Abstract
There is a growing consensus that the auditory system is dynamic in its representation of behaviorally relevant sounds. The auditory cortex in particular seems to be an important locus for plasticity that may reflect the memory of such sounds, or functionally improve their processing. The mechanisms that underlie these changes may be either intrinsic because they depend on the receiver’s physiological state, or extrinsic because they arise from the context in which behavioral relevance is gained. Research in a mouse model of acoustic communication between offspring and adult females offers the opportunity to explore both of these contributions to auditory cortical plasticity in a natural context. Recent works have found that after the vocalizations of infant mice become behaviorally relevant to mothers, auditory cortical activity is significantly changed in a way that may improve their processing. Here we consider the hypothesis that maternal hormones (intrinsic factor) and sensory experience (extrinsic factor) contribute together to drive these changes, focusing specifically on the evidence that well-known experience-dependent mechanisms of cortical plasticity can be modulated by hormones.
Keywords: auditory cortex, estrogen, ultrasound communication, mouse, neurotransmitter, neuromodulation
Introduction
Early views of the adult auditory system describe it as a static processor organized as a series of filters for spectral and temporal characteristics of sound (Moore, 2003; Pickles, 2008). Ample evidence now demonstrates that the adult auditory system is actually dynamic in the way it encodes sound (Fontanini and Katz, 2008; Fritz et al., 2007; Weinberger, 2007; Weinberger and Diamond, 1987) – changing so that behaviorally relevant signals can be more “powerfully” represented. What mechanisms produce plasticity in sensory processing? Investigating this question is a rich and growing area in sensory neuroscience. Both extrinsic factors, such as experience with sounds, and intrinsic factors, such as the physiological state of the receiver, have been shown to produce systematic changes in sensory representations. For example, the way in which sound experience is gained can affect its neural representation, according to a recent study in the ferret auditory cortex (AC) showing differences depending on whether an animal is trained by conditioned avoidance or positive reinforcement to detect a sound (David et al., 2008). Likewise, an animal’s reproductive status can influence auditory neural responses – as demonstrated in fish (Sisneros and Bass, 2003), frogs (Goense and Feng, 2005; Hillery, 1984; Miranda and Wilczynski, 2009) and birds (Lucas et al., 2002; Lucas et al., 2007) – and improve the processing of species-specific mating calls. In the laboratory, both these extrinsic and intrinsic contributions can be straightforwardly studied in isolation. In natural contexts, however, both factors likely work together, potentially in nonlinear ways. Hence, to understand the natural means by which sensory representational plasticity arises, model systems in which both contributions can be dissected need to be investigated.
In this work, we describe a relatively new, ethologically-motivated model system that provides opportunities to specifically explore interactions between extrinsic sound experience and internal hormonal state. This model focuses on representational plasticity in the mammalian AC, a critical site for encoding behaviorally relevant stimuli in a plastic manner (Buonomano and Merzenich, 1998; Keuroghlian and Knudsen, 2007). Mechanistically, much is already known about the role that specific neurotransmitter systems play in driving experience-dependent cortical plasticity (Gu, 2002). Much less appreciated though is how hormones may modulate these neurotransmitter systems to affect this plasticity.
In our model, AC representational plasticity is observed in the female mouse for the ultrasonic vocalizations of mouse pups. Systematic differences in AC responses between virgin and maternal females lead to a more powerful representation of these calls that can improve their detection and discrimination. Here we will first review the nature of these changes, and then lay out a framework for studying the mechanisms by which they may have occurred. Given the focus of this special issue, we will synthesize what is known about how maternal hormones may interact with neurotransmitter systems underlying cortical plasticity to propose mechanisms for AC representational plasticity in this natural communication context. We aim for this to provide a more integrative framework within which the role of hormones in sensory cortical plasticity may be explored.
The mouse maternal model of auditory cortical plasticity
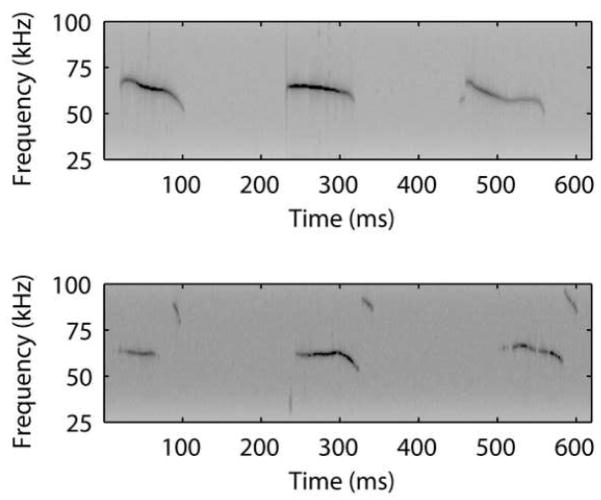
Species-specific vocalizations are one of the most important acoustic signals that any auditory system must process. Hence, to understand the natural, integrative mechanisms of AC plasticity for behaviorally-relevant signals, we have adopted a natural communication context in the mouse as a behavioral model. Specifically, mouse pups emit bouts of high ultrasonic vocalizations (USV), depicted in Fig. 1, that carry communicative significance (Ehret, 2005). These USVs are produced by pups when they are isolated from their nest. The sounds elicit a directed search and retrieval response in the mother. Playback of the call itself has been shown to initiate this behavior, provided the mother is suitably maternally motivated (Haack et al., 1983). In fact, two-alternative choice playback tests indicate mothers prefer to approach to a 50-kHz tone model of a pup call about twice as often as to a neutral sound. Importantly though, virgins that have never cared for pups approach both sounds equally often (Ehret et al., 1987). In other words, these USVs become behaviorally relevant to and are recognized by mothers, but not virgins.
Fig. 1.
Sample spectrograms of two bouts of natural ultrasonic calls emitted by postnatal day 7 pups. Note the rhythmic nature of the calls, which are typically separated by ~180–200 ms (onset to onset).
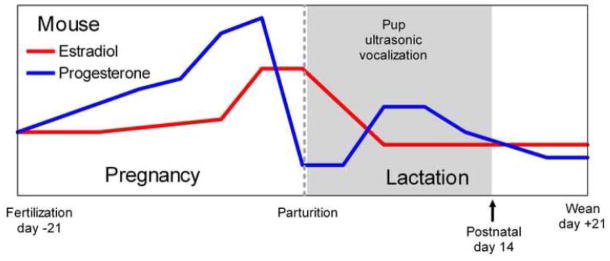
Behavioral studies suggest that both experience with pups and ovarian hormones are involved in acquiring and retaining this call recognition. Circulating estrogen levels in the mouse are generally elevated at parturition (Fig. 2), a time when a mother receives the first sensory stimulation from her own pups. However, estrogen by itself does not enable recognition: if ovariectomized females are given estrogen implants but have no interaction with pups, they do not exhibit pup call recognition (Ehret and Koch, 1989). After five days of pup experience though, these females, like intact virgins with the same amount of pup experience, do prefer the pup-like tone. Ovariectomized females without hormone replacement do not, although continued experience with pups for 21 days can lead to pup call recognition when tested within one week of separation from pups (Ehret and Koch, 1989). These results suggest that estrogen facilitates the acquisition of pup call recognition over short periods of pup experience (Ehret and Koch, 1989), and that long-term pup experience may be able to compensate for its absence. Interestingly, recent studies in estrogen receptor-α and -β knockout mice lend further support for this (Choleris et al., 2006). They found impaired conspecific recognition in both knockout animals, and argued that the learning deficit was specific to social contexts.
Fig. 2.
Schematic representation of estradiol and progesterone profiles during pregnancy and lactation in the mouse (Atkinson and Leathem, 1946; Barkley et al., 1979; Bell and Dawson, 1983; Critser et al., 1982; McCormack and Greenwald, 1974). The vertical dashed line denotes the time of parturition. The grey shaded area encompasses the period in which pups produce ultrasonic vocalizations (Elwood and Keeling, 1982; Graham and Letz, 1979; Pontet et al., 1989; Vieira and Brown, 2002).
Acquiring the behavioral relevance and specific recognition of pup calls must therefore involve both intrinsic (hormonal) and extrinsic (experiential) factors. The same appears to be true of the retention of call recognition. Ovariectomized females tested one month after separation from pups did not prefer pup calls whereas mothers still did. Moreover, experienced mothers that were ovariectomized after pup separation and tested one month later also did not show a preference, in contrast to experienced mothers that were kept gonadally intact (Ehret and Koch, 1989). These data suggests that without gonadal hormones, ovariectomized females have reduced long-term recall of pup call recognition. Thus, hormones present during either pup experience or during retention after separation from pups helps to stabilize the acquired call recognition.
These intrinsic and extrinsic factors could alter just the neural circuitry driving the motivation (component 3 in Fig. 3) or behavioral decision to respond to pup calls (between components 3 and 4). In other words, the sensory perception, and its underlying neural representation, would not necessarily have to change between virgins and mothers to explain these differences in behavioral preference for pup calls. In fact though, recent studies in intact female mice demonstrate that the sensory cortical representation of pup calls also changes, consistent with the idea that plasticity occurs which may improve the encoding of behaviorally relevant signals. The first hint of this came from an immunolabeling study comparing mothers (actively caring for postnatal day 7 pups) and virgins in AC expression of the immediate early gene c-Fos that was induced by playback of pup call models (Fichtel and Ehret, 1999). A significant difference in the spatial pattern of activation of AC neurons was found between animal groups in both hemispheres, concentrated in the ultrasound field (UF) and the secondary auditory field (AII).
Fig. 3.
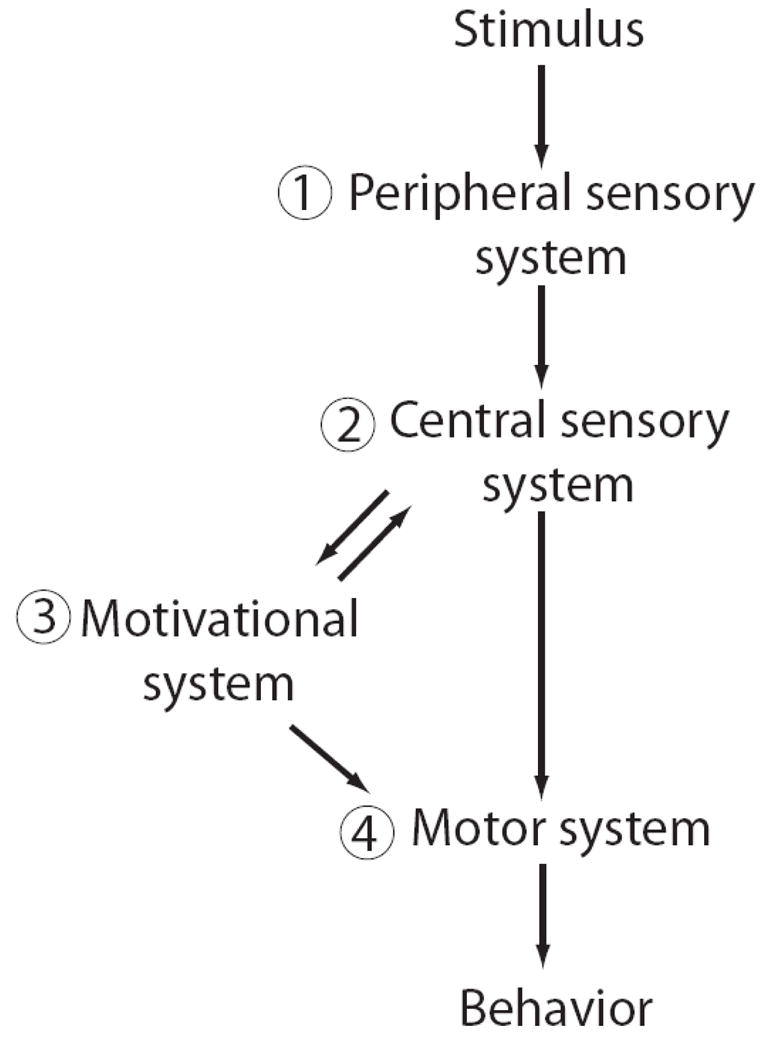
Neuroanatomical model for information flow leading to maternal behavior. Numbers denote components that are referred to in the text. Hormones can act at the numbered levels (1–4).
Since the expression of c-Fos correlates, but is not synonymous, with synaptic stimulation, this result does not necessarily imply differences in how neurons in virgins and mothers actually respond to pup calls. That has been explored in two recent electrophysiological works in ketamine-anesthetized mice that compared similarly aged, gonadally-intact females with different physiological and experiential histories (Liu and Schreiner, 2007; Liu et al., 2006). One group (1–2 week post-weaning mothers) gave birth to pups and cared for them for 21 days, and the other (virgins) had never been pregnant and never cared for pups (although they probably heard pup calls as background noise in the vivarium). Experiments were conducted at a time point when both mothers and virgins had normal estrus cycles, although the precise estrus state of each female was not determined. Both studies relied primarily on multiunit (MU) spiking activity sampled across most regions of the left AC, including UF, primary auditory field (A1) and anterior auditory field (AAF).
Results suggest that AC spiking activity in response to pup calls changes from virgins to mothers in a way that is better adapted for processing the acoustic structure of these natural vocalizations. First, Liu et al. (2006) demonstrated that MUs in mothers follow the temporal modulations in real call bouts better than in virgins. Pup calls naturally occur at a ~5 Hz rate, as Fig. 1 shows. The mother’s AC neural population responded to each component call in a bout fairly well at this modulation rate. This was true regardless of the specific acoustic features of the call, making the AC entrainment less susceptible to natural call variation. This could provide a robust distributed neural representation for downstream detection of pup calls, even in the presence of natural acoustic variability. On the other hand, AC neural populations in virgins could only follow repeated vocalizations if they were presented at rates slower (i.e. 3 Hz) than typically produced by pups naturally. Moreover, this entrainment depended more on the specific acoustic structure of a call (e.g. long or short duration). These results provide the first indication that AC neural population responses to the same infant communication sounds can vary systematically depending on different maternal histories, and that the differences could improve call processing.
A second study provided rigorous quantification of this idea, focusing on the neural response to a variety of individual calls presented in isolation. Liu and Schreiner (2007) found that MU responses in mothers can actually carry more Shannon information (Cover and Thomas, 1991) for both detecting and discriminating pup calls. Loosely speaking, improved detection arises if a MUs call-evoked spiking activity (analyzed here in 2 ms time bins across the duration of the response) is more similar for the different calls and significantly different from spontaneous spiking. Improved discrimination occurs if this call-evoked activity can be more reliably used to distinguish between specific calls, irrespective of the spontaneous activity. Across the population, MU spiking in mothers was better than in virgins at providing information for both these call processing functions. Importantly, mothers and virgins did not differ for a non-behaviorally relevant ensemble of sounds presented at the same average sound level, suggesting a relative specificity in the coding changes for pup USVs. Moreover, the plasticity resulted from a change in the timing of spikes evoked in MUs across the entire auditory cortex, with the improved information in mothers arising earlier in the neural response. This is consistent with a recent study in awake guinea pigs suggesting the temporal discharge pattern of AC neurons is important for conveying information about species-specific vocalizations (Huetz et al., 2009). Experiments in the mouse are now also being extended to the awake preparation (Galindo-Leon et al, submitted).
These studies provide clear evidence for AC plasticity in a natural communication context. Hence, even though the behavioral response to pup calls could have simply been due to changes in a mother’s motivation or decision to respond to calls, we now know that its sensory cortical representation is in fact altered. This result raises two types of questions. First, what does this mean for the “quality” of call perception by virgins and mothers? The behavioral experiments that would be needed to explore this have not been done, and would be challenging to pursue without invoking some operant training paradigm that risks altering the cortical representations. Hence, behavioral analysis may not be able to provide a definitive explanation for the role this plasticity has in maternal care. On the other hand, the computational analyses described in the cited studies at least provide evidence that the neural changes can improve functional processing for downstream decisions to act.
Second, what caused these sensory cortical differences between virgin and mother mice to appear and persist, even weeks after the last experience with pups? We know that hormones alone can affect neural activity throughout the auditory system (see various examples in this special issue). Auditory experience alone can also alter neural representations of sound, particularly at the level of the cortex (Norena et al., 2006; Weinberger, 2004). In this maternal context though, where hormone levels and pup interactions are changing throughout parturition and lactation, these intrinsic and extrinsic factors are probably not independent. Indeed, the behavior itself indicates that both maternal hormones and pup experience contribute to the acquisition and retention of call recognition. Hence, in considering possible experience-dependent mechanisms for natural sensory representational plasticity, the question of how hormones may modulate this should be addressed. In the next section, we begin this by drawing on the neural plasticity and hormone literature to synthesize an understanding of how well-known sites responsible for AC plasticity may be susceptible to hormonal influences during maternal behavior.
Interaction sites between hormones and cortical plasticity
Several hormones are associated with pregnancy and maternal behavior, including estrogen, progesterone and oxytocin. We focus first on estrogen, since a great deal is known about its role in neuroplasticity and its influence on brain areas that mediate auditory cortical plasticity. Estradiol, a naturally-occurring form of estrogen, can generally increase dendritic spine density and neural excitability, enhance long term potentiation, promote neurotrophin activity and increase neurogenesis (Raz et al., 2008; Spencer et al., 2008; Toran-Allerand et al., 1999; Wise et al., 2001). As noted earlier, estrogen levels peak during the latter third of pregnancy (Fig. 2), fall after birth towards baseline, and remain low throughout lactation (Atkinson and Leathem, 1946; Rosenblatt and Siegel, 1981; Taya and Greenwald, 1982). Thus, sensory stimulation by pups actually begins just as estrogen starts to drop. Through the long timescale structural changes it promotes though (Wise et al., 2001), the high pre-parturitional levels of estrogen likely still help prime neural circuits for plasticity during these first several days of pup experience.
The mechanism for these neural changes is believed to involve the engagement of various neurotransmitter systems, including the dopaminergic, noradrenergic, serotonergic and cholinergic systems (Edeline, 2003; Gu, 2002; Thiel, 2007; Xerri, 2008). The main sources of these neurotransmitters lie in brain regions that overlap considerably with those activated during maternal and social behaviors: the ventral tegmental area (VTA), the locus coeruleus (LC), the dorsal raphe nuclei (DR) and the nucleus basalis (NB), respectively. Each of these areas is: 1) significantly activated during maternal behavior and/or sensory learning, 2) important in the mechanisms of AC plasticity and 3) sensitive to estrogens or other hormones. These three characteristics make these neural sites prime candidates for integrated endocrine and neural mechanisms of sensory plasticity in the maternal context.
Dopamine
Dopamine, a neurotransmitter usually associated with operant reinforcement and pleasure, has a well-known role in both maternal responsiveness and AC plasticity. Pups are known to be reinforcing (Hauser and Gandelman, 1985; Lee et al., 1999) and rewarding (Ferris et al., 2005; Mattson et al., 2001) to rodent mothers, and pup-directed behaviors such as retrieval, licking and nursing activate the mesocorticolimbic reward system originating in the VTA (Ferris et al., 2005; Hernandez-Gonzalez et al., 2005). In humans, the VTA is activated while listening to cries or viewing emotional images of an infant (Lorberbaum et al., 2002; Strathearn et al., 2008). This documented role of the VTA in maternal responsiveness opens the possibility that it may also influence infant recognition through projections to sensory processing areas (Campbell et al., 1987; Gu, 2002). Indeed, the dopaminergic system can modulate auditory associative learning in reward contexts to produce persistent perceptual changes. For instance, sound stimuli paired with fluid reward results in discrimination between reinforced and non-reinforced sounds for up to two weeks (Watanabe et al., 2001), and requires dopamine D2 receptor activation in primary AC (Kudoh and Shibuki, 2006). In fact, one study has demonstrated long-term representational plasticity in the AC by electrically stimulating the VTA in conjunction with acoustic tone presentation (Bao et al., 2001), an effect that is blocked by dopamine antagonists. Put together, these results suggest VTA dopamine activity may facilitate sensory cortical plasticity in the maternal context.
Importantly, the hormones of pregnancy and parturition also modulate VTA dopaminergic activity. Estrogen receptors (ER) are found in VTA dopamine neurons (Creutz and Kritzer, 2002; Shughrue et al., 1997) and estradiol administration to ovariectomized female rats stimulates dopaminergic activity by elevating VTA dopamine neuron activity (Zhang et al., 2008). In female rhesus macaques, an ovariectomy reduces the cortical innervation of tyrosine hydroxylase (TH), the enzyme responsible for converting the amino acid L-tyrosine to DOPA, the precursor for dopamine; estradiol replacement maintains it (Kritzer and Kohama, 1998). Similarly, male rats show a decrease in cortical TH innervation shortly after gonadectomy that is attenuated by estrogen treatment (Kritzer, 2000). However, the effect of gonadectomy is time-dependent and region-specific since levels of TH innervation weeks after gonadectomy either return to intact levels, or in some cortical areas, significantly surpass intact levels.
This last result hints that estrogen modulation of dopamine activity is complicated by temporal factors. Elevated dopamine activity after extended periods of low hormone levels may explain some contradictory results found in females where high estradiol is actually correlated with low VTA activity (Dazzi et al., 2007; Sakamoto et al., 1993; Zhang et al., 2008). Hence, in considering how estrogen interacts with the dopamine system around birth, it is important to keep in mind the changing levels of estrogen. Perhaps the elevation in estrogen primes the mesocortical pathway by increasing cortical dopaminergic fiber innervation that then influences sensory cortical plasticity during early mother-pup interactions.
Norepinephrine
Norepinephrine (noradrenaline) – a neurotransmitter generally involved in arousal, attention and vigilance – has an established role both in forming memories of maternal experiences and in AC plasticity. An early study found that pharmacologically activating the noradrenergic system in mothers after a brief maternal experience at birth facilitates the long term retention of the experience (Moffat et al., 1993). More recently, mouse mothers lacking the gene for dopamine beta-hydroxylase (Dbh), which synthesizes norepinephrine, show a profound deficit in maternal responsiveness, resulting in the loss of pups (Thomas and Palmiter, 1997). This can be rescued only if the norepinephrine precursor, DOPS, is given to mothers shortly before birth so that it is present at parturition. Amazingly, once rescued for one litter, a subsequent litter from the same mother also survives, even though she is not given a further injection of DOPS. This suggests norepinephrine may help consolidate the long-term memory of the first maternal experience. What normally drives the release of norepinephrine in the maternal context? Intriguingly, LC neurons have dendrites extending into the rostromedial peri-LC region (Rizvi et al., 1994), which receives a dense, focal input from the medial preoptic area, a key nucleus required for maternal behavior (Numan and Insel, 2003). Medial preoptic area activation may thereby help modulate activity in LC, which provides a major source for noradrenergic afferents to the cortex (Gu, 2002). This anatomical pathway may therefore provide a substrate for the motivational components of maternal behavior (component 3 in Fig. 3) to affect cortical processing.
Indeed, cortical norepinephrine release has been found to induce plasticity in sensory processing (Gu, 2002). In primary auditory cortex, iontophoretic application of norepinephrine inhibits both spontaneous and evoked activity (Manunta and Edeline, 1997; Manunta and Edeline, 1998; Manunta and Edeline, 1999), thus sharpening neural receptive fields without changing the overall signal-to-noise ratio across the population. Moreover, pairing of norepinephrine with tones mostly leads to frequency-selective suppression in the tuning curve (Manunta and Edeline, 2004). These changes could conceivably support neural improvement in frequency discrimination by creating greater differential responses to varying frequencies. Indeed, enhanced information for ultrasound frequency discrimination was observed in post-weaning mothers compared to virgins (Liu and Schreiner, 2007). For norepinephrine to be involved in this, its effects would have to survive much longer than current studies have explored (Manunta and Edeline, 1999).
Evidence does point to the possibility that the noradrenergic system can be facilitated by hormones. LC neurons in the rat concentrate estradiol (Heritage et al., 1980) and contain ER-α and ER-β (Helena et al., 2006; Mitra et al., 2003; Shughrue et al., 1997; Zhang et al., 2002). In fact, estradiol regulates Dbh mRNA expression in LC neurons (Serova et al., 2002) and therefore may help control norepinephrine levels. Hence, the noradrenergic system could be an additional site for hormonal augmentation of sensory plasticity.
Serotonin
Serotonin – a neurotransmitter commonly associated with different moods (stress, anger, aggression, anxiety, depression, sexuality, sleep and appetite) – also affects a mother’s degree of maternal care and facilitates representational plasticity in AC. Normally, serotonin activity and serotonin receptor function vary systematically across pregnancy and the postpartum period (Glaser et al., 1990). In humans, abnormalities in these time courses have been implicated in postpartum mood disorders (Doornbos et al., 2008), and are perhaps a physiological reflection of problems dealing with the stresses of motherhood. Furthermore, a direct role for serotonin in maternal care was recently demonstrated by genetically disrupting the development of serotonergic neurons in the mouse DR (Lerch-Haner et al., 2008). Pups born to these mothers survived less than three days, unlike those of wild type controls. Mothers had a specific deficit in pup retrieval, even though measures of their olfactory, locomotive and fear-related behaviors were normal. Hence, serotonin released through the projections of DR neurons must normally be involved in maternal responsiveness, although the precise site of action is unknown. Nevertheless, since DR projects widely to all areas and layers of the cortex (Campbell et al., 1987; Gu, 2002), its activation during maternal behavior likely also impacts auditory cortical processing of infant cues.
The effects of serotonin on sensory cortical activity are quite varied. Under normal conditions, evoked responses in primary auditory cortex are inversely related to DR serotonergic cell activity (Juckel et al., 1999), and to local serotonin levels in primary auditory cortex (Ji and Suga, 2007). However, exogenous application of serotonin or a serotonin receptor agonist directly onto the AC can produce myriad changes in frequency tuning, which are dose-dependent (Ji and Suga, 2007). This is hypothesized to reflect a changing balance between excitation and inhibition, depending on which serotonin receptors on which types of neurons are activated. As with the noradrenergic system though, we do not yet know the long-term effects of serotonergic modulation of cortical activity.
A wealth of evidence supports the hypothesis that the DR serotonergic pathway is modulated by pregnancy hormones (Bethea et al., 2002; Bodo and Rissman, 2006). Many of the serotonergic neurons in the DR contain ER-α and ER-β (Sheng et al., 2004; Shughrue and Merchenthaler, 2001) suggesting that estradiol could modulate serotonin activity in the synaptic targets of these neurons. Estrogen acts to increase serotonin activity by modulating synthesis, reuptake and degradation, as well as neural firing (Bethea et al., 2002). Thus, the DR may be yet another site for the hormonal changes associated with motherhood to potentially affect sensory cortical plasticity during experience with pups.
Acetylcholine
The basal forebrain NB cholinergic system has not been commonly linked to maternal responsiveness, but is strongly implicated in general associative learning (Gu, 2002). Thus, we consider it here for its potential involvement in the acquisition and memory of sensory cues from infants. The NB contributes to the long-term retention of various forms of conditioned auditory associations (McLin et al., 2002; Miasnikov et al., 2006; Montero-Pastor et al., 2001). It is specifically hypothesized to organize the learned behavioral significance of sounds at the level of the AC (Suga and Ma, 2003; Weinberger, 2003), and thus could play a key role in its plasticity within the maternal context. Indeed, many studies have shown that electrical stimulation of the basal forebrain paired with an acoustic tone can drive frequency-specific changes in AC receptive fields and long-term reorganization of the AC’s map of sound frequency (Bakin and Weinberger, 1996; Chen and Yan, 2007; Kilgard and Merzenich, 1998; Kilgard et al., 2007; Kilgard et al., 2001a; Kilgard et al., 2001b; Yan and Zhang, 2005).
Less well-known is the potential effect that estrogen may have on the NB cholinergic pathway (Gibbs and Aggarwal, 1998). NB neurons contain both ER-α and ER-β in mice (Mitra et al., 2003), rats (Shughrue et al., 1997; Shughrue et al., 2000) and humans (Ishunina and Swaab, 2001; Osterlund et al., 2000). This could allow estradiol to modulate associative learning via the NB cholinergic pathway, as a recent study suggests (Horvath et al., 2002). Rats were conditioned using foot-shock in a two-way active avoidance learning paradigm to move from one compartment of a test box to another upon hearing an auditory stimulus. Estrogen replacement in ovariectomized rats improved the rate of acquisition in this active avoidance task (although retention after 24 hours was not affected). The improved acquisition rate in estrogen treated animals was accompanied by an increase in cholinergic fiber density in the somatosensory cortex (no measurements in AC). This result hints that estrogen may prime the NB to accelerate sensory associative learning, at least in the case of active avoidance responses. If this also applies in the maternal context, perhaps it contributes to the more rapid acquisition of pup call recognition in estrogen-replaced compared to ovariectomized females.
Potential influence of other hormones
Besides estrogen, other hormones are also changing during the maternal experience and can affect the neuromodulatory systems involved in cortical plasticity. For example, progesterone levels in the maternal mouse peak during the latter third of pregnancy, rapidly decrease during the days before birth and peak midway through the lactation period (Fig. 2), which is consistent with other rodents (Atkinson and Leathem, 1946; Rosenblatt and Siegel, 1981; Taya and Greenwald, 1982; Tomogane et al., 1969). Behaviorally, administering progesterone to rats has been found to improve their performance on cognitive and memory related tasks, including those mediated by the prefrontal cortex and hippocampus (Frye, 2007; Frye and Walf, 2008a; Frye and Walf, 2008b). While such behavioral changes do not require any sensory cortical changes, progesterone can nevertheless modulate the same neuromodulatory systems discussed above that affect cortical plasticity. Progesterone receptors and receptor mRNA have been demonstrated in VTA, DR and LC (Curran-Rauhut and Petersen, 2002; Helena et al., 2006). In fact, progesterone along with estradiol can increase dopamine release in several dopamine target areas (Frye, 2007).
Finally, the neuropeptide oxytocin also functions during maternal behavior, peaking at birth and remaining high throughout lactation. Besides its effects on parturition and lactation, oxytocin is hypothesized to play a more general role specifically in social, but not nonsocial, recognition (Bielsky and Young, 2004). This has been mainly tested using olfactory recognition tasks, where male oxytocin-knockout mice fail to recognize a familiar female but not a familiar nonsocial odor (Ferguson et al., 2000). Oxytocin acting in the medial amygdala is believed to mediate this recognition (Ferguson et al., 2001). Whether oxytocin might play a similar role for other sensory modalities, such as the recognition of conspecific vocalizations, is currently unknown. In terms of a potential influence on neuromodulation though, oxytocin fibers do innervate VTA and LC in rodents (Hermes et al., 1988; Rosen et al., 2008) and receptors are present in human NB and DR (Gimpl and Fahrenholz, 2001).
Summary and future
It is clear from decades of research that hormones play an important role, along with infant experience, in regulating the acquisition and retention of maternal responsiveness. Less well appreciated is the influence hormones and experience may have on purely sensory aspects of maternal behavior, such as the recognition and discrimination of behaviorally significant infant cues. In particular, experiments in the auditory system demonstrate that the internal cortical representation of infant stimuli changes over the course of motherhood, irrespective of changes in the neural circuitry for raising motivation or deciding to respond to infants.
This paper draws attention to the potential means by which such changes might naturally occur. We presented a synthesis of known experience-dependent mechanisms for sensory cortical plasticity with known influences by maternal hormones on these mechanisms. On balance, the dopaminergic and cholinergic systems may be the most relevant for driving the documented sensory cortical plasticity. Each has been shown in other contexts to generate long-term physiological or behavioral changes in auditory processing, and both can be directly influenced by estrogen or other hormones. Looking forward, to better understand how these neuromodulatory centers might function in natural contexts to drive sensory representational plasticity, future experiments will need to directly test the effects of hormonal modulation on these areas.
Acknowledgments
Funding for our research is provided by the National Institute of Deafness and Communication Disorders grant DC008343 (JAM and RCL), the Center for Behavioral Neuroscience under the STC Program of the National Science Foundation (IBN-9876754), and the NIH Institutional Research and Academic Career Development Award K12 GM000680 (JAM).
Abbreviations
- A1
primary auditory field
- AAF
anterior auditory field
- AC
auditory cortex
- AII
secondary auditory field
- Dbh
dopamine beta-hydroxylase
- DR
dorsal raphe nuclei
- ER
estrogen receptor
- LC
locus coeruleus
- MU
multiunit
- NB
nucleus basalis
- TH
tyrosine hydroxylase
- UF
ultrasound field
- USV
ultrasonic vocalizations
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cited References
- Atkinson WB, Leathem JH. The day to day level of estrogen and progestin during lactation in the mouse. Anat Rec. 1946;95:147–157. doi: 10.1002/ar.1090950207. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao SW, Chan WT, Merzenich MM. Cortical remodeling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Lewis DA, Foote SL, Morrison JH. Distribution of choline acetyl transferase-immunoreactive, serotonin-immunoreactive, dopamine-beta-hydroxylase-immunoreactive, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–220. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- Chen GL, Yan J. Cholinergic modulation incorporated with a tone presentation induces frequency-specific threshold decreases in the auditory cortex of the mouse. Eur J Neurosci. 2007;25:1793–1803. doi: 10.1111/j.1460-9568.2007.05432.x. [DOI] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Cover TM, Thomas JA. Elements of information theory. John Wiley & Sons, Inc; New York: 1991. [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: Regional, subregional, and cellular localization in the a10, a9, and a8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor mrna in rat brainstem. Gene Expr Patterns. 2002;1:151–7. doi: 10.1016/s1567-133x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- David SV, Fritz JB, Shamma SA. A dynamic network for contrast enhancement in primary auditory cortex during behavior. The Society for Neuroscience; Washington, DC: 2008. Program No. 418.5. [Google Scholar]
- Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2007;32:892–901. doi: 10.1038/sj.npp.1301150. [DOI] [PubMed] [Google Scholar]
- Doornbos B, Fekkes D, Tanke MAC, de Jonge P, Korf J. Sequential serotonin and noradrenalin associated processes involved in postpartum blues. Prog Neuro-Psychoph. 2008;32:1320–1325. doi: 10.1016/j.pnpbp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Edeline JM. The thalamo-cortical auditory receptive fields: Regulation by the states of vigilance, learning and the neuromodulatory systems. Exp Brain Res. 2003;153:554–572. doi: 10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds - a gate to the understanding of sound communication. Behav Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Ehret G, Koch M. Ultrasound-induced parental behavior in house mice is controlled by female sex-hormones and parental experience. Ethology. 1989;80:81–93. [Google Scholar]
- Ehret G, Koch M, Haack B, Markl H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften. 1987;74:47–47. doi: 10.1007/BF00367047. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtel I, Ehret G. Perception and recognition discriminated in the mouse auditory cortex by c-fos labeling. Neuroreport. 1999;10:2341–2345. doi: 10.1097/00001756-199908020-00022. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100:1160–1168. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J Neurophysiol. 2007;98:2337–2346. doi: 10.1152/jn.00552.2007. [DOI] [PubMed] [Google Scholar]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–219. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008a;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone enhances performance of aged mice in cortical or hippocampal tasks. Neurosci Lett. 2008b;437:116–120. doi: 10.1016/j.neulet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: Implications for brain aging and alzheimer’s disease-related cognitive decline. Horm Behav. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glaser J, Russell VA, Devilliers AS, Searson JA, Taljaard JJF. Rat brain monoamine and serotonin s2 receptor changes during pregnancy. Neurochem Res. 1990;15:949–956. doi: 10.1007/BF00965738. [DOI] [PubMed] [Google Scholar]
- Goense JBM, Feng AS. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. J Neurobiol. 2005;65:22–36. doi: 10.1002/neu.20172. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Haack B, Markl H, Ehret G. Sound communication between parents and offspring. In: Willott JF, editor. The auditory psychobiology of the mouse. Charles C Thomas Pub Ltd; Springfield, IL: 1983. pp. 57–97. [Google Scholar]
- Hauser H, Gandelman R. Lever pressing for pups - evidence for hormonal influence upon maternal-behavior of mice. Horm Behav. 1985;19:454–468. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Helena CVV, Poletini MD, Sanvitto GL, Hayashi S, Franci CR, Anselmo-Franci JA. Changes in alpha-estradiol receptor and progesterone receptor expression in the locus coeruleus and preoptic area throughout the rat estrous cycle. J Endocrinol. 2006;188:155–165. doi: 10.1677/joe.1.06268. [DOI] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–9. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- Hermes M, Buijs RM, Massonpevet M, Pevet P. Oxytocinergic innervation of the brain of the garden dormouse (eliomys quercinus l) J Comp Neurol. 1988;273:252–262. doi: 10.1002/cne.902730209. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Process. 2005;70:132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hillery CM. Seasonality of two midbrain auditory responses in the treefrog, hyla chrysoscelis. Copeia. 1984:844–852. [Google Scholar]
- Horvath KM, Hartig W, Van der Veen R, Keijser JN, Mulder J, Ziegert M, Van der Zee EA, Harkany T, Luiten PGM. 17 beta-estradiol enhances cortical cholinergic innervation and preserves synaptic density following excitotoxic lesions to the rat nucleus basalis magnocellularis. Neuroscience. 2002;110:489–504. doi: 10.1016/s0306-4522(01)00560-7. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline JM. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J Neurosci. 2009;29:334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Increased expression of estrogen receptor alpha and beta in the nucleus basalis of meynert in alzheimer’s disease. Neurobiol Aging. 2001;22:417–426. doi: 10.1016/s0197-4580(00)00255-4. [DOI] [PubMed] [Google Scholar]
- Ji WQ, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci. 2007;27:4910–4918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Hegerl U, Molnar M, Csepe V, Karmos G. Auditory evoked potentials reflect serotonergic neuronal activity - a study in behaving cats administered drugs acting on 5-ht1a autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology. 1999;21:710–716. doi: 10.1016/S0893-133X(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Vazquez JL, Engineer ND, Pandya PK. Experience dependent plasticity alters cortical synchronization. Hear Res. 2007;229:171–179. doi: 10.1016/j.heares.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez JL, Rathbun DL, Engineer ND, Moucha R. Spectral features control temporal plasticity in auditory cortex. Audiol Neuro-Otol. 2001a;6:196–202. doi: 10.1159/000046832. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001b;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: Insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427:617–633. [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- Kudoh M, Shibuki K. Sound sequence discrimination learning motivated by reward requires dopaminergic d2 receptor activation in the rat auditory cortex. Learn Mem. 2006;13:690–698. doi: 10.1101/lm.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: Effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. Plos Biol. 2007;5:1426–1439. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiat. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: Correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A. 2002;188:981–992. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Long GR, Krishnan A. Seasonal variation in avian auditory evoked responses to tones: A comparative analysis of carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A. 2007;193:201–215. doi: 10.1007/s00359-006-0180-z. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of rat auditory cortex neurons. Eur J Neurosci. 1997;9:833–847. doi: 10.1111/j.1460-9568.1997.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on rate-level function of auditory cortex neurons: Is there a “Gating” Effect of noradrenaline? Exp Brain Res. 1998;118:361–372. doi: 10.1007/s002210050290. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of auditory cortex neurons during wakefulness and slow-wave sleep. Eur J Neurosci. 1999;11:2134–2150. doi: 10.1046/j.1460-9568.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol. 2004;92:1445–1463. doi: 10.1152/jn.00079.2004. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JJ. Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- McLin DE, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 2002;99:4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiol Learn Mem. 2006;86:47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. J Comp Physiol A. 2009;195:341–349. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Suh EJ, Fleming AS. Noradrenergic involvement in the consolidation of maternal experience in postpartum rats. Physiol Behav. 1993;53:805–811. doi: 10.1016/0031-9384(93)90192-i. [DOI] [PubMed] [Google Scholar]
- Montero-Pastor A, Vale-Martinez A, Guillazo-Blanch G, Nadal-Alemany R, Marti-Nicolovius M, Morgado-Bernal I. Nucleus basalis magnocellularis electrical stimulation facilitates two-way active avoidance retention, in rats. Brain Res. 2001;900:337–341. doi: 10.1016/s0006-8993(01)02325-3. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. An introduction to the psychology of hearing. 5. Academic Press; Boston: 2003. [Google Scholar]
- Norena AJ, Gourevich B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer-Verlag New York, Inc; New York: 2003. [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (er beta) messenger ribonucleic acid (mrna) expression within the human forebrain: Distinct distribution pattern to er alpha mrna. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Pickles JO. An introduction to the physiology of hearing. 3. Academic Press Limited; San Diego: 2008. [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Astonjones G, Jiang MR, Liu WL, Behbehani MM, Shipley MT. Preoptic projections to barringtons nucleus and the pericoerulear region - architecture and terminal organization. J Comp Neurol. 1994;347:1–24. doi: 10.1002/cne.903470102. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–817. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Siegel HI. Factors governing the onset and maintenance of maternal behavior among nonprimate mammals: The role of hormonal and nonhormonal factors. In: Gubernick DJ, Klopfer PH, editors. Parental care in mammals. Plenum Press; New York: 1981. pp. 13–76. [Google Scholar]
- Sakamoto Y, Suga S, Sakuma Y. Estrogen-sensitive neurons in the female rat ventral tegmental area - a dual route for the hormone action. J Neurophysiol. 1993;70:1469–1475. doi: 10.1152/jn.1993.70.4.1469. [DOI] [PubMed] [Google Scholar]
- Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- Sheng ZJ, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mrna in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (er alpha and er beta) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma XF. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Taya K, Greenwald GS. Mechanisms of suppression of ovarian follicular development during lactation in the rat. Biol Reprod. 1982;27:1090–1101. doi: 10.1095/biolreprod27.5.1090. [DOI] [PubMed] [Google Scholar]
- Thiel CM. Pharmacological modulation of learning-induced plasticity in human auditory cortex. Restorative Neurology and Neuroscience. 2007;25:435–443. [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Tomogane H, Ota K, Yokoyama A. Progesterone and 20alpha-hydroxypregn-4-en-3-one levels in ovarian vein blood of rat throughout lactation. J Endocrinol. 1969;44:101–106. doi: 10.1677/joe.0.0440101. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G. Novel mechanisms of estrogen action in the brain: New players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kudoh M, Ohnishi K, Shibuki K. Long-lasting memory of sounds combined with reward in rats. Neurosci Lett. 2001;311:25–28. doi: 10.1016/s0304-3940(01)02121-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Diamond DM. Physiological plasticity in auditory-cortex - rapid induction by learning. Prog Neurobiol. 1987;29:1–55. doi: 10.1016/0301-0082(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: Trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Xerri C. Imprinting of idyosyncratic experience in cortical sensory maps: Neural substrates of representational remodeling and correlative perceptual changes. Behav Brain Res. 2008;192:26–41. doi: 10.1016/j.bbr.2008.02.038. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang YF. Sound-guided shaping of the receptive field in the mouse auditory cortex by basal forebrain activation. Eur J Neurosci. 2005;21:563–576. doi: 10.1111/j.1460-9568.2005.03878.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang CH, Jin GZ, Zhen XC. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology. 2008;199:625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]