Abstract
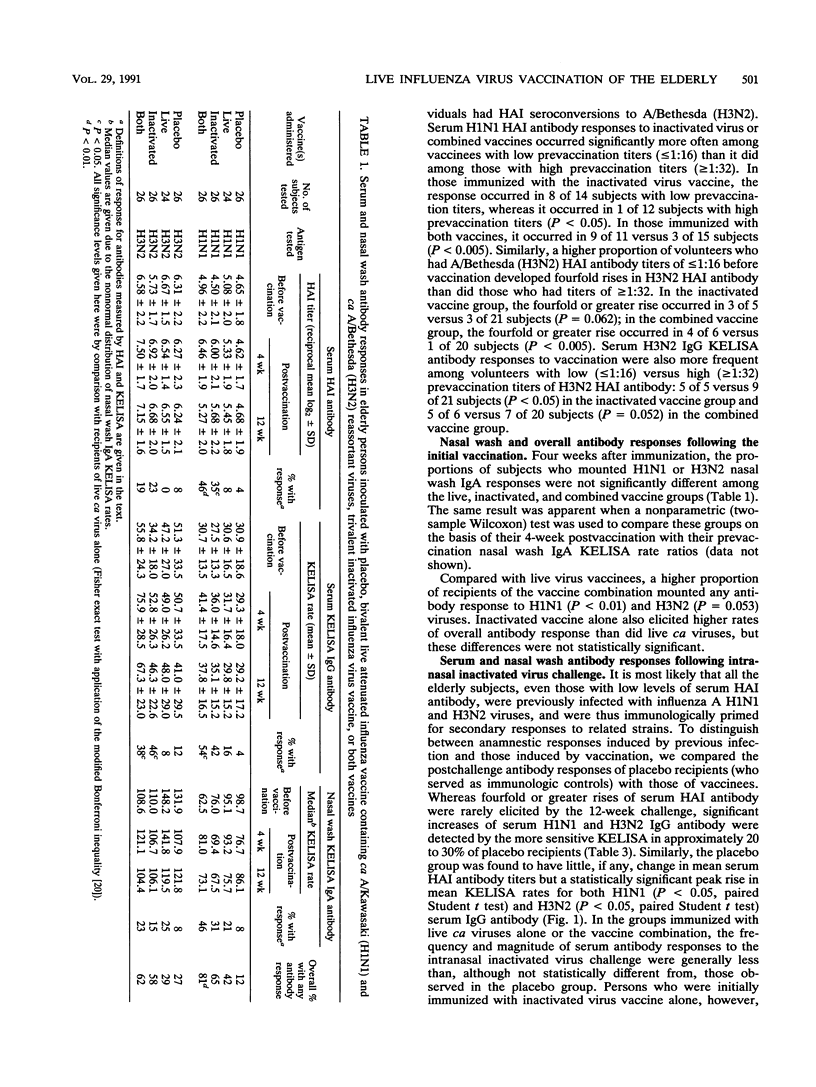
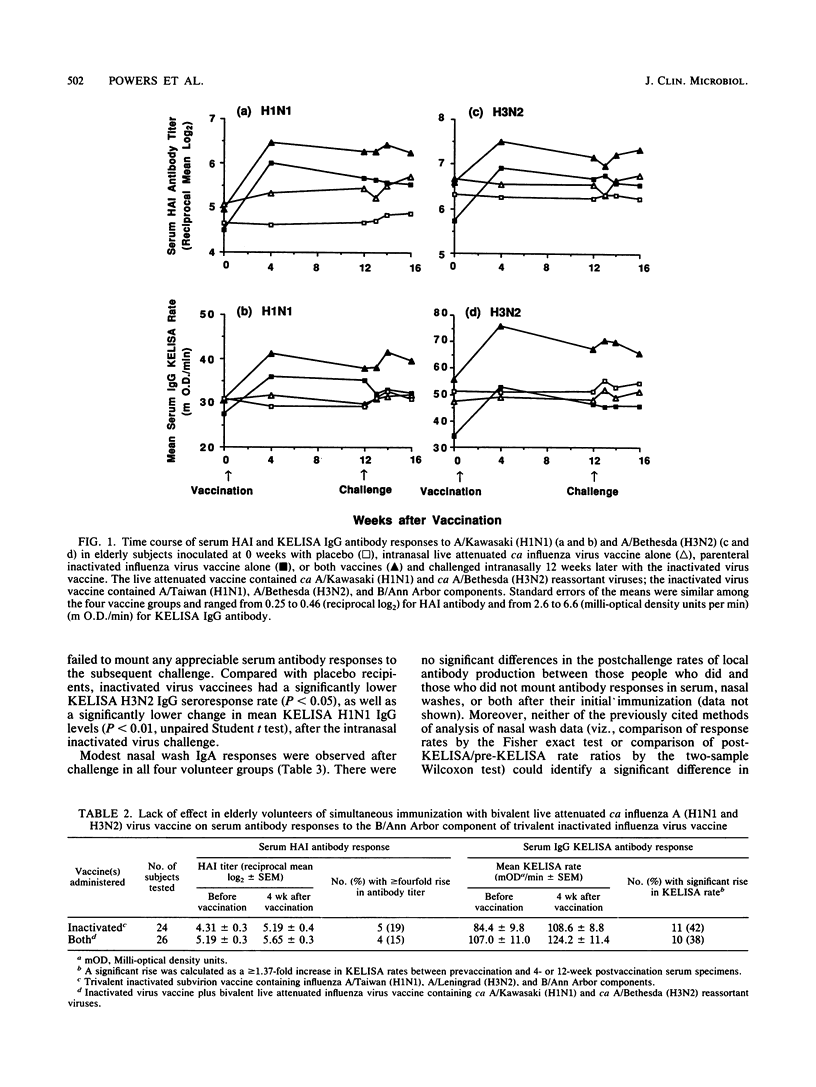
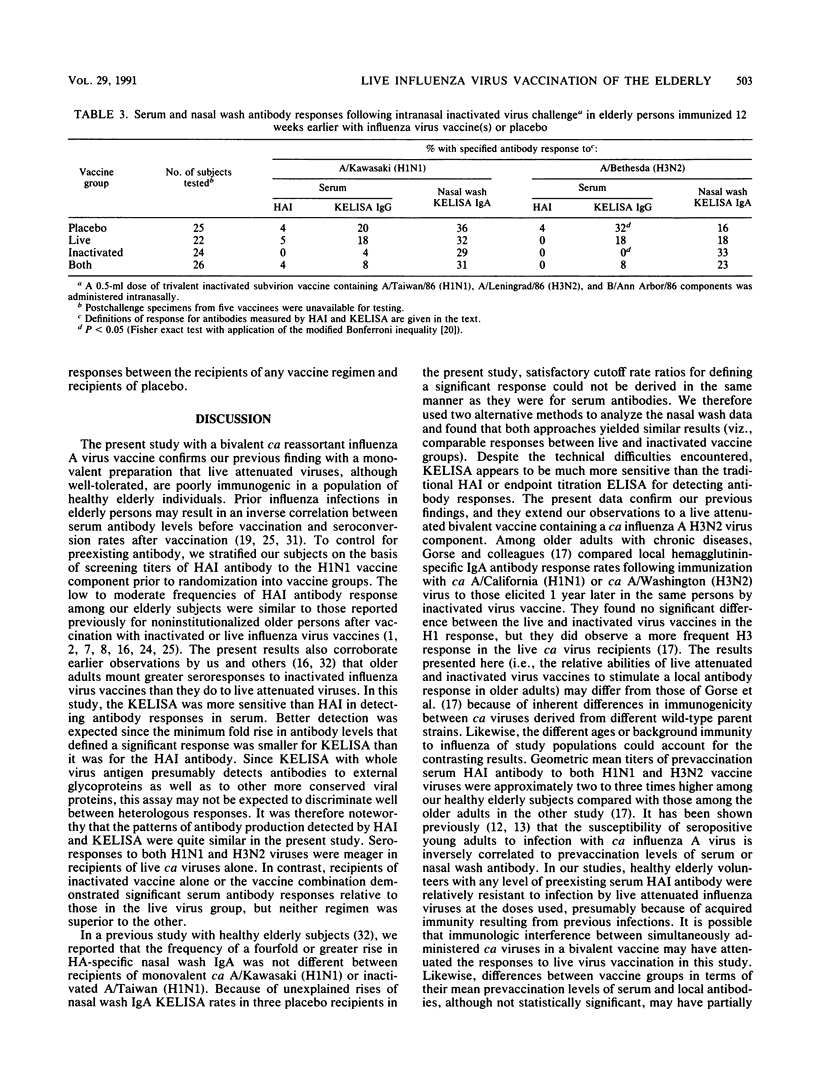
In a double-blind, randomized trial, 102 healthy elderly subjects were inoculated with one of four preparations: (i) intranasal bivalent live attenuated influenza vaccine containing cold-adapted A/Kawasaki/86 (H1N1) and cold-adapted A/Bethesda/85 (H3N2) viruses; (ii) parenteral trivalent inactivated subvirion vaccine containing A/Taiwan/86 (H1N1), A/Leningrad/86 (H3N2), and B/Ann Arbor/86 antigens; (iii) both vaccines; or (iv) placebo. To determine whether local or systemic immunization augmented mucosal immunologic memory, all volunteers were challenged intranasally 12 weeks later with the inactivated virus vaccine. We used a hemagglutination inhibition assay to measure antibodies in sera and a kinetic enzyme-linked immunosorbent assay to measure immunoglobulin G (IgG) and IgA antibodies in sera and nasal washes, respectively. In comparison with the live virus vaccine, the inactivated virus vaccine elicited higher and more frequent rises of serum antibodies, while nasal wash antibody responses were similar. The vaccine combination induced serum and local antibodies slightly more often than the inactivated vaccine alone did. Coadministration of live influenza A virus vaccine did not alter the serum antibody response to the influenza B virus component of the inactivated vaccine. The anamnestic nasal antibody response elicited by intranasal inactivated virus challenge did not differ in the live, inactivated, or combined vaccine groups from that observed in the placebo group not previously immunized. These results suggest that in elderly persons cold-adapted influenza A virus vaccines offer little advantage over inactivated virus vaccines in terms of inducing serum or secretory antibody or local immunological memory. Studies are needed to determine whether both vaccines in combination are more efficacious than inactivated vaccine alone in people in this age group.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker W. H., Mullooly J. P. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980 Dec;112(6):798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Bartram J., Crookshanks F. K. Live attenuated influenza A virus vaccines in children: results of a field trial. J Infect Dis. 1984 Dec;150(6):834–840. doi: 10.1093/infdis/150.6.834. [DOI] [PubMed] [Google Scholar]
- Cate T. R., Couch R. B., Parker D., Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines - 1978. Rev Infect Dis. 1983 Jul-Aug;5(4):737–747. doi: 10.1093/clinids/5.4.737. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Murphy B. R. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet. 1984 Mar 31;1(8379):705–708. doi: 10.1016/s0140-6736(84)92222-0. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Tierney E. L., Murphy B. R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986 Jul;24(1):157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Murphy B. R. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986 Jan;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., O'Donnell S., Levine M. M., Chanock R. M., Murphy B. R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983 Jun;40(3):1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Tierney E. L., Murphy B. R. Response of seronegative and seropositive adult volunteers to live attenuated cold-adapted reassortant influenza A virus vaccine. J Clin Microbiol. 1985 Jun;21(6):997–999. doi: 10.1128/jcm.21.6.997-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio D. J., Cox P. M., Jr, Dick E. C. Failure of inactivated influenza vaccine to protect an aged population. JAMA. 1969 Oct 20;210(3):485–489. [PubMed] [Google Scholar]
- Feery B. J., Evered M. G., Morrison E. I. Different protection rates in various groups of volunteers given subunit influenza virus vaccine in 1976. J Infect Dis. 1979 Feb;139(2):237–241. doi: 10.1093/infdis/139.2.237. [DOI] [PubMed] [Google Scholar]
- Gorse G. J., Belshe R. B., Munn N. J. Local and systemic antibody responses in high-risk adults given live-attenuated and inactivated influenza A virus vaccines. J Clin Microbiol. 1988 May;26(5):911–918. doi: 10.1128/jcm.26.5.911-918.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse G. J., Belshe R. B., Munn N. J. Safety of and serum antibody response to cold-recombinant influenza A and inactivated trivalent influenza virus vaccines in older adults with chronic diseases. J Clin Microbiol. 1986 Sep;24(3):336–342. doi: 10.1128/jcm.24.3.336-342.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. A., Quinnan G. V., Rodstein M., LaMontagne J. R., Kaslow R. A., Saah A. J., Wallenstein S., Neufeld R., Denning C., Gaerlan P. Association of influenza immunization with reduction in mortality in an elderly population. A prospective study. Arch Intern Med. 1988 Mar;148(3):562–565. [PubMed] [Google Scholar]
- Hobson D., Baker F. A., Curry R. L. Effect of influenza vaccines in stimulating antibody in volunteers with prior immunity. Lancet. 1973 Jul 21;2(7821):155–156. doi: 10.1016/s0140-6736(73)93106-1. [DOI] [PubMed] [Google Scholar]
- Howells C. H., Vesselinova-Jenkins C. K., Evans A. D., James J. Influenza vaccination and mortality from bronchopneumonia in the elderly. Lancet. 1975 Feb 15;1(7903):381–383. doi: 10.1016/s0140-6736(75)91291-x. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Feldman S., Thompson J. M., Mahoney J. D., Wright P. F. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986 Jul;154(1):121–127. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Couch R. B., Six H. R., Knight V. Antigenicity of licensed whole virion and subvirion influenza vaccines in "high risk" persons. Proc Soc Exp Biol Med. 1976 Apr;151(4):742–747. doi: 10.3181/00379727-151-39298. [DOI] [PubMed] [Google Scholar]
- MacKenzie J. S. Influenza subunit vaccine: antibody responses to one and two doses of vaccine and length of response, with particular reference to the elderly. Br Med J. 1977 Jan 22;1(6055):200–202. doi: 10.1136/bmj.1.6055.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Holley H. P., Jr, Berquist E. J., Levine M. M., Spring S. B., Maassab H. F., Kendal A. P., Chanock R. M. Cold-adapted variants of influenza A virus: evaluation in adult seronegative volunteers of A/Scotland/840/74 and A/Victoria/3/75 cold-adapted recombinants derived from the cold-adapted A/Ann Arbor/6/60 strain. Infect Immun. 1979 Feb;23(2):253–259. doi: 10.1128/iai.23.2.253-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P. A., Weber J. A., Parker R. A., Hall W. N., Kendal A. P., Bregman D. J., Schonberger L. B. Efficacy of influenza vaccine in nursing homes. Reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985 Feb 22;253(8):1136–1139. [PubMed] [Google Scholar]
- Peters N. L., Meiklejohn G., Jahnigen D. W. Antibody response of an elderly population to a supplemental dose of influenza B vaccine. J Am Geriatr Soc. 1988 Jul;36(7):593–599. doi: 10.1111/j.1532-5415.1988.tb06152.x. [DOI] [PubMed] [Google Scholar]
- Powers D. C., Sears S. D., Murphy B. R., Thumar B., Clements M. L. Systemic and local antibody responses in elderly subjects given live or inactivated influenza A virus vaccines. J Clin Microbiol. 1989 Dec;27(12):2666–2671. doi: 10.1128/jcm.27.12.2666-2671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears S. D., Clements M. L., Betts R. F., Maassab H. F., Murphy B. R., Snyder M. H. Comparison of live, attenuated H1N1 and H3N2 cold-adapted and avian-human influenza A reassortant viruses and inactivated virus vaccine in adults. J Infect Dis. 1988 Dec;158(6):1209–1219. doi: 10.1093/infdis/158.6.1209. [DOI] [PubMed] [Google Scholar]
- Snyder M. H., Banks S., Murphy B. R. Determination of antibody response to influenza virus surface glycoproteins by kinetic enzyme-linked immunosorbent assay. J Clin Microbiol. 1988 Oct;26(10):2034–2040. doi: 10.1128/jcm.26.10.2034-2040.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburg M. A., Greenland S., Sorvillo F. J., Lieb L. E., Habel L. A. Influenza in the elderly: report of an outbreak and a review of vaccine effectiveness reports. Vaccine. 1986 Mar;4(1):38–44. doi: 10.1016/s0264-410x(86)80002-0. [DOI] [PubMed] [Google Scholar]
- Treanor J. J., Roth F. K., Betts R. F. Use of live cold-adapted influenza A H1N1 and H3N2 virus vaccines in seropositive adults. J Clin Microbiol. 1990 Mar;28(3):596–599. doi: 10.1128/jcm.28.3.596-599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. F., Murphy B. R., Kervina M., Lawrence E. M., Phelan M. A., Karzon D. T. Secretory immunological response after intranasal inactivated influenza A virus vaccinations: evidence for immunoglobulin A memory. Infect Immun. 1983 Jun;40(3):1092–1095. doi: 10.1128/iai.40.3.1092-1095.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradnik J. M., Kasel J. A., Martin R. R., Six H. R., Cate T. R. Immune responses in serum and respiratory secretions following vaccination with a live cold-recombinant (CR35) and inactivated A/USSR/77 (H1N1) influenza virus vaccine. J Med Virol. 1983;11(4):277–285. doi: 10.1002/jmv.1890110403. [DOI] [PubMed] [Google Scholar]


