Abstract
Background. The study was performed to investigate the prevalence, awareness and the risk factors of chronic kidney disease (CKD) in the community population in Shanghai, China.
Methods. A total of 2596 residents were randomly recruited from the community population in Shanghai, China. All were screened for albuminuria, haematuria, morning spot urine albumin-to-creatinine ratio and renal function. Serum creatinine, uric acid, cholesterol, triglyceride and haemoglobin were assessed. A simplified MDRD equation was used to estimate the glomerular filtration rate (eGFR). All studied subjects were screened by kidney ultrasound. Haematuria, if present in the morning spot urine dipstick test, was confirmed by microscopy. The associations among the demographic characteristics, health characteristics and indicators of kidney damage were examined.
Results. Two thousand five hundred and fifty-four residents (n = 2554), after giving informed consent and with complete data, were entered into this study. Albuminuria and haematuria were detected in 6.3% and 1.2% of all the studied subjects, respectively, whereas decreased kidney function was found in 5.8% of all studied subjects. Approximately 11.8% of subjects had at least one indicator of kidney damage. The rate of awareness of CKD was 8.2%. The logistic regression model showed that age, central obesity, hypertension, diabetes, anaemia, hyperuricaemia and nephrolithiasis each contributed to the development of CKD.
Conclusion. This is the first Shanghai community-based epidemiological study data on Chinese CKD patients. The prevalence of CKD in the community population in Shanghai is 11.8%, and the rate of awareness of CKD is 8.2%. All the factors including age, central obesity, hypertension, diabetes, anaemia, hyperuricaemia and nephrolithiasis are positively correlated with the development of CKD in our studied subjects.
Keywords: awareness, chronic kidney disease, epidemiology, prevalence, risk factors
Introduction
Chronic kidney disease (CKD) is a common health problem imposing demand on healthcare service and cost to the patients. The 2006 USRDS report indicated that the number of cases of end-stage renal disease (ESRD) worldwide had grown from 970 436 in 1999 to 1 172 655 in 2004, representing a 21% increase. In the USA, the adjusted rate for new cases of ESRD had reached 339 per million population in 2004. The adjusted prevalence rate of ESRD had surged to 1542 per million population in 2004 that was 5.4 times higher than that in 1980 and 1.4 times higher than that in 1994 [1]. The total Medicare expenditure for the ESRD in 2004, which was 18.5 billion USD, 16.3 billion USD were spent on the provision of dialysis alone and the dialysis cost per patient year was reported to be USD 66 650 [1]. In China, the prevalence rate of ESRD was 33.16 per million population in 1999. The prevalence of ESRD in Shanghai in 1992 was 180 per million population [2], but rose to 240 per million population in 2002 [3].
As healthcare resources are scarce, understanding its prevalence, disease awareness and identification of the early risk factors of CKD is mandatory and beneficial in determining the most cost-effective strategies for the prevention of ESRD. A few studies have demonstrated that between 58.7% and 89.7% of CKD patients can be detected earlier from the general population screening [4,5]. It has also been widely accepted that early and regular nephrology care will result in a decreased morbidity and mortality, as well as improving the patients’ quality of life [6]. Given the high prevalence and the low awareness of CKD, it becomes inevitably necessary for many countries, including China, to formulate appropriate strategies for the prevention of the ESRD to control the escalating healthcare cost. Since there can be a difference in the causative factors leading to the development of the CKD in our community, we undertook a community-based study on CKD subjects in Shanghai from March to July 2006 to evaluate its prevalence and awareness among the general public and its potential risk factors. We hope our study will allow us to identify the appropriate interventional strategies towards managing both the health costs and quality of life for CKD patients more effectively and efficiently.
Material and methods
Study population
Shanghai is one of the most affluent cities in China with a total population of about 19 million. It consists of eight districts of equally dense populations. Sampling came from a 3-stage study. First, the Changning district was randomly selected. Secondly, a suburban area within the Changning district was assigned to this study. Thirdly, random blocks within this suburban area with a reasonable number of residents (n = 2596 residents) were randomly recruited. The subjects studied must all have resided in the Changning district for 6 months or more and be aged 18 years or older. A total of 2554 individuals who had completed data collection were entered into the study. Among them, 911 were males and 1643 were females, aged between 18 and 104 years (median age 58.4 ± 15).
Screening protocol
The screening was conducted by the trained nephrologists and professional volunteers. After giving their informed consent, individuals completed a standardized questionnaire including information with regard to their socio-demographic status (e.g. age, gender, education, occupation), personal and family health history (e.g. hypertension, diabetes mellitus, chronic kidney disease, cardiovascular disease, cerebrovascular disease and tumours) and lifestyle habits (e.g. smoking, alcohol consumption). Their height, weight, body mass index (BMI), waist circumference and hip circumference were recorded. Blood pressures were measured as stipulated in the guidelines of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure [7]. Fasting blood glucose and 2-h postpandrial glucose tests were performed. The serum creatinine, uric acid, cholesterol, triglyceride, haemoglobin as well as urinary albumin-to-creatinine ratio after an overnight fast were also evaluated. Morning spot urine specimens were collected to obtain the microalbumin-to-creatinine ratio. Also, positive haematuria and pyuria morning spot urine dipstick results were confirmed by microscopy. Kidney function was measured using the simplified Modification of Diet in Renal Disease (MDRD) equation to obtain estimated glomerular filtration rate (eGFR). Nephrolithiasis was screened by ultrasound echogram of kidneys in all studied subjects.
Evaluation criteria
CKD was assessed based on the following: (1) the presence of the albuminuria, haematuria and pyuria, (2) eGRF and (3) morning spot urinary microalbumin-to-creatinine ratio by dipstick (Bayer Diagnostics Manufacturing, Ltd).
Haematuria and pyuria were confirmed by urine microscopy for RBC >3/HP and WBC >5/HP after excluding for urinary tract infection and menstruation. Those who had haematuria or pyuria were to be tested again in three months.
Elevated serum creatinine values were defined as eGFR (mL/min/ 1.73 m2) that was calculated by the simplified MDRD equation: eGFR = [186 × (serum creatinine − 1.154) × (age − 0.203)] [8]. However, for female study subjects, the calculated values were multiplied by 0.742. If individuals’ eGFR was under <60 mL/min/1.73 m2, they were classified as having decreased kidney function. Also, the Kidney Disease Outcomes Quality Initiative (K/DOQI) classification system was employed to evaluate reduced kidney function based on eGFR for stage 1 disease (eGFR ≥90 mL/min) to stage 5 (eGFR <15 mL/min) [9].
The morning spot urinary microalbumin-to-creatinine ratio was obtained by a half-quantization test as described above. Those who had a positive morning spot urinary microalbuminuria dipstick test were to be tested again three months later by a quantization test of the microalbumin-to-creatinine ratio using the Unical DxC 800 auto-analyser (Beckman Coulter Company). Only those positive at the baseline screening and with individual urinary albumin-to-creatinine ratio >3.5 mg/mmol (for males) and 2.5 mg/mmol (for females) 3 months later would be considered positive.
Participants who currently required antihypertensive therapy to control their blood pressure or those with a systolic blood pressure (SBP) of 140 mmHg or greater and/or diastolic blood pressure (DBP) of 90 mmHg or greater at screening had to be reconfirmed after a third measurement (repeated every 15 min after rest) before they were accepted as hypertensive [7]. Subjects with a diabetic history or those with fasting blood glucose >7 mmol/L or 2-h postprandial blood glucose >11 mmol/L were categorized as diabetic [10]. An equation [(weight in kilograms)/(square of the height in meters)] was used to calculate the BMI. BMI of 30 or greater was defined as obesity, and those between 25 and 30 were considered overweight [11]. Subjects had abdominal obesity or central obesity if the waist-to-hip ratio was over 0.9 for males and 0.85 for females [11], or if the waist circumference was 102 cm or greater for males and 88 cm or greater for females [12]. Aaemia was defined by a haemoglobin <130 g/L for men and women older than 50 years and <120 g/L for women 50 years or younger [13]. Those with serum cholesterol >5.72 mmol/L and serum triglyceride >1.7 mmol/L at screening were noted to have hypercholesteraemia and hypertriglyceridaemia, respectively [14]. Those with serum uric acid of 420 μmol/L (males) and of 360 μmol/L (females) at screening were considered hyperuricaemic [15].
Statistical analysis
All the statistical data entering the EpiData 3.0 had an error checking before the SPSS 11.50 analysis. Descriptive analyses were used to characterize the participant population by sociodemographic data (e.g. age, gender and education), health status (obesity, central obesity, hypertension, diabetes, albuminuria, haematuria, pyuria, eGFR, urinary albumin-to-creatinine, hypercholesteraemia, hypertriglyceridaemia, hyperuricaemia and nephrolithiasis) and lifestyle factors (smoking, alcohol consumption). Measurement data in mean and standard error were also presented. Numeration data were analysed by the chi-square test. Multivariate analyses were performed to assess the association of the screening demographic and health characteristics with the presence of the urinary albumin-to-creatinine ratio or decreased kidney function or CKD. A P-value < 0.05 was considered to be statistically significant.
Results
Demographic characteristics of the participants
Of 2596 individuals attending the screening programme, only 2554 had completed the data entry (of which 911 were males and 1643 were females). They ranged between 18 and 104 years, the mean age being 58.4 ± 15.3 years. A total of 27.8% of participants (n = 710) were 50 years old and younger. Most of the participants were well-educated university graduates and students: 9.2% (n = 235); 18.7% of participants (n = 478) were college graduates and students: 18.7% (n = 478); secondary high school graduates and students: 53.1% (n = 1357). Eighty-six percent of the participants had some form of health insurance. Among all the 2554 participants, 53.1% had hypertension, 12.0% diabetes, 12.6% anaemia, 11.1% hypercholesterolaemia, 16.6% hypertriglyceridaemia, 9.8% hyperuricaemia, 4.8% gout and 2.2% had nephrolithiasis. In our study, 7.05% (180/2554) of participants had an abnormal waist-to-hip ratio, 30.2% (772/2554) of participants were overweight while 4.6% (118/2554) were obese, and abnormal waist circumference was present in 14.4% of 2554 participants.
Among all hypertensive subjects, 80.2% noted that they had hypertension but only 53% of all the hypertensives received antihypertensive therapy. However, 9.7% of all the hypertensive subjects had albuminuria but only 8.8% of all hypertensive subjects also had decreased kidney function. Diabetes awareness was 76.1% among all studied subjects but only 64.7% of them had treatment. Of those diabetics, only 14.7% had albuminuria, 11.1% had decreased kidney function. Nephrolithiasis was detected in 56 subjects but over 65% of them were males. A total of 14.3% of nephrolithiasis participants had albuminuria but only 14.3% had decreased kidney function.
Kidney damage
Kidney damage was evaluated depending upon whether there was the presence or the absence of albuminuria, haematuria, decreased kidney function and urinary albumin-to-creatinine ratio. Routine urinalysis at baseline showed that 8.9% of the participants (n = 228) tested postive, including 2.0% (n = 52) with proteinuria, 1.2% (n = 30) with haematuria and 5.7% (n = 146) with pyuria. Three months later, 228 participants with abnormal urinalysis at baseline screening should have been retested again, but only 86.8% attended (n = 198). At the follow-ups, the incidence of an abnormal urinary finding dropped to 3.1%, including 1.2%, 1.2% and 0.7% for those with initial proteinuria, haematuria and pyuria, respectively. There was no difference between males and females in haematuria (P > 0.05), but there was a significant difference between males and females in pyuria (P < 0.01) and there was clearly a much higher prevalence in females with pyuria.
In the half-quantization of the urinary albumin-to-creatinine ratio test (ACR), 18.7% of the participants (n = 478) were positive, and 93% (n = 444) of them were tested again by the quantization of ACR. Of all participants, 6.3% (n = 161) had albuminuria. Of these albuminuria participants, 80% (n = 129) had a hypertensive history. Of all albuminuria participants, 28% (n = 45) had diabetes.
Among all the participants, 5.8% (n = 149) had decreased kidney function of which 140 participants had an eGFR between 30 and 59.9 mL/min/1.73 m2, eight with an eGFR between 15 and 29.9 mL/min/1.73 m2 and one with an eGFR <15 mL/min/1.73 m2. However, if the decreased kidney function were defined by the elevated serum creatinine >115 μmol/l, the prevalence of the decreased kidney function would reduce to 2.4%. In the renal morphological examination, among all the studied subjects, 9.0% (n = 231) had renal cysts. Of these, 2.2% (n = 56) had nephrolithiasis and 1.8% (n = 46) had other abnormalities (e.g. hamartoma, double pelvic and polycystic kidney).
The prevalence of CKD
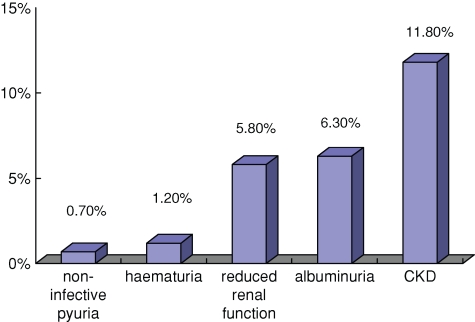
In accordance with the guidelines of the Kidney Disease Outcome Quality Initiative set out by the National Kidney Foundation in 1999 [9], CKD takes place in the presence of albuminuria, haematuria and the decreased kidney function. A total of 11.8% of our studied subjects (n = 302) had CKD, of which 2.4% were at stage 1, 3.6% at stage 2, 5.5% at stage 3, 0.3% at stage 4 and 0.04% at stage 5 (Figure 1 and Table 1). In our study, the most common community subjects studied fall at the CKD stage 3 category.
Fig. 1.
Biomeasures of renal disease in the current study.
Table 1.
Distribution of decreased kidney function and CKD among the surveyed community population
| Decreased kidney function | CKD | |||||
|---|---|---|---|---|---|---|
| Total number of | Prevalence (%) | Number of | Number of | Number of | Prevalence (%) | |
| participants | participants with albuminuria at | participants with continuous | participants | |||
| MDRD-eGFR | baseline screening | albuminuria at the | ||||
| (mL/min/1.73 m2) | repeated test | |||||
| ≥90 | 1189 | 46.6 | 188 | 49 | 61 | 2.4 |
| 60–89.9 | 1216 | 47.6 | 237 | 79 | 92 | 3.6 |
| 30–59.9 | 140 | 5.5 | 53 | 27 | 140 | 5.5 |
| 15–29.9 | 8 | 0.3 | 8 | 5 | 8 | 0.3 |
| <15 | 1 | 0.04 | 1 | 1 | 1 | 0.04 |
| Total | 2554 | 100 | 487 | 161 | 302 | 11.8 |
A total of 302 participants with CKD were identified, of which, 98 were males and 204 were females. The CKD prevalence for our male subjects was 10.8% and 12.4% for females, but there was no significant difference between them (P > 0.05). Our CKD participants were aged from 10 to 104 years, an average of 68.9 ± 13.2 years. The CKD prevalence was 0.9% for the group of 18–29 years old, 4.6% for 30–39 years old, 5.1% for 40–49 years old, 7.6% for 50–59 years old, 9.9% for 60–69 years old, 22.9% for 70–79 years old and 37.1% for 80 years old and above (Table 2). A significant statistical difference was noted among every age group (P < 0.01), and obviously as the age advances, the CKD prevalence also rises.
Table 2.
Distribution of the community CKD participants in various age groups
| 18–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–104 | |
|---|---|---|---|---|---|---|---|
| Stage 1 | 1.5% | 7.5% | 16.4% | 29.9% | 14.9% | 22.4% | 7.5% |
| Stage 2 | 0.0% | 1.0% | 7.8% | 22.3% | 14.6% | 42.7% | 11.7% |
| Stage 3 | 0.0% | 0.7% | 2.9% | 5.7% | 11.4% | 52.1% | 27.1% |
| Stage 4 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 50.0% | 50.0% |
| Stage 5 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 100.0% | 0.0% |
| Total | 0.9% | 4.6% | 5.1% | 7.6% | 9.9% | 22.9% | 37.1% |
Multivariate analyses
The logistic multiple regression analysis in our community study demonstrated that the following were the risk factors of CKD: (1) age (OR = 1.062, P < 0.001); (2) central obesity (OR = 1.631, P = 0.045); (3) anaemia (OR = 2.745, P < 0.001); (4) hypertension (OR = 1.463, P = 0.037); (5) diabetes (OR = 1.970, P < 0.001); (6) hyperuricaemia (OR = 3.084, P < 0.001) and (7) nephrolithiasis (OR = 2.922, P = 0.018) (Table 3).
Table 3.
The logistic multiple regression analysis of the risk factors of community CKD
| Risk factors | Coefficient of regression | Standard error | Chi-square value | OR | P-value |
|---|---|---|---|---|---|
| Age | 0.060 | 0.005 | 137.533 | 1.062 | <0.001 |
| Male | 0.258 | 0.134 | 3.725 | 1.295 | 0.054 |
| Overweight | −0.071 | 0.206 | 0.120 | 0.931 | 0.729 |
| Obesity | −0.338 | 0.369 | 0.841 | 0.713 | 0.359 |
| Central obesity | 0.489 | 0.243 | 4.036 | 1.631 | 0.045 |
| Waist-to-hip ratio | 0.222 | 0.191 | 1.351 | 1.249 | 0.245 |
| Hypertension | 0.381 | 0.183 | 4.335 | 1.463 | 0.037 |
| Diabetes | 0.678 | 0.212 | 10.273 | 1.970 | 0.001 |
| Anaemia | 1.010 | 0.199 | 25.686 | 2.745 | <0.001 |
| Hypertriglyceridaemia | 0.099 | 0.204 | 0.238 | 1.105 | 0.626 |
| Hyperuricaemia | 1.126 | 0.229 | 24.217 | 3.084 | <0.001 |
| Nephrolithiasis | 1.072 | 0.455 | 5.558 | 2.922 | 0.018 |
| Gout | 0.472 | 0.331 | 2.037 | 1.604 | 0.154 |
| Cerebral vascular accident | 0.254 | 0.271 | 0.877 | 1.289 | 0.349 |
| Cardiovascular disease | −0.003 | 0.191 | 0.000 | 0.997 | 0.989 |
Likewise, for the development of albuminuria, we found that all the following were risk factors, namely hypertension (OR = 1.972, P = 0.014), diabetes (OR = 2.139, P = 0.004), anaemia (OR = 2.093, P = 0.007) and cerebral vascular accident (CVA) (OR = 1.918, P = 0.043).
Also it was shown that age (OR = 1.090, P < 0.001), hypertension (OR = 1.012, P = 0.039), anaemia (OR = 2.398, P < 0.001), gout (OR = 1.892, P = 0.033), nephrolithiasis (OR = 2.615, P = 0.049), hyperuricaemia (OR = 4.976, P < 0.001) and albuminuria (OR = 2.301, P = 0.002) were the risk factors for the development of decreased kidney function.
The awareness of CKD
Among all the community CKD participants, 38.4% (n = 116) had never had a urinary test before and 94.7% (n = 286) never had a serum creatinine test before. Among all, only 25 participants had a history of kidney damage of which 11 had decreased kidney function, 5 had chronic nephritis, 1 had polycystic renal disease, 1 had a transplant kidney, 6 had proteinuria and 1 had haematuria. Hence, the awareness of CKD among the CKD participants was 8.2% and there was no difference between males and females (P > 0.05). Most were well educated but no significant difference was detected among the different levels of education (P > 0.05). The awareness of stage 1–5 CKD was 4.5%, 8.7%, 7.1%, 37.5%, 100%, respectively, but statistically significant among all five different stages (P < 0.01). Apparently, deteriorated renal function with symptomatic manifestation did raise the awareness of CKD in our studied subjects. Participants with a family history of CKD also had an increased awareness (22.2%) compared to those without a family history of CKD (7.3%). The difference between the two groups aforesaid was statistically significant (P < 0.01). The awareness of CKD of those with comorbidity (e.g. hypertension, diabetes, cardiovascular disease, cerebral vascular accident) was 2.0% as compared with those without comorbidity (0.3%). Again, there was a significant difference between the two groups mentioned above (P < 0.01), but there was no difference among the type of comorbid disease (P > 0.05).
Discussion
This is the first study in Shanghai on surveillance of community CKD patients. However, before this study, little was known about the prevalence, disease awareness and its risk factors leading to the development of CKD in Shanghai. Our primary objective is to examine the CKD prevalence in Shanghai, which has about 20 million inhabitants, the disease awareness and its potential risk factors that will lead to CKD in the public. Our secondary objective is to detect the earlier cases of the CKD and those populations at high risk of developing CKD. Our third objective is, of course, to determine the most appropriate management strategies to improve the prognosis of our CKD patient population in Shanghai and ultimately improve their quality of life.
The epidemiological screening of the population in Beijing, examining only those aged 40 and above, revealed a 9.4% prevalent rate of CKD [16]. In this study, our studied subjects ranged from the age of 18 to 104 years and 10.3% of our studied subjects were <40 years old. After studying 2554 adult participants randomly, it is clear that the prevalence of community CKD in Shanghai is 11.8%, which is very similar to the recent survey in America [17]. The prevalence of CKD in Australia, by testing albumin and serum creatinine, is 16% among those aged 25 years and older [18].
In our study, we used the staging of CKD recommended by KDOQI to categorize all the participants with kidney damage. However, it was proposed recently that stages 1 and 2 of CKD should be eliminated because they did not reflect the severity and complications of CKD accurately in elderly people [19]. As the definition of CKD in elderly patients is controversial, further study is necessary to evaluate the staging of CKD in those populations.
In China, nephritis followed by diabetic nephropathy has been well documented as the second most leading cause of the CKD [3]. Hence, the indicators that we used in this study to screen for the kidney damage evidence were albuminuria, haematuria and decreased kidney function.
Examining albuminuria in the routine analysis, in our view, is not only a relatively simple, non-invasive and cost effective measure to give the qualitative and quantitative assessment of the early stage of CKD, and to reveal those at high risk of developing CKD, but also is informative in the prediction of hypertension, diabetes and cardiovascular disease [20]. We re-examined all our participants three months later if their baseline urinalysis was positive at screening. A total of 94.6% of those with albuminuria and 86.8% of those with haematuria or pyuria at the baseline screening undertook their repeated urinalysis. Results show that the prevalence of proteinuria is 1.2% in our study. In Singapore, the prevalence of proteinuria is 1.1% [5]. In our study, the prevalence of albuminuria is 6.3% by the urinary albumin-to-creatinine ratio. In America [17] and the Dutch PREVEND study, the prevalence of albuminuria is 9.3% and 7.2% by the urinary albumin-to-creatinine ratio, respectively. In Japan, it is 4.7% and 3.5% for males and females, respectively [22]. Obviously, the urinary albumin-to-creatinine ratio could improve the detection rate and reduce the rate of misdiagnosis.
The K/DIGO guidelines also recommended examining the urinary albumin-to-creatinine ratio as the best index for the evidence of the early vascular endothelial injury [23]. In our study, the prevalence of the urinary albumin-to-creatinine ratio is 6.3% compared to 7.2% reported in the PREVEND study [21].
Our result shows that the prevalence of haematuria is 1.2% as compared with the 4.7% being reported in the AusDiab survey [18]. The prevalence of pyuria in our study is 0.7%. We did exclude urinary infection or urinary stones in subjects with pyuria to avoid false positive interpretation.
Serum creatinine has been widely used to measure individual renal function but its assessment can be affected by age, gender, racial difference and GFR. Because our kidneys have a powerful compensatory function, serum creatinine will only begin to cripple up when our renal function has >50% deterioration. Hence, many stage 3 CKD patients do not necessarily have an abnormally high serum creatinine level. If the decreased kidney function is defined by the presence of the serum creatinine reaching 115 μmol/L, then we could only identify a 2.4% prevalence of the decreased kidney function in our study. We feel that measurement of the serum creatinine alone as the only criteria for the CKD diagnosis may be misleading. Hence, in this study, we detected the prevalent rate of decreased kidney function at 5.8% after using the simplified MDRD equation whereas 6.8% and 11.2% have been reported in the USA and Australia, respectively.
In our survey, it was shown that several clinical variables were associated with CKD. Those variables include hyperuricaemia, nephrolithiasis, anaemia, diabetes, central obesity, hypertension and age. Ageing has been described as a non-regulatory factor for the development of CKD [24]. It has also been universally recognized to be in positive association with an arterial atherosclerotic change.
In recent years [25–27], excessive abdominal fat (central obesity) has made more hazards to health than the fatty bottom and limbs. Now it is generally accepted that the waist circumference is the most simple and practical indicator to measure abdominal fat. The level of abdominal fat is an independent risk factor predictor of many diseases. It is more sensitive than the waist-to-hip ratio in estimating the abdominal fat. Our survey showed that elevated waist circumference is a risk factor for the development of CKD. Also, its prevalence is high at 14.4%. It is not related to general obesity, overweight and elevated waist-to-hip ratio. This is different from that observed in other countries [28]. It may be that the cause is of ethnic origin. It also showed that central obesity is related not only to diabetes and cardiovascular disease but also to CKD.
The K/DOQI guidelines include the imaging examination in the definition of CKD. First we perform renal ultrasound examination in the epidemiological screening. In our study, the prevalence of renal cysts is 9.0%. At the same time, the prevalence of nephrolithiasis is higher in CKD patients (5.6%) than in non-CKD patients (1.7%). This is similar to the results being reported in America [29]. Also in other studies, nephrolithiasis has been documented as a risk factor for the development of CKD [30,31]. In 3.0% patients, ESRD was caused by nephrolithiasis. In China, the cause of nephrolithiasis is mainly due to dietary intake [32]. The formation of calculus will be reduced by adjusting the diet structure. Then reducing prevalence of nephrolithiasis might be helpful to reduce prevalence of CKD. We first concentrate on the nephrolithiasis in the epidemiological screening. It will be further observed in the subsequent study. As the renal ultrasound examination is simple, non-invasive and of low cost, we think that it could be applied to the epidemiological screen of CKD to scree for nephrolithiasis.
In our study, hyperuricaemia is associated with CKD. In the studies published elsewhere, hyperuricaemia was reported to be a risk factor for the development of CKD and elevated levels of uric acid independently increase the risk for new-onset kidney disease [33–35]. As CKD could cause elevated levels of uric acid while hyperuricaemia could also contribute to the progression of CKD, the predictive value of hyperuricaemia in CKD needs further study. Earlier management of the anaemia has been documented to retard the development of CKD [36]. Our study shows that anaemia is another variable associated with CKD.
Some studies suggest that it is more cost-effective to screen among the high-risk population than among the general population [33]. However, we find that the risk factor of CKD among the Chinese population is different from that in the USA and Europe. Maybe it is related to the spectrum of disease, race, diet and living habits. Therefore, we could not simply assume the risk factors of CKD among the US people to be the same as among the Chinese population. It is necessary to screen among the general population in China.
The awareness of CKD in the US is low [37]. In our study, many patients with CKD have fewer clinical symptoms, which perhaps helps to explain why the disease awareness of CKD is low in spite of high prevalence rates, but this could be tested by regular laboratory examination. Even if we already have a simple test to detect the early stage of CKD, it has not been widely used clinically. Even if the participants had a health examination, half of them did not have a regular urine test. The awareness of CKD is increased with the decline in renal function, the development of CKD, complicated by hypertension, diabetes and cardiovascular disease.
In conclusion, the prevalence of CKD is 11.8% among the community population in Shanghai. It is 2.4%, 3.6%, 5.5%, 0.3% and 0.04% in stages 1–5, respectively. As it is more prevalent in the early stage of CKD in America, we should concentrate on the early stages of CKD in epidemiological survey and young people. Also the population shows decreased kidney function by the MDRD equation with a normal urine test. The screening was effective in identifying the CKD population and high-risk CKD population.
Acknowledgments
This work was supported by grants from the Leading Academic Discipline Project of Shanghai Health Bureau (05III001 and 2003ZD002) and Shanghai Leading Academic Discipline Project (T0201). We thank Dr Dong Xiuzhen and Dr Jiang Mingxia from Shanghai Municipal Centre for Disease Control and Prevention for their assistance and Professor Li Guohong from Department of Statistics and Department of Clinical Epidemiology, Shanghai Jiaotong University, School of Medicine for their excellent statistical assistance. We thank all participants and volunteers from Jiangsu Community for their excellent help to our survey.
Conflict of interest statement. None declared.
References
- 1.US Renal Data System (USRDS) 2006. ADR (http://www.usrds.org/adr.htm. )
- 2.Dialysis and Transplantation Registration Group The report about the registration of dialysis and transplantation in China. Chin J Nephrol. 1999;17:77–78. [Google Scholar]
- 3.Shanghai Dialysis and Transplantation Registration Group The report about the registration of dialysis and transplantation in China. Chin J Nephrol. 1999;17:77–78. [Google Scholar]
- 4.Garg AX, Kiberd BA, Clark WF, et al. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez SP, McClellan W, Port FK, et al. Risk factors for proteinuria in a large, multiracial, southeast Asian population. J Am Soc Nephrol. 2002;13:1907–1917. doi: 10.1097/01.asn.0000018406.20282.c8. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Vecchio LD, Pozzoni P. The importance of early detection of chronic kidney disease. Nephrol Dial Transplant. 2002;17(Suppl 11):2–7. doi: 10.1093/ndt/17.suppl_11.2. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 10.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 11.Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894 i–xii1, 1–253. [PubMed] [Google Scholar]
- 12.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 13.WHO/UNICEF/UNU . Iron Deficiency Anemia: Assessment, Prevention, and Control: A Guide for Program Managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L-X, Zuo L, Xu G-B, et al. Community-based screening for chronic kidney disease among population older than 40 years in Beijing. Chin J Nephrol. 2006;22:67–71. doi: 10.1093/ndt/gfl763. [DOI] [PubMed] [Google Scholar]
- 17.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 18.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 19.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol. 2008;19:844–846. doi: 10.1681/ASN.2008010110. [DOI] [PubMed] [Google Scholar]
- 20.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 21.Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 22.Iseki K, Iseki C, Ikemiya Y, et al. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49:800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Levin A. Identification of patients and risk factors in chronic kidney disease—evaluating risk factors and therapeutic strategies. Nephrol Dial Transplant. 2001;16(Suppl 7):57–60. doi: 10.1093/ndt/16.suppl_7.57. [DOI] [PubMed] [Google Scholar]
- 25.Pi-Sunyer FX. Obesity. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Diseases, 9th edn. New York: Williams & Wilkins; 1999. pp. 1395–1418. [Google Scholar]
- 26.Physical status: the use and interpretation of anthropometry Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 27.Group Working Group on Obesity in China Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin J Epidemiol. 2002;23(1):5–10. [PubMed] [Google Scholar]
- 28.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 29.Stankus N, Hammes M, Gillen D, et al. African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res. 2007;35:83–87. doi: 10.1007/s00240-007-0079-3. [DOI] [PubMed] [Google Scholar]
- 30.Jungers P, Joly D, Barbey F, et al. ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis. 2004;44:799–805. [PubMed] [Google Scholar]
- 31.Vupputuri S, Soucie JM, McClellan W, et al. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol. 2004;14:222–228. doi: 10.1016/S1047-2797(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 32.Chang S, Li L, Huang X. A Population-based case-control study on the risk factors of urinary calculi. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23(4):273–276. [PubMed] [Google Scholar]
- 33.Boulware LE, Jaar BG, Tarver-Carr ME, et al. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 34.Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J Am Soc Nephrol. 2005;16:791–799. doi: 10.1681/ASN.2004030208. [DOI] [PubMed] [Google Scholar]
- 35.Weiner DE, Tighiouart H, Elsayed EF, et al. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouva C, Nikolopoulos P, Ioannidis JP, et al. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–760. doi: 10.1111/j.1523-1755.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 37.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among US adults,1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]