Abstract
Objective
To describe the anatomical variation occurring in intrahepatic bile ducts (IHDs) in terms of their branching patterns, and to determine the frequency of each variation.
Materials and Methods
The study group consisted of 300 consecutive donors for liver transplantation who underwent intraoperative cholangiography. Anatomical variation in IHDs was classified according to the branching pattern of the right anterior and right posterior segmental duct (RASD and RPSD, respectively), and the presence or absence of the first-order branch of the left hepatic duct (LHD), and of an accessory hepatic duct.
Results
The anatomy of the intrahepatic bile ducts was typical in 63% of cases (n=188), showed triple confluence in 10% (n=29), anomalous drainage of the RPSD into the LHD in 11% (n=34), anomalous drainage of the RPSD into the common hepatic duct (CHD) in 6% (n=19), anomalous drainage of the RPSD into the cystic duct in 2% (n=6), drainage of the right hepatic duct (RHD) into the cystic duct (n=1), the presence of an accessory duct leading to the CHD or RHD in 5% (n=16), individual drainage of the LHD into the RHD or CHD in 1% (n=4), and unclassified or complex variation in 1% (n=3).
Conclusion
The branching pattern of IHDs was atypical in 37% of cases. The two most common variations were drainage of the RPSD into the LHD (11%) and triple confluence of the RASD, RPSD and LHD (10%).
Keywords: Bile ducts, anatomy; Bile ducts, abnormalities; Bile ducts, radiography
When radiologists interpret a cholangiogram or perform percutaneous drainage of the bile duct, anomalous drainage of the segmental biliary ducts can lead to difficulties in opacification or drainage of the entire ductal system (1). Surgical procedures such as liver resection and partial liver transplantation are, moreover, increasing in frequency and complexity (2-4), and in hepatic resection for living donor liver transplantation (LDLT), an accurate knowledge of the anatomy of intrahepatic bile ducts (IHDs) is thus critical if the liver is to be successfully harvested and postoperative complications minimized (2, 3, 5). While several reports have described the anatomic variation in IHDs seen at direct or MR cholangiography, they have included patients in whom pancreatobiliary disease was suspected.
The purpose of this study is to describe anatomic variation in IHDs in terms of the branching patterns observed, and to determine the frequency of each variation. To this end, we retrospectively evaluated the intraoperative cholangiograms of 300 consecutive living donors for liver transplantation.
MATERIALS AND METHODS
The database of our institution's organ transplantation center relating to the period November 1999 to July 2002 was searched for LDLT donors. The 357 identified had undergone partial hepatic resection after selection on the basis of an adequate technical study involving (1) two-phase dynamic CT for the evaluation of liver parenchyma, liver volume, and the anatomy of hepatic vessels; (2) Doppler US for the evaluation of liver parenchyma and the anatomy and status of hepatic vascular flow; (3) plain chest radiography to determine current thoracic disease. Liver was defined as 'normal' if there was neither a history nor clinical findings of hepatic or other systemic disease (based on laboratory, US, CT or pathological findings). All donors underwent intraoperative cholangiography to determine the presence or absence of anatomic variation in IHD branching patterns; the cholangiograms obtained were adequate in 308 donors, an 'adequate' image being defined as one in which there was opacification of every second-order IHD. Eight donors were excluded because of difficulty in determining the branching patterns of IHDs due to their incomplete opacification. Our eventual study group comprised 300 donors, 229 men and 71 women aged 16-60 (mean, 30) years.
For intraoperative cholangiography, a 3- to 5- Fr catheter was used prior to lobectomy or segmentectomy of the donor's liver. After cholecystectomy and clamping of the proximal common bile duct, 25-30mL of meglumine, an ionic contrast material (Telebrix 30; Guerbet, France), was injected through the cystic duct to opacify the IHDs. Using a mobile X-ray imaging unit (Shimadzu MU-125M; Shimadzu, Tokyo, Japan), two anteroposterior plain radiographic images of the right upper abdomen were obtained. If these failed to depict all IHDs, additional anteroposterior images were acquired until the branching pattern of the IHDs was identified.
Intraoperative cholangiograms were retrospectively evaluated by two radiologists (T.K.K., J.W.C.), and a consensus was reached as to the to branching pattern of the right anterior segmental duct (RASD), right posterior segmental duct (RPSD), and the presence or absence of a first-order branch of the left hepatic duct (LHD) and an accessory hepatic duct. In each subtype, we also measured the length of the first order branch of the right hepatic duct (RHD).
RESULTS
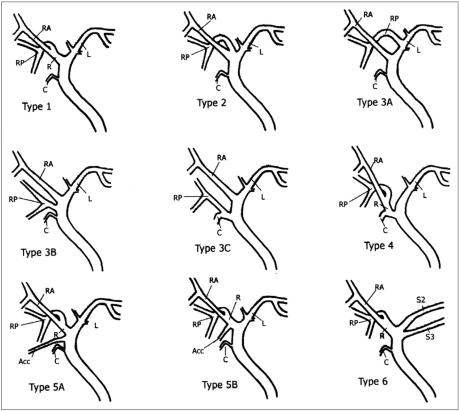
The branching patterns of IHDs were classified as one of seven types (Fig. 1). The anatomy of type 1 is typical, i.e. a common hepatic duct is formed by fusion of the RHD and LHD (Fig. 2). The RHD arises through fusion of the RASD, which drains anterior segments V and VIII, and the RPSD, which drains posterior segments VI and VII. Type 2 involves triple confluence, the simultaneous emptying of the RASD, RPSD and LHD into the common hepatic duct (CHD) (Fig. 3). Type 3, representing anomalous drainage of the RPSD, is subdivided into types 3A, 3B, and 3C, according to the drainage pattern of the RPSD. In type 3A, this drains into the LHD (Fig. 4a); in type 3B, into the CHD (Fig. 4b); and in type 3C, into the cystic duct. Type-4 IHD systems are those in which the RHD drains into the cystic duct (Fig. 5). Type 5, in which an accessory duct is present, is subdivided into types 5A and 5b according to the drainage pattern of duct: in type 5A, it drains into the CHD (Fig. 6a), and in type 5B, into the RHD (Fig. 6b). A type 6 is one in which segments II and III of the segmental duct drain individually into the RHD or CHD (Fig. 7), while a type 7 shows unclassified or complex variation (Fig. 8).
Fig. 1.
Schematic drawing of IHD anatomy. Type 1 is typical. Type 2 involves triple confluence, the simultaneous emptying of the RASD, RPSD and LHD into the CHD. In type 3, the RPSD drains anomalously, and in type 4, the RHD drains into the cystic duct. In type 5, an accessory duct is present, and in type 6, segments II and III drain individually into the RHD or CHD. Type 7 shows unclassified or complex variation.
R=right hepatic duct, L=left hepatic duct, RA=right anterior segmental duct, RP=right posterior segmental duct, C=cystic duct, Acc=accessory duct
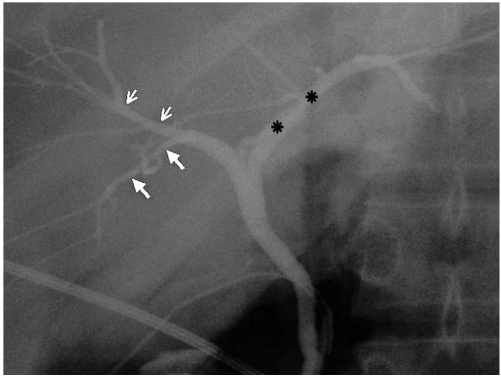
Fig. 2.
Typical IHD anatomy (type 1). Operative cholangiogram shows that the CHD is formed by fusion of the RHD and LHD (asterisks). The RHD is formed by fusion of the RASD (small arrows), which drains anterior segments V and VIII, and the RPSD (large arrows), which drains posterior segments VI and VII.
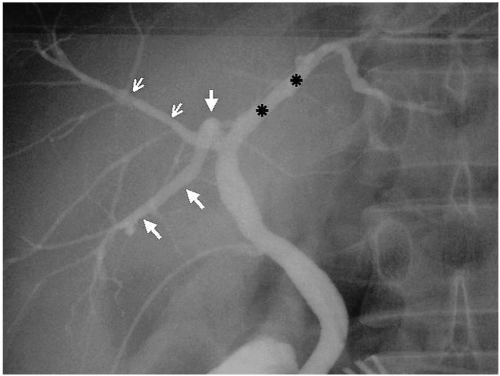
Fig. 3.
Triple confluence (type 2). Operative cholangiogram demonstrates simultaneous emptying of the RASD (small arrows), RPSD (large arrows) and LHD (asterisks) into the CHD.
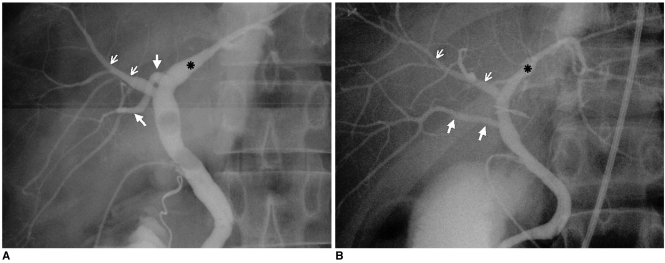
Fig. 4.
Anomalous drainage of the RPSD (type 3).
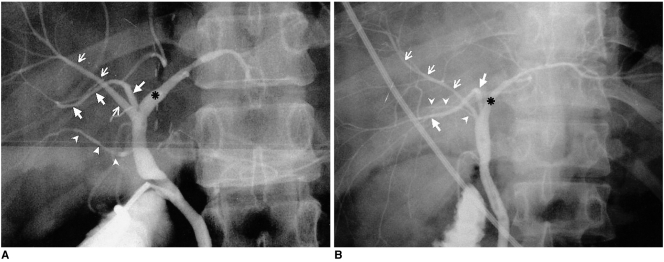
A. Drainage of the RPSD into the LHD (type 3A).
B. Drainage of the RPSD into the CHD (type 3B). Each operative cholangiogram depicts drainage of the RPSD (large arrows) into the LHD (asterisk) and CHD, respectively. Small arrows=RASD
Fig. 5.
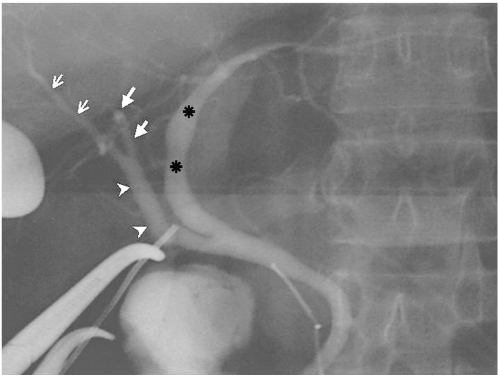
Drainage of the RHD into the cystic duct (type 4). Operative cholangiogram shows the RHD (arrowheads), formed by fusion of the RASD (small arrows) and RPSD (large arrows), into the cystic duct. Asterisks=LHD
Fig. 6.
Accessory hepatic ducts (type 5).
A. Drainage of an accessory hepatic duct into the CHD (type 5A).
B. Drainage of an accessory hepatic duct into the RHD. Operative cholangiograms indicate that accessory hepatic ducts (arrowheads) drain into the CHD and RHD, respectively. Small arrows=RASD, large arrows=RPSD
Fig. 7.
Segments II and III of the segmental duct drain individually into the RHD or CHD (type 6). Operative cholangiogram shows that segmental duct branches S2 (large arrows) and S3 (arrowheads) drain into the CHD. There is no left main duct.
Fig. 8.
Unclassified or complex variation (type 7). Cholangiogram shows type-3 trifurcation, with the accessory right posterior segmental duct (arrowheads) pouring into the caudate branch of the bile duct. Large arrows=RPSD, small arrows=RASD, asterisks=LHD
The frequencies of each type were as the follows: type 1, 63% (n=188); type 2, 10% (n=29); type 3A, 11% (n=34); type 3B, 6% (n=19); type 3C, 2% (n=6), type 4, 0% (n=1); type 5A, 3% (n=8); type 5B, 3% (n=8); type 6, 1% (n=4); type 7, 1% (n=3).
In eight type-5A cases, the accessory duct was combined with either type 1 (n=3), type 2 (n=3) or type 3A (n=2), and in eight type-5B cases, with either type 1 (n=6) or type 3A (n=2).
One of the three type-7 patterns, exhibiting complex variation, included a type 3A, with the accessory duct pouring into the RPSD; in the second, the first-order branch of the left hepatic duct was absent, and the RPSD drained directly into the S3 branch of the LHD; in the third case there was trifurcation, with an accessory RPSD which poured into the caudate branch of the bile duct.
In donors with a type-1 pattern, the length of the first-order branch was 2.4-30 (mean, 12.8) mm; in 64 of the 188, it was less than 10 mm.
DISCUSSION
Variations in the anatomy of the intrahepatic bile ducts have long been recognized. Serious consideration of the surgical anatomy of the liver began, however, with the advent of minimally invasive therapeutic intervention for bile duct or hepatic resection, or partial liver transplantation. Thus, accurate knowledge of the anatomy of IHDs is critical.
Previous studies have reported that anatomic variants of IHDs were detected at ERCP or MRCP (2-4, 6, 7, 15); however, the conclusions to be drawn from these studies might be limited by the fact that the study groups involved included selected patients, referred for radiologic studies, and it was difficult to use ERCP or MRCP for detailed analysis of the bile duct anatomy. In our study, evaluation focused on the intraoperative cholangiograms of 300 consecutive LDLT donors, who might represent a normal population.
We arbitrarily classified the branching pattern of IHDs according to the Cauinaud nomenclature, based on the relationship between the hepatic segmental duct, cystic duct, and the presence of an accessory duct. Our results showed that in the majority of the subjects (63%), the anatomy of the IHDs was type 1, or typical, a finding similar to that of earlier studies, in which the figure for this was 57-63% (2-4, 6, 7). While this type is considered the simplest, and ideal for harvesting where a right or left lobe is required for LDLT, the length of the RHD is an important factor. If this is short, the bile duct is likely to be easily injured during hepatic resection, and anastomosis between the donor's liver and recipient's bile duct or bowel is also likely to be difficult. In our study, the length of the first order branch ranged from 2.4 to 30 mm, and was less than 10 mm in 34% of donors.
Among the seven types of anatomic variant, type 3A (drainage of the RPSD into the LHD) was the most common, followed by type 2 (trifurcation), and these were present in 11% and 10% of donors, respectively. This finding is similar to that reported in previous studies: drainage of the RPSD into the LHD before its confluence with the RASD was found to occur in 13-19% of the population (7, 8). A knowledge of this anatomic variation is important, especially in performing percutaneous biliary procedures. Since it can result in drainage of the left side of the liver and posterior segment of right side (6), left-sided percutaneous transhepatic biliary drainage is preferable in patients with periportal metastatic disease, in whom there is a risk of multiple segmental ductal obstructions. Moreover, in biliary disease such as hepatolithiasis, it is theorized that the ramification pattern of IHDs may affect hepatic biliary flow, leading to biliary stasis and subsequent secondary bacterial infection and recurrent pyogenic cholangitis (9). The fact that hepatolithiasis is more prevalent in the left lobe may well support this hypothesis (10). The LHD joins the CHD at a more acute angle than the RHD, and because the most acute angle is created between the RPSD and LHD in type 3A, such a patient is, in theory, likely to experience more biliary stasis and a greater incidence of hepatic stone than those with other types.
Accessory hepatic ducts have been reported in approximately 2% of donors and may originate from either the left or right ductal system, along which they run. They may present as a solitary finding or in conjunction with other types of IHD variation (4). In our study, accessory hepatic ducts, which included type 5A, type 5B and two type-7, were observed in 18 patients (6%). Although accessory ducts are a minor aspect of variation, they should not be overlooked in liver transplantation or hepatic resection performed for other reasons. Intraoperative identification of accessory ducts and appropriate tailoring of the surgical technique are important if serious complications such as biloma or bile leakage are to be avoided. Because electrocautery may seal an accessory duct temporarily, even with careful inspection of the cut margin of the liver, an awareness of possible variation in an accessory duct is important (5).
The first-order branch of the LHD was absent in 1% of our subjects, in whom bile from segment II and III drained independently into the RHD and CHD, respectively. At hepatic surgery, separate anastomoses are also needed, and require an exact understanding of this variant, in which segment III of the bile duct is intraparenchymally located, as is segment IV. During hepatic hilar dissection, the extrahepatic portion of segment II is visualized at the hilar region, and can easily be misinterpreted as the LHD. Without knowledge of this variation, segment III of the bile duct may be ligated and sacrificed (3).
In six cases, we encountered a variant form in which the RPSD drained into the cystic duct, and one in which the RHD drained into that same duct. It has been reported in the literature that the incidence of this anatomic variation, known as "cysticohepatic ducts", is 1-2% (11, 12). Huang et al. stated that in 2% of the cases they encountered, the RPSD drained into the cystic duct (2, 3), and in a review of the literature, Hamlin reported that in his experience, an anomalous right hepatic duct emptying into the common hepatic or cystic duct was the most common biliary anomaly (13). Reid et al. (14) reported that three of 267 cholangiograms depicted an anomalous right hepatic duct which emptied into the cystic duct. It is crucial that in laparoscopic cholecystectomy, this variation is recognized: ligation or resection of an aberrant duct will lead to complications such as biloma, biliary cirrhosis, or bile leakage (4). When cholecystectomy is performed in patients with this variation, the cystic duct must be ligated between the gallbladder and the point at which the duct joins the anomalous RHD.
In this study, there is some degree of selection bias. This was because LDLT donors were chosen only from among those without complicated vascular variation and with sufficient hepatic volume for lobectomy or segmentectomy. In patients with complicated vascular variation, the possibility of accompanying bile duct variation is high, and the actual percentage of bile duct variation might thus be underestimated.
In summary, atypical branching patterns of IHDs were found in 37% of donors. The two most common variations were RPSD, draining directly into the LHD (11%), and trifurcation of the RASD, RPSD and LHD (10%). In hepatic surgery, a preoperative understanding of bile duct variation will help avoid possible complications and help achieve the most effective relief of CHD obstruction.
References
- 1.Clemett AR. Operative and postoperative cholangiography. In: Berk BN, Clemett AR, editors. Radiology of the gallbladder and bile ducts. 1st ed. Philadelphia: Saunders; 1977. pp. 272–284. [Google Scholar]
- 2.Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc. 1996;28:1669–1670. [PubMed] [Google Scholar]
- 3.Cheng YF, Huang TL, Chen CL, Chen YS, Lee TY. Variants of the intrahepatic bile ducts: application in living-related liver transplantation and splitting liver transplantation. Clin Transplant. 1997;11:337–340. [PubMed] [Google Scholar]
- 4.Mortele KJ, Ros PR. Anatomic variants of the biliary tree: MR cholangiographic findings and clinical applications. AJR Am J Roentgenol. 2001;177:389–394. doi: 10.2214/ajr.177.2.1770389. [DOI] [PubMed] [Google Scholar]
- 5.Nery JR, Fragulidis GP, Scagnelli T, et al. Donor biliary variation: an overlooked problem? Clin Transplant. 1997;11:582–587. [PubMed] [Google Scholar]
- 6.Gulliver DJ, Cotton PB, Baillie J. Anatomic variants and artifacts in ERCP interpretation. AJR Am J Roentgenol. 1991;156:975–980. doi: 10.2214/ajr.156.5.2017963. [DOI] [PubMed] [Google Scholar]
- 7.Gazelle GS, Lee MJ, Mueller PR. Cholangiographic segmental anatomy of the liver. RadioGraphics. 1994;14:1005–1013. doi: 10.1148/radiographics.14.5.7991810. [DOI] [PubMed] [Google Scholar]
- 8.Puente SG, Bannura GC. Radiological anatomy of the biliary tract: variation and congenital abnormalities. World J Surg. 1983;7:271–276. doi: 10.1007/BF01656159. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Sekijima J, Lee SF. Primary intrahepatic stones. Am J Gastroenterol. 1995;90:540–548. [PubMed] [Google Scholar]
- 10.Kim HJ, Kim MH, Lee SK, et al. Normal structure, variations and anomalies of the pancreaticobiliary ducts of Koreans: a nationwide cooperative prospective study. Gastrointest Endosc. 2002;55:889–896. doi: 10.1067/mge.2002.124635. [DOI] [PubMed] [Google Scholar]
- 11.Turner MA, Fulcher AS. The cystic duct: normal anatomy and disease processes. RadioGraphics. 2001;21:3–22. doi: 10.1148/radiographics.21.1.g01ja093. [DOI] [PubMed] [Google Scholar]
- 12.Champetier J, Letoublon C, Alnaasan I, Charvin B. The cysticohepatic ducts: surgical implications. Surg Radiol Anat. 1991;13:203–211. doi: 10.1007/BF01627988. [DOI] [PubMed] [Google Scholar]
- 13.Hamlin JA. Biliary ductal anomalies. In: Berci G, Hamlin JA, editors. Operative biliary radiology. 1st ed. Baltimore: Williams & Wilkins; 1981. pp. 110–116. [Google Scholar]
- 14.Reid SH, Cho SR, Shaw CI, Turner MA. Anomalous hepatic duct inserting into the cystic duct. AJR Am J Roentgenol. 1986;147:1181–1182. doi: 10.2214/ajr.147.6.1181. [DOI] [PubMed] [Google Scholar]
- 15.Park CH, Cho HJ, Kwack EY, Choi CS, Kang IW, Yoon JS. Intrahepatic biliary duct anatomy and its variations. J Korean Radiol Soc. 1991;27:827–831. [Google Scholar]