Abstract
Objective
To evaluate the incidence and angiographic findings of the collateral pathway involving the internal thoracic artery in patients with chronic aortoiliac occlusive disease.
Materials and Methods
Between March 2000 and Februrary 2001, 124 patients at our hospital underwent angiographic evaluation of chronic aortoiliac occlusive disease, and in 15 of these complete obstruction or severe stenosis of the aortoiliac artery was identified. The aortograms and collateral arteriograms obtained, including internal thoracic arteriograms, as well as the medical records of the patients involved, were evaluated.
Results
In nine patients there was complete occlusion of the infrarenal aorta, or diffuse stenosis of 75% or more in the descending thoracic aorta, and in the other six, a patent aorta but complete occlusion or stenosis of 75% or more of the common iliac artery was demonstrated. Collateral perfusion via hypertrophied internal thoracic arteries and rich anastomoses between the superior and inferior epigastric arteries, reconstituting the external iliac artery, were noted in all fifteen patients, regardless of symptom duration, which ranged from six months to twelve years.
Conclusion
In patients with chronic aortoiliac occlusive disease, the internal thoracic artery, along with visceral collaterals and those from the contralateral side, is one of the major parietal collateral pathways.
Keywords: Arteries, internal thoracic; Aorta, stenosis or obstruction; Angiography
In chronic aortoiliac occlusive disease (CAOD), a variety of collateral perfusions can develop, reconstituting the arterial system of the pelvis and lower extremities (1-5). In this, visceral and parietal collateral pathways as well as collaterals arising from opposing vessels are involved.
One parietal collateral pathway consists of body-wall blood vessels and involves the internal thoracic artery (ITA), the superior epigastric artery (SEA), and the inferior epigastric artery (IEA), which eventually reconstitute the external iliac artery (1, 2). Several reports describing acute postoperative crural ischemia have indicated that ITA-SEA-IEA collateral perfusion to the lower extremities may not be uncommon (6-14). Our purpose was to determine that incidence, according to the level of stenosis or obstruction of the aortoiliac artery, and to assess the related angiographic findings.
MATERIALS AND METHODS
Between September 2000 and August 2001, we prospectively evaluated the ITA-SEA-IEA collateral pathways of 124 CAOD patients who were undergoing angiographic evaluation of clinically suspected chronic occlusive disease of the aorta and/or the arteries of the lower extremities. CAOD of the aorta or iliac arteries, involving stenosis of more than 75%, was identified in 15 patients [M:F=11:4; age, 30-75 (mean, 56) years] who later underwent selective ITA angiography. Among the 15, atherosclerosis was diagnosed in eleven and Takayasu's arteritis (on the basis of the American College of Rheumatology's 1990 criteria) in the remaining four.
Aortography and selective ITA angiography were performed using digital subtraction angiography and a percutaneous transbrachial or transfemoral catheterization technique. The catheter was positioned at or slightly above the origin of the renal artery when CAOD of the abdominal aorta was suspected, and at the proximal descending aorta when CAOD of the thoracic aorta was suspected. When CAOD was confirmed at aortography, selective angiography of the subclavian artery and ITA were performed. When CAOD of the descending thoracic or abdominal aorta was identified, the patient underwent bilateral ITA angiography, and when CAOD of the iliac artery was present, ITA angiography was performed ipsilaterally. Other possible collateral perfusion was also investigated.
When stenosis affected more than 75% of the aorta or common iliac artery, the peak systolic pressure gradient was determined. According to the level of obstruction and the patency of other collaterals, aortograms, ITA angiograms and medical records were reviewed to evaluate the extent to which the arteries of the pelvis and/or lower extremities had been reconstituted by the ITA-SEA-IEA collateral pathway.
RESULTS
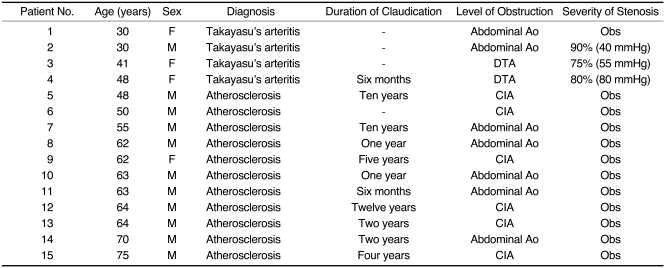
The patients' clinical histories indicated that 11 of the 15 had suffered claudication of the lower extremities for periods ranging from six months to 12 years, while the other four had presented with other symptoms of chronic arterial occlusive disease (Table 1). CAOD was diagnosed in two of the 15 during the investigation of differences in blood pressure between the upper extremities; in one, the diagnosis was established during coronary angiography; and in the other, incidentally during renal angiography prior to renal allograft. At physical examination, the absence or weakness of femoral pulses of the involved extremities was noted. Pulse volume recording amplitudes were diminished, and the Doppler ankle/brachial indices of the involved sites were below 0.7 in all cases.
Table 1.
Patient Characteristics and Angiographic Findings
Note.-Numbers in parentheses indicate peak systolic pressure gradients.
DTA=descending thoracic aorta, Ao=aorta, Obs=obstruction, CIA=common iliac artery
ITA-SEA-IEA pathways serving as collateral feeders were identified in all fifteen patients (Figs. 1, 2). In nine, CAOD had affected the descending thoracic or abdominal aorta, and in six, the common iliac artery was affected and the aorta was patent. Hypertrophy of the ITA and rich anastomosis between the SEA and IEA, by which means the external iliac artery was reconstituted, was noted, regardless of the level of aortic occlusion. Among the nine patients with CAOD of the descending thoracic or abdominal aorta, in whom bilateral ITA angiography was performed, bilateral ITA-SEA-IEA collaterals were present in six, whereas three patients showed only one ITA-SEA-IEA collateral on either side. In one of the three, Takayasu's arteritis had caused unilateral subclavian arterial obstruction. At ITA angiography, visualization of the contralateral ITA via the mediastinal collateral was possible in only three cases.
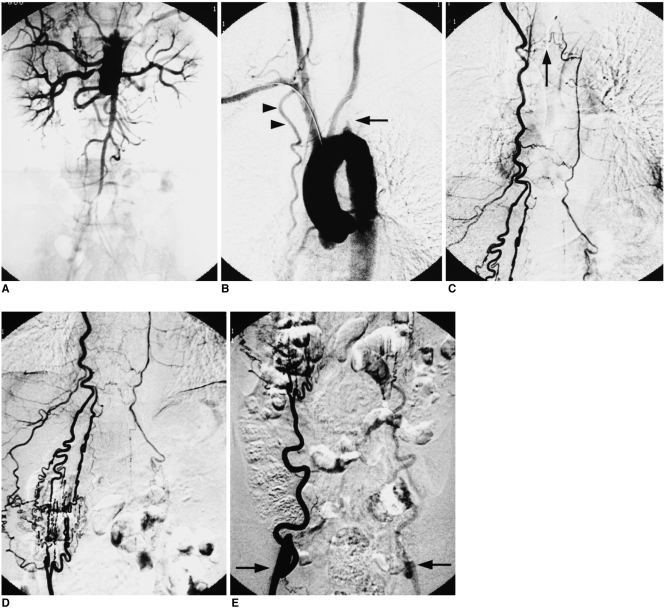
Fig. 1.
A 63-year-old man with atherosclerosis.
A. Aortogram reveals infrarenal aortic obstruction.
B. Aortogram obtained after retrieval of the catheter to the ascending aorta shows left subclavian arterial obstruction (arrow) and a hypertrophic right internal thoracic artery (arrowheads).
C. Selective right internal thoracic arteriogram depicts mediastinal collaterals from the right to the left internal thoracic artery (arrow).
D, E. Selective right internal thoracic arteriogram demonstrates rich anastomosis between the superior and inferior epigastric artery, reconstituting the external iliac artery on both sides (arrows).
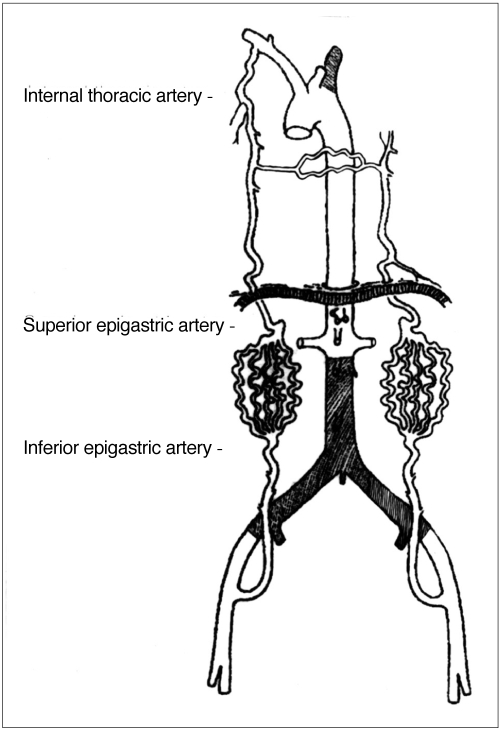
Fig. 2.
Schematic view of Fig. 1. In a patient with total occlusion of the infrarenal aorta and left subclavian artery, the internal thoracic artery provided total collateral perfusion to both lower extremities via the superior and inferior epigastric artery to the external iliac artery. The internal thoracic artery also provided perfusion to the opposing side via mediastinal collaterals.
In the aorta or common iliac artery, stenosis of more than 75% was found in three patients, excluding those in whom there was complete obstruction. In these cases, peak systolic pressure gradients were above 40 mmHg.
In all 15 patients with CAOD, various pelvic collaterals were identified. These involved the middle and inferior hemorrhoidal branch of the hypogastric artery, the iliolumbar branch of the hypogastric artery, and the deep iliac circumflex branch of the external iliac artery. Hemorrhoidal branches of the hypogastric artery predominated.
DISCUSSION
Reports dealing with CAOD have described various collateral pathways that can reconstitute the arteries of the pelvis and lower extremities (1-5). There are three principal pathways, namely the visceral collateral, parietal collateral, and collaterals arising from opposing arteries (15). The arteries that commonly contribute to the visceral collateral pathways are the superior and inferior mesenteric artery, and the middle and inferior hemorrhoidal branches of the hypogastric artery, while parietal collateral pathways commonly involve the intercostal, subcostal, lumbar, and middle sacral branches of the aorta; the ITA branches of the subclavian arteries; the iliolumbar, lateral sacral, superior gluteal, and obturator branches of the hypogastric arteries; the deep iliac circumflex and inferior epigastric branches of the external iliac arteries; and the medial and lateral femoral circumflex branches of the deep femoral arteries. The parietal collateral pathway generally supplies the side of the body on which it is located, whereas the visceral collateral pathway may furnish critical collateral arterial flow to both sides (1, 6, 7, 16). The ITA-SEA-IEA collateral is easily overlooked due to its remoteness from the aortoiliac artery, though together with pelvic collaterals, is known to be a major parietal collateral in CAOD patients (6-14).
In reports describing CAOD cases in which coronary artery bypass grafting involving the ITA was anticipated, preoperative Doppler assessment of inferior epigastric flow, or angiography of the abdominal aorta and lower extremities, was proposed (6, 8-11, 17). It has been demonstrated that when flow reversal in the inferior epigastric artery is suspected, or when perfusion to either limb is not fully established, selective injection of the ipsilateral ITA avoids the postoperative risk of an acute ischemic limb. Limb-threatening ischemia, it has been reported, can also be precipitated by the disruption of either the superior or inferior epigastric artery by transverse abdominal incisions (2).
Contributions from different collaterals depend on various factors: the level of aortic occlusion, the severity of the obstructive process, and the patency of other possible collaterals (18). A 50% reduction in the luminal diameter of a vessel corresponds to a 75-80% decrease in cross-sectional area, a degree of stenosis we consider hemodynamically significant (19).
Where arterial occlusion is distal to the common iliac artery, collaterals from either the inferior mesenteric artery to the middle and inferior hemorrhoidal branches of the hypogastric artery, or from the visceral circulation of the opposite side are possible. In patients with aortoiliac arterial occlusion, on the other hand, collaterals from the marginal artery of Drummond, or from the inferior mesenteric artery or parietal collaterals, which may be obstructed due to arteriosclerosis, seem to be insufficient to reconstitute the arteries of the pelvis and lower extremities, and the ITA forms a major collateral pathway.
To the best of our knowledge, this is the first prospective study to identify the role of the ITA-SEA-IEA pathway, which in all 15 patients with severe CAOD served as an important collateral pathway which fed the arteries of the lower extremities. The incidence of ITA-SEA-IEA collaterals was high, and comparable to that of visceral collaterals or those arising from opposing arteries. The extent to which these vessels had hypertrophied, as demonstrated at angiography, emphasizes their importance (1, 6, 8, 12).
If coronary artery bypass surgery involving the ITA is anticipated in patients with CAOD, we suggest that in order to identify which vessel is hypertrophied and thus clinically significant, bilateral ITA angiography is first performed. Grafting involving the contralateral ITA can then be carefully considered.
In one patient in whom Takayasu's arteritis obstructed the celiac trunk, small branches of the SEA provided collateral supply to the hepatic artery via the falciform artery, demonstrating that when visceral blood supply is insufficient, the parietal collaterals may also supply the internal organs.
In patients with longstanding obstruction (more than 6 months), there was no significant correlation between the duration of claudication and the severity of obstruction or development of the ITA. This finding may be related to the prior existence of alternative provision for collateral circulation, the patients' previous medicosurgical illnesses, or differences in terms of physical activity or age.
In summary, along with other visceral and parietal collateral pathways, the ITA-SEA-IEA route is a major collateral pathway to the arteries of the lower extremities in patients with CAOD. Low extremity run-off is better visualized during subclavian and/or internal thoracic arteriography than during aortography, and, furthermore, a much smaller amount of contrast material is needed. If, for example, coronary artery bypass surgery involving harvesting of the ITA is anticipated, the successful delineation of ITA collaterals requires further investigation using an imaging approach such as selective angiography and Doppler study, regardless of the duration of symptoms.
References
- 1.Chait A. The internal mammary artery: an overlooked collateral pathway to the leg. Radiology. 1976;121:621–624. doi: 10.1148/121.3.621. [DOI] [PubMed] [Google Scholar]
- 2.Krupski WC, Sumchai A, Effeney DJ, Ehrenfeld WK. The importance of abdominal wall collateral blood vessels. Arch Surg. 1984;119:854–859. doi: 10.1001/archsurg.1984.01390190092021. [DOI] [PubMed] [Google Scholar]
- 3.Vine HS, Sacks BA. Visualization of the distal arterial vessels in complete aortic occlusion. AJR Am J Roentgenol. 1980;134:847–848. doi: 10.2214/ajr.134.4.847. [DOI] [PubMed] [Google Scholar]
- 4.Grollman JH., Jr Winslow's pathway-it's not the only way. Catheter Cardiovasc Interv. 2000;49:445–446. doi: 10.1002/(sici)1522-726x(200004)49:4<445::aid-ccd21>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Edwards EA, Lemay M. Occlusion patterns and collaterals in arteriosclerosis of the lower aorta and iliac arteries. Surgery. 1955;38:950–963. [PubMed] [Google Scholar]
- 6.Tsui SSL, Parry AJ, Large SR. Leg ischemia following bilateral internal thoracic artery and inferior epigastric artery harvesting. Eur J Cardiothorac Surg. 1995;9:218–220. doi: 10.1016/s1010-7940(05)80150-2. [DOI] [PubMed] [Google Scholar]
- 7.Dietzek A, Goldsmith J, Veith F, Sanchez L, Gupta S, Wengerter K. Interruption of critical aortoiliac collateral circulation during nonvascular operations: a cause of acute limb-threatening ischemia. J Vasc Surg. 1990;12:645–653. doi: 10.1067/mva.1990.25254. [DOI] [PubMed] [Google Scholar]
- 8.Parashara DK, Kotler MN, Ledley GS, Yazdanfer S. Internal mammary artery collateral to the external iliac artery: an angiographic consideration prior to coronary bypass surgery. Catheter Cardiovasc Diagn. 1994;32:343–345. doi: 10.1002/ccd.1810320411. [DOI] [PubMed] [Google Scholar]
- 9.Arnold JR, Greenberg J, Reddy K, Clements S. Internal mammary artery perfusing Leriche's syndrome in association with significant coronary arteriosclerosis: four case reports and review of literature. Catheter Cardiovasc Interv. 2000;49:441–444. doi: 10.1002/(sici)1522-726x(200004)49:4<441::aid-ccd20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Arnold JR, Greenberg JD, Clements S. Internal mammary artery perfusing the Leriche's syndrome. Ann Thorac Surg. 2000;69:1244–1246. doi: 10.1016/s0003-4975(99)01457-5. [DOI] [PubMed] [Google Scholar]
- 11.Arnold JR. Leriche's syndrome with total perfusion of the left lower extremity by way of the left internal mammary artery. Am J Cardiol. 1998;82:997–999. doi: 10.1016/s0002-9149(98)00517-7. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura S, Inoue K, Kawachi K, et al. Lower extremity ischemia secondary to internal thoracic-coronary artery bypass grafting. Ann Thorac Surg. 1993;56:157–159. doi: 10.1016/0003-4975(93)90423-f. [DOI] [PubMed] [Google Scholar]
- 13.Melissano G, Di Credico G, Chiesa R, Grossi A. The use of internal thoracic arteries for myocardial revascularization may produce acute leg ischemia in patients with concomitant Leriche's syndrome. J Vasc Surg. 1996;24:698. doi: 10.1016/s0741-5214(96)70087-3. [DOI] [PubMed] [Google Scholar]
- 14.Adar R, Rubinstein Z, Hirshberg A. Internal mammary artery coronary bypass and leg ischemia. J Vasc Surg. 1998;7:820–821. doi: 10.1016/0741-5214(88)90054-7. [DOI] [PubMed] [Google Scholar]
- 15.Muller RF, Figley MM. The arteries of the abdomen, pelvis and thigh: I. Normal roentgenographic anatomy; II. Collateral circulation in obstructive arterial disease. Am J Roentgenol Radium Ther Nucl Med. 1957;77:296–311. [PubMed] [Google Scholar]
- 16.Prager RJ, Akin JR, Akin GC, Binder RJ. Winslow's pathway: a rare collateral channel in infrarenal aortic occlusion. AJR Am J Roentgenol. 1977;128:485–487. doi: 10.2214/ajr.128.3.485. [DOI] [PubMed] [Google Scholar]
- 17.Kwann JHM, Connolly JE. Doppler assessment of the inferior epigastric artery flow patterns as a screening test for aortoiliac obstruction. Am J Surg. 1979;137:250–251. doi: 10.1016/0002-9610(79)90156-9. [DOI] [PubMed] [Google Scholar]
- 18.Bron KM. Thrombotic occlusion of the abdominal aorta. AJR Am J Roentgenol. 1966;96:887–895. doi: 10.2214/ajr.96.4.887. [DOI] [PubMed] [Google Scholar]
- 19.Isner JM, Rosenfeld K. Redefining the treatment of peripheral artery disease: Role of percutaneous revascularization. Circulation. 1993;88:1534–1557. doi: 10.1161/01.cir.88.4.1534. [DOI] [PubMed] [Google Scholar]