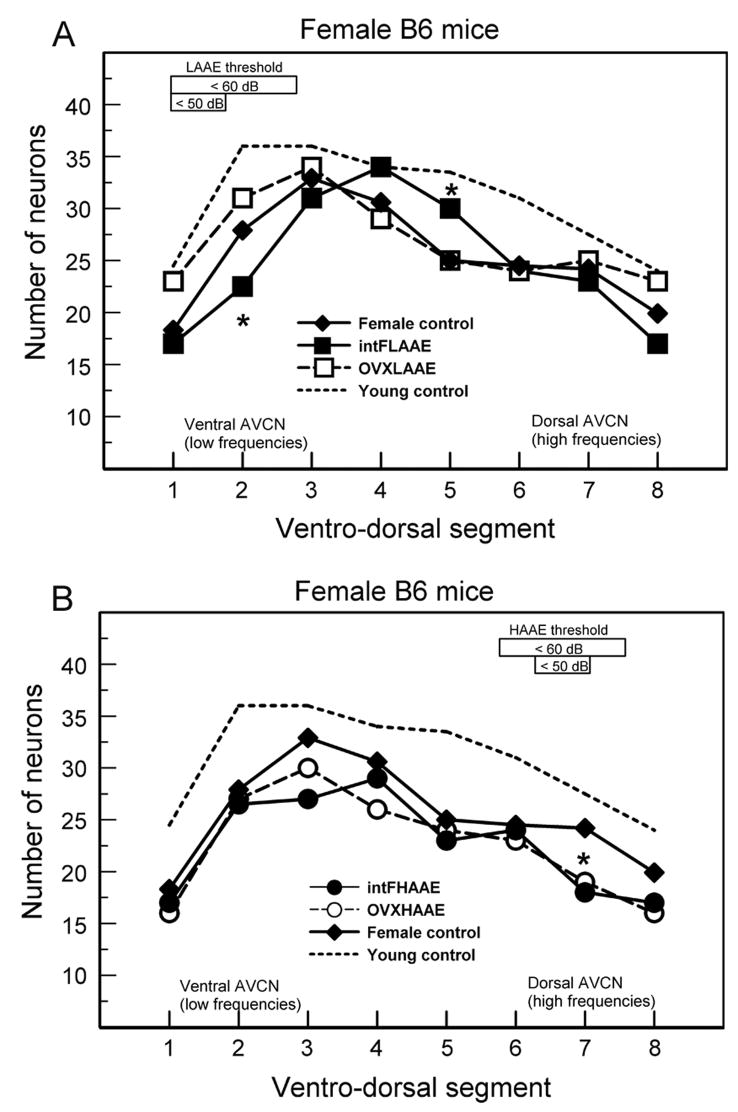
ABR thresholds for female B6 mice. The frequency bands of the AAE noise are shown by the shaded bars. A. Gonadally intact females. B. OVX females. L = intact LAAE-treated females differed significantly from controls; H = intact HAAE-treated females differed significantly from controls. There were 5 to 9 mice per group (see Willott et al., 2006b and 2008 for ANOVAs and SEMs).
Males exposed to the LAAE, whether ORX or intact, exhibited threshold elevations from 8 to 24 kHz (Fig. 2: squares; L = significant difference from controls). HAAE exposure (Fig. 2: circles) resulted in elevation of thresholds at 16 kHz for intact and OVX males compared to controls, with the elevation at 24 kHz also significant for intact males (H = significant difference from controls). The ORX and intact male groups did not differ significantly from one another.
Females (Fig. 3: circles) exhibited threshold elevations for tones of 12 and 16 kHz after HAAE treatment whose relative magnitude was not affected by ovariectomy. Differing from males, however, LAAE exposure did not cause significant ABR threshold elevations in intact or OVX females (Fig. 3: squares). However, because male controls had lower thresholds than female controls to begin with (Fig. 1), the absolute post-LAAE-exposure thresholds did not differ between sexes for tones of 12 kHz and 16 kHz. Indeed, LAAE-treated females had higher thresholds than LAAE-treated males for 24 kHz tones.
2.3. Summary
In non-exposed female control mice, ovariectomy lessened ABR threshold elevations only for lower frequencies. Gonadectomy had no significant effect on male controls or on the relative magnitude of threshold differences between exposed and control mice of either sex. Males treated with the LAAE exhibited threshold elevations for lower frequencies, but the absolute thresholds still ended up being similar to or lower than those of LAAE-treated females.
3. Cytocochleograms
Cytocochleograms were constructed by plotting the percentage of missing OHCs or IHCs (re: laboratory norms for young mice; Sponger et al., 1977) as a function of distance from the apex of the cochlea. Cytocochleograms for 9-month-old mice are presented in Figures 4 and 5.
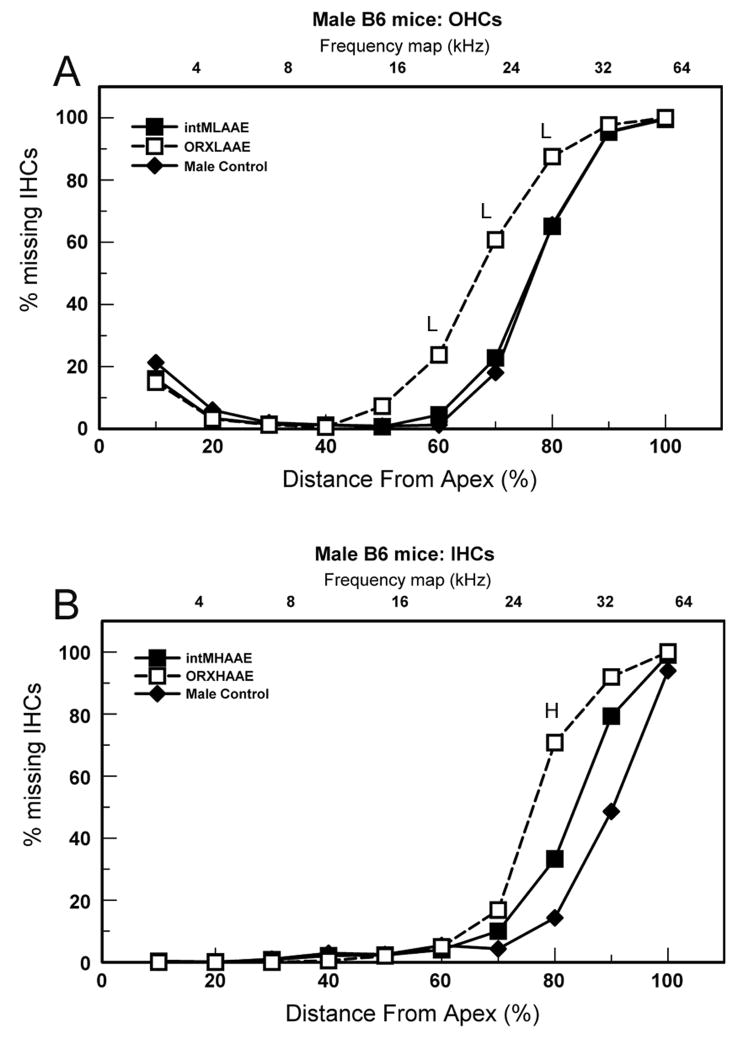
Figure 4.
Cytocochleograms (% missing hair cells) for 9-month-old males. A. OHCs. L = LAAE-treated ORX males differed significantly from controls. B. IHCs. H = AAE-treated ORX males differed significantly from controls. Young B6 mice have near 0% missing hair cells.
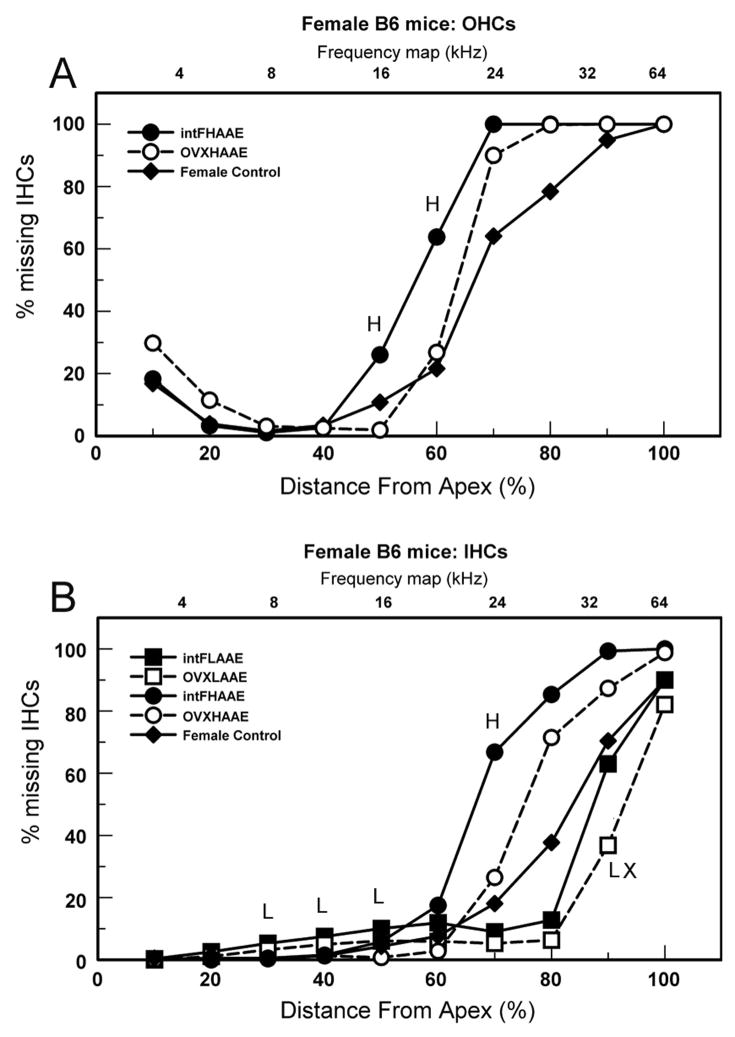
Figure 5.
Cytocochleograms (% missing hair cells) for 9-month-old females. A. OHCs. H = intact HAAE-treated females differed significantly from controls. B. IHCs. H = intact HAAE-treated females differed significantly from controls; L = intact LAAE-treated females differed significantly from controls. LX= OVX LAAE-treated females differed significantly from controls. Young B6 mice have near 0% missing hair cells.
3.1. Non-exposed controls
Gonadectomy had no significant effect on cytocochleograms in non-exposed control mice of either sex. Irrespective of gonadectomy, females had a greater loss of hair cells than males in cochlear regions roughly coinciding with the ABR threshold elevations. The sex effect can be seen by comparing control values of males and females (Figs 4 and 5: filled triangles; the control data for gonadectomized and control mice of each sex have been combined for these figures).
Willott and Bross (2004) evaluated hair cells in mice that were 12–14 months old. At this older age, OHC and IHC loss were more severe than they were at age 9 months in both sexes. Female mice continued to have more severe loss of OHCs than males, but the sex difference for IHCs had all but disappeared (i.e., the males “caught up”).
3.2. Effects of AAE exposure
The ABR threshold elevations caused by LAAE and HAAE exposure were paralleled by increased hair cell loss in cytocochleograms. However, gonadectomy appears to have played a stronger role in modulating the hair cell losses from AAE exposure than was apparent in ABR thresholds. Figures 4 and 5 show comparisons that were statistically significant (2-way Group X Segment mixed ANOVAs, followed by Fisher’s LSD protected t-tests) from which several observations can be gleaned.
In males, orchidectomy potentiated the loss of hair cells caused by LAAE and HAAE treatment. This was observed for OHCs after LAAE treatment (Fig. 4A) where ORX males (unfilled squares) exhibited more severe loss of OHCs than both controls (diamonds) and intact LAAE mice (filled squares) in the 60–80 percent distance from apex. Similarly, HAAE exposure resulted in a greater loss of IHCs compared to controls in the 80 percent distance in ORX males but not in intact males (Fig. 4B, unfilled squares). Other comparisons (OHCs with HAAE treatment and IHCs with LAAE treatment) were not statistically significant and are not shown.
Cytocochleograms indicated that intact females were more vulnerable to detrimental AAE effects (exacerbated cochlear damage) than OVX females. A significant effect of ovariectomy was observed in the apical half of the cochlea after LAAE treatment, as intact females had the greatest loss of IHCs in the 30–50 percent distance from apex (Fig. 5B). The small differences were significant because virtually all control mice had no loss of IHCs in these segments, whereas exposed mice had some losses. With HAAE treatment, intact females exhibited more severe loss of OHCs (Fig. 5A) and IHCs (Fig. 5B) than OVX females within the 50–70 percent distance.
3.3. Summary
Although gonadectomy did not affect the severity of hair cell loss in non-exposed control mice, it was a factor with LAAE and HAAE exposure. Orchidectomy resulted in more severe loss of hair cells in AAE-exposed males. In contrast, ovariectomy resulted in less severe loss of hair cells in exposed females. It would appear that the presence of male gonadal hormones was a mitigating factor with respect to the damaging effects of LAAE and HAAE treatment on hair cells, whereas the presence of ovarian hormones exacerbated the negative effects.
HAAE exposure affected cochlear regions roughly corresponding to the HAAE frequency spectrum. This was not the case with respect to the LAAE spectrum and the locus of cochlear damage. In males, frequencies within the spectrum of the LAAE are represented in the apical third of the cochlea, yet significant loss of OHCs associated with LAAE treatment occurred in more basal cochlear segments of ORX males (Fig. 4A, unfilled squares: 60–80 percent distance from apex). However, because male controls had significantly more OHCs than females to begin with, the post-LAAE counts were still greater than those of females. This is the same situation as occurred for ABR thresholds and LAAE treatment, discussed earlier. In essence, LAAE treatment diminished the male advantage at high frequencies but did not erase it.
An effect of LAAE exposure on high frequencies occurred in the opposite direction in females. LAAE-treated OVX females had less severe IHC loss than female controls in the 90 percent distance from apex (Fig. 5B: unfilled squares, “LX”). However, here too, the IHC loss did not differ significantly from that of male controls (Fig. 4B: filled triangles), which was less severe than that of female controls. Nonetheless, this represents an ameliorative effect of ovariectomy.
One way to view these findings is that the basal cochlea of female B6 mice was affected less severely by LAAE exposure because the cochlea of non-exposed females is more impaired than that of males. Paradoxically, the basal cochlea of females could not be over-stimulated sufficiently to cause as much additional damage. What LAAE exposure did to males was to make cochlear damage more similar to that occurring in females.
4. Spiral ganglion cells
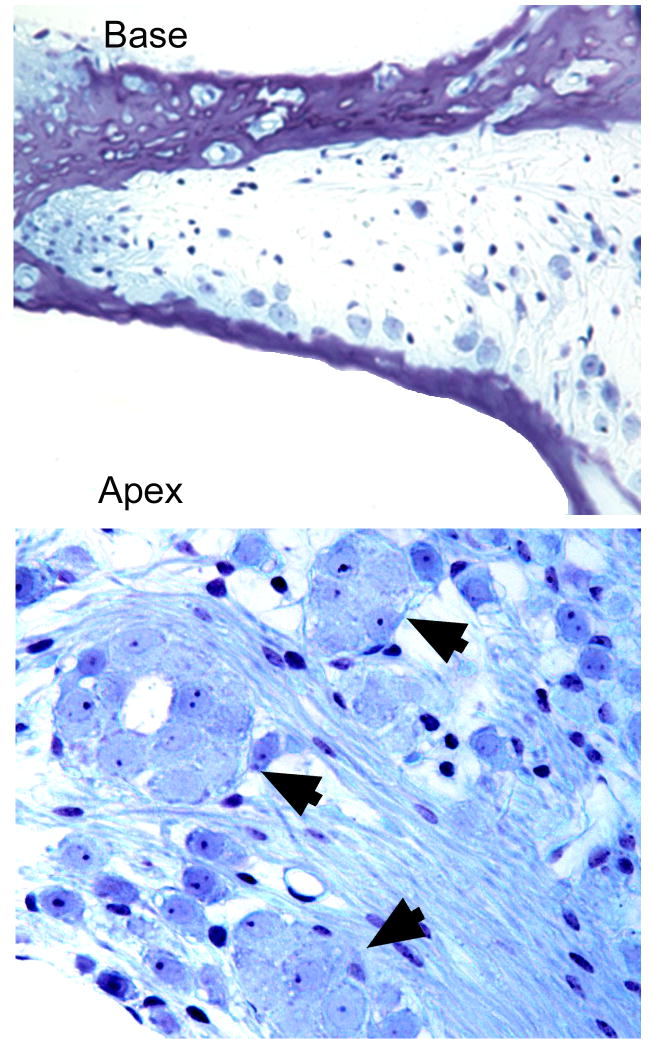
The two types of age-related SGC pathology that occur in B6 mice are shown in Figure 6. Most ubiquitous is a loss of SGCs in the basal cochlea (upper part of Fig. 6), a histopathological feature also observed in other strains with sensorineural or noise-induced damage (Willott, 1996; Willott et al., 1995). The second type of histopathology (lower part of Fig. 6) is unusual and consists of demyelination, fusion, and clumping of SGC somata in the extreme cochlear apex (Cohen and Grasso, 1987; Cohen and Bullers, 1990; Willott, 1996). One suspects that the two types of SGC are caused by different genes, but this has not been verified.
Figure 6.
Spiral ganglion cell histopathology in B6 mice. A. cochlear base is almost devoid of SGCs. B. SGCs clumping together in cochlear apex (arrows).
Recently, SGC histological material was prepared for the various subject groups used in the LAAE and HAAE studies, in collaboration with Drs. Richard Salvi and Dalian Ding at the University at Buffalo. SGCs from 15 intact and 17 gonadectomized mice, representing the various groups, were counted at four baso-apical locations, and the packing densities were computed. The variance within groups was high, and there was no evidence that SGC densities differed as a function of gonadectomy. The presence of SGC clumping in the apex was observed in 10 of the 32 mice, but these were not concentrated in any particular group either. While the sample is limited, it appears that SGC loss was not strongly regulated by gonadal hormones.
5. Possible mechanisms for sex/hormone effects in the cochlea
5.1. Sex differences in control (non-exposed) mice and the effects of gonadectomy
During the first 9 months of life in B6 mice, the male and female reproductive systems are functioning at a high level, and gonadectomy has to have a major effect on gonadal hormone levels (Nelson et al., 1975, 1981, 1982, 1992, 1995). That said, the present findings do not provide evidence for a necessary or sufficient role of hormones as being responsible for the sex differences in the usual course of B6 progressive hearing loss at high frequencies. In the ABR data, thresholds of both intact and OVX females were higher than those of males for tones of 16–32 kHz, and intact and OVX thresholds did not differ from one another. There was, however, evidence for a role of ovarian hormone(s) at low frequencies, as the sex difference only occurred for intact females (Fig. 1). This is suggestive of a hormonal effect specific to age-related, low-frequency hearing loss, and this is discussed later in this review.
Two caveats need to be mentioned. First, it is important to note that the present experiments only speak to adult levels of hormones, because the gonadectomized mice were intact prior to surgery at 2–3 weeks of age. Thus, it is possible that early, organizational hormonal effects occurred prior to surgery. Second is the possibility that there were some hormonal effects, but they were too subtle to be revealed by ABRs, cytocochleograms, or SGC counts.
Caveats notwithstanding, the present findings make feasible the possibility that some sex differences in progressive hearing loss in B6 mice (e.g., high-frequency hearing loss) were, at least in part, controlled by mechanisms other than gonadal hormones. There is evidence that sex differences in brain and behavior may be mediated by genetic mechanisms that do not involve gonadal hormones (Arnold, 1996; Blecher et al., 2007; Bocklandt and Vilain, 2007; Toran-Allerand et al., 1999), giving credence to this possibility.
5.2. AAE effects
The cytocochleograms provide evidence for a hormonal a role in modulating the detrimental effects of LAAE and HAAE exposure. Androgen may be protective, as intact exposed males exhibited less hair cell loss than ORX mice (Fig. 4). In contrast, estrogen and/or progesterone may facilitate the negative effects of LAAE and HAAE treatment in females (Fig. 5). LAAE- and HAAE- treated intact females exhibited more hair cell loss than their OVX counterparts.
The major question raised by these observations is: what is the mechanism for damaging LAAE and HAAE effects upon which hormones may be working? It has been hypothesized that vulnerability to noise-induced hearing loss (NIHL) may be responsible for the negative effects of LAAE and HAAE exposure in B6 mice (Willott et al., 2008). Studies have shown that B6 mice are exceptionally vulnerable to NIHL, and this has been linked to the Ahl gene which is also responsible for age-related hearing loss (Davis, 2001; Davis et al., 2003; Harding et al., 2004 Holme and Steel, 2003; Hultcrantz and Li, 1993; Li et al., 1993; Shone et al., 1991; Vazquez et al., 2004). The research has shown that exposures to intense noise cause more severe or long-lasting hearing loss in B6 mice than in strains of mice that do not possess the Ahl gene. These studies have used noise intense enough to cause NIHL in any strain of mouse, rather than determining if the “threshold” is lowered for the noise intensity sufficient to cause NIHL (e.g., Table 1 in Davis, 2001). Nonetheless, it is reasonable that a noise level that is not quite intense enough to cause NIHL in a non-vulnerable strain (e.g., 70 dB SPL noise bursts used for AAEs) would be able to do so in B6 mice when the exposures are 12 hours per night for several months. This being the case, an attractive hypothesis is that detrimental effects of LAAE and HAAE treatment in B6 mice are related to noise-induced damage from over-stimulation of vulnerable ears.
Ovarian hormones certainly have potential to affect the cochlea in various species (e.g., Coleman et al., 1994; Hultcrantz et al., 2006; Laugel et al., 1987, 1988; Lee and Marcus, 2001; Stenberg et al., 1999), but their effect on NIHL has not been a focus of research. In one potentially relevant study on B6 mice, Shen et al. (2007) presented evidence that threshold shifts after noise exposure were reduced by calcium channel blockers in male B6 mice but not in females, suggesting female vulnerability.
NIHL typically affects cochlear regions that map to frequencies at and above the noise frequency spectrum (e.g., Colvin and Luxon, 2007). This was indeed the pattern seen with HAAE exposure and ABR thresholds (Figs. 2, 3) and hair cell loss (Figs. 4A,B; 5B). However in males, LAAE exposure included elevation of high frequency ABR thresholds (Fig. 2: 24 kHz, intact and ORX mice) and loss of basal OHCs (Fig. 4A, ORX males). This may be related to the considerable vulnerability of the basal half of the cochlea in B6 mice, which undergoes the earliest and most severe sensorineural degeneration. In this regard, Ou et al. (2000) exposed hybrids of B6 and a normal-hearing CBA strain to low-frequency octave band noises of 100–105 dB SPL. The mice were young adults, and the data were not analyzed as a function of sex. Despite the fact that the hybrids have normal hearing and are not abnormally susceptible to NIHL, an octave band with a 4 kHz center frequency caused significant threshold elevations both low and high frequencies. An 8 kHz-centered noise caused threshold elevations at all tested frequencies. This study lends more weight to the notion that LAAE exposure could affect high frequencies in the NIHL-vulnerable B6 mice.
One can speculate about mechanisms that might increase cochlear damage by over-stimulation from AAEs and provide a pathway for hormonal influence. A number of variables have been implicated as mechanisms contributing to cochlear damage and NIHL as a result of intense noise exposure in otherwise healthy ears. These include noise-induced oxidative stress (damage from reactive oxygen species [ROS], free radicals), vasoconstriction, glutamate excitotoxicity (disruption of intracellular calcium homeostasis), apoptosis, and interactions among them (see reviews by Henderson et al., 2006; Le Prell et al., 2003). A breakdown of antioxidant defenses has been implicated in age-related and other types of hearing loss (Henderson et al., 2006; Jiang et al., 2006; McFadden et al., 1999; Seidman, 2000; Seidman et al., 1993), and evidence has been presented linking the Ahl gene to oxidative stress in B6 mice (Staecker et al., 2001). So, it is feasible that B6 mice would be vulnerable to additional oxidative damage from prolonged, moderately intense AAE exposure. Brown et al. (1995) observed altered responsiveness in cochlear vascular reactivity in B6 mice, suggesting another source of vulnerability. Excitotoxicity occurs when there is an abnormal influx of cellular calcium at synapses using the neurotransmitter glutamate. Work by Pujol and colleagues (Pujol et al., 1990; Pujol and Puel., 1999) has shown excitotoxic damage at glutamatergic IHC synapses following acoustic stimulation. As is the case for oxidative stress, it is possible that excitotoxicity from chronic acoustic stimulation could damage the vulnerable cochlea.
Efferent systems from brain to cochlea provide another interesting possibility for modifying progressive sensorineural hearing loss by the acoustic environment. Most notably, the olivocochlear efferent systems, if activated by the acoustic environment, might modulate cochlear sensitivity (see review by Le Prell et al., 2003). Interestingly, B6 mice showed altered efferent dynamics that preceded age-related hearing OHC loss, with the medial olivocochlear system, which modulates OHC function, declining rapidly during young adulthood (Zhu et al., 2007). This suggests that a malfunction of this system could play a role in the AAE effects observed in B6 mice, although a role of sex has not yet been shown.
It is clear that many potential pathways can be considered by which gonadal hormones might modify the effects of LAAE and/or HAAE exposure in B6 mice. At this time, however, we have little insight into which, if any, of these might be actually involved.
6. Central effects of AAE treatment: AVCN
The central auditory system has little in common with the cochlea with respect to its neurons, neurotransmitters (excitatory and inhibitory), neural circuits, central versus peripheral milieu, and functions. Thus, understanding the effects of sex, hormones, and the acoustic environmental on the central auditory system requires a rather different –probably multifaceted -- approach from what is relevant to the cochlea. To address the issue, this review focuses on histological work on the AVCN. This structure is relatively less complex than higher-order auditory nuclei, receives direct input from the cochlea, and is tonotopically organized. The working assumption is that the effects of AAE treatment on AVCN tissue are primarily related to AAE-evoked neural stimulation arriving from the cochlea via synaptic input from the eighth nerve. The impact, whether positive or negative, is potentially modulated by gonadal hormones and other factors (e.g., genes, development, aging), although likely in a rather different manner than such variables might affect the cochlea.
B6 mice normally lose about 20 to 25 % of their AVCN neurons during the period that sensorineural hearing loss occurs, and the process is largely completed by 9 months of age (Willott and Bross, 1996). The degenerative changes in AVCN are assumed to be triggered by deprivation/denervation secondary to the attenuation or loss of auditory nerve input (see Willott and Bross, 1996; 2004). The diminution of activity-evoked up-regulation of blood supply, oxygen, neurotrophins, or other beneficial substances puts the surviving neurons at a metabolic risk (see Smittkamp and Durham, 2004 and Saunders et al., 1998 for examples in birds). With severe or total cochlear damage, adult mammals including mice (Willott et al., 1994) exhibit denervation-induced changes such as shrinkage of neurons and neuropil, but neurons tend to survive (Harris and Rubel, 2006). Paradoxically, a loss of input restricted to high frequencies (e.g., B6 mice) is more conducive to neuron death than complete denervation (Willott and Bross, 1996).
6.1. Tonotopic organization
The AVCN is tonotopically organized because the ventral, low-frequency region of AVCN receives input from the apical (low-frequency) cochlea, the ventro-dorsal middle region receives input from mid-cochlea, and the dorsal region of AVCN receives input from the basal (high frequency) cochlea. Thus, age-related or noise-induced damage to the basal cochlea reduces auditory evoked input to the dorsal AVCN, whereas apical damage alters input to the ventral AVCN. By the same token, exposures to LAAE and HAAE increase auditory-evoked neural input to the ventral and dorsal regions of the AVCN, respectively.
In order to take tonotopic organization into account, the histological analysis of AVCN sections has focused on morphological properties in the ventro-dorsal (tonotopic) dimension. To reflect AVCN tonotopicity, imaging software was used to separate frontal sections into 8 ventro-dorsal segments. Thus, the ventro-dorsal segments roughly paralleled tonotopicity. Morphometric measurements were made within each ventro-dorsal segment of frontal sections, but the discussion here is limited to the number of surviving neurons in a representative “standard” AVCN section (see Willott et al., 2008 for additional data). Mice were 9 months old.
The data on AVCN neuron counts have been organized and analyzed to clarify sex and hormonal effects, and several key comparisons among groups are presented in Figures 7 and 8. Within each sex, AVCN neuron counts for intact and gonadectomized, non-exposed control groups did not differ significantly (Group X Segment mixed ANOVA), so they have been combined in each figure (diamonds). As a reference, values for young B6 mice are shown in dotted lines; sexes were combined for young mice because they did not differ significantly in young B6 mice. Group comparisons that were not significant are not shown.
Figure 7.
Number of AVCN neurons in male B6 mice. Asterisks: counts differed significantly from non-exposed controls. A. After LAAE treatment intact males treated with the LAAE (filled squares) had more neurons than controls in most segments; ORX mice treated with the LAAE (unfilled squares) had fewer neurons than controls in segment 8. B. After HAAE treatment ORX males had fewer neurons than controls in segments 2 and 8. There were 4 to 8 mice per group (see Willott et al., 2008 for ANOVAs and SEMs).
Figure 8.
Number of AVCN neurons in female B6 mice. Asterisks: counts differed significantly from non-exposed controls. A. After LAAE treatment intact females treated with the LAAE had fewer neurons than controls in segment 2 but more neurons in segment 5. B. After HAAE treatment intact and OVX females had fewer neurons than controls in segment 7. There were 4 to 8 mice per group (see Willott et al., 2008 for ANOVAs and SEMs).
6.2 Hypotheses and the AVCN data
Because there were no comparisons of interest among control groups, the discussion focuses on effects of LAAE and HAAE treatment. In doing so, the findings are framed in the context of three hypotheses: the deprivation hypothesis, unfair competition hypothesis, and excitotoxicity hypothesis.
6.2.1. The deprivation hypothesis
As mentioned, it is assumed that, in non-exposed control mice, the loss of neurons is secondary to the deprivation of auditory-evoked neural activity brought about by sensorineural damage. If deprivation is causing a loss of AVCN neurons in control B6 mice, would AAE- treatment provide sufficiently increased neural input to the AVCN to reverse the deprivation and result in a savings of neurons compared to controls? This was indeed the finding of two studies that did not use B6 mice. Willott et al. (2006a) used the same HAAE treatment in mice of the DBA/2J strain, which exhibit very early severe hearing loss. High-frequency hearing loss was ameliorated, as was the loss of neurons in the dorsal AVCN. In a study of cats by Noreña and Eggermont (2005), cats were exposed to traumatizing noise, then treated for several weeks with an HAAE. Noise-induced hearing loss in the treated cats was ameliorated, and central changes that normally accompany hearing loss were prevented.
The problem with respect to overcoming deprivation with HAAE exposure in B6 mice is that the treatment itself caused substantial high-frequency hearing loss (Figs. 2 and 3). This would compromise the ability of the HAAE to overcome deprivation. For example, thresholds for 24 kHz tones (near the middle of the HAAE spectrum) were all above 60 dB SPL after HAAE treatment, so that the cochlea would not be able to deliver much additional auditory-evoked neural input to the dorsal AVCN from the barely suprathreshold 70 DB SPL HAAE. This appears to be the case, because in no condition did HAAE treatment produce savings of neurons in the dorsal AVCN compared to controls (Figs 7B, 8B). Indeed, there were fewer neurons in the dorsal region in some cases. If the HAAE was ineffective in stimulating AVCN neurons, the deprivation hypothesis holds.
After LAAE treatment, ABR thresholds for tones within a good portion of the LAAE spectrum were still below 50 dB SPL (Figs. 2 and 3). Therefore, one would expect the LAAE to readily evoke auditory activity in the ventral AVCN (unlike the situation with the HAAE). Intact males treated with the LAAE did have more neurons than control mice (Fig. 7A), suggesting a reversal of any low-frequency deprivation that occurred. In this case, the deprivation hypothesis holds. Beyond this, however, the hypothesis fails. The benefits of LAAE treatment to neuron survival extended into ventro-dorsal regions that respond poorly if at all to the LAAE. Moreover, ORX males and females did not show any diminished neuron loss in the ventral AVCN after LAAE treatment. Indeed, intact females had fewer neurons. Interestingly, the three groups without high levels of androgen did not benefit from LAAE treatment.
6.2.2. The unfair competition hypothesis
The high-frequency tonotopic region of the AVCN has two disadvantages when high-frequency cochlear hearing loss occurs. First, neurons are deprived of normal afferent input from high-frequency sounds and put at metabolic risk, as mentioned. Second, if neural activity is increased in the adjacent, healthy middle-frequency region, the available supply of blood, oxygen, and beneficial substances (which is locally limited) could be usurped at the expense of the already-deprived high-frequency region. This has been dubbed the “limited resources” effect (Willott and Bross, 2004). Moreover, neurons in the healthy region might exert inhibitory influences or compete for synaptic sites from other neurons to further reduce neural activity in the deprived region. The competitive advantage hypothesis suggests that this “one-two punch” causes increased degeneration of the deprived region. This hypothesis was developed to account for data on MAAE (middle-frequency AAE) treatment in older (12–14 month-old) male B6 mice (Willott and Bross, 2004). Despite ameliorative effects on OHC survival in mid-cochlea and middle frequency ABR thresholds, MAAE-treated males had an increase in the loss of neurons in the dorsal and ventral extremes of AVCN. At this age, male B6 mice continue to have relatively good sensitivity to middle frequencies, but high- and low-frequency sensitivity has declined greatly, thereby depriving ventral and dorsal regions of input. MAAE stimulation would still deliver increased excitation to neurons in the middle region of AVCN, gaining a competitive edge at the expense of the under-stimulated regions. Older female B6 mice did not exhibit the negative effect of MAAE treatment in ventral and dorsal AVCN. They exhibit more severe hearing loss than males during middle age, as discussed earlier. This would limit the ability of the MAAE to stimulate the mid-AVCN and prevent the usurpation of resources away from the dorsal and ventral regions (i.e., closer to complete deprivation that typically does not kill neurons).
A variant of the unfair competition hypothesis might be applied to the finding of savings of AVCN neurons in ventro-dorsal segment 5 of intact females exposed to the LAAE (Fig. 8A: filled squares). The loss of neurons is pronounced in more ventral segments, resulting in less demand of resources. This could provide more for the neurons in segments 4 and 5 and, coupled with stimulation from ambient sound (including some effect of the LAAE), have a protective effect compared to controls.
6.2.3. Excitotoxicity hypothesis
The excitotoxicity hypothesis provides an amendment to the deprivation hypothesis to explain some results. The notion of excitotoxicity arises from a large body of evidence showing that the excitatory amino acid neurotransmitter glutamate is implicated in damage to neurons linked to aging, neurodegenerative diseases, or other conditions (see Mishizen et al., 2001 for a review). Directly relevant are studies showing that auditory over-stimulation can damage cochlear nucleus neurons via excitotoxicity (Kim et al., 1997; 2004; Muly et al., 2004; Potashner et al., 1997).
In the present context, activation of auditory nerve - AVCN glutamatergic synapses by HAAE stimulation would cause excitotoxic damage to AVCN neurons if they are vulnerable in some way. The deprivation hypothesis would explain this if one aspect of deprivation-induced changes in AVCN neurons is increased vulnerability to excitotoxicity. The introduction of HAAE stimulation each night (after a 12-hr day of high-frequency deprivation) would cause excitotoxic damage in the corresponding tonotopic region. There is, however, a delicate balance: hearing loss needs to be sufficient to cause denervation-induced vulnerability during the daytime periods when the HAAE is off, but not so severe that auditory input from the nighttime HAAE does not cause excitotoxicity. With too much hearing loss, deprivation would predominate, and auditory-evoked excitotoxicity would not occur.
The excitotoxicity hypothesis provides a second potential mechanism to account for the loss of neurons in the dorsal AVCN from HAAE treatment (Figs 7B, 8B). The neurons were put at risk by auditory deprivation, and the ability of the HAAE to drive excitatory responses was sufficient to cause additional excitotoxic damage and neuron loss.
Because the hypothesis relies on deprivation to make the neurons vulnerable to excitotoxicity, it is not necessarily at odds with the deprivation hypothesis. The empirical issue, which cannot be answered at this time, is whether the degree of hearing loss allows the excitotoxic process to occur. This is not an unimportant distinction. If we are to understand and treat the processes underlying central effects of hearing loss, the occurrence of excitotoxicity would require a different ameliorative approach than deprivation alone.
6.2.4. Integration of the three hypotheses
For all three hypotheses, damage or vulnerability resulting from hearing-loss-induced deprivation of central neurons is a key element. For the deprivation hypothesis, the vulnerability is sufficient for neuron loss. For the unfair competition hypotheses, the vulnerability from deprivation is coupled with the usurpation of metabolic resources by adjacent healthy, AAE-stimulated AVCN tissue. For the excitotoxicity hypothesis, deprivation increases vulnerability to excitotoxicity, which then induces additional damage. Obviously, the phenomena are not mutually exclusive, and one can envision the mechanisms acting in concert. For example, negative effects of reduced high-frequency input to the dorsal AVCN (cell loss or damage) could be exacerbated by unfair competition, with middle-frequency input provided by ambient sounds. This, in turn could increase the vulnerability of the neurons to auditory-evoked excitotoxicity. In any event, it would appear that there are different pathways by which hearing loss and/or the acoustic environment can negatively affect the AVCN. A possibility for hormonal influence lies in the vulnerability of neurons in the face of deprivation.
6.2.5. Hormone effects
As mentioned earlier, at least some differences in AVCN cell loss between intact and gonadectomized mice cannot be adequately explained by differences in cochlear sensitivity between the groups. Intact males exposed to the LAAE had more AVCN neurons than controls (Fig. 7A), even in high-frequency, dorsal segments. This finding suggests that the combination of androgen and acoustic stimulation had a protective effect that was not frequency specific. A neuroprotective effect of androgen is also suggested by the finding that the number of surviving neurons in intact males treated with the HAAE did not differ from that of controls, but ORX males had fewer neurons (Fig. 7B). This protective effect was also manifested in tonotopic regions not stimulated by the AAE.
Little is known about the influence of androgen on the mammalian auditory system. Simerly et al. (1990) demonstrated the presence of androgen receptors in the AVCN of rats, suggesting the possibility of direct effects on AVCN tissue. Thus, it is of interest that androgens can have neuroprotective or neurotrophic effect on brain tissue against excitotoxicity, apoptosis, and oxidative stress (e.g. Barron et al., 2006; Charalampopoulos et al., 2006; Dluzen et al., 1994; Kimonides et al., 1998, 1999; Li et al., 2001; Pike, 2001; Pouliot et al., 1996).
Evidence reviewed here suggests that ovarian hormones increase the vulnerability of low-frequency regions of the female B6 auditory system. Intact females had fewer neurons in the ventral (low frequency) AVCN than OVX females after LAAE treatment (Fig. 8A). IHCs exhibited differences in the same direction after LAAE treatment, but as mentioned earlier, the small effect did not seem sufficient to limit stimulation from the LAAE. In non-exposed control mice, ABR thresholds of intact females were higher than those of OVX females at low frequencies (Fig. 1). Finally, the study of 12–14 month-olds B6 mice by Willott and Bross (2004) found female control mice to have fewer OHCs and SGCs than males in the apical (low-frequency) half of the cochlea but not in the base.
How might one explain the apparent negative ovarian hormonal effect? Estrogen can have neuroprotective effects in various systems (e.g., Garcia-Segura et al., 2001; Picazo et al., 2003; Wise et al., 2001), and can be ameliorative for the auditory system of humans (Hultcrantz et al, 2006; Kilicdag et al., 2004; Kim et al., 2002). This would seem at odds with the findings. However, estrogen and progesterone can also have detrimental effects on brain tissue even when receptors are not present (Scallet, 1999). Thus, it is conceivable that estrogen and/or progesterone increased the vulnerability of neurons in the ventral AVCN of intact females (compared to OVX mice) such that the effect of LAAE treatment was harmful to neurons. Along these lines, Guimaraes et al. (2006) found that postmenopausal women treated with estrogen plus progestin (synthetic progesterone) had greater hearing loss and perceptual deficits than those taking estrogen alone or a non-hormone replacement. They concluded that progestin affected both the peripheral and central auditory system negatively. Other evidence also suggests that estrogen plus progesterone can lead to a decline in hearing and/or central changes (Caruso et al., 2003a,b; McFadden, 2002).
Another avenue to consider stems from the well known fact that ovarian hormones modulate female behaviors, including maternal responses to pup calls in mice (Easton et al., 2006; Numan, 1985; Rosenblatt et al., 1985). Gonadal hormones have substantial effects on the central auditory system of some songbird species (e.g., Maney et al., 2006) suggesting the potential power of hormones to alter the responsiveness of the central auditory system. Mouse pups emit several communicative vocalizations that elicit responses in lactating dams. All of these calls have substantial high-frequency content, and “isolation calls” are completely ultrasonic (Haack et al., 1983). Koch and Ehret (1989) showed that estradiol in particular plays a key role in eliciting pup retrieving by female mice. From an evolutionary, adaptive perspective, it might be expected that the low-frequency regions the female central auditory system would have diminished importance, especially when hormonal physiology is in place. Diminished importance could be associated with less protective resource allocation (neurotrophins, antioxidants, etc.), and in turn, greater vulnerability to LAAE exposure. The applicability of a direct estrogen effect on the vulnerability of low-frequency regions of the female B6 AVCN is speculative, but worthy of consideration.
7. Conclusions and future research
This review has presented evidence that gender and gonadal hormones can influence sensorineural hearing loss in B6 mice. The ovarian hormonal influence is presumably interacting with the actions of the Ahl gene and perhaps other background genes that may be specific to the B6 strain. As such, it remains to be determined how broadly the findings generalize to other species or causes of hearing loss. Nonetheless, the B6 mouse provides a unique model to investigate contributions and interactions among gender, gonadal hormones, genes, and hearing loss. Anything that can be learned about what can and does occur in a mammalian cochlea will advance our knowledge of hearing loss.
AVCN degeneration is presumed to be a secondary effect of deprivation from cochlear damage. The central changes probably have nothing to do with the peripherally-acting Ahl gene per se, as any variable that causes sensorineural damage would also deprive the AVCN of input. Thus, the hormonal effects may be more readily applied to the auditory system of rodents and, perhaps mammals in general. The findings on B6 mice suggest a hormonal influence on the health of central auditory neurons, particularly when they are vulnerable to harmful factors.
Studies have found that audiograms obtained from older women may have elevated thresholds for low-frequency tones compared to older men, even though men have worse high-frequency hearing (Glorig, 1957; Hinchcliffe and Jones, 1968; Moscicki et al., 1985). The apparent negative effect of ovarian hormone(s) on the low-frequency regions of the female B6 auditory system may prove to be a valuable model for investigating this sex effect.
The ability of the Ahl gene to increase vulnerability to NIHL is not unique. A number of other factors can be harmful to the cochlea (e.g., Humes, 1984), so it is feasible that cochlear damage from moderately intense, chronic AAEs occurs in other species and settings. An example that has been used is the use of hearing aid amplification with ears made vulnerable by age, existing pathology, or other factors. It is important to understand the negative potential of amplification and possible roles of sex, age, and hormones.
Some of the sex differences in B6 mice appeared to be independent of gonadal hormone influence. Little attention has been given to the possibility of non-hormonal sex effects in the auditory system, yet this may be an important issue.
These examples suggest that much may be learned about sex and hearing loss from the B6 model.
Acknowledgments
This research was supported by R01 AG07554 (JFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- Barron AM, Fuller SJ, Verdile G, Martins RN. Reproductive hormones modulate oxidative stress in Alzheimer’s disease. Antioxid Redox Signal. 2006;11–12:2047–2059. doi: 10.1089/ars.2006.8.2047. [DOI] [PubMed] [Google Scholar]
- Blecher SR, Erickson RP. Genetics of sexual development: a new paradigm. Amer J Med Genet A. 2007;143:3054–3068. doi: 10.1002/ajmg.a.32037. [DOI] [PubMed] [Google Scholar]
- Bocklandt S, Vilain E. Sex differences in brain and behavior: hormones versus genes. Adv Genet. 2007;59:245–66. doi: 10.1016/S0065-2660(07)59009-7. [DOI] [PubMed] [Google Scholar]
- Brown JN, Miller JM, Nuttall AL. Age-related changes in cochlear vascular conductance in mice. Hear Res. 1995;86:189–194. doi: 10.1016/0378-5955(95)00070-k. [DOI] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Serra A. Auditory brainstem response in premenopausal women taking oral contraceptives. Hum Reprod. 2003a;18:85–89. doi: 10.1093/humrep/deg003. [DOI] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Agnello A, Garozzo A, Di Mari L, Serra A. Effects of patch or gel estrogen therapies on auditory brainstem response in surgically postmenopausal women: a prospective, randomized study. Fertil Steril. 2003b;79:556–561. doi: 10.1016/s0015-0282(02)04763-5. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Alexaki VI, Tsatsanis C, Minas V, Dermitzaki E, Lasaridis I, Vardouli L, Stournaras C, Margioris AN, Castanas E, Gravanis A. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci. 2006;1088:139–152. doi: 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- Cohen GM, Grasso JS. Further observations on the degeneration of spiral ganglia in aging C57BL/6 mice. Assoc Res Otolaryngol Abstr. 1987;10:12. [Google Scholar]
- Cohen GM, Bullers S. Age- and condition-related changes in levels of neuron specific enolase in spiral ganglion neurons of C57BL/6 mice. Assoc Res Otolaryngol Abstr. 1989;12:357. [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Colvin I, Luxon LM. Clinical diagnosis of noise-induced hearing loss. In: Luxon LM, Prasher D, editors. Noise and its effects. Whurr; Chichester: 2007. pp. 182–231. [Google Scholar]
- Davis RR. Noise-induced hearing loss. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 477–488. [Google Scholar]
- Davis RR, Kozel P, Erway LC. Genetic influences in individual susceptibility to noise: a review. Noise Health. 2003;5:19–28. [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, Steel KP, Noben-Trauth K. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001a;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- Di Palma F, Pellegrino R, Noben-Trauth K. Genomic structure, alternative splice forms and normal and mutant alleles of cadherin 23 (Cdh23) Gene. 2001b;281:31–41. doi: 10.1016/s0378-1119(01)00761-2. [DOI] [PubMed] [Google Scholar]
- Dluzen D, Jain R, Liu B. Modulatory effects of testosterone on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurochem. 1994;62:94–101. doi: 10.1046/j.1471-4159.1994.62010094.x. [DOI] [PubMed] [Google Scholar]
- Easton A, Dwyer E, Pfaff DW. Estradiol and orexin-2 saporin actions on multiple forms of behavioral arousal in female mice. Behav Neurosci. 2006;120:1–9. doi: 10.1037/0735-7044.120.1.1. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison D. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Erway LC, Zheng QY, Johnson KR. Inbred strains of mice for genetics of hearing in mammals: searching for genes for hearing loss. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 429–440. [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton: 2001. pp. 339–380. [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Glorig A. Wisconsin state fail hearing survey. American Academy of Opthalmology and Otolaryngology; 1957. [PubMed] [Google Scholar]
- Guimaraes P, Frisina ST, Mapes F, Tadros SF, Frisina DR, Frisina RD. Progestin negatively affects hearing in aged women. Proc Natl Acad Sci U S A. 2006;103:14246–14249. doi: 10.1073/pnas.0606891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack B, Markl H, Ehret G. Sound communication between parent and offspring. In: Willott JF, editor. The Auditory Psychobiology of the Mouse. Charles C. Thomas; Springfield: 1983. pp. 57–97. [Google Scholar]
- Harding GW, Bohne BA, Vos JD. The effect of an age-related hearing loss gene (Ahl) on noise-induced hearing loss and cochlear damage from low-frequency noise. Hear Res. 2004;204:90–100. doi: 10.1016/j.heares.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Harris JA, Rubel EW. Afferent regulation of neuron number in the cochlear nucleus: cellular and molecular analyses of a critical period. Hear Res. 2006;216–217:127–137. doi: 10.1016/j.heares.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Henry KR. Ageing and audition. In: Willott JF, editor. The Auditory Psychobiology of the Mouse. Charles C. Thomas; Springfield: 1983. pp. 470–493. [Google Scholar]
- Henry KR. Sex- and age-related elevation of cochlear nerve envelope response (CNER) and auditory brainstem response (ABR) thresholds in C57BL/6 mice. Hear Res. 2002;170:107–115. doi: 10.1016/s0378-5955(02)00391-x. [DOI] [PubMed] [Google Scholar]
- Henry KR. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear Res. 2004;190:141–148. doi: 10.1016/S0378-5955(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe R, Jones WI. Hearing levels of a suburban Jamaican population. Int Audiol. 1968;7:239–258. [Google Scholar]
- Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol. 2003;5:66–79. doi: 10.1007/s10162-003-4021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz M, Li HS. Inner ear morphology in CBA/Ca and C57BL/6J mice in relationship to noise, age and phenotype. Eur Arch Otorhinolaryngol. 1993;250:257–264. doi: 10.1007/BF00186222. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Humes LE. Noise-induced hearing loss as influenced by other agents and by some physical characteristics of the individual. J Acoust Soc Amer. 1984;76:1318–1329. doi: 10.1121/1.391447. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Bogdanovic N, Viberg A, Canlon B. Auditory peripheral influences on calcium binding protein immunoreactivity in the cochlear nucleus during aging in the C57BL/6J mouse. Hear Res. 2003;179:33–42. doi: 10.1016/s0378-5955(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erikan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. Amer J Obstet Gynecol. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kim J, Morest DK, Bohne BA. Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation. Hear Res. 1997;103:169–191. doi: 10.1016/s0378-5955(96)00173-6. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Morest DK, Potashner SJ. Quantitative study of degeneration and new growth of axons and synaptic endings in the chinchilla cochlear nucleus after acoustic overstimulation. J Neurosci Res. 2004;77:829–842. doi: 10.1002/jnr.20211. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang BM, Chae HD, Kim CH. The association between serum estradiol level and hearing sensitivity in postmenopausal women. Obstet Gynecol. 2002;99:726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandosterone (HEA) and DHEA-sulphate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Nat Acad Sci USA. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonides VG, Spillantini MG, Sofroniew MV, Fawcett JW, Herbert J. Dehydroepiandosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. 1999;89:429–436. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- Koch M, Ehret G. Estradiol and parental experience, but not prolactin are necessary for ultrasound recognition and pup-retrieving in the mouse. Physiol Behav. 1989;45:771–776. doi: 10.1016/0031-9384(89)90293-x. [DOI] [PubMed] [Google Scholar]
- Laugel GR, Dengerink HA, Wright JW. Ovarian steroid and vasoconstrictor effects on cochlear blood flow. Hear Res. 1987;31:245–251. doi: 10.1016/0378-5955(87)90194-8. [DOI] [PubMed] [Google Scholar]
- Laugel GR, Wright JW, Dengerink HA. Angiotensin II and progesterone effects on laser Doppler measures of cochlear blood flow. Acta Otolaryngol. 1988;106:34–39. doi: 10.3109/00016488809107368. [DOI] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Estrogen acutely inhibits ion transport by isolated stria vascularis. Hear Res. 2001;158:123–130. doi: 10.1016/s0378-5955(01)00316-1. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Dolan DF, Schacht J, Miller JM, Lomax MI, Altschuler RA. Pathways for protection from noise induced hearing loss. Noise Health. 2003;5:1–17. [PubMed] [Google Scholar]
- Li H, Klein G, Sun P, Buchan AM. Dehydroepiandosterone (DHEA) reduces neuronal injury in a rat model of global cerebral ischemia. Brain Res. 2001;888:263–266. doi: 10.1016/s0006-8993(00)03077-8. [DOI] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Li HS, Hultcrantz M, Borg E. Influence of age on noise-induced permanent threshold shifts in CBA/Ca and C57BL/6J mice. Audiol. 1993;32:195–204. doi: 10.3109/00206099309072935. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinization effects in the auditory system. Arch Sex Behav. 2002;31:99–111. doi: 10.1023/a:1014087319682. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Henselman LW, Zheng XY. Sex differences in auditory sensitivity of chinchillas before and after exposure to impulse noise. Ear Hear. 1999;20:164–174. doi: 10.1097/00003446-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Mishizen A, Ikonomovic M, Armstrong DA. Glutamate receptors in aging and Alzheimer’s disease. In: Hof PR, Mobbs CV, editors. Functional neurobiology of aging. Academic Press; San Diego: 2001. pp. 283–314. [Google Scholar]
- Moscicki EK, Elkins EF, Baum HM, McNamara P. Hearing loss in the elderly: An epidemiologic study of the Framingham heart study cohort. Ear Hear. 1985;6:184–190. [PubMed] [Google Scholar]
- Muly SM, Gross JS, Potashner SJ. Noise trauma alters D-[3H]aspartate release and AMPA binding in chinchilla cochlear nucleus. J Neurosci Res. 2004;75:585–596. doi: 10.1002/jnr.20011. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrus cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasm estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinol. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrus cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: Effects of age and disease. Acta Endodrinol. 1975;80:744–742. doi: 10.1530/acta.0.0800744. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci. 2005;25:699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Brain mechanisms and parental behavior. In: Adler N, Pfaff D, Goy RW, editors. Handbook of behavioral neurobiology. Vol. 7. New York: Plenum Press; 1985. pp. 537–605. [Google Scholar]
- Ohlemiller KK, Gagnon PM. Apical-to-basal gradients in age-related cochlear degeneration and their relationship to “primary” loss of cochlear neurons. J Comp Neurol. 2004;479:103–116. doi: 10.1002/cne.20326. [DOI] [PubMed] [Google Scholar]
- Ou HC, Bohne BA, Harding GW. Noise damage in the C57BL/CBA cochlea. Hear Res. 2000;145:111–122. doi: 10.1016/s0378-5955(00)00081-2. [DOI] [PubMed] [Google Scholar]
- Picazo O, Azcoitia I, Garcia-Segura LM. Neuroprotective and neurotoxic effects of estrogens. Brain Res. 2003;990:20–27. doi: 10.1016/s0006-8993(03)03380-8. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1997;148:222–235. doi: 10.1006/exnr.1997.6641. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Pujol R, Rebillard G, Puel J, Lenoir M, Eybalin M, Recasens M. Glutamate neurotoxicity in the cochlea: a possible consequence of ischaemic or anoxic conditions occurring in aging. Acta Otolaryngol Suppl. 1990;476:32–36. doi: 10.3109/00016489109127253. [DOI] [PubMed] [Google Scholar]
- Pouliot WA, Handa RJ, Beck SG. Androgen modulates N-methyl-D-aspartate- mediated depolarization in CA1 hippocampal pyramidal cells. Synapse. 1996;23:10–19. doi: 10.1002/(SICI)1098-2396(199605)23:1<10::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Mayer AD, Siegel HI. Maternal behavior among the nonprimate mammals. In: Adler N, Pfaff D, Goy RW, editors. Handbook of behavioral neurobiology. Vol. 7. New York: Plenum Press; 1985. pp. 229–298. [Google Scholar]
- Saunders JC, Adler HJ, Cohen YE, Smullen S, Kazahaya K. Morphometric changes in the chick nucleus magnocellularis following acoustic overstimulation. J Comp Neurol. 1998;390:412–426. [PubMed] [Google Scholar]
- Scallet AC. Estrogens: neuroprotective or neurotoxic? Ann N Y Acad Sci. 1999;890:121–132. doi: 10.1111/j.1749-6632.1999.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngosc. 2000;110:727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Shivapuja BG, Quirk WS. The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol Head Neck Surg. 1993;109:1052–1056. doi: 10.1177/019459989310900613. [DOI] [PubMed] [Google Scholar]
- Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao X, Wang Q, Ohlemiller KK, Piccirillo J, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shone G, Altschuler RA, Miller JM, Nuttall AL. The effect of noise exposure on the aging ear. Hear Res. 1991;56:173–178. doi: 10.1016/0378-5955(91)90167-8. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smittkamp SE, Durham D. Contributions of age, cochlear integrity, and auditory environment to avian cochlear nucleus metabolism. Hear Res. 2004;195:79–89. doi: 10.1016/j.heares.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Amer. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;211:666–672. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, Hultcrantz M. Mapping of estrogen receptors alpha and beta in the inner ear of mouse and rat. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G., Jr Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Vazquez AE, Jimenez AM, Martin GK, Luebke AE, Lonsbury-Martin BL. Evaluating cochlear function and the effects of noise exposure in the B6. CAST+Ahl mouse with distortion product otoacoustic emissions. Hear Res. 2004;194:87–96. doi: 10.1016/j.heares.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the auditory system. In: Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, editors. Pathobiology of the Aging Mouse, ILSI Monographs on the Pathobiology of Aging Animals. Washington, D.C: ILSI Press; 1996. pp. 179–204. [Google Scholar]
- Willott JF, Bross LS. Morphological changes in the anteroventral cochlear nucleus that accompany sensorineural hearing loss in DBA/2J and C57BL/6J mice. Brain Res Dev Brain Res. 1996;91:218– 226. doi: 10.1016/0165-3806(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Willott JF, Bross LS. Effects of prolonged exposure to an augmented acoustic environment on the auditory system of middle-aged C57BL/6J mice: cochlear and central histology and sex differences. J Comp Neurol. 2004;472:358–370. doi: 10.1002/cne.20065. [DOI] [PubMed] [Google Scholar]
- Willott JF, Bross LS, McFadden SL. Morphology of the cochlear nucleus in CBA/J mice with chronic, severe sensorineural cochlear pathology induced during adulthood. Hear Res. 1994;74:1–21. doi: 10.1016/0378-5955(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Willott JF, Erway LC, Archer JR, Harrison D. Genetics of age-related hearing loss in mice: II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear Res. 1995;88:143–155. doi: 10.1016/0378-5955(95)00107-f. [DOI] [PubMed] [Google Scholar]
- Willott JF, Jackson LM, Hunter KP. Morphometric study of the anteroventral cochlear nucleus of two mouse models of presbycusis. J Comp Neurol. 1987;260:472–480. doi: 10.1002/cne.902600312. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hear Res. 1999;135:78–88. doi: 10.1016/s0378-5955(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Willott JF, VandenBosche J, Shimizu T, Ding D. Effects of exposing DBA/2J mice to a high-frequency augmented acoustic environment on the cochlea and anteroventral cochlear nucleus. Hear Res. 2006a;216–217:138–145. doi: 10.1016/j.heares.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Willott JF, VandenBosche J, Shimizu T, Ding D, Salvi R. Effects of exposing gonadectomized and intact C57BL/6J mice to a high-frequency augmented acoustic environment: auditory brainstem response thresholds and cytocochleograms. Hear Res. 2006b;221:73–81. doi: 10.1016/j.heares.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, VandenBosche J, Shimizu T, Ding D, Salvi R. Effects of exposing C57BL/6J mice to high- and low-frequency augmented acoustic environments: auditory brainstem response thresholds, cytocochleograms, anterior cochlear nucleus morphology and the role of gonadal hormones. Hear Res. 2008;235:60–71. doi: 10.1016/j.heares.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M. Minireview: neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinol. 2001;142:969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Yan D, Ouyang XM, Du LL, Yu H, Chang B, Johnson KR, Liu XZ. Digenic inheritance of deafness caused by mutations in genes encoding cadherin 23 and protocadherin 15 in mice and humans. Hum Mol Genet. 2005;14:103–111. doi: 10.1093/hmg/ddi010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007;503:593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]